Question
Topic:
Given: $0.10 \mathrm{~mol}$ of hydrochloric acid is mixed with $0.10 \mathrm{~mol}$ of calcium carbonate.
$$
2 \mathrm{HCl}(\mathrm{aq})+\mathrm{CaCO}_3(\mathrm{~s}) \rightarrow \mathrm{CaCl}_2(\mathrm{aq})+\mathrm{H}_2 \mathrm{O}(\mathrm{l})+\mathrm{CO}_2(\mathrm{~g})
$$
Discuss: Which is correct?
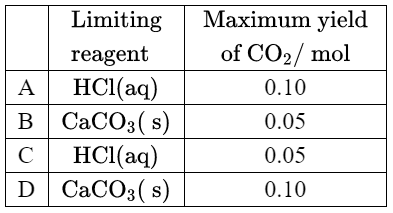
▶️Answer/Explanation
Ans:C
Solution:
To determine the limiting reagent, we can compare the number of moles of each reactant to the stoichiometric coefficients in the balanced chemical equation.
From the equation, we see that 2 moles of $\mathrm{HCl}$ react with 1 mole of $\mathrm{CaCO_3} to produce 1 mole of $\mathrm{CO_2}$. Therefore, the number of moles of $\mathrm{CO_2}$ that can be produced is limited by the reactant that is present in the lesser amount relative to its stoichiometric coefficient.
For $\mathrm{HCl}$: $\frac{0.10~\mathrm{mol}}{2} = 0.05~\mathrm{mol}$
For $\mathrm{CaCO_3}$: $\frac{0.10~\mathrm{mol}}{1} = 0.10~\mathrm{mol}$
Since $\mathrm{HCl}$ has a smaller value, it is the limiting reagent. Thus, the correct answer is option C.
To calculate the maximum yield of $\mathrm{CO_2}$, we use the amount of limiting reagent as the basis for our calculation. From the equation, we see that 1 mole of $\mathrm{CO_2}$ is produced for every 2 moles of $\mathrm{HCl}$ reacted. Therefore, the maximum yield of $\mathrm{CO_2}$ is:
$\mathrm{Maximum~yield~of~CO_2} = \frac{0.05~\mathrm{mol~HCl}}{2} = 0.025~\mathrm{mol}$
Therefore, the correct answer is also option C, with a maximum yield of 0.025 mol of $\mathrm{CO_2}$.
Question
Topic:
Discuss: What is the sum of the coefficients when the equation is balanced with whole numbers?
$$
\ldots{\mathrm{MnO}_2(\mathrm{~s})+\ldots} \mathrm{HCl}(\mathrm{aq}) \rightarrow\ldots\mathrm{MnCl}_2(\mathrm{aq})+\ldots \mathrm{H}_2 \mathrm{O}(\mathrm{l})+\ldots \mathrm{Cl}_2(\mathrm{~g})
$$
A. $6$
B. $7$
C. $8$
D. $9$
▶️Answer/Explanation
Ans:D
Solution:
First, let’s identify the unbalanced elements in the equation: $\mathrm{Mn, Cl, ~and~ H.}$
Next, we can begin balancing the equation by adding coefficients to the reactants and products to equalize the number of each type of atom on both sides of the equation.
$$
\mathrm{MnO}_2(\mathrm{s}) + 4\mathrm{HCl}(\mathrm{aq}) \rightarrow \mathrm{MnCl}_2(\mathrm{aq}) + 2\mathrm{H}_2\mathrm{O}(\mathrm{l}) + \mathrm{Cl}_2(\mathrm{g})
$$
Now the equation is balanced, and we can see that the sum of the coefficients is $1+4+1+2+1 = 9.$
Therefore, the correct answer is D, $9.$
Question
Topic:
Discuss: Which is correct?
A. Mixtures are either homogeneous or heterogeneous and their chemical properties are an average of the individual component properties.
B. Mixtures are never heterogeneous and their chemical properties are an average of the individual component properties.
C. Mixtures are either homogeneous or heterogeneous and the components retain their individual chemical properties.
D. Mixtures are never homogeneous and the components retain their individual chemical properties.
▶️Answer/Explanation
Ans:C
Solution:
The correct statement is:
C. Mixtures are either homogeneous or heterogeneous and the components retain their individual chemical properties.
Explanation:
A mixture is a combination of two or more substances that are not chemically bonded together. Mixtures can be either homogeneous or heterogeneous.
In a homogeneous mixture, the components are evenly distributed throughout the mixture, and the mixture has a uniform composition. Examples of homogeneous mixtures include saltwater and air.
In a heterogeneous mixture, the components are not evenly distributed, and the mixture does not have a uniform composition. Examples of heterogeneous mixtures include a salad or a mixture of oil and water.
The chemical properties of a mixture are determined by the chemical properties of the individual components, and these properties are retained in the mixture. For example, if you mix water and salt, the chemical properties of the water and the salt are retained in the mixture. The mixture will still have the chemical properties of water and salt.
Question
Topic:
Discuss: Which contains the greatest number of moles of oxygen atoms?
A. $ 0.05 \mathrm{~mol}~ \mathrm{Mg}\left(\mathrm{NO}_3\right)_2$
B. $0.05 \mathrm{~mol} ~\mathrm{C}_6 \mathrm{H}_4\left(\mathrm{NO}_2\right)_2$
C. $0.1 \mathrm{~mol} ~\mathrm{H}_2 \mathrm{O}$
D. $0.1 \mathrm{~mol}~ \mathrm{NO}_2$
▶️Answer/Explanation
Ans:A
Solution:
To determine the number of moles of oxygen atoms in each compound, we need to count the number of oxygen atoms in each formula and multiply by the number of moles given.
A. $0.05 \mathrm{~mol} \mathrm{Mg}\left(\mathrm{NO}_3\right)_2$ contains $2 \times 3 \times 0.05 = 0.3$ moles of oxygen atoms.
B. $0.05 \mathrm{~mol} \mathrm{C}_6 \mathrm{H}_4\left(\mathrm{NO}_2\right)_2$ contains $2 \times 2 \times 0.05 = 0.2$ moles of oxygen atoms.
C. $0.1 \mathrm{~mol} \mathrm{H}_2 \mathrm{O}$ contains $1 \times 0.1 = 0.1$ moles of oxygen atoms.
D. $0.1 \mathrm{~mol} \mathrm{NO}_2$ contains $2 \times 0.1 = 0.2$ moles of oxygen atoms.
Therefore, compound A contains the greatest number of moles of oxygen atoms, and the correct answer is A.
Question
Topic:
Discuss: What is represented by $\mathrm{A}$ in ${ }_\mathrm{Z}^\mathrm{A} \mathrm{X}^{2-}$ ?
A. Number of electrons
B. Number of neutrons
C. Number of nucleons
D. Number of protons
▶️Answer/Explanation
Ans:C
Solution:
In the symbol ${ }_\mathrm{Z}^\mathrm{A} \mathrm{X}^{2-}$:
${ }_\mathrm{Z}$ represents the atomic number, which is the number of protons in the nucleus of the atom.
${ }^\mathrm{A}$ represents the mass number, which is the sum of the number of protons and neutrons in the nucleus of the atom.
$\mathrm{X}^{2-}$ represents the ion charge, which indicates the number of electrons that the ion has gained or lost.
Therefore, the correct answer is:
C. ${}^\mathrm{A}$ represents the mass number, which is the sum of the number of protons and neutrons in the nucleus of the atom. Therefore, the number of nucleons (protons and neutrons) in the nucleus
The symbol ${ }_\mathrm{Z}^\mathrm{A} \mathrm{X}^{2-}$ represents an ion of the element X with a charge of 2- and a mass number of A, which means it has Z protons in its nucleus.
