Question-1(a) :2019-Nov-Chemistry_paper_2__TZ0_SL
Topic:
Given: The equations show steps in the formation and decomposition of ozone in the stratosphere, some of which absorb ultraviolet light.
Step $1 \quad \mathrm{O}_2 \rightarrow 2 \mathrm{O}\cdot$
Step $2 \quad \mathrm{O} \cdot+\mathrm{O}_2 \rightarrow \mathrm{O}_3$
Step $3 \quad \mathrm{O}_3 \rightarrow \mathrm{O} \cdot+\mathrm{O}_2$
Step $4 \quad \mathrm{O} \cdot+\mathrm{O}_3 \rightarrow 2 \mathrm{O}_2$
Draw: the Lewis structures of oxygen, $\mathrm{O}_2$, and ozone, $\mathrm{O}_3$
▶️Answer/Explanation
Solution:
Here are the Lewis structures of oxygen molecule ($\mathrm{O}_2$), and ozone ($\mathrm{O}_3$):
Oxygen molecule ($\mathrm{O}_2$):
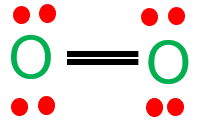
Ozone ($\mathrm{O}_3$):
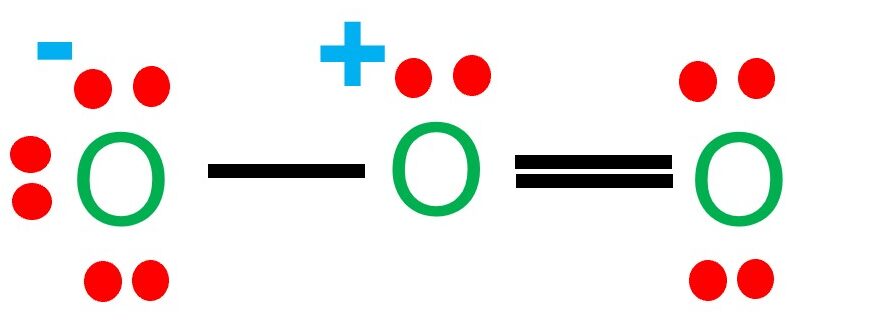
Note that in the Lewis structure of ozone, there is a resonance structure where the double bond can be located between any of the three oxygen atoms.
Question-1(b) :2019-Nov-Chemistry_paper_2__TZ0_SL
Topic:
Discuss: why both bonds in the ozone molecule are the same length and predict the bond length in the ozone molecule. Refer to section 10 of the data booklet.
Reason:
Length:
▶️Answer/Explanation
Solution:
Both bonds in the ozone molecule are the same length because the molecule has a resonance structure. This means that the double bond character is shared equally among the three oxygen atoms, and the molecule has a bond length that is intermediate between a single bond and a double bond.
According to section 10 of the data booklet, the bond length of the O-O bond in the ozone molecule is approximately 121 pm to 148 pm. (picometers).
Question-1(c) :2019-Nov-Chemistry_paper_2__TZ0_HL
Topic:
Discuss: about the bond angle in the ozone molecule.
▶️Answer/Explanation
Solution:
The bond angle in the ozone molecule can be predicted using the VSEPR (Valence Shell Electron Pair Repulsion) theory. In ozone, there are three bonding pairs and one lone pair of electrons around the central oxygen atom. These electron pairs will repel each other and adopt a geometry that minimizes their repulsions.
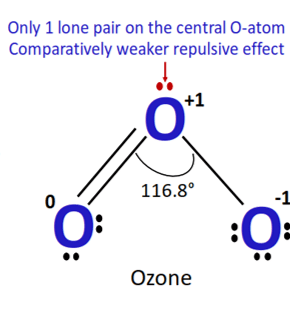
The geometry of the ozone molecule is trigonal planar, with the three oxygen atoms forming a flat triangle. The bond angle between any two oxygen atoms is therefore approximately 117 degrees.
Question-1(d) :2019-Nov-Chemistry_paper_2__TZ0_SL
Topic:
Discuss: how the different bond strengths between the oxygen atoms in $\mathrm{O}_2$ and $\mathrm{O}_3$ in the ozone layer affect radiation reaching the Earth’s surface.
▶️Answer/Explanation
Solution:
The different bond strengths between the oxygen atoms in $\mathrm{O}_2$ and $\mathrm{O}_3$ play a crucial role in the ability of the ozone layer to absorb radiation and protect the Earth’s surface.
The bond in $\mathrm{O}_2$ is a double bond with a bond energy of 495 kJ/mol, which makes it relatively strong and not easily broken by ultraviolet radiation. On the other hand, the bond in $\mathrm{O}_3$ is a weaker double bond with a bond energy of 366 kJ/mol. This makes $\mathrm{O}_3$ more susceptible to breaking upon exposure to ultraviolet radiation.
When ultraviolet radiation with wavelengths shorter than 320 nm is absorbed by the $\mathrm{O}_3$ molecules in the ozone layer, the $\mathrm{O}_3$ molecules can break apart into $\mathrm{O}_2$ and $\mathrm{O}$ atoms, as shown in the equations given in part (a). These $\mathrm{O}$ atoms can then react with other $\mathrm{O}_3$ molecules to reform $\mathrm{O}_2$ and create more $\mathrm{O}_2$ molecules that can absorb additional ultraviolet radiation.
In this way, the weaker bond in $\mathrm{O}_3$ allows the ozone layer to absorb a significant amount of the harmful ultraviolet radiation that would otherwise reach the Earth’s surface and cause damage to living organisms and the environment.
Question-2[(a) (i)] :2019-Nov-Chemistry_paper_2__TZ0_SL
Topic:
Given: The biochemical oxygen demand of a water sample can be determined by the following series of reactions. The final step is titration of the sample with sodium thiosulfate solution, $\mathrm{Na}_2 \mathrm{~S}_2 \mathrm{O}_3(\mathrm{aq})$
$$
\begin{gathered}
2 \mathrm{Mn}^{2+}(\mathrm{aq})+\mathrm{O}_2(\mathrm{aq})+4 \mathrm{OH}^{-}(\mathrm{aq}) \rightarrow 2 \mathrm{MnO}_2(\mathrm{~s})+2 \mathrm{H}_2 \mathrm{O}(\mathrm{l}) \\
\mathrm{MnO}_2(\mathrm{~s})+2 \mathrm{I}^{-}(\mathrm{aq})+4 \mathrm{H}^{+}(\mathrm{aq}) \rightarrow \mathrm{Mn}^{2+}(\mathrm{aq})+\mathrm{I}_2(\mathrm{aq})+2 \mathrm{H}_2 \mathrm{O}(\mathrm{l}) \\
2 \mathrm{~S}_2 \mathrm{O}_3{ }^{2-}(\mathrm{aq})+\mathrm{I}_2(\mathrm{aq}) \rightarrow 2 \mathrm{I}^{-}(\mathrm{aq})+\mathrm{S}_4 \mathrm{O}_6{ }^{2-}(\mathrm{aq})
\end{gathered}
$$
A student analysed two $300.0 \mathrm{~cm}^3$ samples of water taken from the school pond: one immediately (day 0), and the other after leaving it sealed in a dark cupboard for five days (day 5). The following results were obtained for the titration of the samples with $0.0100 \mathrm{~mol} \mathrm{dm}^{-3} \mathrm{Na}_2 \mathrm{~S}_2 \mathrm{O}_3(\mathrm{aq})$.

Calculate: the mole ratio of $\mathrm{S}_2 \mathrm{O}_3{ }^{2-}$ to $\mathrm{O}_2$, using the balanced equations.
▶️Answer/Explanation
Solution:
The balanced equations are given as:
$$
\begin{aligned}
&2 \mathrm{Mn}^{2+}(\mathrm{aq})+\mathrm{O}_2(\mathrm{aq})+4 \mathrm{OH}^{-}(\mathrm{aq}) \rightarrow 2 \mathrm{MnO}_2(\mathrm{~s})+2 \mathrm{H}_2 \mathrm{O}(\mathrm{l}) \\
&\mathrm{MnO}_2(\mathrm{~s})+2 \mathrm{I}^{-}(\mathrm{aq})+4 \mathrm{H}^{+}(\mathrm{aq}) \rightarrow \mathrm{Mn}^{2+}(\mathrm{aq})+\mathrm{I}_2(\mathrm{aq})+2 \mathrm{H}_2 \mathrm{O}(\mathrm{l}) \\
&2 \mathrm{~S}_2 \mathrm{O}_3{ }^{2-}(\mathrm{aq})+\mathrm{I}_2(\mathrm{aq}) \rightarrow 2 \mathrm{I}^{-}(\mathrm{aq})+\mathrm{S}_4 \mathrm{O}_6{ }^{2-}(\mathrm{aq})
\end{aligned}
$$
$\begin{aligned} & n\left(\mathrm{MnO}_2\right): n\left(\mathrm{O}_2\right)=2: 1 \\ & n\left(\mathrm{I}_2\right): n\left(\mathrm{MnO}_2\right)=1: 1 \\ & n\left(\mathrm{~S}_2 \mathrm{O}_3^{2-}\right): n\left(\mathrm{I}_2\right)=2: 1\end{aligned}$
From this , $n\left(\mathrm{~S}_2 \mathrm{O}_3^{2-}\right): n\left(\mathrm{O}_2\right)=4: 1$
