Question-1[(a) (i)] :2020-nov-Chemistry_paper_2__TZ0_HL
Topic:
Given: Chlorine undergoes many reactions.
Discuss: the full electron configuration of the chlorine atom.
Answer/Explanation
Solution:
Electronic configuration of $\mathrm{Cl} 1 s^2 2 s^2 2 p^6 3 s^2 3 p^5$
Question-1[(a) (ii)] :2020-nov-Chemistry_paper_2__TZ0_HL
Topic:
Discuss: giving a reason, whether the chlorine atom or the chloride ion has a larger radius.
Answer/Explanation
Solution:
Chloride ion has larger radius as chloride ion has one extra electron and nuclear charge is same so nuclear charge per electron is less so force of attraction per electron will be less and size will be more and also electron-electron repulsion will result in larger size.
Question-1[(a) (iii)] :2020-nov-Chemistry_paper_2__TZ0_HL
Topic:
Discuss: why the chlorine atom has a smaller atomic radius than the sulfur atom
Answer/Explanation
Solution:
Chlorine atom has smaller atomic radius than the sulfur atom.
When we move across the period size decreases and sulfur and chlorine are in the same period and when we move from sulfur to chlorine electron is added in the same shell so screening effect remains same.
Effective nuclear charge increases so size decreases.
Question-1[(a) (iv)] :2020-nov-Chemistry_paper_2__TZ0_HL
Topic:
Given: The mass spectrum of chlorine is shown.
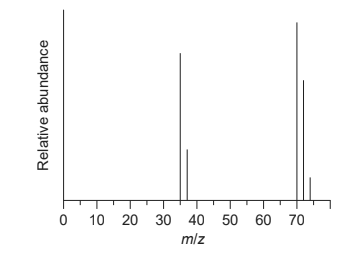
Discuss: the reason for the two peaks at $m / z=35$ and 37 .
Answer/Explanation
Solution:
Reason for the two peaks at $\mathrm{m} / \mathrm{z} 35$ and 37 is that chlorine has two isotopes of atomic mass of 35 and 37 .
Question-1[(a) (v)] :2020-nov-Chemistry_paper_2__TZ0_HL
Topic:
Discuss: the presence and relative abundance of the peak at $m / z=74$
Answer/Explanation
Solution:
Presence of peak at $\mathrm{m} / \mathrm{z} =74$ is due to molecule formed by two $\mathrm{Cl}-37$ and Cl-37 molecule and peak is very low as chlorine isotope of 37 mass is very less abundant and chances of formation of chlorine molecule of 37 mass number is further less.
Question-1[(b) (i)] :2020-nov-Chemistry_paper_2__TZ0_HL
Topic:
Given: $2.67 \mathrm{~g}$ of manganese(IV) oxide was added to $200.0 \mathrm{~cm}^3$ of $2.00 \mathrm{~mol} \mathrm{dm}^{-3} \mathrm{HCl}$.
$$
\mathrm{MnO}_2(\mathrm{~s})+4 \mathrm{HCl}(\mathrm{aq}) \rightarrow \mathrm{Cl}_2(\mathrm{~g})+2 \mathrm{H}_2 \mathrm{O}(\mathrm{l})+\mathrm{MnCl}_2(\mathrm{aq})
$$
Calculate: the amount, in mol, of manganese(IV) oxide added.
Answer/Explanation
Solution:
$\text{Moles of} ~\mathrm{MnO}_2=\frac{2.67}{ \mathrm{mol~ mass}}=\frac{2.67 }{ 86.94}=0.0307~\text{moles}$
