Question-1(a) :2021-may-Chemistry_paper_2__TZ1_HL
Topic:
Given: Iron may be extracted from iron (II) sulfide, $\mathrm{FeS}$.
Discuss: why metals, like iron, can conduct electricity.
Answer/Explanation
Solution:
Metals like iron can conduct electricity because they have a unique atomic structure in which the valence electrons, the outermost electrons in the atom, are delocalized and can move freely throughout the metal lattice. This allows the electrons to carry an electric current from one end of the metal to the other.
In more detail, the atoms in a metal are arranged in a regular, repeating lattice structure. The valence electrons of the metal atoms occupy a band of energy levels, called the conduction band, which overlaps with the next highest energy level, called the valence band. This means that the valence electrons can easily move from one atom to another, and as a result, a flow of electrons, or an electric current, can be established.
Furthermore, the delocalized electrons also interact with positively charged metal ions in the lattice, creating a “sea” of electrons that is free to move throughout the metal. This is known as metallic bonding and contributes to the high electrical conductivity of metals like iron.
Question-1(b) :2021-may-Chemistry_paper_2__TZ1_HL
Topic:
Discuss: Justify why sulfur is classified as a non-metal by giving two of its chemical properties.
Answer/Explanation
Solution:
Sulfur is classified as a non-metal because it exhibits several characteristic properties of non-metals, including:
Electronegativity: Non-metals tend to have a higher electronegativity, meaning they have a greater attraction for electrons in chemical bonds. Sulfur has an electronegativity of 2.58, which is higher than that of many metals, including iron (1.83). This makes sulfur more likely to form covalent bonds with other non-metals rather than forming ionic bonds with metals.
Non-metallic character: Non-metals tend to be brittle, have low melting and boiling points, and are poor conductors of heat and electricity. Sulfur is a brittle solid at room temperature and has a melting point of 115 degrees Celsius and a boiling point of 444 degrees Celsius. It is also a poor conductor of heat and electricity, further supporting its classification as a non-metal.
In addition, sulfur is located on the right side of the periodic table, along with other non-metals such as oxygen, nitrogen, and chlorine. It also tends to form oxides and acids, which are typical of non-metals rather than metals.
Question-1(c) :2021-may-Chemistry_paper_2__TZ1_HL
Topic:
Sketch: the first eight successive ionisation energies of sulfur.
Answer/Explanation
Solution:
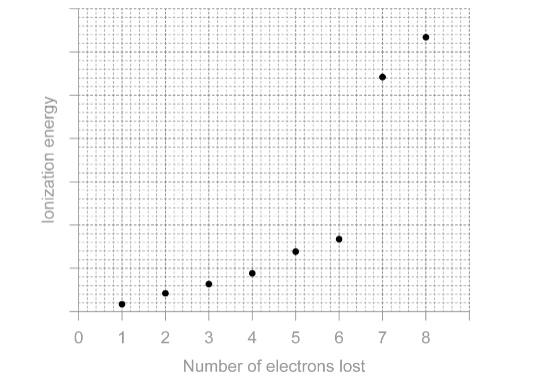
As can be seen from the values, there are two regions of small increases in ionisation energies, corresponding to the removal of electrons from the 3p orbital. However, there is a large increase in ionisation energy between the 6th and 7th ionisation energies, which corresponds to the removal of an electron from the 3s orbital. This large increase in ionisation energy indicates that the 3s electron is held more tightly by the sulfur atom than the 3p electrons.
Question-1[(d) (i)] :2021-may-Chemistry_paper_2__TZ1_HL
Topic:
Given: Iron (II) sulfide, FeS, is ionically bonded.
Describe: the bonding in this type of solid.
Answer/Explanation
Solution:
In ionic bonding, the atoms transfer electrons to each other to form ions with opposite charges that are held together by the electrostatic attraction between them. In the case of iron (II) sulfide, FeS, iron (Fe) loses two electrons to become a positively charged ion ($\mathrm{Fe}^{2+}$) and sulfur (S) gains two electrons to become a negatively charged ion ($\mathrm{S}^{2-}$). The resulting ionic compound is held together by the attraction between the oppositely charged ions.
Question-1[(d) (ii)] :2021-may-Chemistry_paper_2__TZ1_HL
Topic:
Discuss: a technique that could be used to determine the crystal structure of the solid compound.
Answer/Explanation
Solution:
X-ray diffraction is a commonly used technique to determine the crystal structure of a solid compound like FeS. In this technique, a beam of X-rays is directed at a crystal, and the diffraction pattern of the X-rays that are scattered by the atoms in the crystal is recorded. From this diffraction pattern, the positions of the atoms in the crystal can be determined, allowing the crystal structure to be deduced.
Question-1[(d) (iii)] :2021-may-Chemistry_paper_2__TZ1_HL
Topic:
Discuss: the full electron configuration of the sulfide ion.
Answer/Explanation
Solution:
The electron configuration of a sulfide ion, $\mathrm{S}^{2-}$, can be determined by adding two electrons to the neutral sulfur atom’s electron configuration, which is 1s2 2s2 2p6 3s2 3p4. Therefore, the full electron configuration of the sulfide ion is:
$\mathrm{1s^2 2s^2 2p^6 3s^2 3p^6}$
