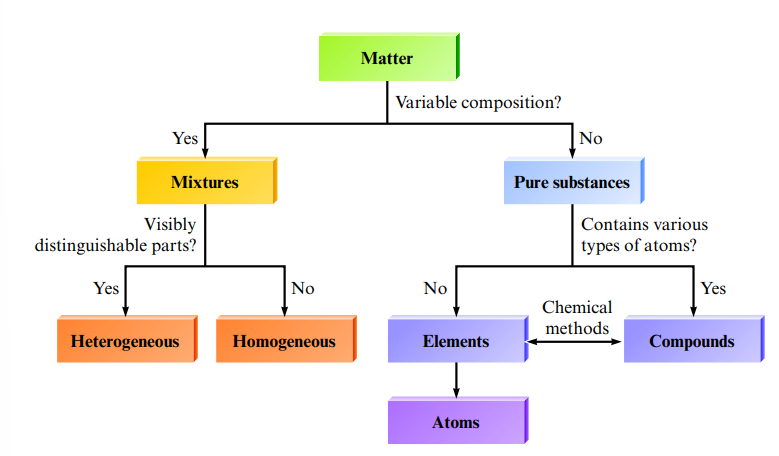
Matter:
- Matter is made up of tiny particles called atoms; anything that has mass and takes up space
- Atom: smallest part of a chemical element that retains the property of the element
- Elements: substances that cannot be broken into simpler substances by chemical or physical means
- Is made up of one type of atom
- Molecule: two or more atoms joined → one unit
- Can be the same or different atoms
- Compound: a substance with constant composition that can be broken down into elements by chemical processes.
- Is made up of different elements/atoms → all compounds are molecules, but not all molecules are compounds
- Diatomic (two atom) molecules
- Ex: oxygen, hydrogen, nitrogen, chlorine, fluorine, bromine, iodine,
Units of Measurement
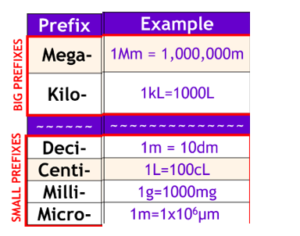
Volume
- Not a fundamental SI Unit → derived from length
- 1 L = 1000 cm^3 = 1000 mL
- 1 cm^3 = 1 milliliter (mL)
- Mass is the amount of matter in an object
- Weight: the force that gravity exerts on an object
- 1 L = 1000 cm^3 = 1000 mL
General Notes
- Qualitative Observations: descriptive
- Quantitative Observations: are numerical
- A measuring system and units must be used
- A law summarizes what happens
- A theory (model) is an attempt to explain why it happens.
Uncertainty in Precision
- Report a measurement by recording all the certain digits plus the first uncertain digit.
- These numbers are called the significant figures of a measurement
Exact vs Inexact Numbers
- Exact: value with no uncertainty
- Definitions, counting, whole numbers, and simple fractions
- Inexact: value with uncertainty
- Any measurement
Measurement of Volume using a Buret 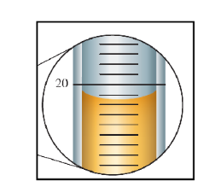
- The volume is read at the bottom of the liquid curve (meniscus).
- Meniscus of the liquid occurs at about 20.15 mL.
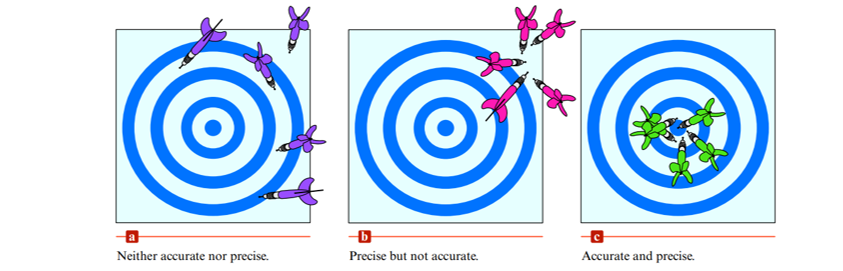
Precision and Accuracy
- Precision: degree of agreement among several measurements of the same quantity
- Reflects the reproducibility of a given type of measurement.
- Accuracy: the agreement of a particular value with the true value
Significant Figures and Calculations
- Certain Digits: Numbers that stay the same no matter who measures them
- Uncertain digits: digits that must be estimated and therefore vary
Rules for Counting Significant Figures
- Nonzero integers always count as significant figures.
- Zeros. There are three classes of zeros:
a) Leading zeros are zeros that precede (to the left) all the nonzero digits. These do not count as significant figures
- 0.032 has 2 significant figures
b) Captive zeros are zeros between nonzero digits. These always count as significant figures
- 19.04 has 4 significant figures
c) Trailing zeros are zeros at the right end of the number. They are significant only if the number contains a decimal point.
- 200 has 4 significant figures; 500. has 3 significant figures
- 6200 has 2 significant figures
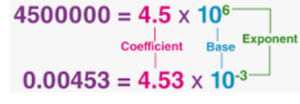
Exponential Notations
Rules for Rounding
1. For multiplication or division, round the answer to the least number of significant figures
a) Ex: 
2. For addition or subtraction, round the answer to the least number of decimal places
a) Ex:
- In a series of calculations, carry the extra digits through to the final result, then round.
Temperature Density

Mixtures
- Mixture: has a variable composition (more than one substance)
- Mixtures can be classified as homogeneous (having visibly indistinguishable parts) or heterogeneous (having visibly distinguishable parts)
- A homogeneous mixture is called a solution.
- Heterogeneous mixtures usually can be separated into two or more homogeneous mixtures or pure substances
- Pure substance: has constant composition
- Are either compounds or free elements
Composition of Pure Substances
- All pure substances have a fixed composition: the elements present and the ratio of those element’s atoms is the same for every sample of the compound →
- Ex: every sample NaCl has a 1:1 ratio of sodium atoms and chlorine atoms
- Fixed ratio of atoms of each element of a compound means there is a constant mass ratio of elements in every compound
- Ex: every sample NaCl has same 40% Na and 60.66% Cl by mass
Understanding Percent Composition 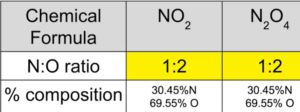
If diff compounds have the same smallest whole number ratio of atoms, the composition by mass of those compounds is the same
Separating Techniques
- Pure substances can be isolated by separation methods
- Filtration: filter particles based on their size
- Fractional Crystallization: evaporate solvent (ex: salt water)
- Distillation: (works for vinegar)
Atomic Structure & Mass Spectroscopy
Mass Spectroscopy
- Why are atomic masses not whole numbers? Because they are weighted averages of isotopes
- Mass spectroscopy: study of the manner in which substances interact with electromagnetic radiation
- Isotopes: atoms with the same number of protons (same element) but different number of neutrons
- Have almost the same properties because chemistry comes from the number of electrons
- Elements occur in nature as mixtures of isotopes
Fundamental Chemical Laws
- Law of Conservation of mass: mass is neither created nor destroyed [in a reaction]
- Law of Definite proportion: A compound always contains exactly the same proportion of elements by mass
- Law of Multiple Proportions: when two elements combine with each other to form more than one type of compound, the weight of one element that combines with a fixed rate of the other can always be reduced to small whole numbers
- Mass percent of an element:

Dalton’s Atomic Theory
- Each element is made up of tiny particles called atoms.
- The atoms of a given element are identical; the atoms of different elements are different
- Chemical compounds are formed when atoms of different elements combine with each other.
- Chemical reactions involve reorganization of the atoms—changes in the way they are bound together.
Characterizing the Atoms
Structure
- Nucleus: protons and neutrons
- Nucleus is very small but accounts for almost all of an atom’s mass
- Protons are positive; neutrons are neutral
- Protons (+) = Electrons (-)
- So that atom is stable and electrically neutral
The Electron
- Mostly energy, negligible mass
- Electrons have potential energy that increases the further away they are from the nucleus → The energy an electron has depends on its distance from the nucleus
- Valence electrons in outermost s and p shell is where the chemistry of an atom takes place
- Determines how atoms react with other atoms
- Electrons (-) orbit the nucleus
- Adding energy to element excites electrons
- Electron moves to higher energy lvl
- To fall back electron releases energy as light, heat, sound etc
Notes
- The number of protons determines the type of element an atom is and the number of electrons determines how the atom will react
- Gaining/losing protons changes element
- Atomic Number: number of protons
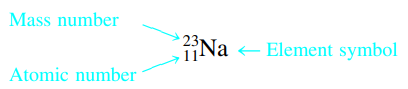 Atomic Mass/Mass Number: total number of protons and neutrons and average of isotopes, expressed in atomic mass units (u)
Atomic Mass/Mass Number: total number of protons and neutrons and average of isotopes, expressed in atomic mass units (u)- Also called mass-to-charge ratio
- Molar Mass: atomic mass of an atom expressed in grams, is equal to one mole (units: g/mol)
- The molar mass of diff elements are different bcuz constituent particles are different but all equal one mole
- Mole: 022× 10^23 of some chemical unit
- Can be atoms, particles, people etc
- Electrons are repelled by other electrons, an electron between a valence and nucleus causes the valence to be weaker (called shielding)
- Octet Rule: in order to be stable an atom must have 8 electrons in its outermost s and p shells
- Stable: unreactive, lowest energy lvl → everything in nature wants to be stable
J.J Thompson 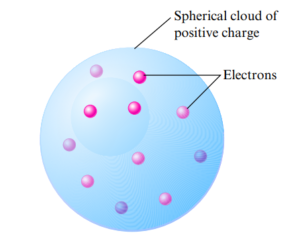
- His model of the atom had a spherical cloud of positive charge with negative electrons randomly embedded in it
One Model of The Nuclear Atom
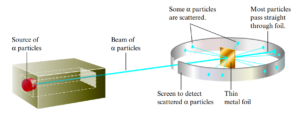
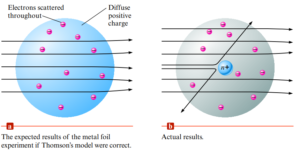
- Results could be explained only in terms of a nuclear atom—an atom with a dense center of positive charge (the nucleus) with electrons moving around the nucleus at a distance that is large relative to the nuclear radius.
Mass Spectroscopy
Three Types of Questions on the AP exam
1. Calculate avg atomic mass from mass spectrum (might be fictitious element)
- avg AM = (abundance of 1st isotope × its atomic mass) + (abundance of 2nd isotope × its atomic mass) / 100
2. Identify element from mass spectrum
- Identify isotopes masses and approximate abundances from the graph → estimate the avg atomic mass → compare estimate to elements on periodic table
3. Identify isotope from mass spectrum
- Determine the element represented by the graph using technique above → determine the number of neutrons by subtracting atomic number (protons) from mass number (protons + neutrons)
Graph Analysis
- Each bar represents a different isotope
- The height of the bars represents the relative abundance
- X-axis may be labeled mass, m/z, mass charge, or atomic mass
Notes
- Reaction Stoichiometry: mass relationships between reactants and products in a chemical reaction
- Mole Ratio: conversion factor that relates amount in moles of two substances in chemical reaction
- Coefficients convey ratio of substances needed for the reaction to occur (in terms of moles)
- One mole water = 18 grams; 1 molecule of water = 18 amu
- Oxygen Ratio: 1 mole = 16 grams = 6.02 x 10^23 atoms
- Round up to 100th place
- Ex: 4:5:6:4 can mean 4 molecules react with 5 molecules to produce 6 molecules and 4 molecules or 4 moles react
Molar Mass
Molar Mass of a Compound
- Determine how many of each atom
- Multiply each element’s number of atoms by atomic mass
- Add up total mass to find molar mass (don’t forget to write grams/mol)
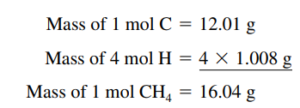
Molar Mass of a Compound Containing Polyatomic Ions
- Number under parenthesis belongs to ALL atoms so multiply to find how many
Determining the Formula of a Compound
- Molecular Formula: the actual number of atoms of each element in a compound
- Ex: C6H12O6
- Empirical Formula: the lowest whole number ratio of elements
- Ex: : CH2O
Determining Empirical and Molecular Formula for a Compound
- Use process when asked to find subscripts of an element in compound
- Multiply given percent with element’s molar mass to find gram of element → convert grams into moles (divide each element by atomic mass) → divide by lowest number of moles = empirical formula
- If given only percentages, assume 100g sample and convert to grams → follow above steps
- If you do not have a whole number, multiply by the smallest whole # to get a whole # ratio → whole #s = subscripts for each element
- To find the molecular formula, divide the molecular molar mass by the empirical molar mass. Then multiply each of the subscripts by that answer
Empirical Formula on the AP Exam
- Straightforward mass or % composition data → empirical formula
- Combustion analysis: burning a sample in O2 and analyzing products to determine relative amounts of C and H
- Hydrate analysis: heating hydrated ionic solid and analyzing mass change to determine mole ratio of water to solid
- Hydrate is heated → water is driven off → mass of sample decreases; hydrate is no longer a hydrate when mass is not decreasing
- To determine formula of hydrated compound → determine moles of water → determine moles of hydrate → find ratio of moles of water / moles of compound → answer equals the number of H2O
The Meaning of a Chemical Equation 
- The chemical equation for a reaction gives 2 types of info: the nature of the reactants and products and the relative numbers of each.
Balancing Chemical Equations
- Atoms are conserved in a chemical reaction.
- Only the coefficients can be changed; the subscripts in a formula cannot be changed, nor can atoms be added or subtracted from a formula.
- Process in balancing elements: Metals → Nonmetals → Hydrogen → Oxygen
For Combustion Reactions (Fuel + O2 → CO2 + H2O)
- Balance carbon → hydrogen
- Count the number of oxygen atoms on the right
- Take half that number and make it the coefficient of the oxygen on the left
- If you now have a fraction, multiply all coefficients by 2
Stoichiometric Calculations: Amounts of Reactants and Products
- Moles to particles → use avogadro’s number
- Moles to mass (grams) → use molar mass from periodic table
- Moles to Volume (liters) → use 22.4 liters/mol but only gasses at standard temp and pressure
Calculating Masses of Reactants and Products in Reactions
- Balance the equation for the reaction
- Convert the known masses of reactant or product to moles
- Use balanced equation to set up mole ratios
- Use the mole ratios to calculate the number of moles of desired reactant or product.
- If required, convert moles back to grams
The Limiting Reactant
- Limiting Reactant: the one that is consumed first and thus limits the amount of product
- You know you have a limiting reactant problem anytime you are given amounts of both reactants
- To determine how much product can be produced in a reaction, we have to look for the reactant that is limiting
- Theoretical yield: The amount of a product formed when the limiting reactant is completely consumed
- What you should have gotten if everything was perfect
- Actual yield: what you actually get from a reaction
- AP exam will never give you the theoretical yield → will always have to calculate it
- Given mass of reactant
- Write balanced equation
- Process: mass of reactant given x (grams of product/grams of reactant from balanced equation
Determination of Limiting Reactant U sing Reactant Quantities
- Balance the equation
- Do two stoichiometry problems
- Figure out how much product each reactant makes
- The one that makes the least is the limiting reagent (the other is the excess reagent)
- The lesser amount of product is the true amount made
Atomic Structure and Periodicity
Electromagnetic Radiation (EMR)
- EMR: energy that exhibits wavelike behavior and travels thru space at the speed of light in a vacuum
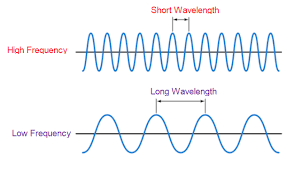
- Ex: light from the sun, X-rays
- All EMR travel at speed of light
- Each form of EMR is only different in wavelength
- Photon: tiny particle of light that acts as a carrier of energy
- Shorter wavelength = higher frequency = more energy
- So there is an inverse relationship between wavelength and frequency
Essential Formulas
- Formula:
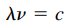
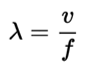
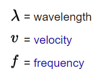
3 Characteristics of Waves
| Wavelength ( | Frequency (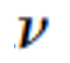 ) ) | Speed of Light ( |
|
|
|
Albert Einstein
- The intensity of light is a measure of the number of photons in a beam
- Greater intensity = more photons are available to release electrons
 → Energy has mass!
→ Energy has mass! 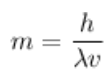
- Mass (kg) of a particle:
- v = velocity
- Can also use equation to calculate wavelength of a particle
- The Dual Nature of Light: EMR can show both wave properties and particulate matter propertiesElectrons seem to move in an interference pattern (like a wave) and have the ability to carry energy and momentum when in motion (like a particle) → an electron is both a wave and particle
The Bohr Model 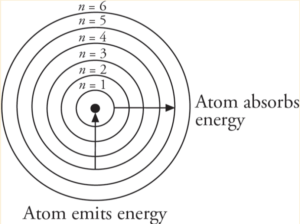
- Predicted that electrons orbit the nucleus at fixed radii
- Atoms absorb energy in the form of electromagnetic radiation → electrons move to higher energy lvl
- As electrons become more tightly bound, its energy becomes more negative
- As the electron is brought closer to the nucleus, energy is released from the system
- Is wrong bcuz does not take into account for sublevels (s, p, d, f), orbitals, or electron spin → electrons aren’t actually locked into orbits
The Quantum Mechanical Model of the Atom
- Quantum Mechanical Model: specifies the probability of finding an electron in the 3D space
- Wave function of an electron: the amplitude where an electron can stay in an atom
- Square of the wave function = the probability of the electron in the orbital
| Shell | Subshell | Orbital |
Pathway followed by an electron around the atom’s nucleus Given n Can hold max 32 e- | Pathway an electron moves inside the shell Given l Maximum e- depends on type of subshell | Most probable location to find an electron and its spatial distribution Given mₗ Can hold max 2 e- Energy of a orbital is determined by its value of n → all orbitals with the same value of n have the same energy (are said to be degenerate) Orbital configurations determine the shape of a molecule → determines their properties and how they behave |
Heisenberg Uncertainty Principle
- Formula:
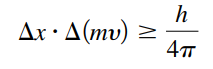
- Δx : is the uncertainty in a particle’s position
- Δ(mv) : is the uncertainty in a particles momentum
- h: Planck’s constant
- Says that it’s impossible to know both the position and momentum of an electron at a particular instant → can’t know the exact motion of the electron as it moves around the nucleus
- Two types of electron motions in an atom
- Orbit motion around a nucleus
- Magnetic motion created by the spin on its axis
- The probability of finding the electron at a specific position is greatest the closer it is to the nucleus
Different Types of Wave Functions
| S Sublevel | P Sublevel | D Sublevel | F Sublevel |
1 orbital = max 2 electrons → the simplest Spherical pattern of standing waves around the nucleus | 3 orbitals = max 6 e- There are 3 P orbitals bcuz are talking about 3D space → can be one orbital on the x-axis, y-axis, and z-axis Each axis can hold a total of 8 electrons (s=2 & p=6)→ octet rule | 5 orbitals → max 10 electrons Four of the d orbitals (xz, yz, xy, x2-y2) have four lobes centered in the plane indicated in the orbitals label. | 7 orbitals → max 14 electrons Orbital shapes are very complex |
Nodes 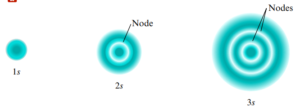
- Point where the probability of finding an electron is 0
- The number of nodes is always one less than the principal quantum number
- # number of nodes increases with n
- Standing wave can have a different number of nodes→ allows patterns to repeat themselves when there are more electrons around the nucleus
Quantum Numbers
- Each of these orbitals is characterized by a series of numbers called quantum numbers

Example: 2p5 → find n, l , mₗ, mₛ
- N =2
- L =1
- mₗ→ draw our number of orbitals → place electrons → 0
- Ms → -½
Important Principles 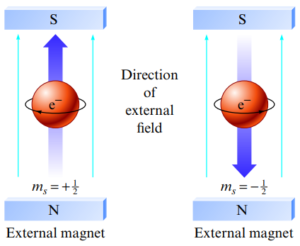
- Pauli Exclusion Principle: two electrons which share an orbital cannot have the same spin → have different values of mₛ
- Aufbau principle: electrons are placed in orbitals, shells, and subshells of increasing energy
- Hund’s rule: Every orbital in a sublevel is singly occupied before any orbital is doubly occupied + All of the electrons in singly occupied orbitals have the same spin
- Because of the symmetrical distribution of electrons, orbitals in which the subshell is exactly half-filled or filled are more stable → so removing an electron from these atoms requires more energy
Radial Probability, Penetration, and Electron Repulsion
- Electrons are attracted to the nucleus at the same time as electrons repel each other.
- Penetration: The ability of an electron to get close to the nucleus
- Penetration depends on the shell (n) and subshell (ml)
- Within same shell value (nP, penetrating power follows trend in subshells (ml)
- s>p>d>f
- For different shall values and subshell (l), penetrating power follows this trend
- 1s>2s>2p>3s>3p>4s>3d>4p>5s>4d>5p>6s>4f….
- Within same shell value (nP, penetrating power follows trend in subshells (ml)
- Penetration depends on the shell (n) and subshell (ml)
- The closer an electron comes to the nucleus/more it penetrates → the stronger its attraction to the nucleus
- Penetration and shielding result in an Effective force that holds the outer electrons to the atom
- Electrons in different orbitals have different wave functions → have different radial distributions and probabilities
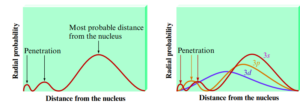
- Each peak in the diagram corresponds to a subshell with different values of n
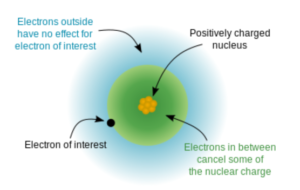
Electron Shielding
- Shielding: describes the blocking of the attraction between valence electrons and nucleus by the inner-shell electrons
- Results from balance between attractive and repulsive forces
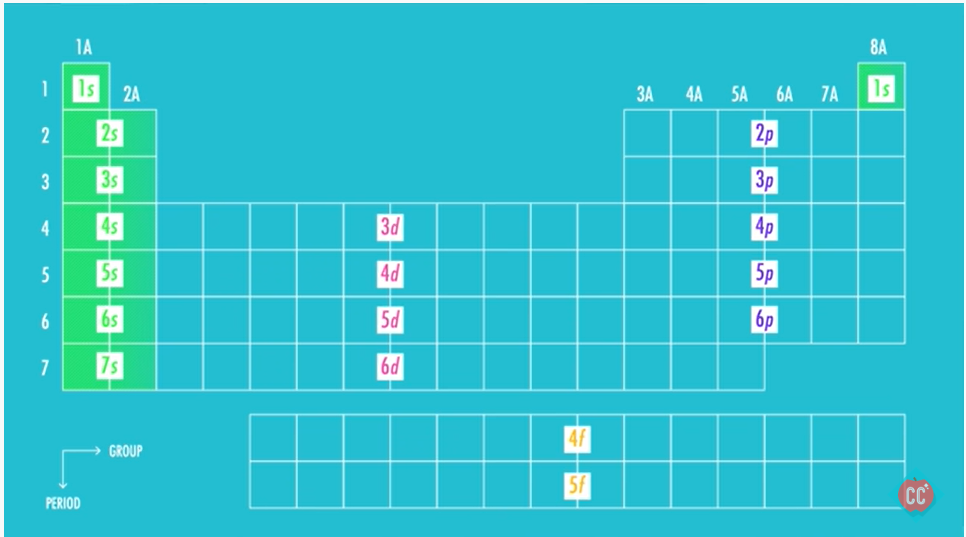
Writing electron configuration
- Electron configuration: tell us where electrons are approx
- For D block elements, count starting from S block
- Noble gas Configuration: consists of the elemental symbol of the last noble gas prior to that atom, followed by the configuration of the remaining electrons
- Use as shortcut → on AP exam can always use
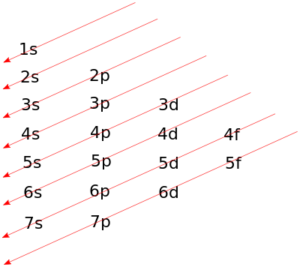
- Use as shortcut → on AP exam can always use
- Ex:

Ion Configurations
- Electrons are removed/added to the valence energy lvl first → only then can e- be removed/added from the d sublvl
- Ex:
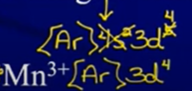
- Ex:
- Write out configuration for pure element first and then remove/add electrons
- Can’t just keep noble gas in brackets → have to back up to noble gas before

Periodic Table and Trends
An Introduction to the Periodic Table
Column/Families/Groups
Rows/Periods
Groups of Periodic Table Group 1: Alkali Metals
Group 1: Alkali Earth Metals
Group 3-12: Transition metals
Diagonal row: Metalloids
Next: Nonmetals
Group 7: Halogens
Group 8: Noble Gasses
Polyelectronic Atoms
Effective Nuclear Charge
Weakens moving down a group
Strengthens from left to right across a period
Atomic Radius
Atomic Radius Trends
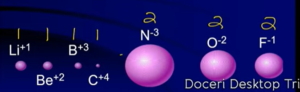
Ionic Radius
Ionic Radius Trend
Isoelectronic Ions
Ionization Energy
Ionization Energy Trend
Electronegativity
Electronegativity Trend
Electron Affinity
Metals
Reactivity
Properties of Nonmetals
Metalloids
The Properties of a Group: The Alkali Metals Alkali Metals
|