Question
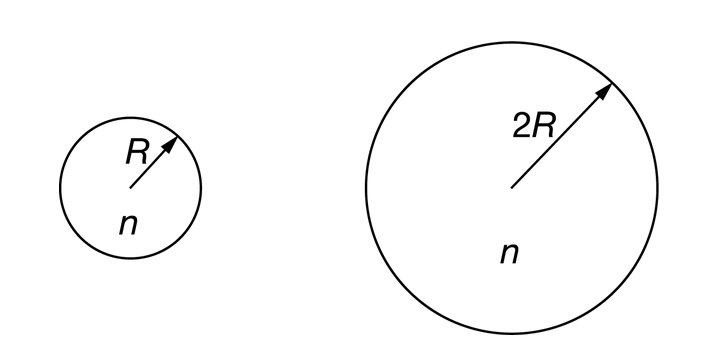
A sphere of radius R is filled with n moles of helium gas at a constant temperature T. A second sphere of radius 2R is filled with the same amount of helium gas at the same temperature. Which of the following is a reason why the pressure in the second sphere is less than that in the first sphere?
A The average speed of the molecules in the second sphere is less than the average speed of the molecules in the first sphere.
B The molecules in the second sphere have to go travel a longer distance between collisions with the walls of the sphere than the molecules in the first sphere do.
C The molecules in the second sphere hit a smaller percent of the area of the sphere walls than the molecules in the first sphere do.
D The molecules in the second sphere bounce off the walls of the sphere with a smaller speed than the molecules in the first sphere do.
▶️Answer/Explanation
Ans:B
The second sphere is larger, and because the molecules have the same temperature, they have the same average speed. So, it takes a molecule longer to travel across the diameter of the sphere, and it hits the sphere wall less frequently than it would in the first sphere. That means less force; thus, less pressure is exerted on the second sphere.
Question
Which of the following correctly explain why the pressure of a gas in a rigid container increases with increasing temperature? Select two answers.
A The average molecular kinetic energy increases with temperature, so the molecules exert a larger average force on the walls of the container when they collide with the walls of the container.
B The average molecular kinetic energy increases with temperature, so the molecules exert a larger average force on each other when they collide with each other.
C The average molecular speed increases with temperature, so the molecules collide with the walls of the container more frequently.
D The average molecular speed increases with temperature, so the molecules collide with each other more frequently.
▶️Answer/Explanation
Ans:A , C
The pressure of a gas is the force it exerts on the walls of the container divided by the area of the walls. If the molecules on average are moving faster because of increased temperature, then they exert a greater force on the container walls.
The pressure of a gas is the force it exerts on the walls of the container divided by the area of the walls. If the molecules on average are moving faster because of increased temperature, then they collide with the walls more frequently, increasing the average force exerted on the walls.
Question
A gas enclosed in a cylinder has a pressure of \(2.0×10^5Pa\). The ends of the cylinder have a diameter of 0.40m and the cylinder has a height of 0.30m
. The magnitude of the force exerted by the gas on the wall at one end of the cylinder is most nearly
A \(7.5×10^3N\)
B \(2.5×10^4N\)
C \(7.5×10^4N\)
D \(1.0×10^5N\)
▶️Answer/Explanation
Ans: B
The force on one end wall is related to the pressure by \(F=PA=P(πr^2)\) . Substituting values gives \(F=(2.0×10^5Pa)(π[0.40m/2]^2)=2.5×104N\).
Question
Compressed air in a vertical cylinder with a piston of radius 0.30m is used to lift a crate. The minimum pressure of the air in the cylinder needed for the piston to lift the crate is 1.61×105N/m2, and the pressure in the room outside the piston is \(1.01×105N/m^2\).
If the mass of the piston is negligible, the weight of the crate is most nearly
A \(1.7×10^4N\)
B \(4.6×10^4N\)
C \(7.4×10^4N\)
D \(2.1×10^5N\)
▶️Answer/Explanation
Ans:A
Pressure and force are related by \(P=F/A\) . The difference in pressure between the gas inside the cylinder and the outside air causes an upward force. The force exerted by the pressure difference is \(F=ΔP⋅A=(1.61×105N/m^2−1.01×105N/m^2)(π)(0.3m)^2=1.7×104N\).
Question
A student has a sample of gas with a known temperature and volume in an insulated cylinder with a piston and wants to investigate the relationship between temperature and volume. The student quickly pushes on the piston to reduce the volume a small amount and measures the new temperature and volume of the gas. This procedure is repeated to get measurements for increasingly smaller volumes of the gas. The data show that as the volume of the gas decreases, its temperature increases, which appears to contradict the ideal gas law. Which of the following modifications to the experiment would demonstrate the relationship between temperature and volume in the ideal gas law?
A Repeat the experiment with a number of different gases.
B Repeat the experiment, but compress the gas more quickly.
C Perform a new experiment in which the piston is fixed and energy is added to the gas by heating.
D Perform a new experiment with hot gas that uses an object on top of the piston to keep the pressure of the gas constant while the gas is allowed to cool.
▶️Answer/Explanation
Ans:D
The flaw in the investigation was that the pressure was not held constant. This new procedure will allow the student to determine the relationship between volume and temperature.
