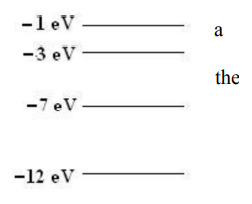Question
An atomic particle of mass m moving at speed v is found to have wavelength λ. What is the wavelength of a second particle with three times the speed and twice the mass?
A) 3λ/2 B) 2λ/3 C) 6λ D) λ/6
▶️Answer/Explanation
Ans:D
Solution: The de Broglie wavelength is given by p = h / λ. Since p = mv, λ = h / mv. Therefore, λnew =h/[(3m)(2v)] = λ/6.
Question
According to the Bohr theory of the hydrogen atom, electrons starting in the 4th energy level and eventually ending up in the ground state, could produce a total of how many lines in the hydrogen spectra?
A) 3 B) 4 C) 5 D) 6
▶️Answer/Explanation
Ans:
D
Solution: 4–3,3–2,2–1 … or 4–2, 2–1 … or 4–3, 3–1 … or 4–1 … this is a total of 6 different transitions.
Question
According to the Bohr theory of the hydrogen atom, electrons starting in the 4th energy level and eventually ending up in the ground state, could produce a total of how many lines in the hydrogen spectra?
A) 3 B) 4 C) 5 D) 6
▶️Answer/Explanation
Ans:
D
Solution: 4–3,3–2,2–1 … or 4–2, 2–1 … or 4–3, 3–1 … or 4–1 … this is a total of 6 different transitions.
Question
In the photoelectric effect experiment, a stopping potential of Vstop is needed when light of frequency fo shines on the electron-emitting metal surface. If the metal surface on which the light shines is replaced with a new material that has half the work function, what is the new stopping potential, Vnew , for light of frequency shining on it?
A) Vnew > 2Vstop B) Vnew = 2 Vstop C) Vstop < Vnew < 2Vstop D) It is indeterminate from the given information
▶️Answer/Explanation
Ans:D
Solution: Stopping potential is given by. K=Vstopq … combined with K = hf – ϕ , we see the stopping potential is related to the incoming light energy minus the work function. However, none of the choices give a proper result. The answer depends on what that actual incoming energy hf and work function are. Here is an example. Lets say hf was 3eV and the ϕ was 2 eV initially. From
Vq=hf– ϕ … the stopping potential for an electron (1e) would be equal to 1eV. Now if we were to half the work function, the new stopping potential would be 3eV – 1eV = 2 eV so it appears that the stopping potential doubled. But that result only works for those sample numbers. Lets assume instead that hf = 10eV and ϕ = 2eV. Now initially the Vstop = 8eV. Then we again half the work function … 10eV – 1 eV and we get a stopping potential of 9V .. not nearly doubled this time. The results depend on the actual numbers used because of the minus sign in the equation and not a simple multiplier effect.
Question
The diagram to the right shows the lowest four energy levels for an electron in hypothetical atom. The electron is excited to the −1 eV level of the atom and transitions to the lowest energy state by emitting only two photons. Which of the following energies could not belong to either of the photons?
(A) 2 eV (B) 4 eV (C) 5 eV (D) 6 eV
▶️Answer/Explanation
Ans:B
Solution: To transition to the –12eV state with only two photon emissions, the only options are for the electron to make the following transitions: –1eV → – 3eV → –12eV giving us photons of energy 2eV and 9eV or –1eV → – 7 eV → – 12eV giving photons of energy 6eV and 5eV . This means that the 4 eV photon is not possible with only two transitions.