Periodic Classification of
Elements
In this Chapter...
!
Earlier Attempts at the Classification of Elements
!
Mendeleev’s Periodic Table
! Modern Periodic Table
All substances are made up of elements. At present, there are
elements in the same slot and also put some unlike elements
118 elements known, out of which 98 are naturally occurring.
under the same column which have very different properties
In order to study the properties of all these elements
than other elements.
separately, scientists felt the necessity to group elements
having similar characteristics together.
Mendeleev’s Periodic Table
According to this, the physical and chemical properties of the
Earlier Attempts at the
elements are periodic function of their atomic masses, i.e. on
Classification of Elements
arranging the elements in increasing order of their atomic
masses, the similar properties were repeated after regular
Several attempts have been made to classify the elements
intervals.
according to their properties. Later, many classifications were
tried. Some important of them are discussed below
He took the formulae of the hydrides and oxides formed by
an element as one of the basic properties of an element for its
Dobereiner’s Triads
classification. e.g. Hydride of carbon, CH4 as RH4 and its
He arranged three elements with similar properties into
oxides, CO2 as RO2 .
groups which are known as triads and showed that when
He then arranged 63 elements in the increasing order of
three elements in a triad were arranged in order of increasing
their atomic masses and found that there was a periodic
atomic masses, the atomic mass of middle element was
recurrence of elements with similar physical and chemical
roughly the average of atomic masses of other two elements.
properties. He observed that elements with similar properties
He could identify only three triads from the elements known
fall in the same vertical column. These vertical column are
at that time which are
called groups and horizontal rows of elements are called
Li, Na, K; Ca, Sr, Ba; Cl, Br, I
periods.
Newland’s Law of Octaves
Features of Mendeleev’s Periodic Table
Newland arranged the known elements in order of increasing
● It consists of 8 vertical columns, called groups and 6 horizontal
atomic masses and found that every eighth element had
rows, called periods.
properties similar to that of the first. This law was applicable
● In every period, elements are arranged in increasing order of
only upto calcium and he assumed that there were only 56
their atomic masses.
elements. To fit elements into his table, he adjusted two
● He left gaps for the elements not discovered at that time and
● One of the strengths of Mendeleev’s periodic table was that,
named such elements by prefixing a Sanskrit numeral
when noble gases like helium, neon were discovered, they
Eka (one), divi (two) to the name of the preceding similar
could be placed in a new group without disturbing the existing
element in the same group. e.g. Eka-boron, Eka-aluminium,
order.
which after their discovery were named as scandium, gallium.
Limitations of Mendeleev’s Periodic Table
● He also predicted the atomic masses and properties of several
elements that were not known at that time.
● Elements with dissimilar properties were kept in same
group.
Properties of Eka-aluminium and Gallium
● Position of hydrogen was not fixed in periodic table.
Property
Eka-aluminium
Gallium
● Elements with similar properties were kept in different
Atomic mass
68
69.7
groups.
Formula of oxide
E2O
3
Ga O
2
3
● Heavier elements were kept before the lighter elements.
Formula of chloride
ECl3
GaCl3
● Position of isotopes and isobars could not be explained.
Mendeleev’s Periodic Table (Published in a German journal in 1872)
In the formula of oxides and hydrides at the top of the columns, the letter ‘R’ is used to represent any of the elements in the
group.
Group →
I
II
III
IV
V
VI
VII
VIII
Oxide
R2O
RO
R2O
3
RO2
R2O
5
RO3
R2O
7
RO4
Hydride
RH
RH2
RH3
RH
RH
RH4
3
RH2
Periods ↓
A
B
A
B
A
B
A
B
A
B
A
B
A
B
Transition series
1
H
1.008
2
Li
Be
B
C
N
O
F
6.939
9.012
10.81
12.011
14.007
15.999
18.998
3
Na
Mg
Al
Si
P
S
Cl
22.99
24.31
29.98
28.09
30.974
32.06
35.453
4 First series
K
Ca
Sc
Ti
V
Cr
Mn
Fe
Co
Ni
39.102
40.08
44.96
47.90
50.94
50.20
54.94
55.85
58.93
58.71
Second series
Cu
Zn
Ga
Ge
As
Se
Br
63.54
65.37
69.72
72.59
74.92
78.96
79.909
5 First series
Rb
Sr
Y
Zr
Nb
Mo
Tc
Ru
Rh Pd
85.47
87.62
88.91
91.22
92.91
95.94
99
101.07
102.91
106.4
Second series
Ag
Cd
In
Sn
Sb
Te
I
107.87
112.40
114.82
118.69
121.75
127.60
126.90
6 First series
Cs
Ba
La
Hf
Ta
W
Os Ir
Pt
132.90
137.34
138.91
178.49
180.95
183.85
190.2
192.2
195.09
Second series
Au
Hg
Tl
Pb
Bi
196.97
200.59
204.37
207.19
208.98
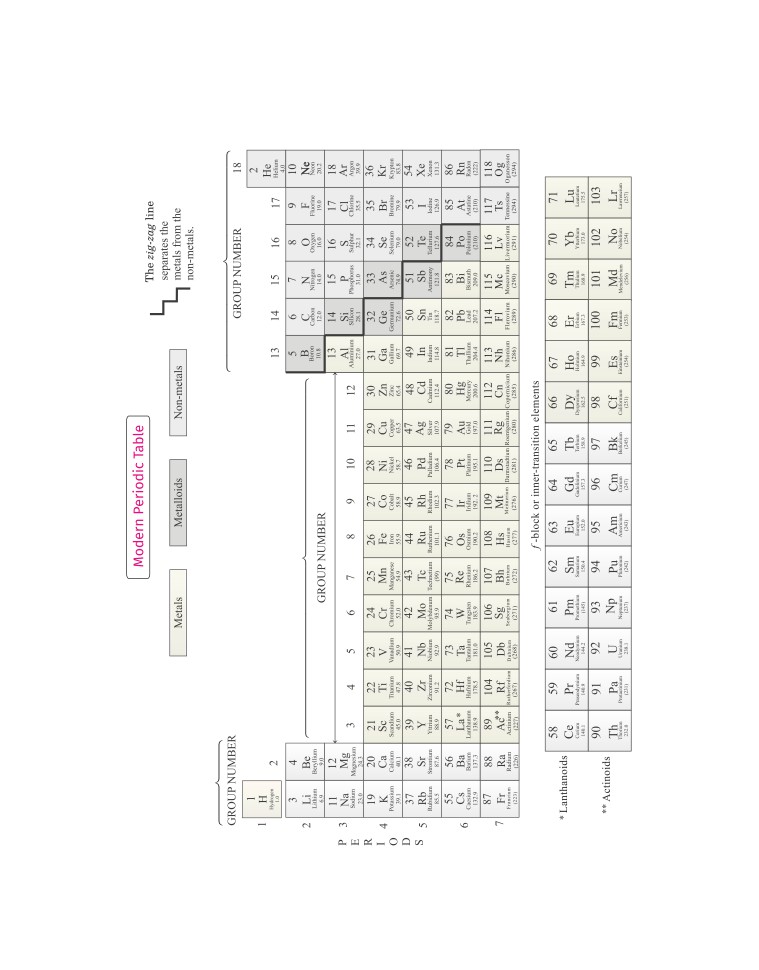
Modern Periodic Table
When the elements were arranged in the increasing order of
their atomic number, the obtained table is called modern
In 1913, Henry Moseley showed that the atomic number of
periodic table.
an element is a more fundamental property. On the basis of
this, he modified Mendeleev’s periodic law as “physical and
In this periodic table, hydrogen is kept at the top left corner
chemical properties of the elements are a periodic function of
because of its unique characteristics. The position of cobalt
and nickel is also justified.
their atomic number”. This is called modern periodic law.
electron, it acquires a stable configuration, hence its valency
Features of Modern Periodic Table
is also 1.
This table has 18 vertical columns, known as groups and
7 horizontal rows, known as periods.
Trends in Modern Periodic Table
A few important features of the elements present in groups
(i)
Valency In a period, it increases with respect to
and periods are as follows
hydrogen from 1 to 4 after that it decreases. On the other
● The groups are not divided into sub-groups.
hand with respect to oxygen, valency increases from 1
● The elements present in a group have the same number of
to 7. In a group, valency remains same as outer
valence electrons and valency.
electronic configuration is same.
● The number of shells increases as we go down the group.
(ii)
Atomic size Atomic size decreases on moving from left
● The elements present in a group have identical chemical
to right in a period due to increase in nuclear charge.
properties and their physical properties like density, melting
It increases down the group as new shells are being
point vary gradually.
added.
● Elements of a period have the same number of shells but they
(iii)
Metallic and non-metallic properties Effective nuclear
do not contain the same number of valence electrons. So, their
charge acting on the valence shell electrons increases
chemical properties are also different.
across a period and decreases down the group.
● The number of valence shell electrons increases by one unit as
Therefore, metallic character decreases across a period
the atomic number increases by one unit on moving from left
and increases down a group. Non-metallic character,
to right in a period.
however increases across a period and decreases down a
● In this table, elements of group 13, 14, 15, 16 and 17 are called
group.
normal elements which includes metals, non-metals and
Metals like Na, Mg are present on left side of periodic
metalloids and elements of group 3, 4, 5, 6, 7, 8, 9, 10, 11 and
table, whereas non-metals like S, Cl are present on right
12 are called transition elements.
side of periodic table.
● In this periodic table, elements from atomic number 58 to 71
There are some metals which exhibits both the
called as lanthanides and elements from 91 to 103 called as
properties of metal and non-metals. These are called
actinoids are kept out of the table.
metalloids like Po, Te, Sb, etc.
Position of Elements in the Modern
(iv)
Electronegativity The electronegativity of the elements
increases along a period, since the non-metallic
Periodic Table
character increases. Similarly, it decreases down the
For this, first of all write electronic configuration of the given
group, since the non-metallic character decreases.
element. Number of shells present in the electronic
(v)
Nature of oxides On moving from left to right in a
configuration shows the period number of that element.
period, due to increase in non-metallic character,
Number of valence electrons present in the electronic
basic nature of oxides decreases while acidic nature
configuration show the group number of the element.
increases.
e.g. Electronic configuration of the element with atomic
Na O MgO
Al O
2
3
, SiO2
, P O SO
5
2
, Cl O
2
7
number 19 is 2, 8, 8, 1, since it has four shells, thus it is
14
4
2
4 ,
14243
Amphoteric
Weakly
Strongly
Strongly basic
Acidic
element of fourth period. Due to presence of one electron in
acidic
acidic
the last shell, its group number is 1. After donating one
On going down the group, the order is reversed.