Dual Nature of Matter and Radiation : Notes and Study Materials -pdf
- Concepts of Dual Nature of Matter and Radiation
- Dual Nature of Matter and Radiation Master File
- Ncert Solution
- NCERT Exemplar Solution
- Important Questions Dual Nature Of Radiation And Matter
- Past Many Years CBSE Questions and Answer: Matter Wave
- Past Many Years CBSE Questions and Answer: Photoelectric Effect
- Test Paper 1
- Test Paper 2
CBSE chapter wise notes based on chapter 11, Dual Nature of Radiation and Matter of NCERT textbook are available in this article.
In this article, students will get important key notes on the topics given below
| Electron Emission |
Methods of Electron Emission • Thermionic emission • Field emission • Photo-electric emission |
| Photoelectric Effect |
| Hertz’s Observation |
| Hallwachs’ and Lenard’s Observations |
| Experimental Study of Photoelectric Effect |
| Effect of intensity of light on photocurrent |
| Effect of potential on photoelectric current |
| Effect of frequency of incident radiation on stopping potential |
| Laws of Photoelectric Effect |
The complete notes are as follows
Electron Emission
Free electron inside the metal surface by the attractive forces of the ions, the electron can come out of the metal surface only if it has got sufficient energy to overcome the attractive pull. A certain minimum amount of energy is required to be given to an electron to pull it out from the surface of the metal. This minimum energy required by an electron to escape from the metal surface is called the work function of the metal.
Methods of Electron Emission
Thermionic emission
By supplying heat, sufficient thermal energy can be imparted to the free electrons which enable them to come out of the metal.
Field emission
By applying a very strong electric field (of the order of 108 V m–1) to a metal, electrons can be pulled out of the metal, as in a spark plug.
Photo-electric emission
When light of suitable frequency falls on a metal surface, electrons are emitted. These photo (light) generated electrons are called photoelectrons.
Photoelectric Effect
Metallic surfaces when illuminated by ultraviolet radiation then electrons are emitted from them. This effect is known as the photoelectric effect. By absorbing energy from the incident electromagnetic radiation, the electrons in the metal escape the attraction of ions in the metal.
Hertz’s Observation:
In 1887 by Heinrich Hertz observed that when light falls on a metal surface, some electrons near the surface absorb enough energy from the incident radiation to overcome the attraction of the positive ions in the material of the surface. After gaining sufficient energy from the incident light, the electrons escape from the surface of the metal into the surrounding space.
Hallwachs’ and Lenard’s Observations
By a series of experiments, Hallwachs’ and Lenard’s observed that there is a certain minimum frequency, known as threshold frequency, below which no electrons were emitted.
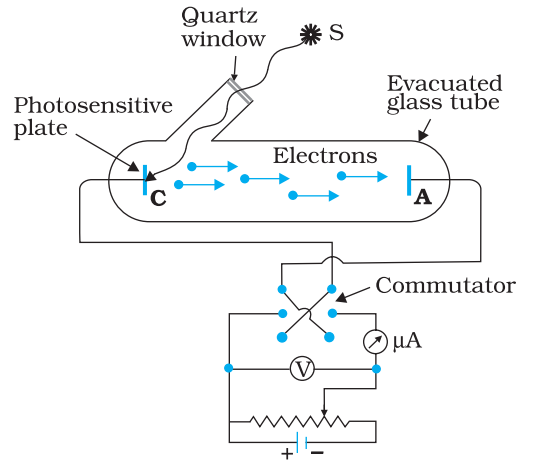
Experimental Study of Photoelectric Effect
Schematic diagram to study of phenomenon of Photoelectric Effect is given above. The main observations of the experiments are given below
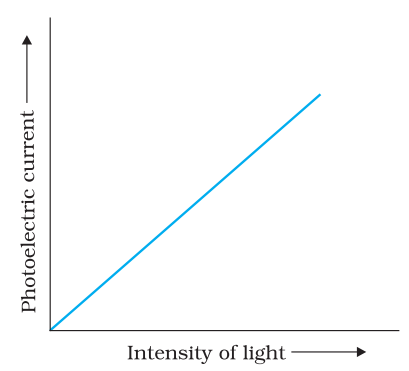
Effect of intensity of light on photocurrent
The photocurrent is directly proportional to the number of photoelectrons emitted per second. This implies that the number of photoelectrons emitted per second is directly proportional to the intensity of incident radiation.
Effect of potential on photoelectric current
For a given frequency of the incident radiation, the stopping potential is independent of its intensity. In other words, the maximum kinetic energy of photoelectrons depends on the light source and the emitter plate material, but is independent of intensity of incident radiation.
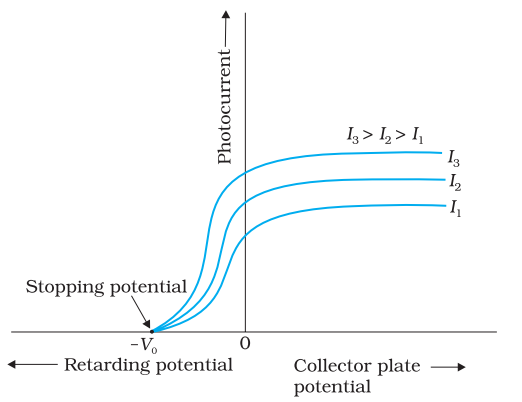
In the above figure, for a particular frequency of incident radiation, the minimum negative (retarding) potential V0 given to the plate A for which the photocurrent stops or becomes zero is called the cut-off or stopping potential.
Effect of frequency of incident radiation on stopping potential
This implies that greater the frequency of incident light, greater is the maximum kinetic energy of the photoelectrons. Consequently, we need greater retarding potential to stop them completely
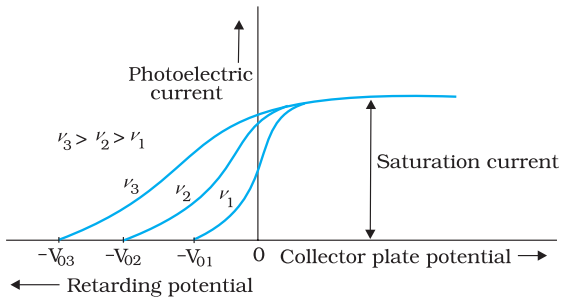
Variation of photoelectric current with collector plate potential for different frequencies of incident radiation is shown in the figure given above.
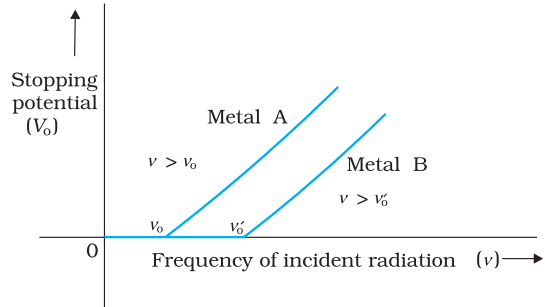
Variation of stopping potential V0 with frequency ν of incident radiation for a given photosensitive material is given above.
Laws of Photoelectric Effect
(i) For a given photosensitive material and frequency of incident radiation (above the threshold frequency), the photoelectric current is directly proportional to the intensity of incident light.
(ii) For a given photosensitive material and frequency of incident radiation, saturation current is found to be proportional to the intensity of incident radiation whereas the stopping potential is independent of its intensity.
(iii) For a given photosensitive material, there exists a certain minimum cut-off frequency of the incident radiation, called the threshold frequency, below which no emission of photoelectrons takes place, no matter how intense the incident light is. Above the threshold frequency, the stopping potential or equivalently the maximum kinetic energy of the emitted photoelectrons increases linearly with the frequency of the incident radiation, but is independent of its intensity.
(iv) The photoelectric emission is an instantaneous process without any apparent time lag (∼10– 9s or less), even when the incident radiation is made exceedingly dim.
In part I, we have studied about electron emission, methods of electron emission, photoelectric effect, Hertz’s observation, Hallwachs’ & Lenard’s observations and experimental study of photoelectric effect etc. Now in part II we will study about the topics given below
| Photoelectric Effect and Wave Theory of Light |
| Photon |
| Einstein’s Photoelectric Equation: Energy Quantum of Radiation |
| Threshold frequency |
| Intensity of Light |
| Kinetic Energy of Photoelectron |
| Number of Photoelectrons Emitted |
| Photoelectric Current |
| Einstein Photoelectric Equation and Stopping Potential |
| Photoelectric Cell |
| Wave Nature of Matter |
| Davisson’s and Germer’s Experiment |
The complete notes are given below
Photoelectric Effect and Wave Theory of Light
The phenomena of interference, diffraction and polarisation were explained by the wave picture of light. But the experimental study of photoelectric effect cannot be explained with the help of wave theory of light. The wave picture is unable to explain the most basic features of photoelectric emission. So, photon picture of light (a new theory) was proposed to explain the phenomenon of photoelectric effect.
Photon
Photon is a packet of energy or quanta of energy associated with electromagnetic radiation. The energy of a photon is given by E = h v, where, v is frequency associated with photon and h is Planck’s constant.
Einstein’s Photoelectric Equation: Energy Quantum of Radiation
In 1905, Albert Einstein proposed a radically new picture of electromagnetic radiation (quanta of energy of radiation) to explain photoelectric effect.
Each quantum of radiant energy has energy hν, where h is Planck’s constant and v is the frequency of light.
In photoelectric effect, an electron absorbs a quantum of energy (hv) of radiation. If this quantum of energy absorbed exceeds the minimum energy needed for the electron to escape from the metal surface (work function ϕ0), the electron is emitted with maximum kinetic energy given by
Kmax = h v ‒ ϕ0
The value of work function for a particular material is constant and it depends on nature of material.
Threshold frequency
For a given photosensitive material, there is a certain minimum cut off frequency vo for which stopping potential is zero this minimum cut off frequency vo is known as threshold frequency.
Intensity of Light
Intensity of light depends upon the number of photons present in it. It does not depend on the frequency of incident light.
Kinetic Energy of Photoelectron
Kinetic energy of photoelectron depends on frequency of incident light as Kmax = h v ‒ ϕ0 or Kmax ∝ v. It does not depend on intensity of radiation.
Number of Photoelectrons Emitted & Frequency of Incident Light
In photoelectric effect, photoelectrons emitted depend only on the intensity of light. It is independent of the frequency of incident light, provided v > vo.
Photoelectric Current
Photoelectric Current is defined as the rate of emission of photoelectrons from the metal surface. It is directly proportional to the intensity of incident radiation.
Einstein Photoelectric Equation & Stopping Potential
Stopping potential is the minimum retarding potential which should be applied across a photoelectric tube in order to make photoelectric current zero.
Suppose, VS is required stopping potential, e is charge on electron then eVo = ½ m (vmax)2.
Now, Einstein equation can be rewritten as h (v ‒ vo) = ½ m (vmax)2 = eVo.
From above equation we can easily observe that,
Stopping potential is directly proportional to the frequency of incident light and inversely proportional to work function.
Stopping potential is independent of
• Intensity of incident light
• Distance of the source from metal surface
• Illuminating power of the source
Photoelectric Cell
A photocell is a technological application of the photoelectric effect. It is a device whose electrical properties are affected by light. It is also sometimes called an electric eye. Photocells are used in the reproduction of sound in motion pictures and in the television camera for scanning and telecasting scenes. They are used in industries for detecting minor flaws or holes in metal sheets.
Wave Nature of Matter
The wave nature of light shows up in the phenomena of interference, diffraction and polarisation. On the other hand, in photoelectric effect and Compton effect which involve energy and momentum transfer, radiation behaves as if it is made up of a bunch of particles – the photons.
A natural question arises: If radiation has a dual (wave-particle) nature, might not the particles of nature (the electrons, protons, etc.) also exhibit wave-like character?
de Broglie hypothesis attributes a wave like character to matter. If radiation shows dual aspects, so should matter.
De Broglie proposed that the wave length λ associated with a particle of momentum p is given by, λ = h/p = h/(m v), where m is the mass of the particle and v its speed.
The above equation is known as the de Broglie relation and the wavelength λ of the matter wave is called de Broglie wavelength.
The above equation shows that λ is smaller for a heavier particle (large m) or more energetic particle (large v).
Davisson’s and Germer’s Experiment
The experiment by Davisson’s and Germer’s provides experimental proof of the concept of wave nature of material particles.
The experimental arrangement used by Davisson and Germer is schematically shown in figure given below:
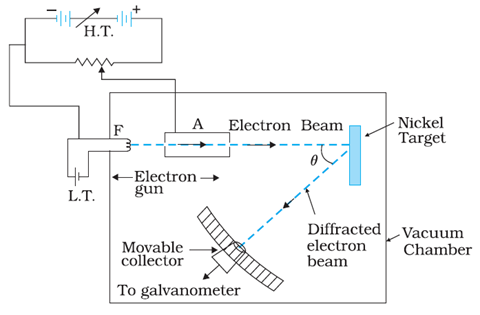
In the experiment, an electron gun was used to produce a beam of electrons. This fine beam of accelerated electrons was made to fall on a nickel crystal and atoms of the crystal scatter these electrons in different directions. The intensity of electrons beam scattered in a given direction was observed by a detector. This detector was made to rotate on a circular scale. From the experiment, scientists observed the intensity of scattered beam of electrons for different values of latitude angles or scattering angles which are the angles between the incident and the scattered electron beams.
The experiment was performed by varying the accelerating voltage from 44 V to 68 V. It was noticed that a strong peak appeared in the intensity (I ) of the scattered electron for an accelerating voltage of 54V at a scattering angle θ = 50º.
The appearance of the peak in a particular direction is due to the constructive interference of electrons scattered from different layers of the regularly spaced atoms of the crystals. From the electron diffraction measurements, the wavelength of matter waves was found to be 0.165 nm.
