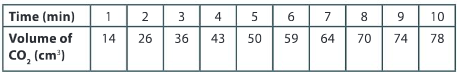
Question:
Plot the volume of carbon dioxide gas evolved V(CO2) against time t. You can do this by hand or by using graphing software. Remember that a smooth curve is required to represent the relationship between the data points.
▶️Answer/Explanation
Ans: 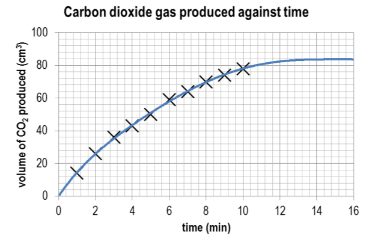
Question:
What conclusions can you draw about the progress of the reaction based on the shape of the curve?
▶️Answer/Explanation
Ans: The initial rate from 0 to 2 minutes is the fastest rate of reaction as this is the steepest part of the curve. As time progresses, the steepness of the curve decreases and therefore the rate of reaction is decreasing.
Question:
Draw a tangent to the curve at t=0 min and calculate the gradient of the tangent. What are the units for this rate?
▶️Answer/Explanation
Ans: Slope of tangent is approximately 19 cm3 min–1. Values given between 17 and 21 cm3 min–1 would be acceptable.
Question:
From the shape of the curve, estimate the time at which the reaction has finished.
▶️Answer/Explanation
Ans: The rate of change in carbon dioxide gas evolved is slowing from minute to minute. It can be estimated that the time at which the reaction will stop would be between 14 to 16 minutes.
Question:
Draw up a data table using the following headings:

▶️Answer/Explanation
Ans: Dependent on the quantitative data collected.
Question:
Calculate the value of 1/ time and record in your data table for each of the individual trials.
▶️Answer/Explanation
Ans: Dependent on the quantitative data collected.
Question:
Plot a graph of rate versus temperature and draw a smooth curve connecting the points.
▶️Answer/Explanation
Ans: Dependent on the quantitative data collected.
Question:
Identify any anomalous data and ignore these data points.
▶️Answer/Explanation
Ans: Dependent on the quantitative data collected.
Question:
Analyze the graph and discuss what effect, if any, an increase in the temperature conditions has on the rate of the reaction.
▶️Answer/Explanation
Ans: An increase in the temperature will result in a decrease in the time taken for the black cross to disappear. This equates to an increase in the rate of the reaction.
Question:
Use your knowledge of the collision theory to explain the effect changing temperature conditions has on the rate of reaction.
▶️Answer/Explanation
Ans: An increase in thermal energy in the reaction mixture will result in an increase in kinetic energy of the reacting particles. This increase in kinetic energy will increase the frequency of collisions between reacting particles and also the proportion of particles that have enough energy to overcome the activation energy barrier.
Question:
Record your data in a table with the following headings:
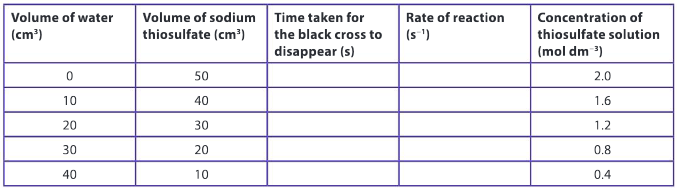
▶️Answer/Explanation
Ans: Dependent on the quantitative data collected.
Question:
Calculate the value of 1/ time and record in your data table for each of the individual trials.
▶️Answer/Explanation
Ans: Dependent on the quantitative data collected.
Question:
Plot a graph of rate versus concentration and draw a smooth curve connecting the points.
▶️Answer/Explanation
Ans: Dependent on the quantitative data collected.
Question:
Suggest why this analytical method of monitoring the rate of the reaction is appropriate for this chemical reaction.
▶️Answer/Explanation
Ans: The formation of solid sulfur is the reason why the solution turns cloudy and eventually the black cross will disappear. Therefore, the time taken for the black cross to disappear is indicative of the rate at which the products of the reaction are formed.
Question:
Identify the independent and dependent variables.
▶️Answer/Explanation
Ans: The independent variable is the concentration of sodium thiosulfate and the dependent variable is the time taken for the black cross to disappear.
Question:
Discuss which variables are being controlled and offer scientific reasons why this is important for this type of reaction.
▶️Answer/Explanation
Ans: The total volume of the reaction mixture is being controlled, as a change in volume between the various trials would change the depth of the solution above the black cross. If the depth of solution was changed, this would affect the qualitative observations of the black cross and in turn, the measured value of time taken for the reaction to occur.
Question:
Use your knowledge of the collision theory to explain the effect concentration has on the rate of reaction.
▶️Answer/Explanation
Ans: Increasing the concentration of a reactant increases the number of particles for a given volume of solution. This results in an increase in the frequency of collisions and in turn an increase in the rate of the reaction.
Question:
Explain why the rate of reaction changes over time. Your answer should include references to the collision theory.
▶️Answer/Explanation
Ans: As time progresses the rate of reaction will decrease until the reaction finishes. The initial rate of reaction is the fastest rate for any chemical reaction. This is because at t = 0, the highest concentration of reactant particles is present in the reaction mixture. Initially there will be a high frequency of collisions. As the reaction progresses, the concentration of reactants decreases and the frequency of collisions decreases.
Question:
What differences were there in the observations you made of the two different combustion reactions?
▶️Answer/Explanation
Ans: The compressed cornstarch on the spoon will slowly burn in the Bunsen burner flame. The cornstarch expelled from the dropping pipette into the Bunsen burner flame bursts into flames.
Question:
Why was there a difference in the rate of reaction? Support your statement with scientific reasoning.
▶️Answer/Explanation
Ans: The rate of reaction increases significantly when the surface area of the reactants is increased. By increasing the number of particles which are exposed to the flame, the rate of combustion of the corn starch also increases. It is analogous to increasing the concentration of a reactant. Increasing the surface area of a reactant is a cheap and effective way that industry increases the overall rate of a reaction.
Question:
Record your data in a table with the following headings:
![]()
▶️Answer/Explanation
Ans: Dependent on the quantitative data collected.
Question:
Prepare a graph with loss of mass of the marble chip on the y-axis and time on the x-axis.
▶️Answer/Explanation
Ans: Dependent on the quantitative data collected.
Question:
Plot the two sets of data on the one set of axes to enable you to make a comparison of the data.
▶️Answer/Explanation
Ans: Dependent on the quantitative data collected.
Question:
Draw smooth curves to match the trends of the data and label the curves.
▶️Answer/Explanation
Ans: Dependent on the quantitative data collected.
Question:
Identify and write a balanced chemical equation for the reaction between calcium carbonate and hydrochloric acid.
▶️Answer/Explanation
Ans: CaCO3(s) + 2HCl(aq) → CaCl2(aq) + CO2(g) + H2O(l)
Question:
Suggest why this analytical method of monitoring the rate of the reaction is appropriate for this chemical reaction.
▶️Answer/Explanation
Ans: In the reaction between calcium carbonate and hydrochloric acid, the gas carbon dioxide is produced. By monitoring the loss of mass of the reaction mixture as carbon dioxide is lost to the
atmosphere, we are able to monitor the rate of this chemical reaction.
Question:
Identify the independent and dependent variables.
▶️Answer/Explanation
Ans: The independent variable is the surface area of calcium carbonate and the dependent variable is the formation of carbon dioxide gas or the rate of loss of mass of the reaction mixture.
Question:
Discuss which variables are being controlled and offer scientific reasons why this is important for this type of reaction.
▶️Answer/Explanation
Ans: The mass of calcium carbonate must remain constant while the surface area changes. This ensures that the number of moles of calcium carbonate reacting remains constant. The volume and concentration of hydrochloric acid in each different experiment must remain constant. The same conical flask must be used in each experiment so that the cross–sectional area of the opening of the flask is constant.
Question:
Use your knowledge of the collision theory to explain the effect surface area has on the rate of reaction.
▶️Answer/Explanation
Ans: An increase in surface area of a reactant has the same effect on the rate of reaction as does increasing the concentration of the reactant. An increasing surface area means that more particles are able to react with the hydrochloric acid thereby increasing the frequency of successful collisions and in turn increasing the rate of the chemical reaction.
Question:
Explain why the rate of reaction for both investigations changes over time.
● Your answer should include references to the collision theory.
● Your answer should include example calculations of the rate of reaction at different times.
▶️Answer/Explanation
Ans: As time progresses the rate of reaction will decrease until the reaction finishes. The initial rate of reaction is the fastest rate for any chemical reaction. This is because at t = 0, the highest concentration of reactant particles is present in the reaction mixture. Initially there will be a high frequency of collisions. As the reaction progresses, the concentration of reactants decreases and the frequency of collisions decreases.
Question:
Give reasons why the final loss of mass in each investigation should be identical.
▶️Answer/Explanation
Ans: The final loss of mass in each investigation should be identical, as the same stoichiometric amounts of calcium carbonate and hydrochloric acid are being used in the different experiments. Only the rate of loss of mass is altered by changing the surface area.
Question:
Calculate the percentage increase in each of these gases for the time periods 1980–1989, 1990–1999, 2000–2009 and 2010–2017.
▶️Answer/Explanation
Ans: 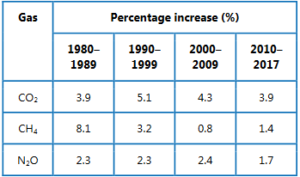
Question:
Compare the percent increase for each time period and discuss the change in concentration of these gases, with time. Is the change constant?
▶️Answer/Explanation
Ans: There is an average increase of 4.3% each decade in the concentration of carbon dioxide. The graph illustrates that there is a steady and constant increase in carbon dioxide in the atmosphere. Overall, there has been a 21% increase in the last 40 years. The concentration of methane in the atmosphere does not change in a consistent manner when compared to carbon dioxide. The average increase for each decade is 3.7%. For dinitrogen oxide, the increase in concentration has been steady.
Question:
Compare the concentration of carbon dioxide recorded in 2017 to the concentration of methane and nitrous oxide in the same year. By what factor do these concentrations differ?
▶️Answer/Explanation
Ans: CO2 400 ppm, CH4 1775 ppb, N2O 328 ppb. Their ratio is 1220 : 5.41 : 1.00.
Question:
What is the main source of nitrous oxide gas in the Earth’s atmosphere? State and justify if there is a link between global development and the concentration of nitrous oxide gas.
▶️Answer/Explanation
Ans: Natural sources of nitrogen oxides include lightning, volcanoes and bacteria. Human–made emissions include fossil fuel combustion from power generation and transportation. The scientific community has proven a link between global development and the increase of greenhouse gas emissions.
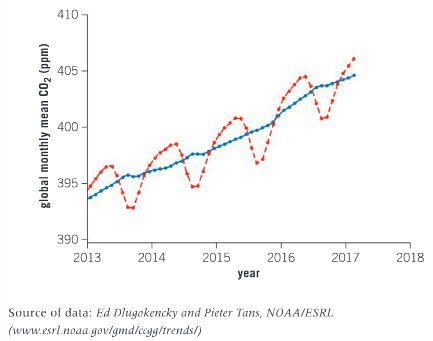
The graph below shows global concentration of carbon dioxide from 2013 to the start of 2017. The dashed red line shows the mean value of carbon dioxide concentration per month. The blue line represents the same, but takes the seasonal cycles of carbon dioxide concentration into account, and is corrected as such.
Question:
Compare the concentration of carbon dioxide in Tasmania and globally over the period 2013–2017. Why are we able to compare this data and make comparisons even though the data originates from different parts of the globe?
▶️Answer/Explanation
Ans: The atmosphere around the globe is not isolated to individual countries. When a country creates a large amount of greenhouse gases, the effects are global as opposed to regional. The fight against increasing greenhouse gas emissions and the accelerated increase in the Earth’s temperature is a global issue.
Summative assessment
The effect of change in concentration on the rate of reaction
Question:
In terms of energy, explain why only a certain proportion of reacting particles in a chemical system will be transformed from reactants into products, at a given temperature
▶️Answer/Explanation
Ans: All chemical reactions have an activation energy that reacting particles must overcome in order for the reaction to occur; at a given temperature a certain proportion of the reacting particles will have sufficient energy to overcome the activation energy barrier.
Question:
Applying your understanding of chemical kinetics, suggest reasons why science needs to find ways to increase the rate of a chemical reaction.
▶️Answer/Explanation
Ans: All matter found on Earth is finite and there is recognition in the scientific community of the need to use important resources efficiently; the cost to industry of increasing the rate of a chemical reaction by increasing the temperature of the reaction mixture or the concentration of the reactants, can be prohibitively expensive; cost effective methods such as catalysts increase the rate of reaction.
Question:
The reaction between calcium carbonate, a metal carbonate, and hydrochloric acid, a strong acid, is a vigorous reaction that produces a gas, carbon dioxide.
a) Write an equation for the reaction between calcium carbonate and hydrochloric acid.
▶️Answer/Explanation
Ans: CaCO3(s) + 2HCl(aq) → CaCl2(aq) + CO2(g) + H2O(l)
(reactant formula; product formula; balanced equation)
A series of simple experiments were performed and the data collected was plotted on a graph:
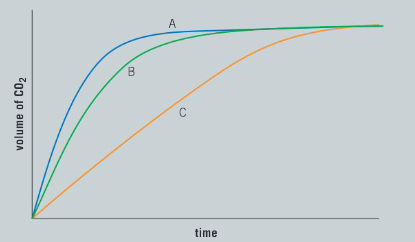
b) The three curves are the result of different concentrations of hydrochloric acid. Which curve represents the highest concentration and which represents the lowest? Justify your answer using scientific reasoning.
▶️Answer/Explanation
Ans: Curve A represents the highest concentration of hydrochloric acid; this curve has the steepest slope over the initial stages of the reaction; Curve C represents the lowest concentration of hydrochloric acid; this curve has the least steep slope over the initial stages of the reaction.
c) All of the curves finish at the same point on the graph. What does this tell us about the reaction?
▶️Answer/Explanation
Ans: All three chemical reactions have the same number of moles of calcium carbonate; the same volume of carbon dioxide is produced in each reaction.
d) Predict the limiting reagent in this reaction.
▶️Answer/Explanation
Ans: Calcium carbonate is the limiting reagent.
The collision theory describes three conditions that need to be met by particles, in order for them to react successfully. When these conditions are met, a reaction will proceed at a rate specific for a
given set of conditions. We have learnt that you can change the rate of a reaction by altering the temperature, concentration and surface area of reactants or by adding a catalyst.
The thiosulfate ion reacts with hydrogen ions supplied by hydrochloric acid, a strong acid. Elemental sulfur forms a suspension and is the reason for a color change in the reaction mixture:
\(S_{2}O_{3}^{2-}(Aq)+2H^{+}(Aq)\rightarrow H_{2}O(l)+SO_{2}(g)+S(s)\)
Question:
Design an experiment between 1.0 mol dm−3 sodium thiosulfate and 1.0 mol dm−3 hydrochloric acid to achieve a color change from colorless to yellow in 60 seconds exactly. You are permitted to change any variable to achieve this outcome. You could use the following points for guidance.
▶️Answer/Explanation
Ans: Design should include clear statement of:
- research question that is focused,
- suitable hypothesis is suggested; hypothesis is testable; hypothesis is based on scientific reasoning
- independent and dependent variables,
- rationale for the method and practical details, including
- correct names of apparatus and volume
- amounts and/or concentration of chemicals being used
- consideration of safety, ethical and environmental issues
- description of the step–by–step methodology for the investigation, including how the variables are controlled
- description of how qualitative observations will be recorded
- identification of quantitative data that will be recorded and the design of data tables to present this information
- analysis of data and the formulation of a conclusion that is described and justified
Marks awarded on a scale from 0 marks for a completely inadequate design to 10 marks for an exemplary design.
Activation energy of a simple chemical reaction
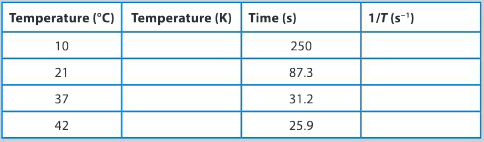
Some students performed the reaction between the thiosulfate ion and hydrogen ions at a range of temperatures. They then recorded their experimental data, using the following table.
Question:
Why it is important that you carefully consider how you will present your quantitative data?
▶️Answer/Explanation
Ans: Quantitative data should be presented in a way that allows the reader to analyze the data and establish if a trend exists; data should be recorded to reflect the level of precision with which it was collected.
Question:
Copy the data table and calculate the temperature in kelvin (K)
▶️Answer/Explanation
Ans: 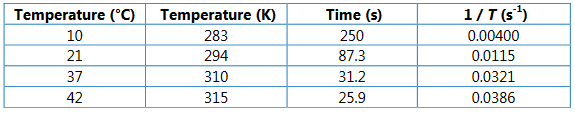
Question:
Construct a temperature (K) versus time (s) graph and draw the appropriate smooth curve.
▶️Answer/Explanation
Ans: 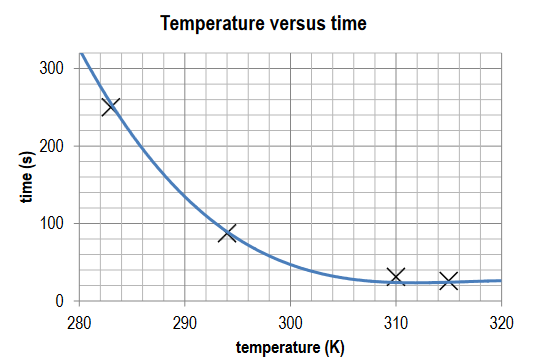
Points plotted accurately; title recorded and axis labelled; smooth curve drawn; anomalous data identified.
Question:
Identify and describe the relationship between temperature and time.
▶️Answer/Explanation
Ans: As the temperature increases, the time taken for the reaction to occur decreases.
Question:
Calculate inverse time (s−1) and record the process data to three significant figures.
▶️Answer/Explanation
Ans: Design should include clear statement of:
- research question that is focused,
- suitable hypothesis is suggested; hypothesis is testable; hypothesis is based on scientific reasoning
- independent and dependent variables,
- rationale for the method and practical details, including
- correct names of apparatus and volume
- amounts and/or concentration of chemicals being used
- consideration of safety, ethical and environmental issues
- description of the step–by–step methodology for the investigation, including how the variables are controlled
- description of how qualitative observations will be recorded
- identification of quantitative data that will be recorded and the design of data tables to present this information
- analysis of data and the formulation of a conclusion that is described and justified
Marks awarded on a scale from 0 marks for a completely inadequate design to 10 marks for an exemplary design.
Question:
Construct a temperature (K) versus 1/T (s−1) graph. State the relationship illustrated by this form of graph.
▶️Answer/Explanation
Ans: 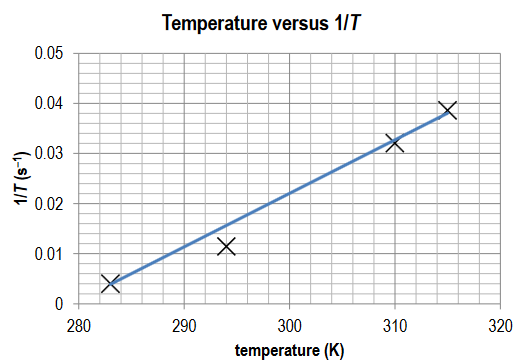
Points plotted accurately; straight line drawn; there is a linear relationship between temperature and 1/T.
Question:
Explain why it is useful for different graphs to be used to represent experimental data.
▶️Answer/Explanation
Ans: A graph is an effective means of communicating the effect the independent variable will have on the dependent variable; sometimes a line of best fit is required to illustrate the relationship between the independent and dependent variable if the data does not follow an exact linear relationship.
Question:
Having examined the data collected, comment on the accuracy of the data given the shape of the curve. Are there any data points that you would consider to be anomalous? Anomalous data is that which does not follow the general trend.
▶️Answer/Explanation
Ans: A comment describing the linear relationship between the independent and dependent variable and how close the data is to the trendline; the identification of anomalous data that lies outside the trendline.
Question:
Evaluate and make a conclusion about the effect of concentration on the rate of reaction and support this conclusion with scientific reasoning.
▶️Answer/Explanation
Ans: As the concentration of a reactant increases the rate of reaction also increases; a large number of reactant particles will result in an increase in the frequency of collisions; the number of particles with sufficient energy to overcome the activation energy barrier will increase with an increase in the concentration.
The importance of catalysts
The article here was written by XiaoZhi Lim and published in the scientific journal Nature, in 2016 (http://www.nature.com/news/the- new-breed-of-cutting-edge-catalysts-1.20538). Read the following extract carefully and then complete the tasks that follow.
Question:
How does the process of catalysis impact on sustainability?
▶️Answer/Explanation
Ans: Catalysis has an important impact on sustainability because it results in less pollution; as by–products are often not produced.
Question:
Discuss the positive implications of the use of catalysts in energy production.
▶️Answer/Explanation
Ans: In energy production, catalysis is allowing energy sources to be used which were previously more difficult to use, to replace dirtier energy sources such as coal, oil or gas; examples are the use of water which can be split into hydrogen fuel and oxygen gas and other materials such as carbon dioxide and biomass.
Question:
Evaluate and explain how advances in computing power are assisting research in the field of catalysis.
▶️Answer/Explanation
Ans: Computers are being used to model catalysts; allowing research and intervention to progress at much faster rates.
Question:
Many catalysts are made of expensive, scarce metals such as palladium, platinum and iridium. Scientists are attempting to address this issue by researching alternative catalysts for industrial
processes. Describe the benefits of the use of cheaper, more abundant metals such as iron, nickel or copper as an alternative to more expensive, rare metals.
▶️Answer/Explanation
Ans: The benefits of using cheaper, more abundant metals are that they will be able to be used more frequently; and as they are more abundant the supply will not be limited.