Question:
Predict the products of each chemical reaction by constructing a word equation.
▶️Answer/Explanation
Ans: sodium chloride + silver nitrate
↓
sodium nitrate + silver chloride
sodium bromide + silver nitrate
↓
sodium nitrate + silver bromide
sodium iodide + silver nitrate
↓
sodium nitrate + silver iodide
Question:
Using the solubility rules, state the salt that forms the insoluble precipitate.
▶️Answer/Explanation
Ans: All nitrates salts are soluble in water therefore the insoluble salts are the silver halides.
Question:
Analyze the colors of the precipitates formed and describe the trend in color of the halide salt as you move down Group 17 from chlorine to bromine to iodine.
▶️Answer/Explanation
Ans: Silver chloride is a white precipitate, silver bromide is a pale yellow precipitate and silver iodide is a cream precipitate. As you move down group 17, the silver halide precipitates become a darker colour.
Question:
Predict the state of matter of each of the products of the following reactions:
a) lead(II) nitrate + sodium sulfate ➝ lead(II) sulfate + sodium nitrate
▶️Answer/Explanation
Ans: lead(II) nitrate(aq) + sodium sulfate(aq)
↓
lead(II) sulfate(s) + sodium nitrate(aq)
b) calcium bromide + sodium carbonate ➝ calcium carbonate + sodium bromide
▶️Answer/Explanation
Ans: calcium bromide(aq) + sodium carbonate(aq)
↓
calcium carbonate(s) + sodium bromide(aq)
c) barium chloride + silver nitrate ➝ barium nitrate + silver chloride
▶️Answer/Explanation
Ans: barium chloride(aq) + silver nitrate(aq)
↓
barium nitrate(aq) + silver chloride(s)
d) potassium bromide + silver nitrate ➝ potassium nitrate + silver bromide
▶️Answer/Explanation
Ans: potassium bromide(aq) + silver nitrate(aq)
↓
potassium nitrate(aq) + silver bromide(s)
e) sodium iodide + silver nitrate ➝ sodium nitrate + silver iodide
▶️Answer/Explanation
Ans: sodium iodide(aq) + silver nitrate(aq)
↓
sodium nitrate(aq) + silver iodide(s)
Question:
Construct the balanced chemical equations for each of the reactions above.
The components of a solution are:
● the solvent—the liquid into which the solid will dissolve
● the solute—the solid substance to be dissolved
● the solution—the resulting combination of solvent and solute, combined in a single phase.
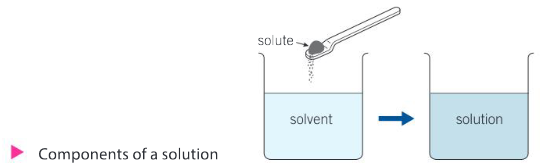
What makes some salts insoluble in water? Some molecules are polar (see Chapter 9, Models), and some are not. A general rule for the formation of solutions is like dissolves like.
▶️Answer/Explanation
Ans: a) Pb(NO3)2 (aq) + Na2SO4(aq)
↓
PbSO4 (s)+ 2NaNO3(aq)
b) CaBr2(aq) + Na2CO3(aq)
↓
CaCO3(s) + 2NaBr(aq)
c) BaCl2(aq) + 2AgNO3(aq)
↓
Ba(NO3)2 (aq) + 2AgCl(s)
d) KBr(aq) + AgNO3(aq)
↓
KNO3 (aq) + AgBr(s)
e) NaI(aq) + AgNO3(aq)
↓
NaNO3(aq) + AgI(s)
The balanced chemical equation for this reaction is:
Pb(NO3)2(aq) + 2KI(aq) ➝ 2KNO3(aq) + PbI2(s)
Question:
What observations did you make? Were they what you expected?
▶️Answer/Explanation
Ans: When you mix two colorless solutions of lead(II) nitrate and potassium iodide, a bright yellow solution rapidly forms.
Question:
What term do we use to describe this type of reaction?
▶️Answer/Explanation
Ans: A precipitation reaction.
Question:
After 10 minutes, what did you observe? What was the color of the precipitate that formed?
▶️Answer/Explanation
Ans: After 10 minutes, the yellow precipitate has settled to the bottom of the beaker and a colorless solution fills the rest of the beaker.
Question:
In this type of reaction we often refer to the presence of spectator ions. These are ions that do not play a direct part in the reaction. Focusing on the formation of the precipitate
lead(II) iodide, what are the spectator ions in this reaction?
▶️Answer/Explanation
Ans: The spectator ions in this reaction are the nitrate ion and the potassium ion.
Question:
Following filtration, what is the color of the lead salt you have made?
▶️Answer/Explanation
Ans: The lead(II) iodide salt is yellow in colour.
Question:
Why are the characteristic colors of transition metal compounds useful for identifying compounds?
▶️Answer/Explanation
Ans: When you have different compounds that are white and cream solids, it is very difficult to identify these compounds visually. However, many of the transition metal compounds have very characteristic colors, for example, copper(II) sulfate is well known and easily identified by its bright blue colour.
Question:
Examine your results. Are there any pigments that are common to two or more food colorings? How did you decide that they were the same pigment?
▶️Answer/Explanation
Ans: There are two pigments that are common to more than two food colorings. They are the same pigment as they have the same Rf value.
Question:
What does the retention value tell you about the solubility of the separated component? Explain your reasoning.
▶️Answer/Explanation
Ans: A pigment with a small Rf value is less soluble in the solvent while a pigment with a large Rf value is more soluble in the solvent. Pigments that are more soluble tend to move with the solvent front higher up the chromatography paper.
Question:
What effect would a different solvent have on the results of this experiment? Compare your ideas with others.
▶️Answer/Explanation
Ans: A different solvent may change the Rf of the pigments as their solubility in the new solvent may differ from their solubility in the original solvent.
Question:
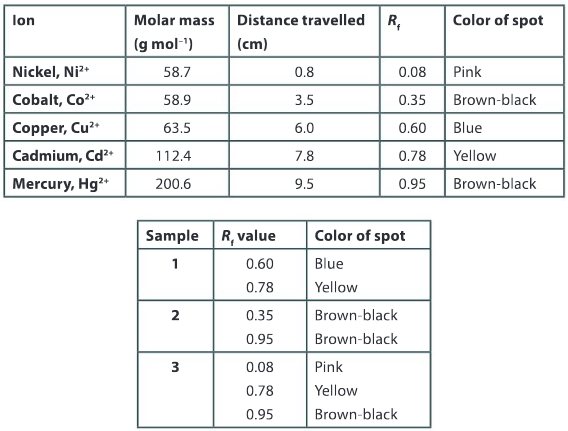
Identify the ions present in Sample 2.
▶️Answer/Explanation
Ans: The metal ions cobalt Co2+ and mercury Hg2+ are present in sample 2.
Question:
Identify the ions present in Sample 3.
▶️Answer/Explanation
Ans: The metal ions nickel Ni2+, cadmium Cd2+ and mercury Hg2+ are present in sample 3.
Question:
Analyze the data and list the ions in the first table from the fastest to the slowest.
▶️Answer/Explanation
Ans: Ni2+, Co2+, Cu2+, Cd2+, Hg2+.
Question:
Sketch the appearance of the chromatography paper of sample 1 after the analysis has been completed.
▶️Answer/Explanation
Ans: The sketch should include a rectangular piece of chromatography paper, with a blue dot positioned 6 cm from the baseline and a yellow dot positioned 7.8 cm from the baseline.
Question:
What do you observe about the relationship between the molar mass and the retention factor?
▶️Answer/Explanation
Ans: In general, the larger the molar mass the greater the retention factor.
Question:
Calculate the molar mass of the following compounds using the values of Ar found in the periodic table.
a) CO2
b) SO3
c) HNO3
d) Na2CO3
e) KMnO4
f) CaCl2
g) Al(NO3)3
h) Fe2(SO4)3
i) (NH4)2SO4
j) Na2S2O3.7H2O
▶️Answer/Explanation
Ans: a) 44.01 g mol–1 f) 110.98 g mol–1
b) 80.07 g mol–1 g) 213.01 g mol–1
c) 63.02 g mol–1 h) 399.91 g mol–1
d) 105.99 g mol–1 i) 132.17 g mol–1
e) 158.04 g mol–1 j) 284.26 g mol–1
Question:
Calculate the number of moles in each of the following masses:
a) 9.8 g of sulfuric acid, H2SO4
b) 25.0 g of calcium carbonate, CaCO3
c) 8.0 g of sodium hydroxide, NaOH
d) 60 g of glucose, C6H12O6
▶️Answer/Explanation
Ans: a) 9.8 g / 98.09 g mol–1 = 0.10 mol
b) 0.250 mol
c) 0.20 mol
d) 0.33 mol
Question:
Calculate the mass (in grams) in each of the following:
a) 0.25 mol of carbon dioxide, CO2
b) 3 mol of ammonia, NH3
c) 0.710 mol of calcium phosphate, Ca3(PO4)2
d) 0.211 mol of iron(III) oxide, Fe2O3
▶️Answer/Explanation
Ans: a) 0.25 mol × 44.01 g mol–1 = 11 g
b) 51.12 g
c) 0.710 mol × 310.18 g mol–1 = 220 g (3 sf)
d) 0.211 mol × 159.7 g mol–1 = 33.7 g (3 sf)
Question:
Calculate the number of particles present in the following:
a) 9.80 g of phosphoric acid, H3PO4
b) 60.77 g of iron(II) sulfate, FeSO4
c) 12.10 g of ethanoic acid, C2H4O2
d) 6.40 g of sulfur dioxide, SO2
▶️Answer/Explanation
Ans: a) 9.80g / 98.0 g mol–1 = 0.100 mol
0.100 × 6.02 × 1023 = 6.02 × 1022 particles
b) 2.41 × 1023 particles
c) 1.21 × 1023 particles
d) 6.01 × 1023 particles
Question:
Calculate the volume of each of the following samples of gases at STP:
a) 7.6 g of argon, Ar
b) 100 g of ethene, C2H4
▶️Answer/Explanation
Ans: a) 7.6 g / 39.95 g mol–1 = 0.19 mol
0.19 mol × 22.7 dm3 mol–1 = 4.3 dm3
b) 80.9 dm3
Question:
Magnesium burns in the presence of oxygen to form the metal oxide, magnesium oxide. This oxidation reaction is commonly performed in a school laboratory. A student weights out 18.0 g of magnesium for the reaction. The equation for the reaction is as follows:
Mg(s) + O2(g) ➝ MgO(s)
a) Balance the chemical equation.
b) Calculate the number of moles of the reactant, magnesium.
c) If oxygen is in excess and all the magnesium is used up, calculate the number of moles of magnesium oxide formed.
d) What mass of magnesium oxide is produced?
▶️Answer/Explanation
Ans: a) 2Mg(s) + O2(g) → 2MgO(s)
b) 18.0 g / 24.31 g mol–1 = 0.740 mol
c) Mg:MgO mole ratio is 1:1
0.740 mol of MgO produced
d) mass = moles × molar mass = 0.740 × 40.31
= 29.8 g
Question:
What mass of barium sulfate would be produced when 10 g of barium chloride is completely consumed in the following reaction?
BaCl2(aq) + H2SO4(aq) ➝ BaSO4(aq) + 2HCl(aq)
▶️Answer/Explanation
Ans: moles of BaCl2 = 10 g / 208.23 g mol–1 = 0.048 mol
mole ratio BaCl2:BaSO4 is 1:1
mass of BaSO4 = 0.048 mol × 233.4 g mol–1 = 11 g
Question:
Copper sulfate reacts with sodium hydroxide to produce a precipitate of copper hydroxide.
CuSO4(aq) + 2NaOH(aq) ➝ Cu(OH)2(s) + Na2SO4(aq)
a) Calculate the mass of sodium hydroxide needed to convert 15.95 g of copper sulfate into copper hydroxide.
▶️Answer/Explanation
Ans: moles of CuSO4 = 15.95 g / 159.62 g mol–1
= 0.09992 mol (4 sf)
mole ratio CuSO4:NaOH is 1:2
mass of NaOH
= 2 × 0.09992 mol × 40.00 g mol–1
= 7.994 g (4 sf)
b) Calculate the mass of copper hydroxide produced.
▶️Answer/Explanation
Ans: mass = moles × molar mass
= 0.09992 × 97.57 = 9.750 g
Summative assessment
Separating the components of sea water
Student A is given the task of designing an experiment to separate and collect the components of a sample of sea water, NaCl(aq). They are provided with standard laboratory
apparatus. Following the experiment, the student recorded the following data for analysis.
Initial volume of sea water = 200.00 cm3
Volume of water recovered = 196 cm3
Mass of salt NaCl recovered = 68.8 g
The student in their research for a laboratory report discovered the following facts.

Question:
Discuss which separation technique the student would have used to separate the sodium chloride from water. Give reasons for your choice of technique.
▶️Answer/Explanation
Ans: Sodium chloride and water have boiling points which are significantly different; the use of a simple distillation technique would result in the water being initially evaporated, then condensed and collected while the sodium chloride would remain in the flask and be crystallized.
Question:
Use the experimental data to determine the amount (in grams) of sodium chloride that was dissolved in 100 cm3 of the salt water sample.
▶️Answer/Explanation
Ans: 68.8 g of NaCl in 196 cm3 of water;
100/196 = 0.510;
0.510 × 68.8 = 35.1 g / 100 cm3
Question:
A saturated solution contains the maximum amount of a solute dissolved for a given solvent at a specific temperature. For this experiment, this amount is 35.9 g/100 cm3.
State if the solution of sea water used by the student is a saturated solution. Show all working to support your answer.
▶️Answer/Explanation
Ans: Amount of salt in solution = 35.1 g / 100 cm3; amount of NaCl in the sea water used is not the maximum possible (35.9 g / 100 cm3); sea water used in this experiment is unsaturated.
Question:
Calculate the number of moles of sodium chloride that was recovered by this separation process.
▶️Answer/Explanation
Ans: Moles of NaCl = 68.8 g / 58.44 g mol–1;
= 1.18 mol
Question:
Suggest reasons why all of the water was not collected during this separation process.
▶️Answer/Explanation
Ans: Water vapor lost from the apparatus; sodium chloride crystals not completely dry on separation; random errors and uncertainties determined by apparatus and human actions.
Question:
Student B, in the same class, only managed to collect a sample of the solid sodium chloride. With the technique chosen by this student, the water is lost to the surroundings.
a) Describe the technique used by student B.
▶️Answer/Explanation
Ans: Saltwater is placed into a piece of apparatus, such as a large beaker, and placed on top of a tripod and gauze mat; Bunsen burner is lit and placed beneath the beaker; when the water evaporates into the surroundings, the sodium chloride crystals remain in the beaker and can be collected.
b) Offer ideas why it is sometimes important to recover the solvent from a solution.
▶️Answer/Explanation
Ans: The release of solvents in their gaseous form into the environment can be harmful to both the environment and people working in the laboratory; solvents can be expensive and it is more cost effective to collect them to be reused again.
Separating mixtures
The American Chemical Society defines analytical chemistry as “the science of obtaining, processing, and communicating information about the composition and structure of matter. In other words, it is the art and science of determining what matter is and how much of it exists”.
Simple separation techniques can be used in the school laboratory to separate substances from one another and help identify the composition of a mixture. Specific analytical tests for the presence of cations and anions can be used to identify the composition of ionic salts.
This investigation has been broken up into three parts.
1. Mixture A is a combination of three different miscible alcohols.
2. Mixture B is a mixture of four different solids.
3. Three different ionic salts, are labelled A, B and C. You are told the possible composition of the salts. You have to identify the contents of A, B and C.
Mixture A

Mixture B
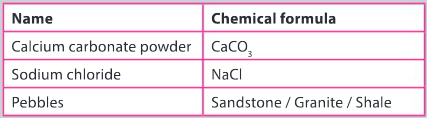
Unknown compounds A, B and C
Three white ionic solids are labelled Solid A, B and C. The identity of each compound is unknown. They could be one of the following compounds:
● Barium iodide
● Potassium bromide
● Sodium chloride
Additional information
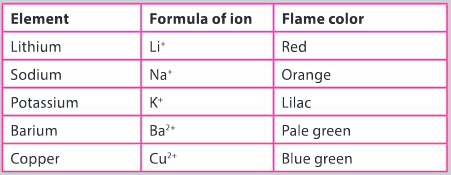
Flame tests are used in the identification of cations.
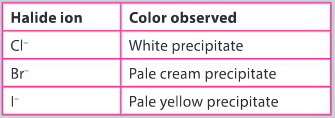
The addition of silver nitrate solution to halide ion solution results in the formation of insoluble salts, each with a characteristic color.
Question:
Design a series of experiments to:
a) Separate the individual fractions of the alcohol mixture and identify the composition of the fraction.
▶️Answer/Explanation
Ans: Physical property of the alcohols which is utilized in the separation technique is stated; choice of separation technique is stated; justification of the use of this technique is outlined; methodology for the separation technique is outlined; use of a thermometer to determine the boiling point of the individual alcohols is clearly stated.
b) Separate and recover the three solids and confirm the identity of sodium chloride and calcium carbonate by performing a number of simple tests.
▶️Answer/Explanation
Ans: Method and separation technique described to initially separate calcium carbonate powder from a sodium chloride and pebble mixture; secondary separation technique described to separate and recover sodium chloride from the pebbles; a description of a chemical reaction involving both solids calcium carbonate and sodium chloride recovered and separated from the pebbles; the gas evolved in one of the reactions can be tested with lime water; perform flame tests on both solids to confirm their identity.
c) Identify solids A, B and C.
▶️Answer/Explanation
Ans: Design should include clear statement of:
- description of the step–by–step methodology for the investigation including how to perform a flame test and testing for the presence of halide ions
- description of how qualitative observations will be recorded and how these observations can be used to identify a positive test for a cation and an anion
Marks awarded on a scale from 0 marks for a completely inadequate design to 5 marks for an exemplary design.
Evaluating the choice of methods
Question:
The behavior of substances under different conditions, how they function, can be investigated to determine the most appropriate method of physical separation technique. Examine the following investigations and using your knowledge of the physical and chemical properties of the substances in the mixture, evaluate the validity of the method chosen to separate the components of the mixture. In each case:
● State the validity of the technique chosen. Is it the most appropriate technique?
● Explain improvements or extensions to the method of separation
▶️Answer/Explanation
Ans: Investigation A
Chromatography is the most appropriate technique for separating and identifying components of a liquid mixture; if there was a need to isolate the individual components, fractional distillation could be used if the boiling points of the individual components were sufficiently different.
Investigation B
The method selected is not the most appropriate; as activated carbon is insoluble in ethanol, filtration will effectively separate the solid activated carbon from liquid ethanol.
Investigation C
The method selected is not the most appropriate; two immiscible liquids can be accurately separated using a glass separatory funnel.
Solar power and tomato farming
This article appeared on the MIT Technology Review website dated October 2016. Read the passage and then answer the questions.
Question:
Identify and list the science and technology being used by Sundrop Farms in South Australia to grow tomatoes.
▶️Answer/Explanation
Ans: Solar power for generating electrical energy; desalination for the extraction of pure water.
Question:
Determine the problem that this technology is being used to solve.
▶️Answer/Explanation
Ans: This technology enables society to grow tomatoes in the dry landscape of South Australia.
Question:
Despite the reported great success of this project, there is criticism of the final outcome—growing tomatoes. Evaluate the proposition made in New Scientist by Paul Kristiansen from the University of New England and debate with your peers if this criticism is justified.
▶️Answer/Explanation
Ans: Students prepare points from the article and then debate them (four).
Question:
The writers of this article suggest that selective breeding and genetic modification of crops will be more practical in poorer, arid regions. What are their arguments to support this?
▶️Answer/Explanation
Ans: Breeding and genetic modification of crops will be more effective and practical in poorer, dry regions, as desalination of water in order to grow existing crops is a very expensive and energy–intensive process; poorer countries would not be able to afford to build sufficient desalination plants.
Question:
Explain why this project is an example of how science and technology can alter the natural relationships that exist between food crops and the natural environment.
▶️Answer/Explanation
Ans: The intervention of science and technology changes the dynamics in this project and others, as plants are grown under artificial conditions such as in greenhouses or hot houses and irrigated with fresh water which is produced from naturally occurring seawater; plants are therefore growing in environments where they do not occur or grow naturally.
Question:
What is your opinion of the Sundrop Farms project in South Australia? Evaluate the benefits and potential downsides of such a project. Structure your answer as a summary that would enable comparison with your peers.
▶️Answer/Explanation
Ans: Identify at least two benefits and two potential downsides of this project in your summary.