CBSE Class 12 Physics MCQs Solution All Chapters
Free PDF Download of CBSE Physics Multiple Choice Questions for Class 12 with Answers Chapter 1 Electric Charges and Fields. Physics MCQs for Class 12 Chapter Wise with Answers PDF Download was Prepared Based on Latest Exam Pattern. Students can solve NCERT Class 12 Physics Electric Charges and Fields MCQs Pdf with Answers to know their preparation level.
Atoms Class 12 Physics MCQs
1. If 13.6 eV energy is required to ionise the hydrogen atom, then energy required to remove an electron from n = 2 is
(a) 10.2 eV
(b) 0 eV
(c) 3.4 eV
(d) 6.8 eV.
Answer/Explanation
Answer: c
Explaination: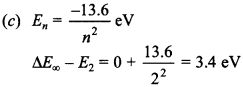
2. In Bohr’s model of an atom which of the following is an integral multiple of \(\frac{h}{2 \pi}\)?
(a) Kinetic energy
(b) Radius of an atom
(c) Potential energy
(d) Angular momentum
Answer/Explanation
Answer:
Explaination:
(d) Angular momentum L = mvr = \(\frac{n h}{2 \pi}\)
3. In Bohr’s model, the atomic radius of the first orbit is rQ. Then, the radius of the third orbit is
(a) r0/9
(b) r0
(c) 9r0
(d) 3r0
Answer/Explanation
Answer: c
Explaination:
rn = rnn²
∴ r3 = 9r0
4. The K.E. of the electron in an orbit of radius r in hydrogen atom is proportional to
Answer/Explanation
Answer: b
Explaination:
(b) \(\frac{e^{2}}{2 r}\), Scince K.E = \(\frac{k e^{2}}{2 r}\)
5. The ratio between Bohr radii is
(a) 1 : 2 : 3
(b) 2 : 4 : 6
(c) 1 : 4 : 9
(d) 1 : 3 : 5
Answer/Explanation
Answer: c
Explaination:
(c) 1 : 4 : 9, In Bohr’s atomic model, rn n²
6. The longest wavelength in Balmer series of hydrogen spectrum will be
(a) 6557 Å
(b) 1216 Å
(c) 4800 Å
(d) 5600 Å
Answer/Explanation
Answer: a
Explaination:
(a) 6557 Å
For longest wavelength in Balmer series n1 = 2 and n2 = 3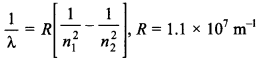
7. In terms of Rydberg constant R, the wave number of the first Balmer line is
(a) R
(b) 3R
(c) \(\frac{5 R}{36}\)
(d) \(\frac{8 R}{9}\)
Answer/Explanation
Answer: c
Explaination:
8. The ionisation energy of hydrogen atom is 13.6 eV. Following Bohr’s theory the energy corresponding to a transition between 3rd and 4th orbits is
(a) 3.40 eV
(b) 1.51 eV
(c) 0.85 eV
(d) 0.66 eV
Answer/Explanation
Answer: d
Explaination: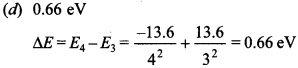
9. The energy of hydrogen atom in the nth orbit is En, then the energy in the nth orbit of single ionised helium atom is
Answer/Explanation
Answer: c
Explaination:
(c) As energy E ∝ Z²
For hydrogen atom Z = 1,
for Helium Z = 2
EHe = 4En.
10. On moving up in the energy states of a H-like atom, the energy difference between two consecutive energy states
(a) decreases.
(b) increases.
(c) first decreases then increases.
(d) first increases then decreases.
Answer/Explanation
Answer: a
Explaination:
(a) As, En = \(\frac{-13.6}{n^{2}}\)
11. The transition of electron from n = 4, 5, 6, ………. to n = 3 corresponds to
(a) Lyman series
(b) Balmer series
(c) Paschen series
(d) Brackettseries
Answer/Explanation
Answer: c
Explaination:
(c) In transition from n1 = 3 and n2 = 4, 5, 6,….
Infrared radiation of Paschen spectral is emitted.
12. As per Bohr model, the minimum energy (in eV) required to remove an electron from the ground state of double ionized Li atom (Z = 3) is
(a) 1.51 eV
(b) 13.6 eV
(c) 40.8 eV
(d) 122.4 eV
Answer/Explanation
Answer: d
Explaination:
(d) Since energy of electron in nth state of hydrogen like atom is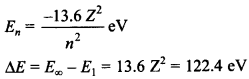
13. Which of the following spectral series in hydrogen atom gives spectral line of 4860 A?
(a) Lyman
(b) Balmer
(c) Paschen
(d) Brackett
Answer/Explanation
Answer: b
Explaination:
(b) Since spectral line of wavelength 4860 A
lies in the visible region of the spectrum which is Balmer series of the spectrum.
14. In terms of Rydberg constant R, the shortest wavelength in Balmer series of hydrogen atom spectrum will have wavelength
Answer/Explanation
Answer: b
Explaination:
(b) For shortest wavelength n1 =∞, n2 = 2![]()
15. The first model of atom in 1898 was proposed by
(a) Ernst Rutherford
(b) Albert Einstein
(c) J.J. Thomson
(d) Niels Bohr
Answer
Answer: c
16. In Geiger-Marsden scattering experiment, the trajectory traced by an a-particle depends on
(a) number of collision
(b) number of scattered a-particles
(c) impact parameter
(d) none of these
Answer
Answer: c
17. In the Geiger-Marsden scattering experiment the number of scattered particles detected are maximum and minimum at the scattering angles respectively at
(a) 0° and 180°
(b) 180° and 0°
(c) 90° and 180°
(d) 45° and 90°
Answer
Answer: a
18. In the Geiger-Marsden scattering experiment, is case of head-on collision the impact parameter should be
(a) maximum
(b) minimum
(c) infinite
(d) zero
Answer
Answer: a
19. Rutherford’s experiments suggested that the size of the nucleus is about
(a) 10-14 m to 10-12 m
(b) 10-15 m to 10-13 m
(c) 10-15 m to 10-14 m
(d) 10-15 m to 10-12 m
Answer
Answer: c
20. Which of the following spectral series falls within the visible range of electromagnetic radiation?
(a) Lyman series
(b) Balmer series
(c) Paschen seriee
(d) Pfund series
Answer
Answer: b
21. The first spectral series was discovered by
(a) Balmer
(b) Lyman
(c) Paschen
(d) Pfund
Answer
Answer: a
22. Which of the following postulates of the Bohr model led to the quantization of energy of the hydrogen atom?
(a) The electron goes around the nucleus in circular orbits.
(b) The angular momentum of the electron can only be an integral multiple of h/2π.
(c) The magnitude of the linear momentum of the electron is quantized.
(d) Quantization of energy is itself a postulate of the Bohr model.
Answer
Answer: b
23. The Bohr model of atoms
(a) assumes that the angular momentum of elec-trons is quantized.
(b) uses Einstein’s photoelectric equation.
(c) predicts continuous emission spectra for at-oms.
(d) predicts the same emission spectra for all types of atoms.
Answer
Answer: a
24. If tt is the orbit number of the electron in a hydrogen atom, the correct statement among the following is
(a) electron energy increases as n increases.
(b) hydrogen emits infrared rays for the electron transition from n = to n = 1
(c) electron energy is zero for n = 1 (<0 electron energy varies as n2.
Answer
Answer: a
25. If the radius of inner most electronic orbit of a hydrogen atom is 5.3 * 10~n m, then the radii of n = 2 orbits is
(a) 1.12 Å
(b) 2.12 Å
(c) 3.22 Å
(d) 4.54 Å
Answer
Answer: b
26. The diagram shows the energy levels for an electron in a certain atom. Which transition shown represents the emission of a photon with the most energy?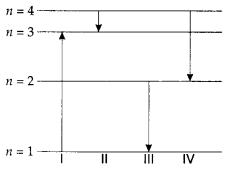
(a) I
(b) II
(c) III
(d) IV
Answer
Answer: c
27. In a hydrogen atom, the radius of nth Bohr orbit is rn. The graph between log(rn/r1) and log n will be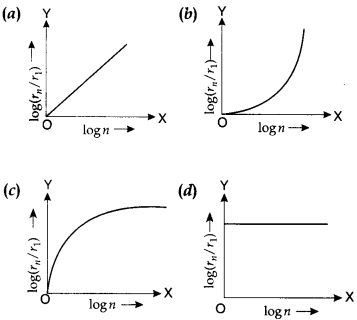
Answer
Answer: a
28. The transition from the state n = 5 to n = 1 in a hydrogen atom results in UV radiation. Infrared radiation will be obtained in the transition
(a) 2 → 1
(b) 3 → 2
(c) 4 → 3
(d) 6 → 2
Answer
Answer: c
29. The hydrogen atom can give spectral lines in the Lyman, Balmer and Paschen series. Which of the following statement is correct?
(a) Lyman series is in the infrared region.
(b) Balmer series is in the visible region.
(c) Paschen series is in the visible region.
(d) Balmer series is in the ultraviolet region.
Answer
Answer: b
30. Which of the relation is correct between time period and number of orbits while an electron is resolving in an orbit?
Answer
Answer: c
31. Energy of an electron in the second orbit of hydrogen atom is E and the energy of electron in 3rd orbit of He will be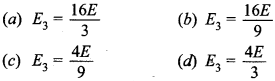
Answer/Explanation
Answer: b
Explaination: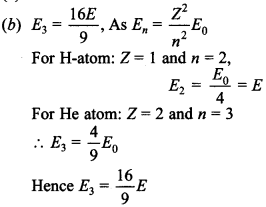
32. The spectral lines in the Brackett series arise due to transition of electron in hydrogen atom from higher orbits to the orbit with
(a) n = 1
(b) n = 2
(c) n = 3
(d) n = 4
Answer
Answer: d
33. Taking the Bohr radius as a0 = 53 pm, the radius of Li++ ion in its ground state, on the basis of Bohr’s model, will be about [NCERT Exemplar]
(a) 53 pm
(b) 27 pm
(c) 18 pm
(d) 13 pm
Answer
Answer: c
34. The simple Bohr model cannot be directly applied to calculate the energy levels of an atom with many electrons. This is because [NCERT Exemplar]
(a) of the electrons not being subject to a central force.
(b) of the electrons colliding with each other.
(c) of screening effects.
(d) the force between the nucleus and an electron will no longer be given by Coulomb’s law.
Answer
Answer: a
35. For the ground state, the electron in the H-atom has an angular momentum = h, according to the simple Bohr model. Angular momentum is a vector and hence there will be infinitely many orbits with the vector pointing in all possible directions. In actuality, this is not true, [NCERT Exemplar]
(a) because Bohr model gives incorrect values of angular momentum.
(b) because only one of these would have a minimum energy.
(c) angular momentum must be in the direction of spin of electron.
(d) because electrons go around only in horizontal orbits.
Answer
Answer: a
36. 02 molecule consists of two oxygen atoms. In the molecule, nuclear force between the nuclei of the two atoms [NCERT Exemplar]
(a) is not important because nuclear forces are short-ranged.
(b) is as important as electrostatic force for binding the two atoms.
(c) cancels the repulsive electrostatic force between the nuclei.
(d) is not important because oxygen nucleus has equal number of neutrons and protons.
Answer
Answer: a
37. Two H atoms in the ground state collide inelastically. The maximum amount by which their combined kinetic energy is reduced is [NCERT Exemplar]
(a) 10.20 eV
(b) 20.40 eV
(c) 13.6 eV
(d) 27.2 eV
Answer
Answer: a
38. A set of atoms in an excited state decays. [NCERT Exemplar]
(a) in general to any of the states with lower energy.
(b) into a lower state only when excited by an external electric field.
(c) all together simultaneously into a lower state.
(d) to emit photons only when they collide.
Answer
Answer: a
39. An ionised H-molecule consists of an electron and two protons. The protons are separated by a small distance of the order of angstrom. In the ground state, [NCERT Exemplar]
(a) the electron would move in circular orbits.
(b) the energy would be (2)4 times that of a H-atom.
(c) the electrons, orbit would go around the protons.
(d) the molecule will soon decay in a proton and a H-atom.
Answer
Answer: c
40. The Balmer series for the H-atom can be observed
(a) if we measure the frequencies of light emitted when an excited atom falls to the ground state.
(b) if we measure the frequencies of light emitted due to transitions between excited states and the first excited state.
(c) in any transition in a H-atom.
(d) as a sequence of frequencies with the lower frequencies getting closely packed.
Answer
Answer: b
41. Let \(E_{n}=\frac{-1}{8 \varepsilon_{0}^{2}} \frac{m e^{4}}{n^{2} h^{2}}\) be the energy of the nth level of H-atom. If all the H-atoms are in the ground state and radiation of frequency (E2 – E1)/h falls on it,
(a) it will not be absorbed at all.
(b) some of atoms will move to the first excited state.
(c) all atoms will be excited to the n = 2 state.
(d) all atoms will make a transition to the n = 3 state.
Answer
Answer: d
42. The Bohr model of an atom
(a) assumes that the angular momentum of electrons is quantised.
(b) uses Einstein’s potoelectric equation.
(c) predicts continuous emission spectra for atoms,
(d) predicts the same emission spectra for all types of atoms.
Answer
Answer: a
43. For ionising an exicited hydrogan atom, the energy required (in eV) will be
(a) a little less than 13.6 eV
(b) 13.6 eV
(c) more than 13.6 eV
(d) 3.4 or less
Answer/Explanation
Answer: d
Explaination:
(d) As the energy of the electron is – -3.4 eV in first excited state and magnitude is less for higher excited state.
44. The electrons in the Bohr’s orbit have
(a) K.E. greater than P.E.
(b) P.E. greater than K.E.
(c) the same values
(d) none of these
Answer/Explanation
Answer: a
Explaination: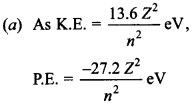
45. The binding energy of a H-atom, considering an electron moving around a fixed nuclei (proton), is
\(B=-\frac{m e^{4}}{8 n^{2} \varepsilon_{0}^{2} h^{2}}\). (m = electron mass)
If one decides to work in a frame of reference where the electron is at rest, the proton would be moving arround it. By similar arguments, the binding energy would be
\(B=-\frac{M e^{4}}{8 n^{2} \varepsilon_{0}^{2} h^{2}}\)(M= proton mass)
This last expression is not correct because [NCERT Exemplar]
(a) n would not be integral.
(b) Bohr-quantisation applies only to electron.
(c) the frame in which the electron is at rest is not inertial.
(d) the motion of the proton would not be in circular orbits, even approximately.
Answer
Answer: c
46. Consider aiming a beam of free electrons towards free protons. When they scatter, an electron and a proton cannot combine to produce a H-atom,
(a) because of energy loss.
(b) without simultaneously releasing energy in the from of radiation.
(c) because of momentum conservation.
(d) because of angular momentum conservation.
Answer
Answer: b
47. The Bohr model for the spectra of a H-atom
(a) will be applicable to hydrogen in the molecular from.
(b) will not be applicable as it is for a He- atom.
(c) is valid only at room temperature.
(d) predicts continuous as well as discrete spectral lines.
Answer
Answer: b
48. Non-radiating electron orbits in an atom are called __________ orbits.
Answer/Explanation
Answer:
Explaination: stationary
49. At distance of closest approach, kinetic energy of a-particle is __________ .
Answer/Explanation
Answer:
Explaination: zero
50. The centripetal force required for revolution of electron in an orbit is provided by __________ between the electron and the nucleus.
Answer/Explanation
Answer:
Explaination: electrostatic attraction
51. __________ is the perpendicular distance of the velocity vector of the a-particle from the centre of the nucleus.
Answer/Explanation
Answer:
Explaination: Impact parameter
52. The number of waves, contained in unit length of the medium is called __________ .
Answer/Explanation
Answer:
Explaination: wave number
53. Angular momentum and energy of an electron in an atom is __________ .
Answer/Explanation
Answer:
Explaination: quantised.
54. Number of possible spectral lines emitted on dexcitation of electron from energy level n to ground state is equal to __________ .
Answer/Explanation
Answer:
Explaination: \(\frac{n(n-1)}{2}\)
55. In the Rutherford scattering experiment, die distance of closest approach for an a-particle is dQ. If an a-particle is replaced by a proton, how much kinetic energy in comparison to a-particle will it require to have the same distance of closest approach d0?
Answer/Explanation
Answer:
Explaination:
At distance of closest approach, the K.E. with the charged particle is converted into electrostatic P.E.
As q is half with a proton in comparison to a-particle, for same d, energy E has to be made half.![]()
56. What is the ratio of radii of the orbits corresponding to first excited state and ground state in a hydrogen atom?
Answer/Explanation
Answer:
Explaination: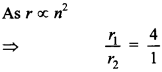
57. Write the expression for Bohr’s radius in hydrogen atom.
Answer/Explanation
Answer:
Explaination:
Bohr’s radius in hydrogen atom,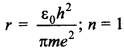
58. Find the ratio of energies of photons produced due to transition of an electron of hydrogen atom from its
(i) second permitted energy level to the first level, and
(ii) the highest permitted energy level to the first permitted level.
Answer/Explanation
Answer:
Explaination:![]()
59. What is the distance of closest approach?
Answer/Explanation
Answer:
Explaination:
The minimum distance up to which an energetic a-particle travelling directly towards a nucleus can reach.
60. An electron in a hydrogen atom is revolving round a positively charged nucleus. Which two physical quantities explain the orbit of an electron?
Answer/Explanation
Answer:
Explaination:
Two physical quantities are:
(i) angular momentum, and
(ii) total energy of electron.
61. What will happen if an electron instead of revolving becomes stationary in H-atom?
Answer/Explanation
Answer:
Explaination:
Then the electrostatic field of the nucleus will attract the electron into the nucleus itself.
62. Calculate the speed of electron revolving around the nucleus of a hydrogen atom in order that it may not be pulled into the nucleus by electrostatic attraction.
Answer/Explanation
Answer:
Explaination:
It is only possible when the centripetal force is equal to electrostatic force of attraction.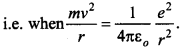
63. What is the value of ionization energy for a hydrogen atom?
Answer/Explanation
Answer:
Explaination: 13.6
Atoms Class 12 MCQs Questions with Answers
Question 1.
A spectral line is emitted when an electron
(a) jumps from lover orbit to higher orbit.
(b) jumps from higher orbit to lower orbit.
(c) rotates in a circular orbit.
(d) rotates in an elliptical orbit.
Answer
Answer: (b) jumps from higher orbit to lower orbit.
Question 2.
The ionisation potential of hydrogen is 13.6 V. The energy of the atom in n = 2 state will be
(a) -10.2 eV
(b) -6.4eV
(c) – 3.4 eV
(d) – 4.4 eV
Answer
Answer: (c) – 3.4 eV
Question 3.
At the time of total solar eclipse, the spectrum of solar radiation would be
(a) a large number of dark Fraunhoffer lines
(b) a small number of dark Fraunhofer lines.
(c) All Fraunhofer lines changed into brilliant colours.
(d) None of these.
Answer
Answer: (c) All Fraunhofer lines changed into brilliant colours.
Question 4.
The adjoining figure indicates the energy levels of a certain atom when the system moves from 2 E to E level, a photon of wavelength λ is emitted. The wavelength of photon produced during its transition from \(\frac {4E}{3}\) to E is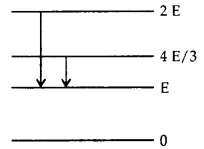
(a) \(\frac {λ}{3}\)
(b) \(\frac {3λ}{4}\)
(c) \(\frac {4λ}{3}\)
(d) 3λ
Answer
Answer: (d) 3λ
Question 5.
A hydrogen atom is in the p-state. For this, values of J are
(a) \(\frac {5}{2}\), \(\frac {3}{2}\), \(\frac {1}{2}\)
(b) \(\frac {3}{2}\), \(\frac {1}{2}\)
(c) –\(\frac {1}{2}\),\(\frac {1}{2}\), \(\frac {3}{2}\)
(d) –\(\frac {1}{2}\), \(\frac {-3}{2}\)
Answer
Answer: (b) \(\frac {3}{2}\), \(\frac {1}{2}\)
Question 6.
Energy levels A, B, C of a certain atom correspond to increasing value of energy i.e., EA > EB > EC. If λ1, λ2 and λ3 are the wavelengths of radiation corresponding to transition C to B, B to A and C to A respectively, which of these of the following is correct?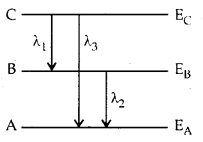
(a) λ3 = λ1 +d λ2
(b) λ3 = \(\frac {λ_1λ_2}{λ_1+λ_2}\)
(c) λ1 + λ2 + λ3 = 0
(d) λ\(_{3}^{2}\) = λ\(_{1}^{2}\) and λ\(_{2}^{2}\)
Answer
Answer: (b) λ3 = \(\frac {λ_1λ_2}{λ_1+λ_2}\)
Question 7.
In Rutherford’s scattering experiment with gold foil, 232 counts per minute are observed at an angle of 60°. The number of counts/min. at an angle of 120° will be
(A) 232
(b) 116
(c) 26
(d) 52
Answer
Answer: (c) 26
Question 8.
In an atom, the two electrons move round the nucleus in circular orbits of radii R and 4R. The ratio of the times taken by them to complete one revolution is
(a) \(\frac {1}{8}\)
(b) \(\frac {1}{4}\)
(c) 4
(d) 8
Answer
Answer: (a) \(\frac {1}{8}\)
Question 9.
The ratio of the energies of the hydrogen atom in its first to second excited state is :
(a) \(\frac {1}{4}\)
(b) \(\frac {4}{9}\)
(c) \(\frac {9}{4}\)
(d) 4.
Answer
Answer: (c) \(\frac {9}{4}\)
Question 10.
In Bohr’s model of the hydrogen atom, the ratio between the period of revolution of an electron in the orbit n = 1 to the period of revolution of electron in the orbit n = 2 is
(a) \(\frac {1}{2}\)
(b) \(\frac {1}{4}\)
(c) \(\frac {1}{8}\)
(d) 2.
Answer
Answer: (c) \(\frac {1}{8}\)
Question 11.
According to Bohr’s theory, the radius of electron in an orbit described by the principal quantum number n and the atomic number Z is propotional to :
(a) Z²n²
(b) \(\frac {Z^2}{n^2}\)
(c) \(\frac {Z^2}{n}\)
(d) \(\frac {n^2}{Z}\)
Answer
Answer: (d) \(\frac {n^2}{Z}\)
Question 12.
The electron in hydrogen atom jumps from the 3rd orbit to second orbit. The wavelength X of the emitted radiations is
(a) \(\frac {36}{5R}\)
(b) \(\frac {5}{36}\)R
(c) \(\frac {5}{R}\)
(d) \(\frac {R}{6}\)
Answer
Answer: (a) \(\frac {36}{5R}\)
Question 13.
To explain fine structure of spectrum of hydrogen atom, we must consider.
(a) a finite size of nucleus.
(b) the presence of neutrons in the nucleus.
(c) spin angular momentum.
(d) orbital angular momentum.
Answer
Answer: (b) the presence of neutrons in the nucleus.
Question 14.
The ratio of the energy of the electron in first orbit to that in the second orbit is
(a) \(\frac {1}{4}\)
(b) \(\frac {1}{2}\)R
(c) 2
(d) 4
Answer
Answer: (d) 4
Question 15.
When an electron jumps from some outer orb it to the innermost orbit in the hydrogen atom, the spectral line belongs to
(a) Lyman series
(b) Balmer series
(c) Paschen series
(d) Pfund series
Answer
Answer: (a) Lyman series
Question 16.
How does the energy difference between two consecutive energy levels vary on the quantum number n increases?
(a) does not change
(b) decrases
(c) increases
(d) may increase or decrease.
Answer
Answer: (b) decrases
Question 17.
According to classical theory, Rutherford atom is
(a) stable
(b) unstable
(c) metastable
(d) semistable
Answer
Answer: (b) unstable
Question 18.
For an electron orbit to be non-radiating, it should be
(a) such that the angular momentum should be integral multiple of h.
(b) circular in nature
(c) elliptical in nature
(d) none of these
Answer
Answer: (a) such that the angular momentum should be integral multiple of h.
Question 19.
Which of the following type ot radiation is not emitted by the electronic structure of atoms :
(a) X-rays
(b) Visible light
(c) γ-rays
(d) Ultraviolet light.
Answer
Answer: (c) γ-rays
Question 20.
If the electron in hydrogen atoms is excited to n = 5 state, the number of different frequencies of radiation which may be emitted is:
(a) 4
(b) 10
(c) 8
(d) 5
Answer
Answer: (b) 10
Question 21.
The ratio of the angular momentum of an electron in first orbit to that in the second orbit is
(a) \(\frac {1}{2}\)
(b) \(\frac {1}{4}\)
(c) 4
(d) 2
Answer
Answer: (a) \(\frac {1}{2}\)
Fill in the Blanks
Question 1
………………… of the electron in the orbit signifies that the electron and nucleus is a bound system.
Answer
Answer: Negative energy.
Question 2.
The ………………… lies in the infrared region of the spectrum.
Answer
Answer: Paschen series.
Question 3.
Lyman series lies in the ………………… region of spectrum and Balmer series lies in the ………………… of the spectrum.
Answer
Answer: Ultraviolet, visible region.
Question 4.
The difference of energy levels goes on ………………… as we move towards higher energy levels.
Answer
Answer: decreasing.
Question 5.
Separation between the orbits goes on ………………… as we move towards higher orbits.
Answer
Answer: increasing.
Question 6.
The radius of the first orbit of hydrogen atom is ………………… times the radius of first orbit of a H-like helium atom.
Answer
Answer: two.
Question 7.
The minimum energy required to excite a hydrogen atom from its ground state is …………………
Answer
Answer: 10.2 eV.
Question 8.
In a hydrogen atom, the electron moves in an orbit of radius 0.5 Å making 1016 revolutions per second. The magnetic dipole moment associated with the orbital motion of the electron is …………………
Answer
Answer: 256 × 10-23 Am².
Question 9.
Band spectrum is produced by the substance in ………………… state.
Answer
Answer: molecular.
Question 10,
Rutherford’s a-particle scattering experiment shows the existence of a ………………… charged nucleus of ………………… size located at the …………………
Answer
Answer: Positively, very small, centre of the atom.
Question 11.
The maximum number of photons emitted when an electron jumps from an energy level n = 4 to n = 1 is …………………
Answer
Answer: 6.
Question 12.
The radius of Bohr’s first orbit is a0. The electron in nth orbit has a radius …………………
Answer
Answer: n² a0
Question 13.
The kinetic energy associated with an electron decreases with an ………………… in the radii of the orbits.
Answer
Answer: increase.
Question 14.
For a given projectile and target, the distance of closest approach ………………… with increase in K.E. of the projectile.
Answer
Answer: decreases.
Question 15.
In scattering of α-particles by nucleus, the distance of closest approach depends upon the charges of ………………… and ………………… as well as ………………… of α-particle.
Answer
Answer: Projectile, target nucleus, kinetic-energy.
Question 16.
The ionisation energy of hydrogen atom is E. When the electron in a hydrogen atom jumps from the state n = 1 to the state n = 2, the energy absorbed by it is …………………
Answer
Answer: \(\frac {3E}{4}\)
Question 17.
The energy of the atom goes on ………………… as we go to higher excited states.
Answer
Answer: increasing.
Question 18.
From Bohr’s theory, when an electron jumps from higher energy orbit to second orbit, the spectral lines that occur belong to ………………… series.
Answer
Answer: Balmer.
Question 19.
When a hydrogen atom is raised from the ground state to an excited state, them P.E ………………… and Kinetic energy …………………
Answer
Answer: increases, decreases.
Question 20.
If elements with principal quantum number n > 4 were not existed in nature then the number of possible electrons would be …………………
Answer
Answer: 60.
