| Alcohols, Phenols and Ethers NEET and AIIMS Special |
| Alcohols, Phenols and Ethers Refresher Course |
| Alcohols, Phenols and Ethers : Master File |
| Alcohols, Phenols and Ethers : Quick Revision Notes |
| Alcohols, Phenols and Ethers : Concepts File |
| Alcohols, Phenols and Ethers : Brain Map |
| Alcohols Reference Book |
| Phenols Reference Book |
| Ethers Reference Book |
About this unit
Alcohols: Nomenclature, methods of preparation, physical and chemical properties (of primary alcohols only); identification of primary, secondary and tertiary alcohols; mechanism of dehydration, uses with special reference to methanol and ethanol. Phenols: Nomenclature, methods of preparation, physical and chemical properties, acidic nature of phenol, electrophillic substitution reactions, uses of phenols. Ethers : Nomenclature, methods of preparation, physical and chemical properties uses.
ALCOHOLS, PHENOLS AND ETHERS
ALCOHOLS
Alcohols are organic compounds containing hydroxyl (OH) group and can be considered analogues of water
CLASSIFICATION OF ALCOHOLS
Alcohols are usually classified as primary, secondary and tertiary
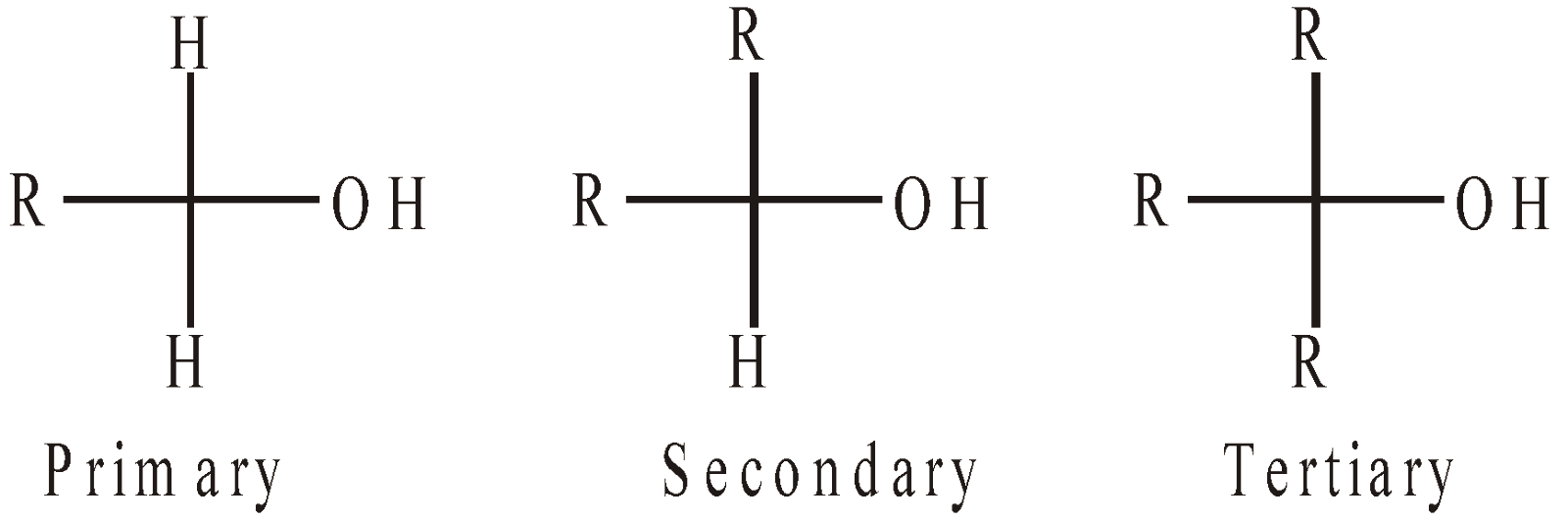
Further they may be
monohydric – containing one OH group
monohydric – containing one OH group
dihydric – containing two OH groups
trihydric – containing three OH groups
Phenols: Compounds with hydroxyl group bound directly to an aromatic (benzene)
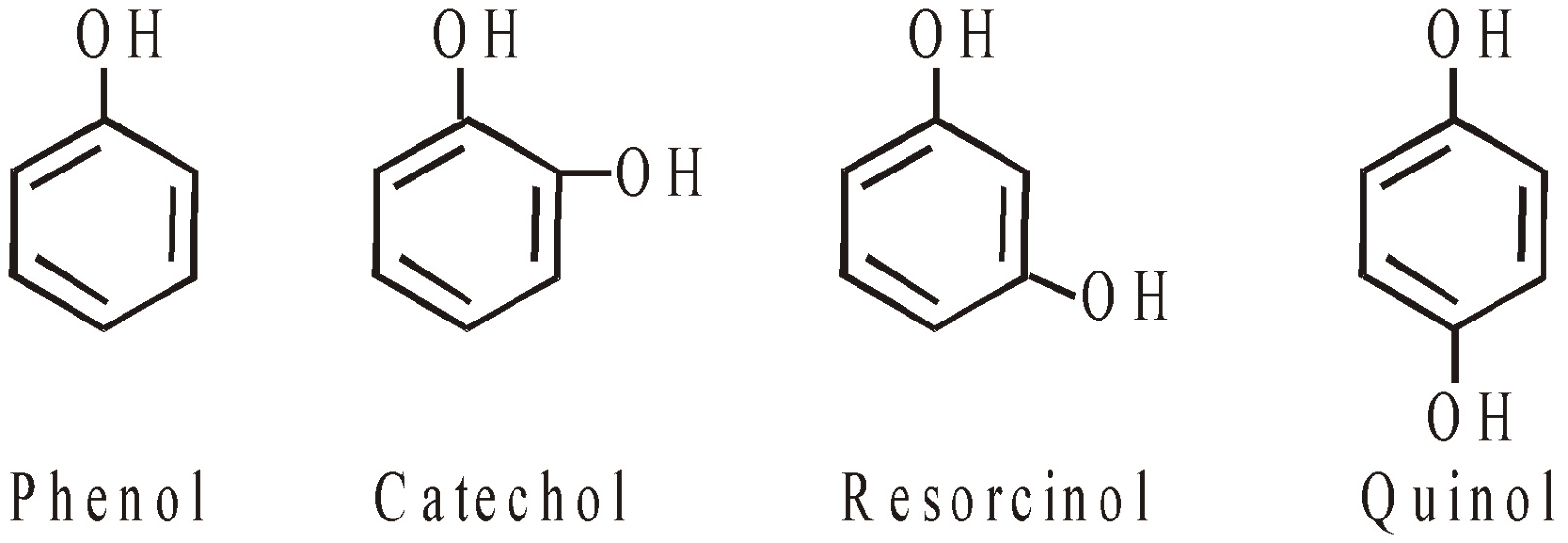
NOMENCLATURE OF ALCOHOLS
There are three systems of nomenclature
COMMON SYSTEM
According to this system alcohols are named as Alkyl alcohols eg.
CARBINOL SYSTEM
In this system the alcohols are regarded as derivatives of methyl alcohol which is expressed as “carbinol”.
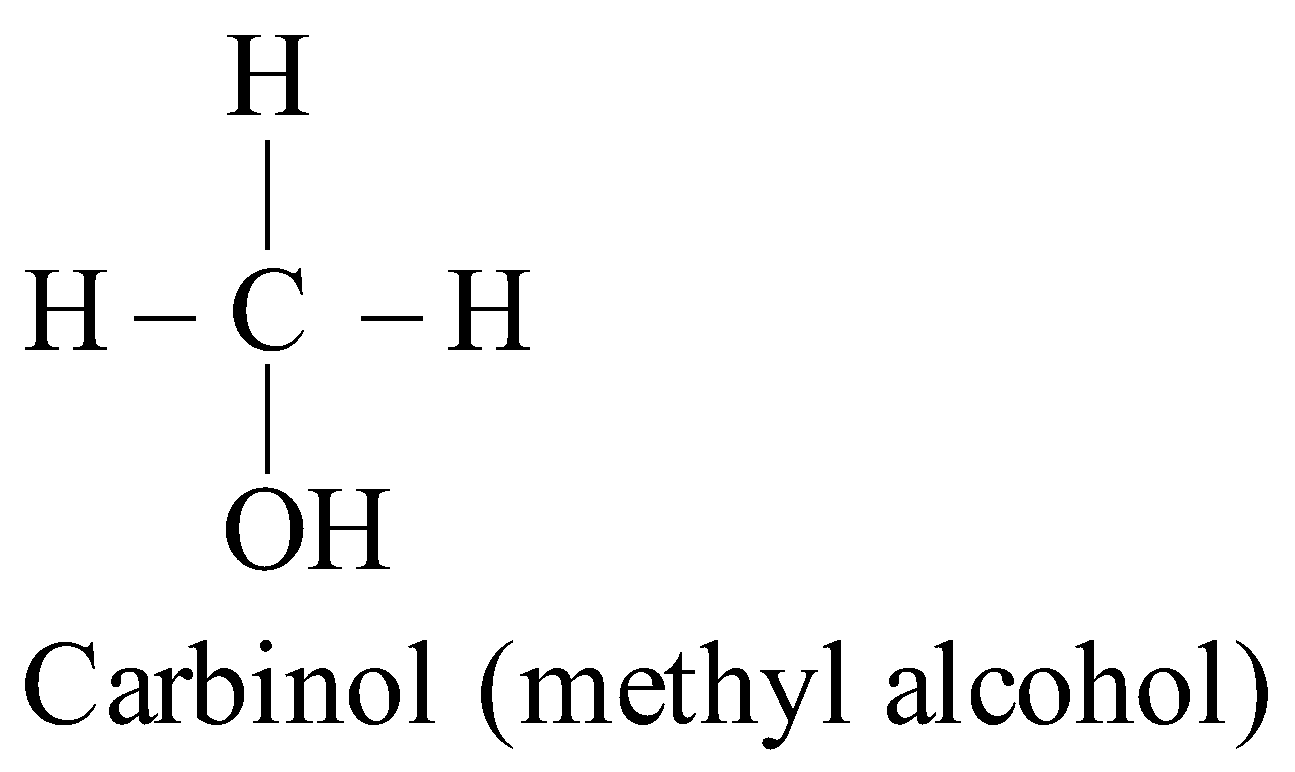
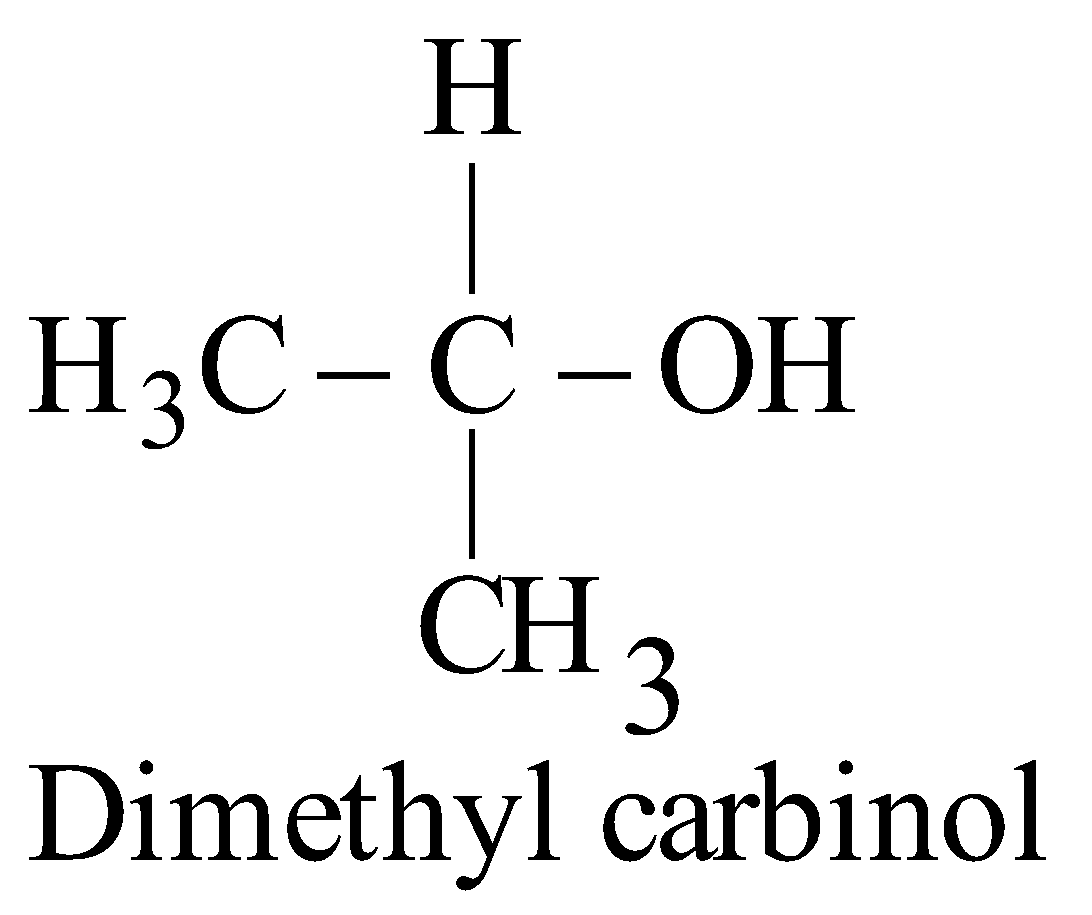
IUPAC SYSTEM
- Drop the last -e from the alkane name and add -ol to obtain the root name.
- Number the longest chain starting at the end nearest the –OH group
- Name the remaining substituents and their numbers as for alkanes and alkenes.
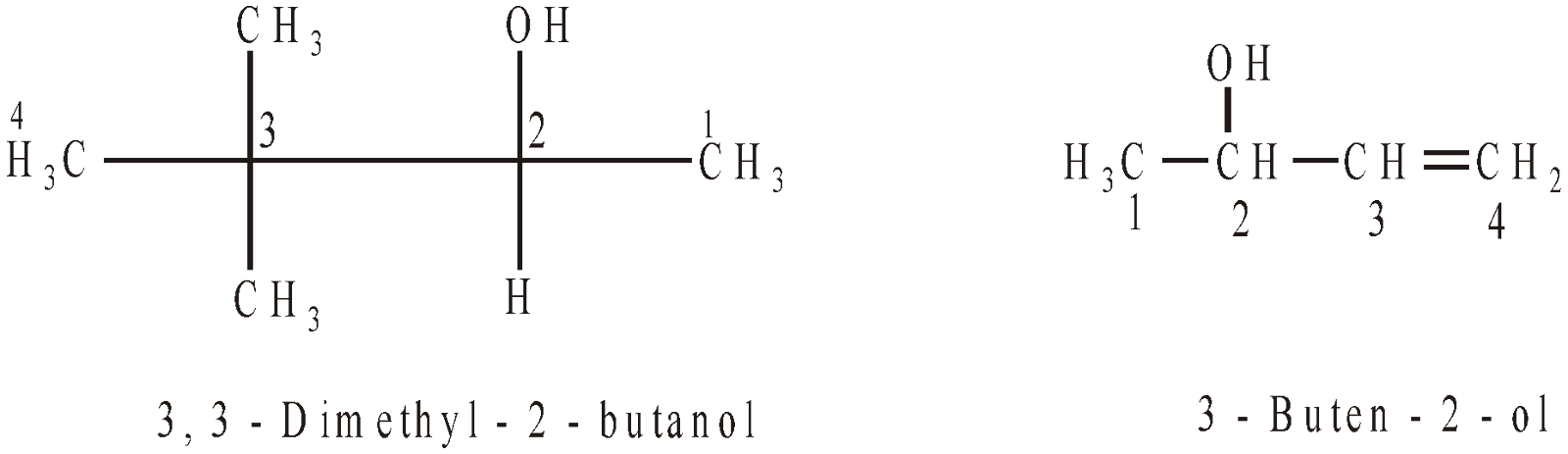
Cyclic alcohols have the prefix cyclo, and the hydroxyl group is deemed to be on C-1
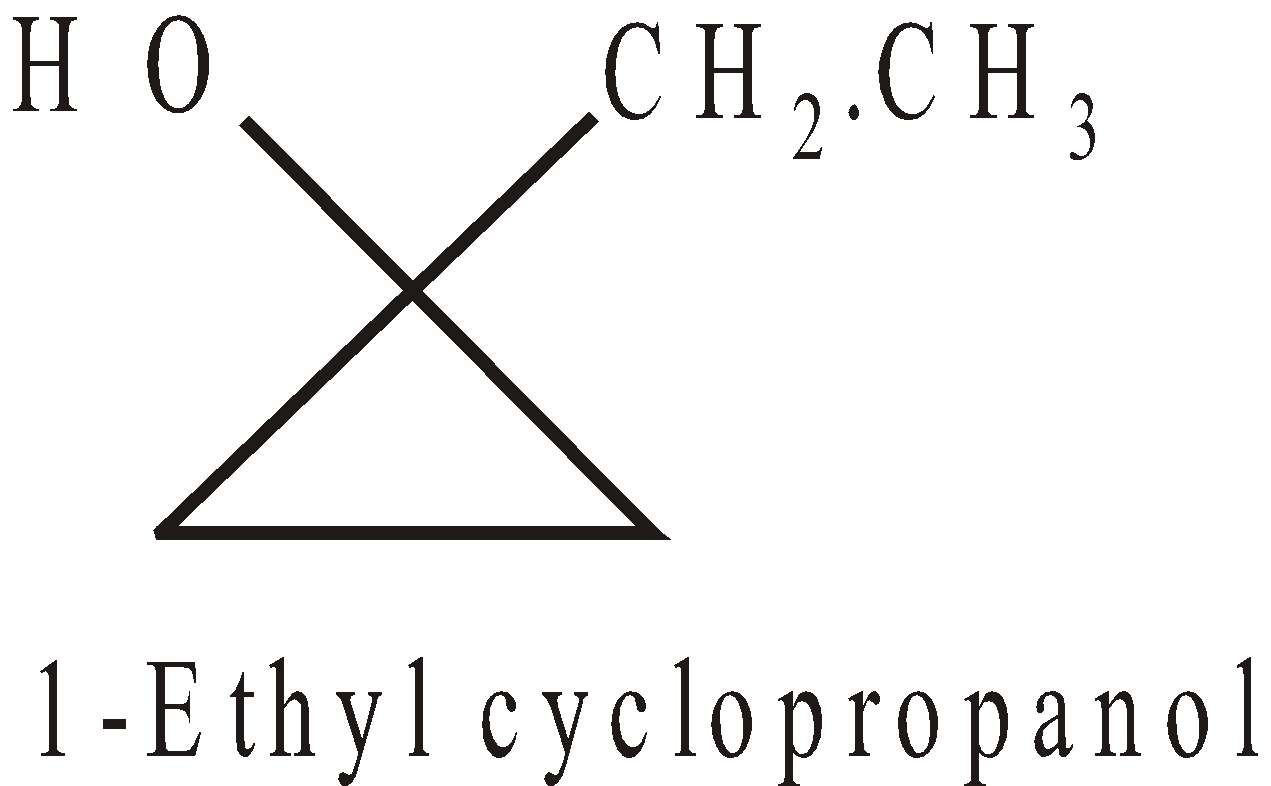
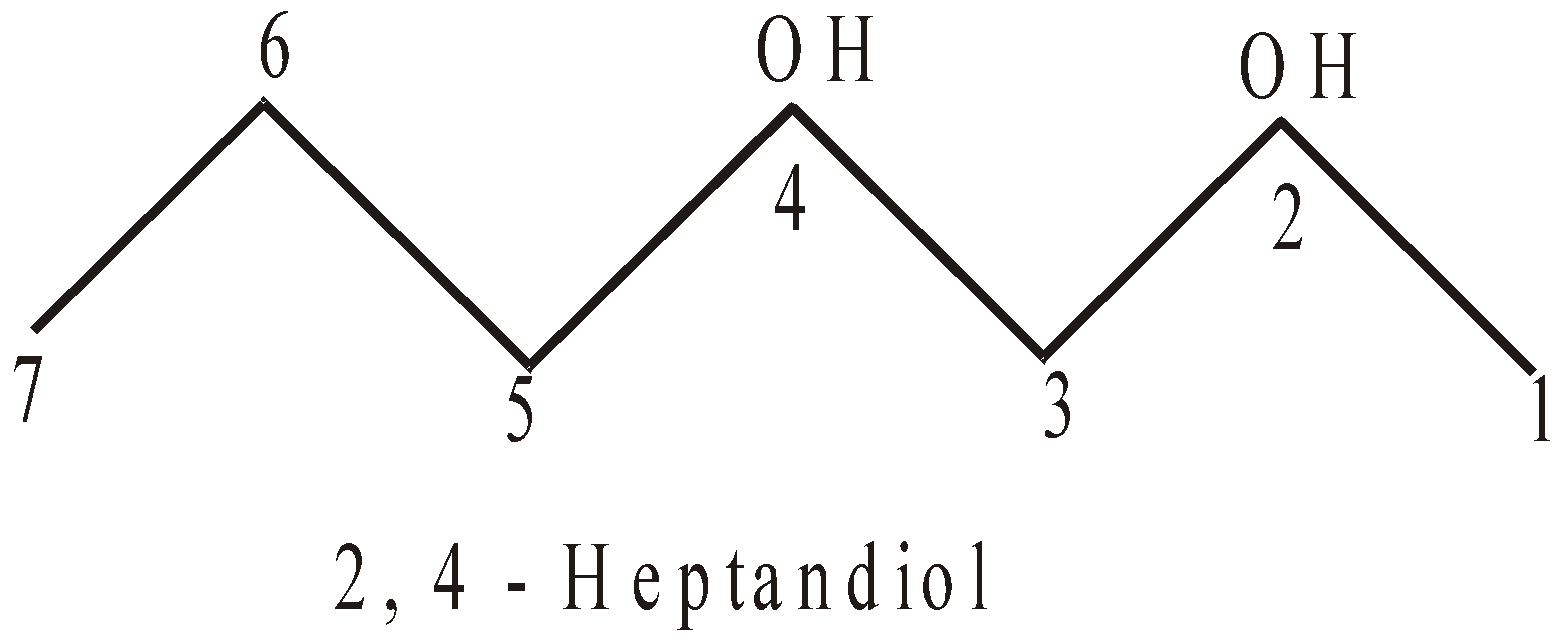
The compounds with two hydroxyl groups are known as diols
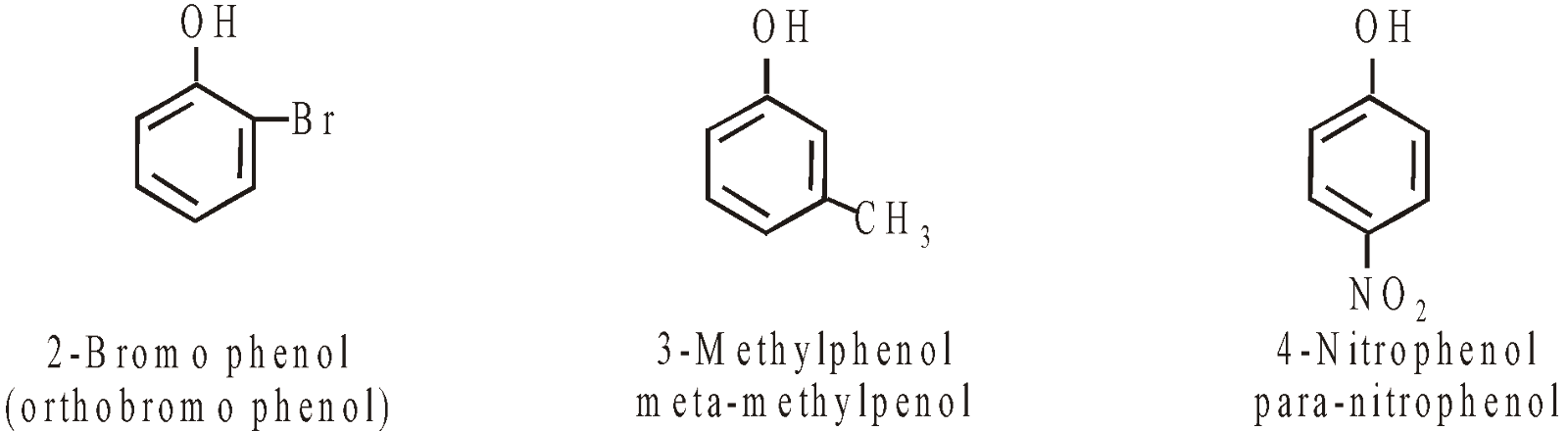
In case of phenols the position of substituents are represented by numbers (the terms ortho, meta and para are non IUPAC)
STRUCTURE
The oxygen atom in alcohols and phenols is sp3 hybridised and they have tetrahedral disposition of hybrid atomic orbitals. The two hybrid atomic orbitals have lone pair of electrons and remaining two are involved in bond formation.
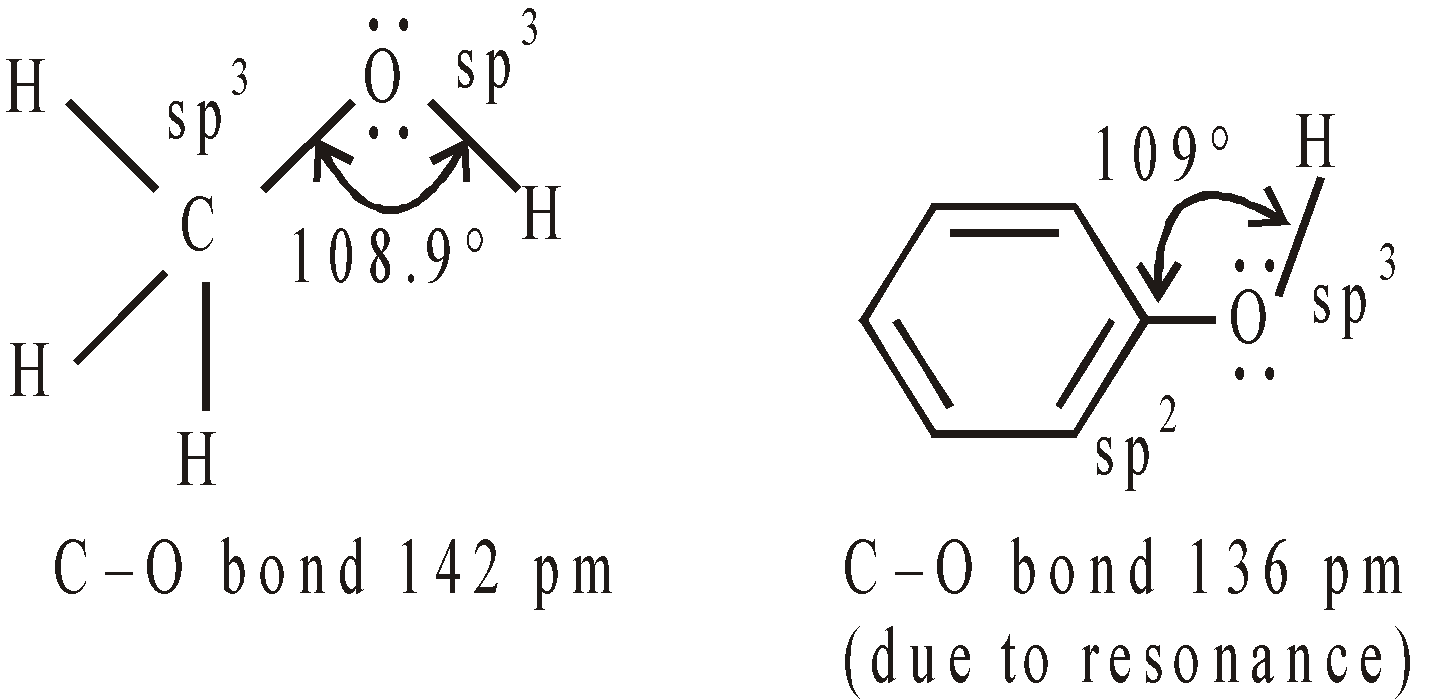
The value of bond angle depends upon the bulk of R group and repulsion between lone pair of electrons on oxygen.
ISOMERISM
The alcohols exhibit
- Position isomerism
- Chain isomerism
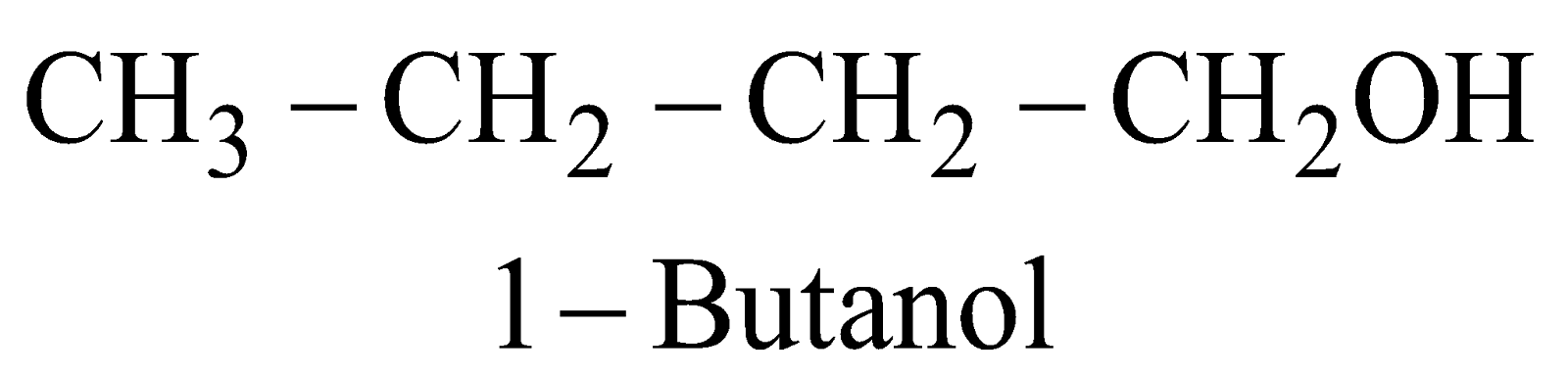
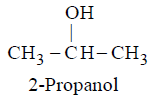
GENERAL METHODS OF PREPARATION
FROM ALKENES
- Acid catalysed hydration of alkenes
Addition of water to an unsymmetrical alkene follows Markovnikov’s rule
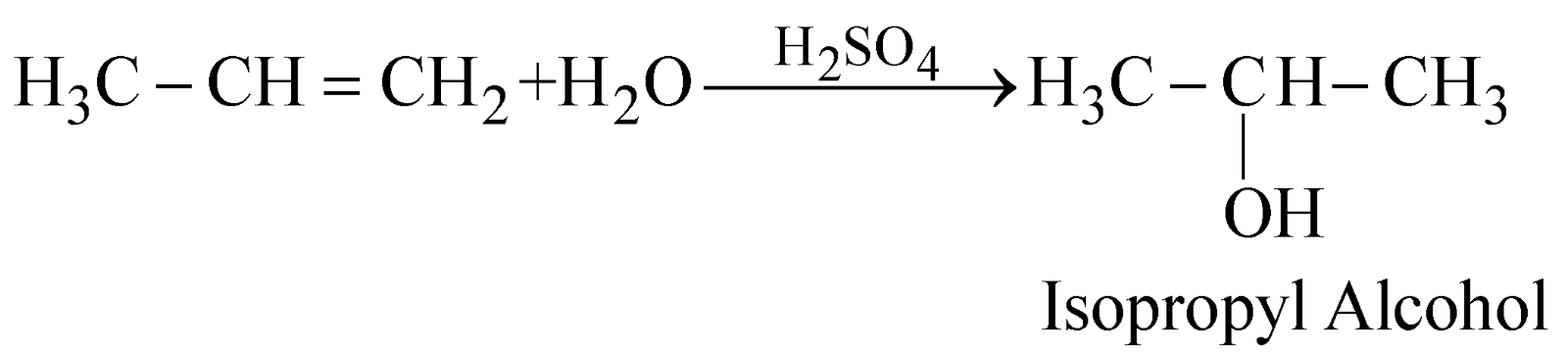
The method is suitable for the preparation of secondary and tertiary alcohols only.
Mechanism :
- Protonation of alkene
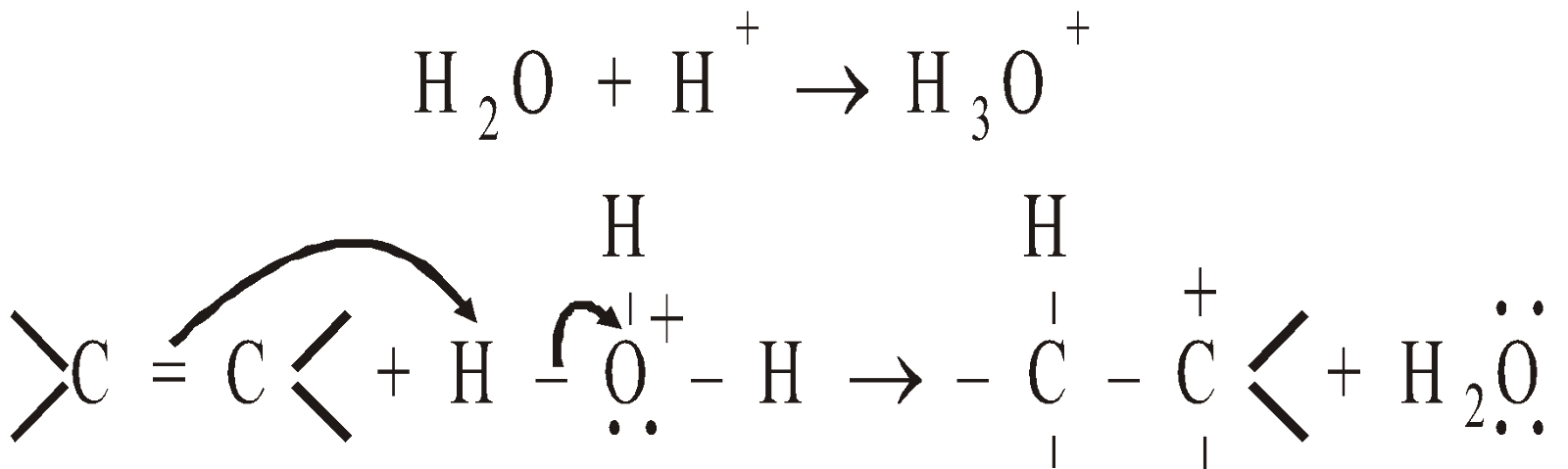
- Nucleophilic attack of H2
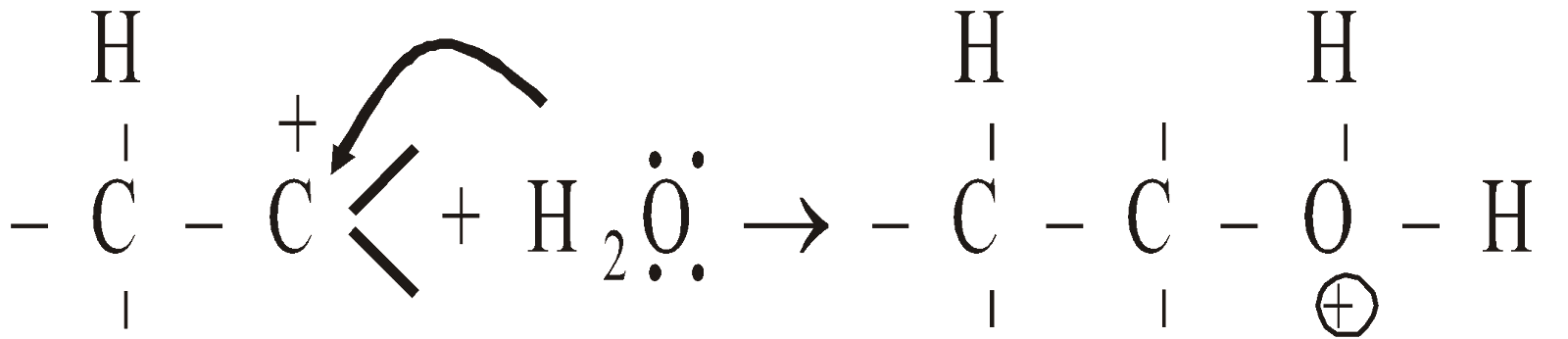
- Deprotonation
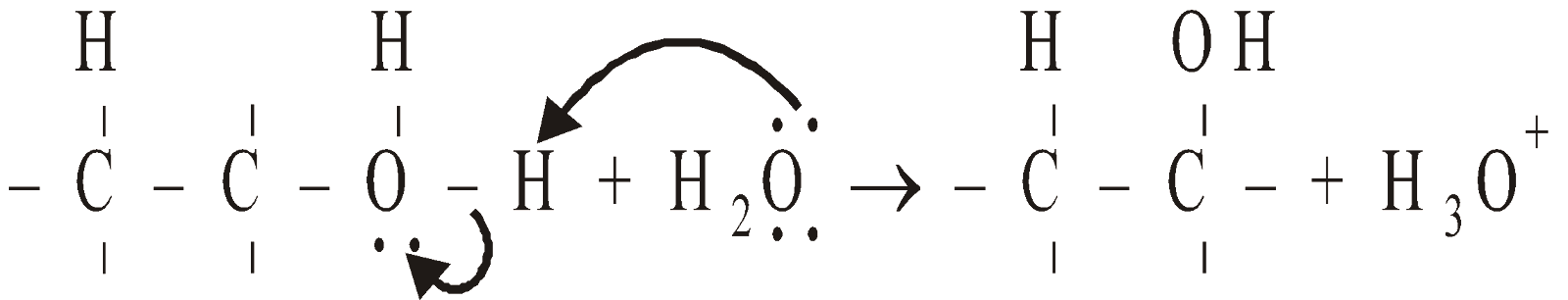
- Oxymercuration-demercuration:
The overall process is the addition of water molecule to unsymmetrical alkene according to Markovnikov’s rule
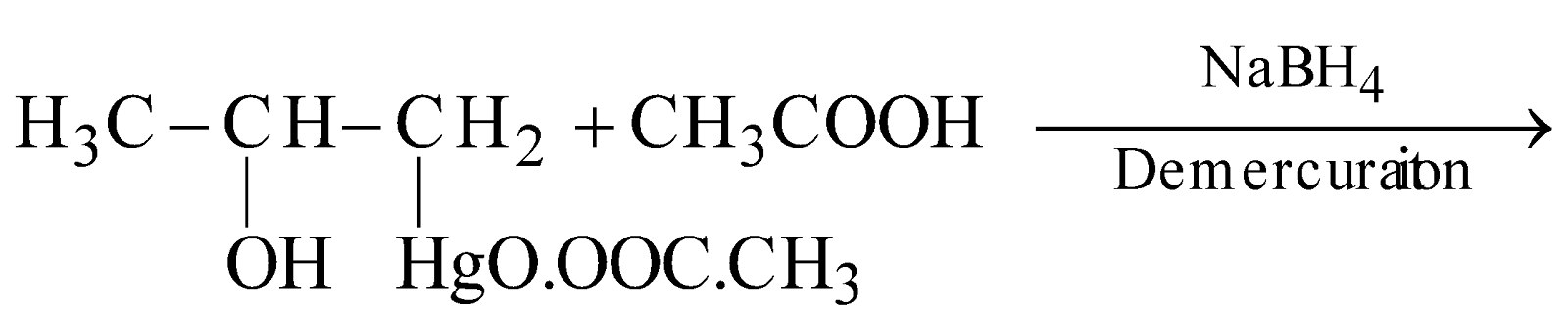
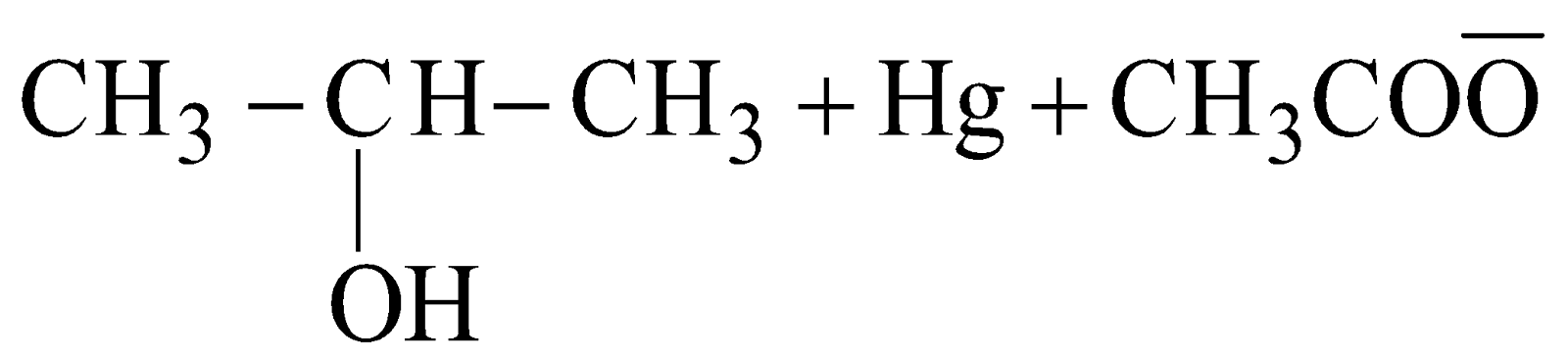
- Hydroboration
Oxidation of alkenes. The overall process is the addition of water molecule to unsymmetrical alkene follow Kharasch effect
FROM CARBONYL COMPOUNDS
- By using Grignard’s reagent
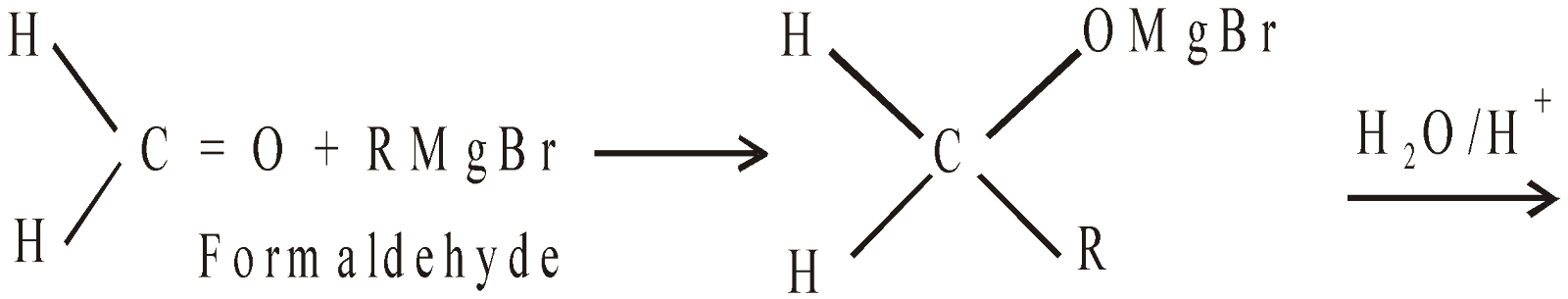
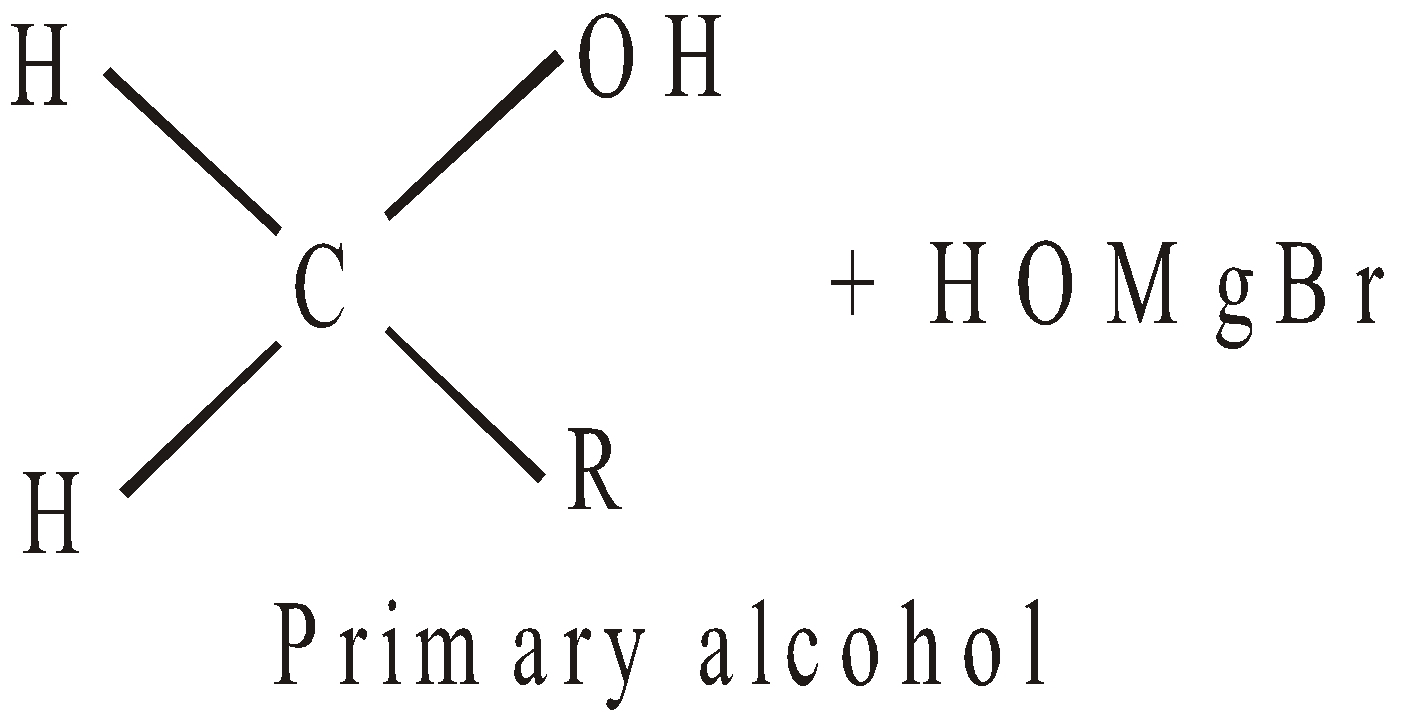
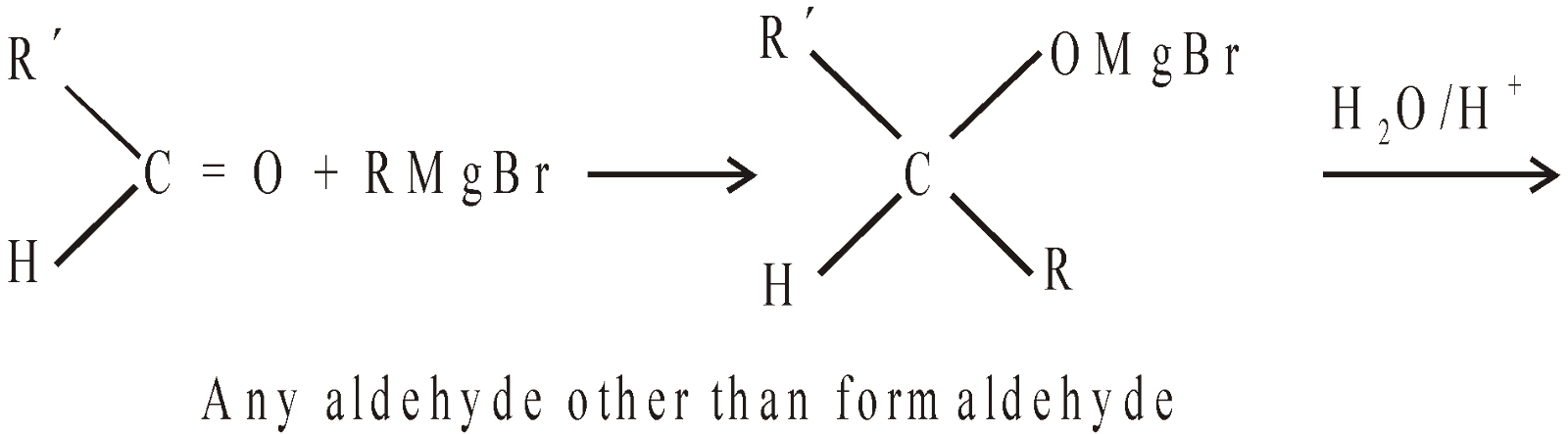
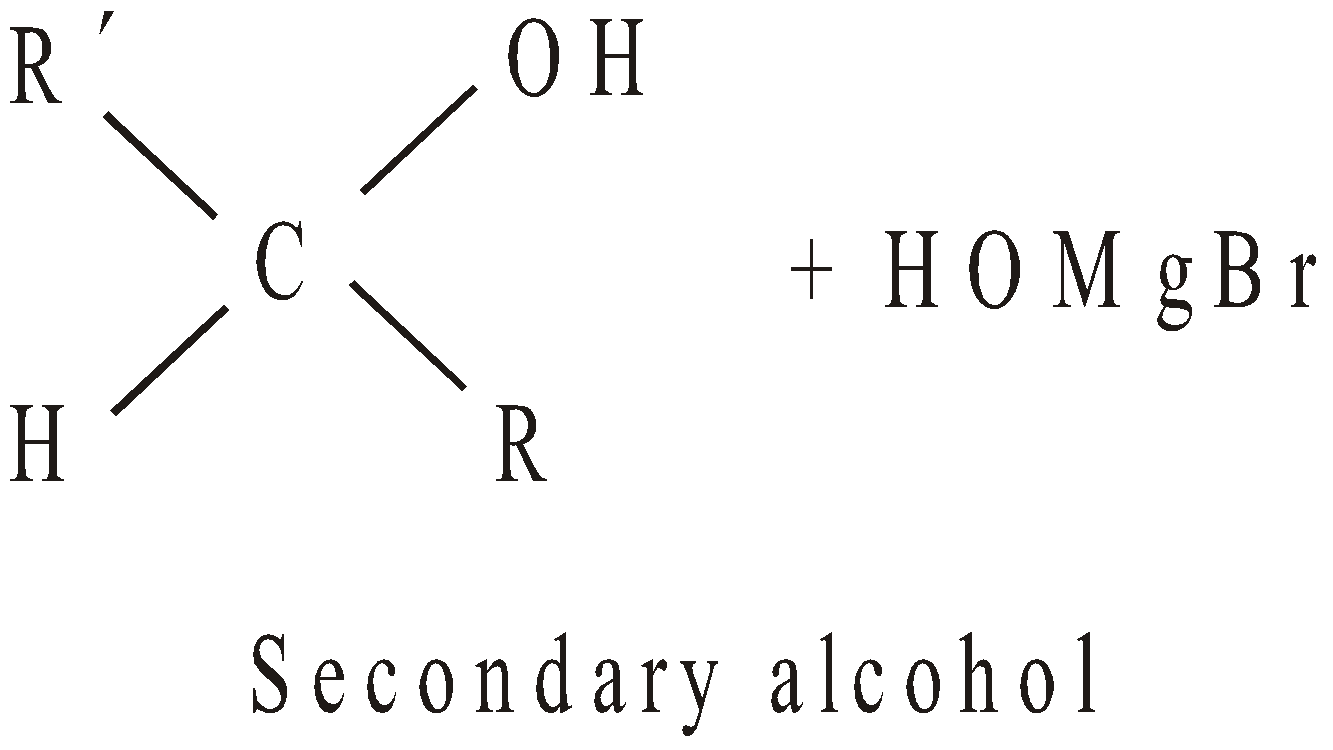
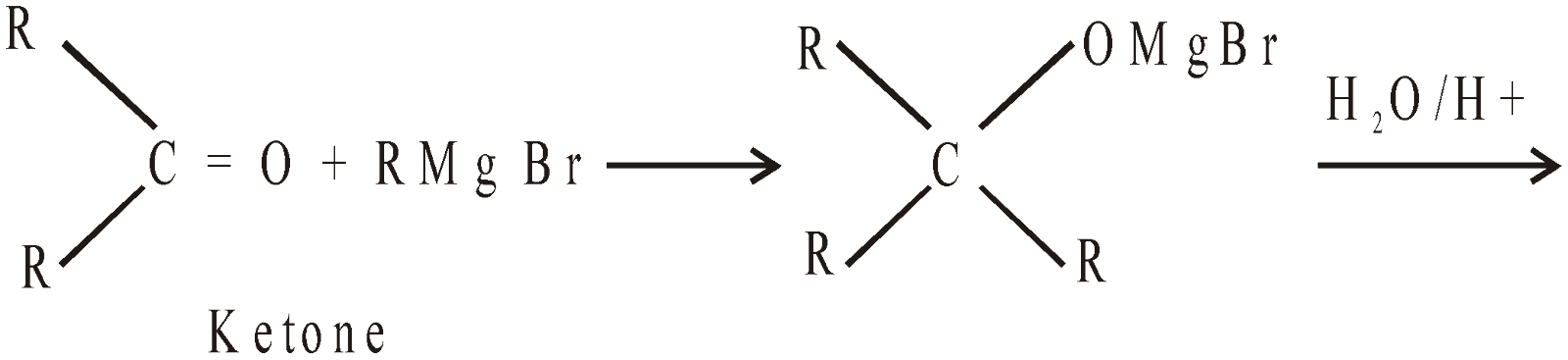
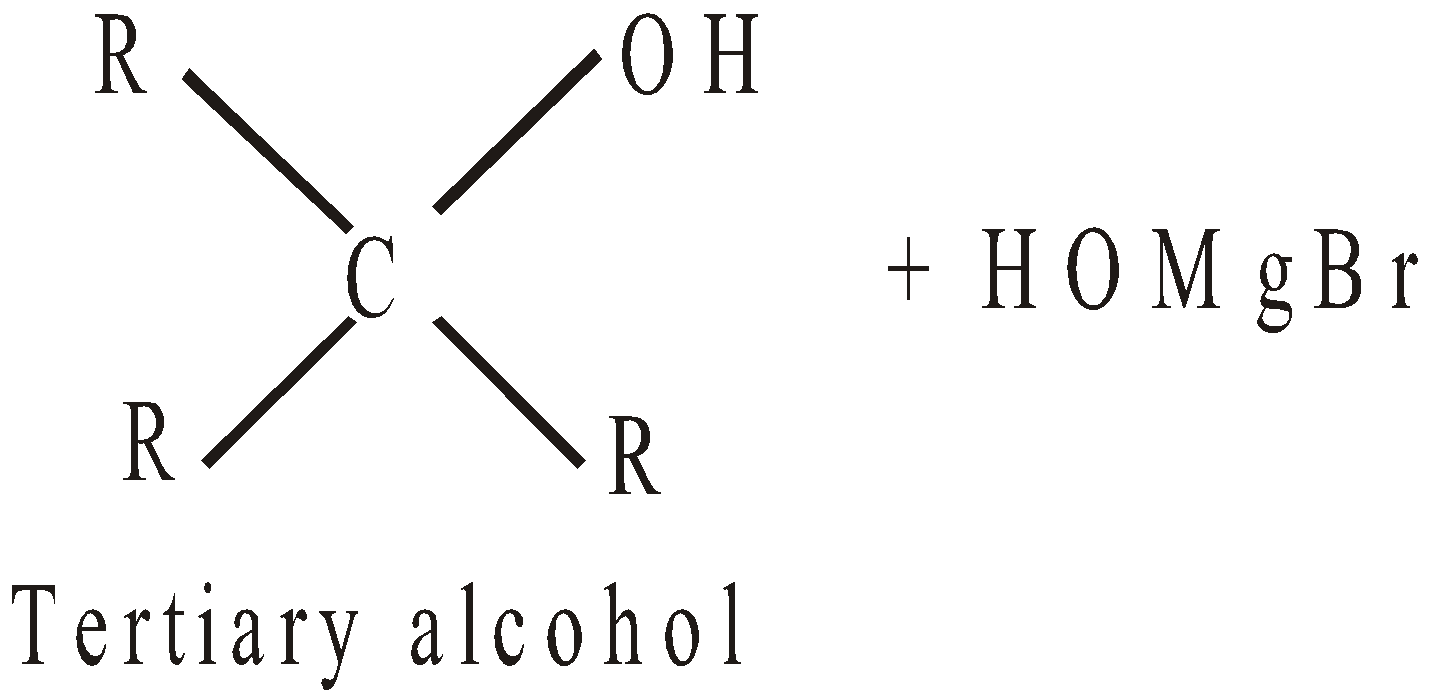
- By reduction (Catalytic hydrogenation)
The reducing agents used are H2/Ni, LiAlH4, NaBH4 or Na/C9H5OH
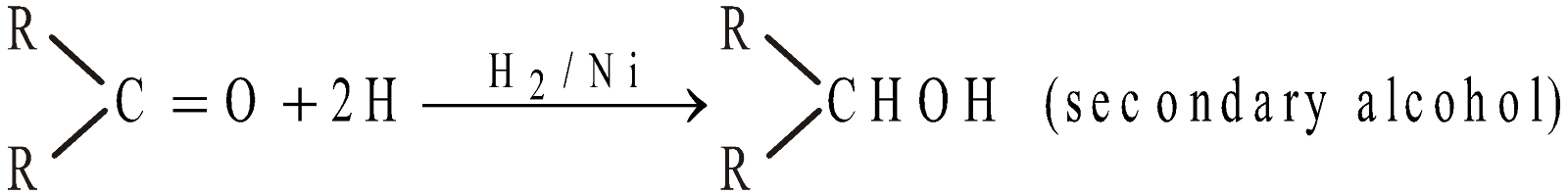
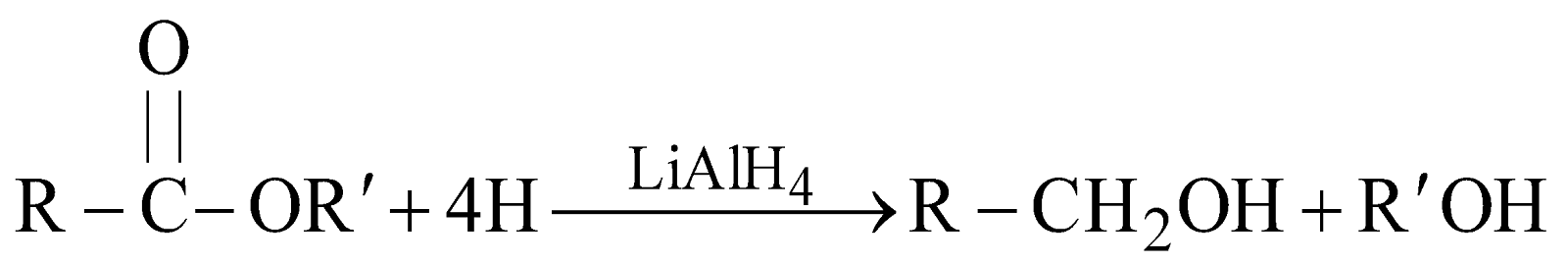
FROM ESTERS
- By reduction with LiAlH4 or Na/C2H5OH (Bouveault-Blanc réduction)
- By using Grignard’s reagent.
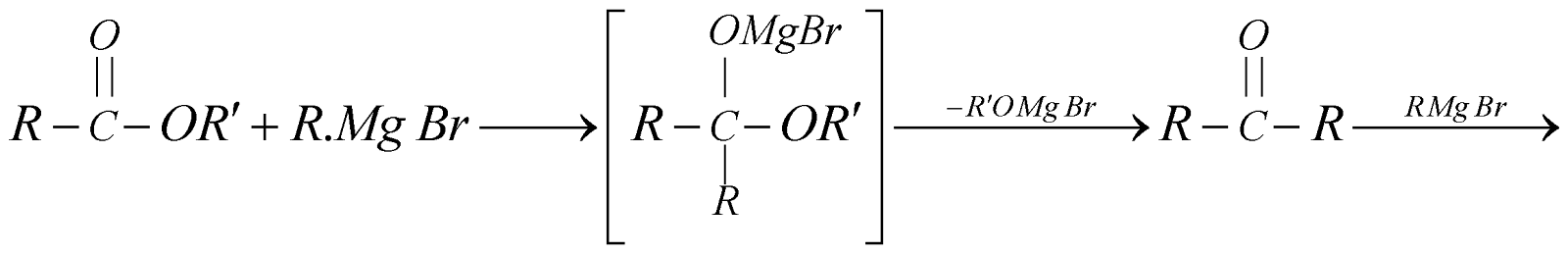
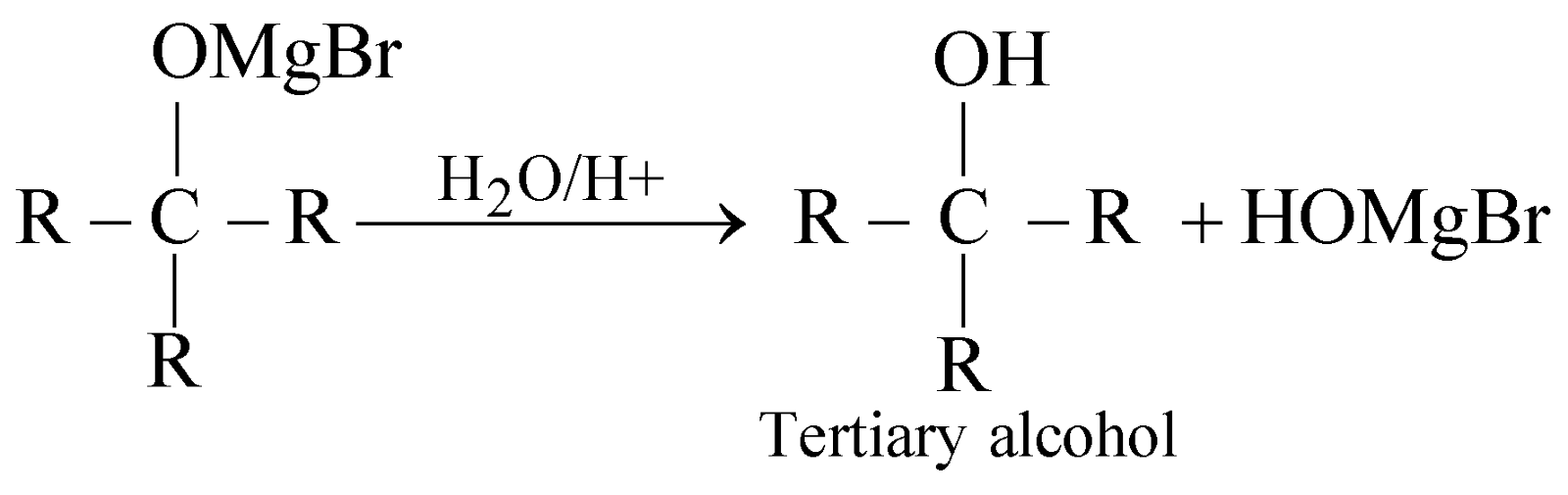
- By hydrolysis of esters:
HYDROLYSIS OF ALKYL HALIDES
To avoid dehydrohalogenation of RX, mild alkalis like moist silver oxide or aqueous potassium carbonate is used
Ease of hydrolysis of alkyl halides RI > RBr > RCl and
t > s > p
t > s > p
BY REDUCTION OF ACIDS AND THEIR DERIVATIVES
BY HYDROLYSIS OF ETHERS
FROM PRIMARY AMINES
By treatment with nitrous acid
Note: Methylamine does not give methyl alcohol when treated with HNO2. It gives CH3OCH3 and CH3ONO
GENERAL PROPERTIES
Alcohols are neutral substances and do not effect litmus. Lower alcohols are colourless toxic liquids, C4 – C11 members are oily liquids and higher alcohols are waxy solids.
SOLUBILITY
The hydroxyl groups in alcohols can form hydrogen bonds with water and many low molecular weight alcohols are miscible with water. The hydroxyl group is said to be hydrophilic (water loving) and alkyl (hydrocarbon) end is hydrophobic (water hating). The solubility decreases with increase in molecular mass. The hydrophobic effect of alkyl group predominates the hydrophilic effect of –OH group. Among isomeric alcohols, the branched chain alcohols are more soluble due to less surface area of hydrophobic part.
ACIDITY
Just like water the hydroxyl groups in alcohols are weakly acidic – strong bases can generate alkoxide ions
The acidities of alcohols vary greatly depending on the substituents.
Alcohol pka
Methanol CH3OH 15.5
Ethanol C2H5OH 15.9
t-Butyl alcohol (CH3)3 C–OH 19.0
2-Chloro ethanol ClC2H4OH 14.3
2, 2, 2-Chloro ethanol Cl3.CCH2OH 12.4
Cyclohexanol C6H11OH 18.0
Phenol C6H5.OH 10.0
Water HOH 15.7
(The lower the pka, the stronger the acid)
Electrons withdrawing groups on an alcohol increase the acidity by stabilising the alkoxide formed.
BOILING POINTS
Due to intermolecular hydrogen bonding the alcohols have higher value for boiling points.
For isomeric alcohols the boiling points follow the order:-
primary alcohol > secondary alcohol > tertiary alcohol
primary alcohol > secondary alcohol > tertiary alcohol
CHEMICAL PROPERTIES
The alcohols contain ionic C–O and O–H bonds
Hence three types of reactions are shown by alcohols.
REACTIONS INVOLVING RUPTURE OF – O – H BOND
- Acidic character of alcohols
The acid strength follows the order
Methyl alcohol > Primary alcohol > Secondary alcohol > Tertiary alcohol
- Ester formation
Alcohols react with acids to give esters in presence of Conc H2SO4 (Fischer Esterification)
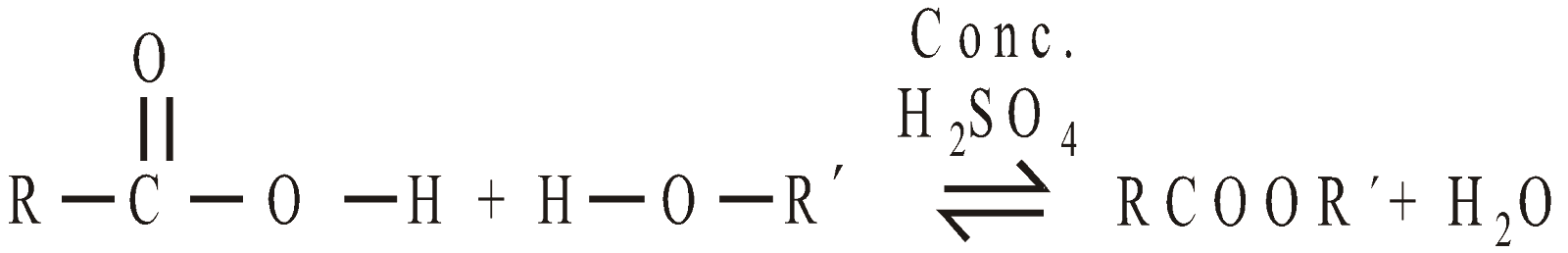
The order of ease of formation of ester
Primary alcohol > Secondary alcohol > Tertiary alcohol
Acid chlorides also produce esters
Esters of Inorganic acids: Alcohols also form esters with inorganic acids
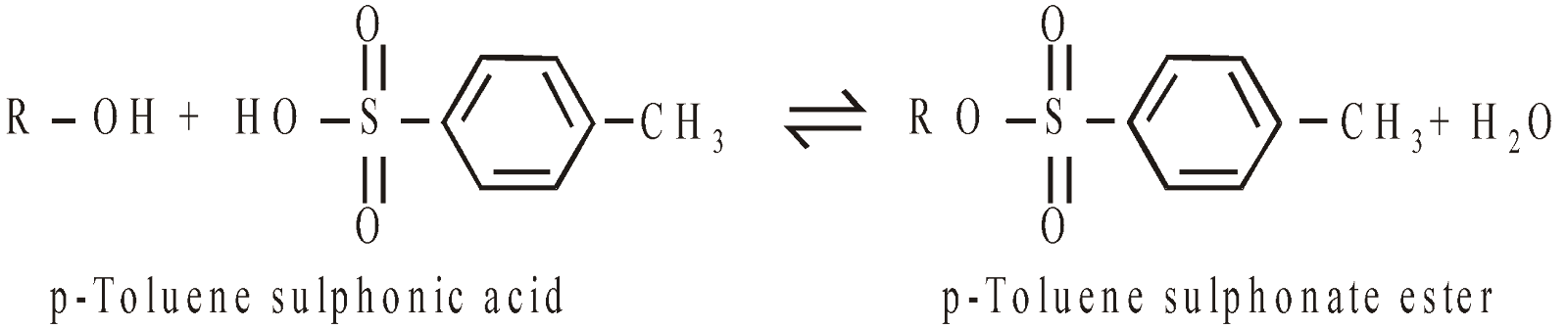
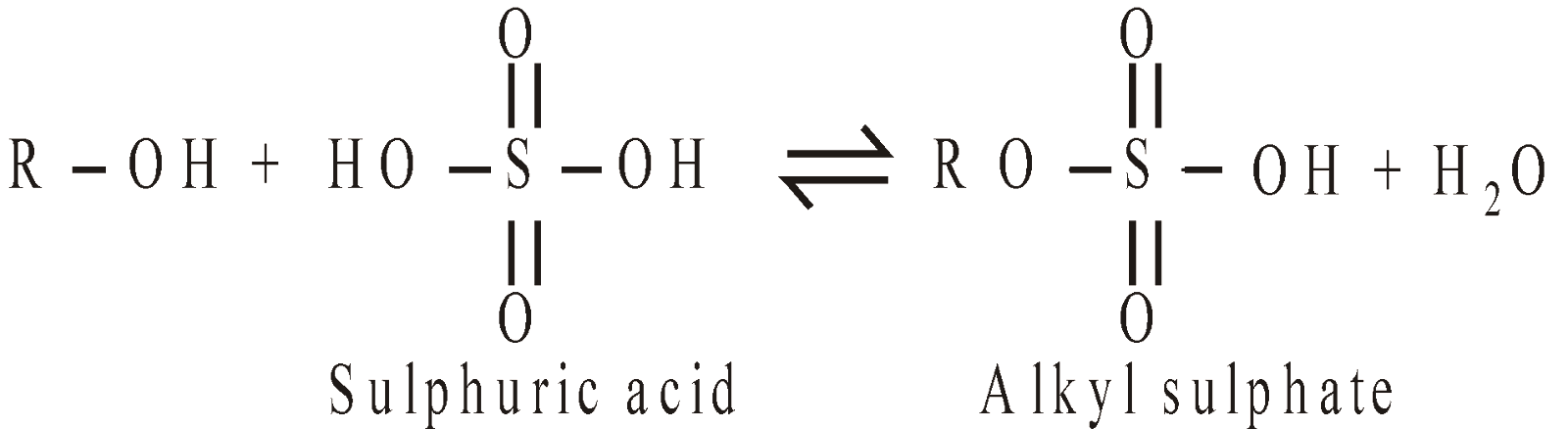
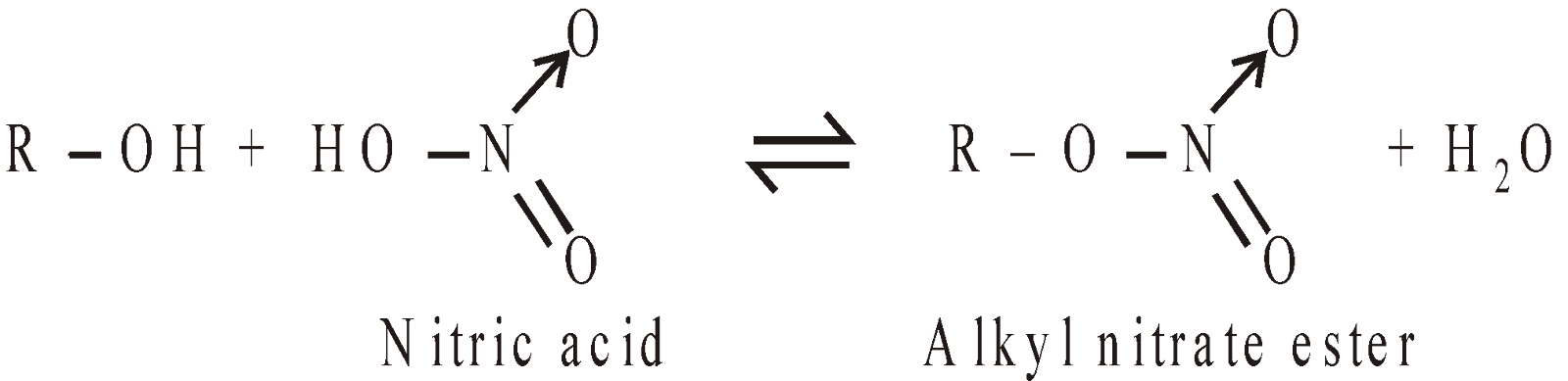
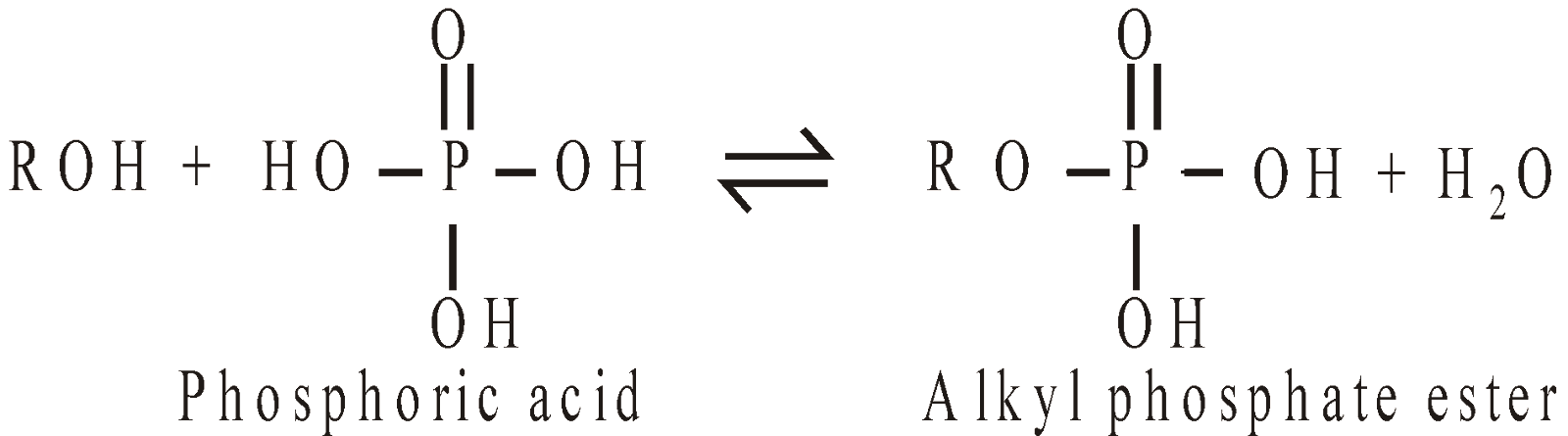
Phosphate esters are important in nature since they link the nucleotide bases together in DNA.
- Reaction with Grignard reagent
Hydrocarbons are formed
- Reaction with metal hydrides or metal amides:
REACTIONS INVOLVING RUPTURE OF –C–O BOND
- Reaction with HX
Alkyl halides are formed. The OH group is a poor leaving group, but is an excellent leaving group. In acidic medium, the alcohol is in equilibrium with protonated form
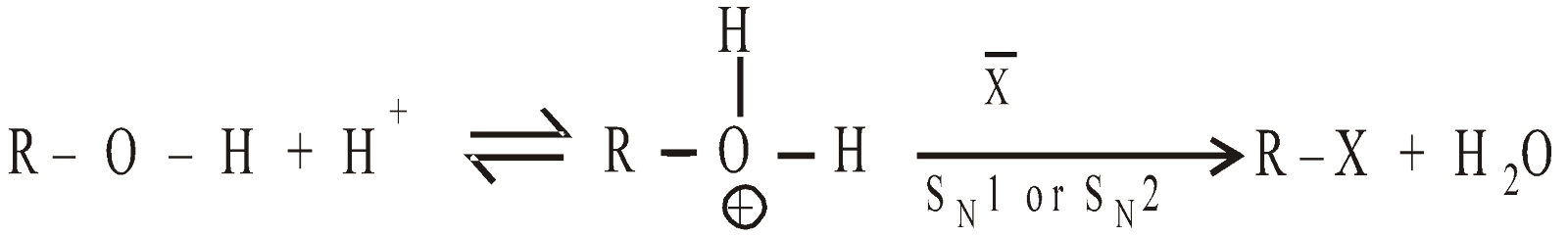
If R is primary alkyl  SN2
SN2
If R is bulky tertiary alkyl  SN1
SN1
The order of reactivity of hydrogen halides HI> HBr > HCl
The order of reactivity of alcohols tertiary > secondary > primary
In case of HCl, ZnCl2 (Lewis acid) is added to help compensate for the lower nucleophilicity of chloride ion.
The mixture of conc. HCl and ZnCl2 is called the Lucas Reagent, secondary and tertiary alcohols react via SN1 mechanism
Limitations of use of HX
- Carbocations can lead to rearranged products
- Reaction can proceed with elimination reaction
- It works better for HCl and HBr.
- Yield is poor in case of primary and secondary alcohols.
- Reaction with phosphorus halides: Phosphorus halides convert alcohols to alkyl halides
- Reaction with thionyl chloride: Alkyl chlorides are obtained
- Dehydration of alcohols: It requires acidic catalyst and the reaction proceeds via intermediate formation of carbonium ion. Acidic catalyst convert hydroxyl group into a good leaving group. Since the rate determining step is the formation of carbocation the ease of dehydration is 3º > 2º > 1º. The free carbocation undergo rearrangement.
Dehydration proceeds by Saytzeff’s rule i.e. more substituted alkene is formed or hydrogen is removed from the C-atom containing lesser number of hydrogen atoms.
The reaction of ethyl alcohol with H2SO4 is very sensitive to reaction conditions
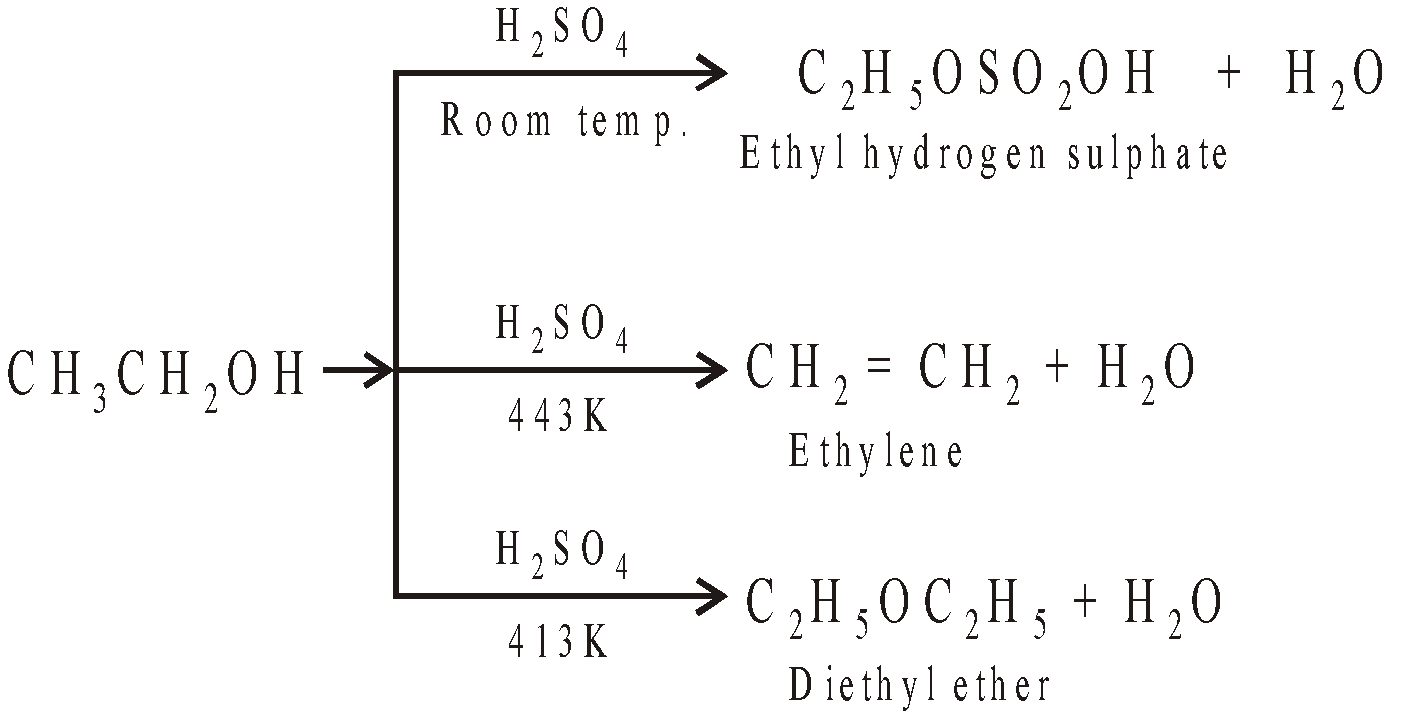
Mechanism


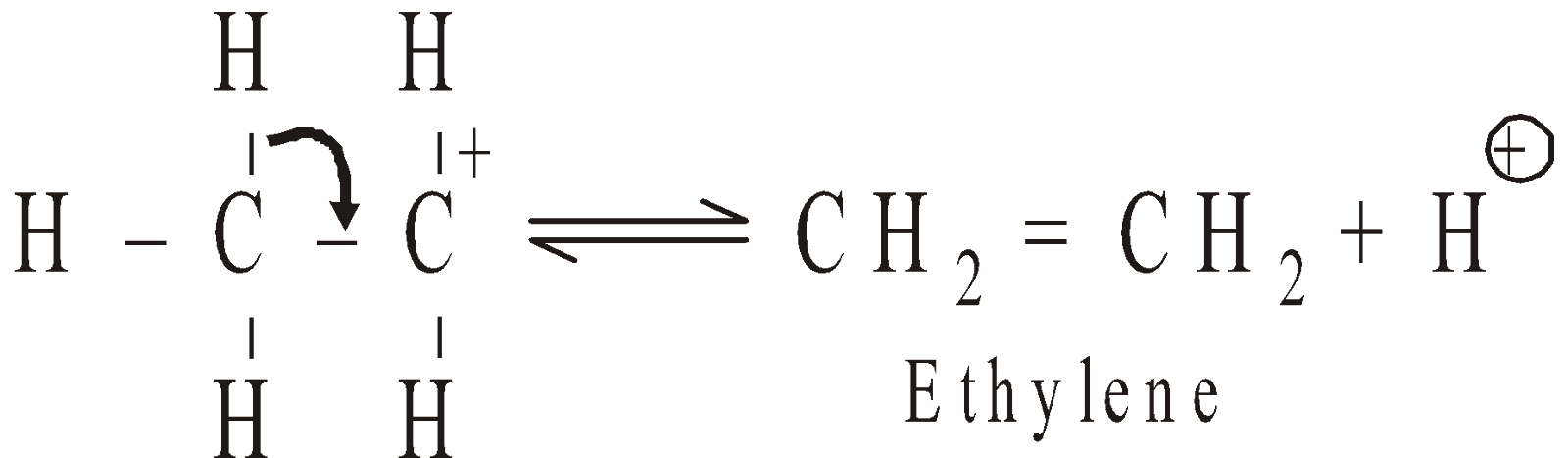
The formation of ether is SN2 reaction (nucleophilic bimolecular)
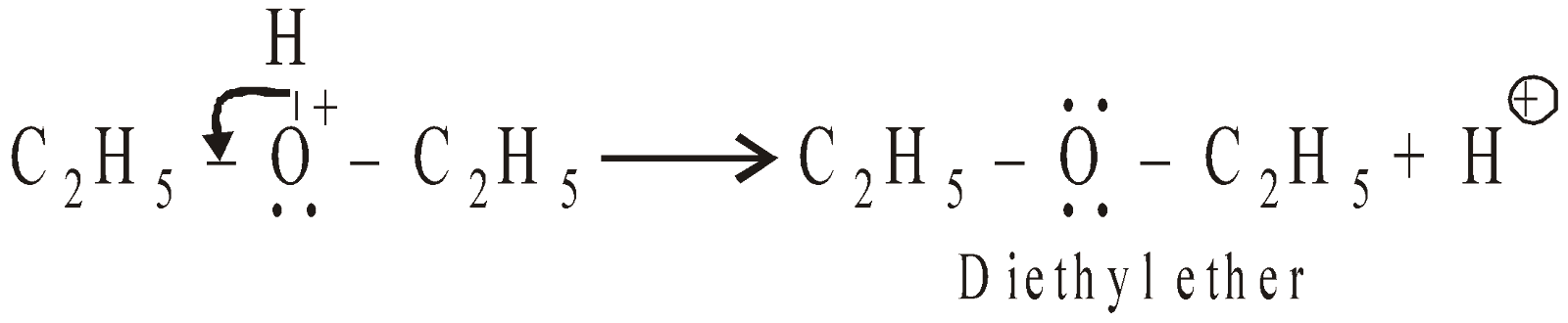
- Reaction with ammonia: A mixture of amines is obtained in presence of a catalyst like anhydrous alumina
OXIDATION
Primary and secondary alcohols are easily oxidised by a variety of reagents eg. acidified potassium dichromate, acidified or alkaline potassium permanganate or dil. nitric acid.
Chromic acid is produced in situ
A common reagent that selectively oxidizes a primary alcohol to an aldehyde (and no further) is pyridinium chlorochromate PCC
Chromate PCC
Tertiary alcohols having no hydrogen atoms attached to the oxygen bearing carbon (carbinol carbon) resist oxidation.
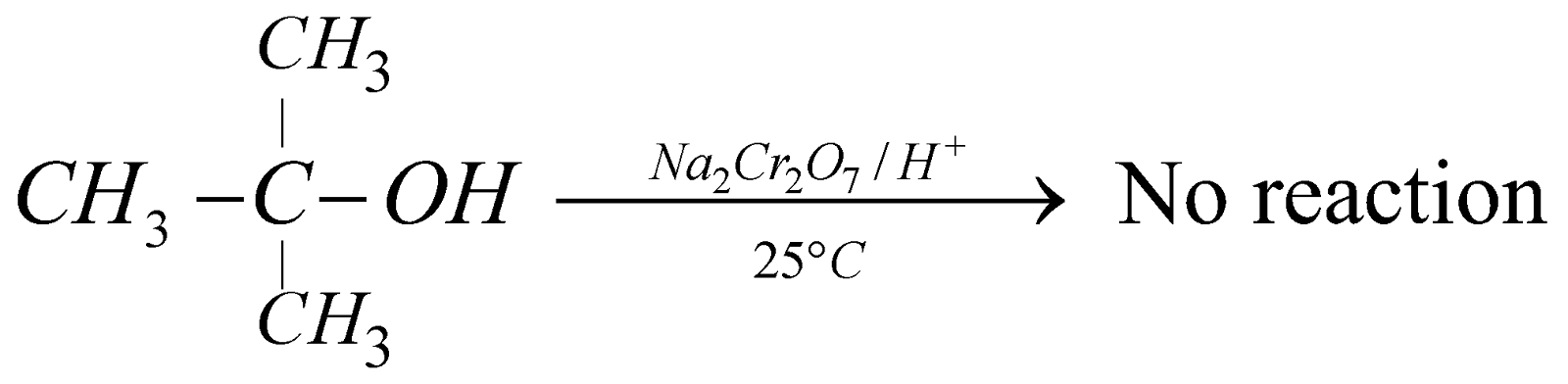
Hence, drastic condition is required.
By oxidation, distinctions are made between 1º, 2º and 3º alcohols
DEHYDROGENATION
When vapours of 1º, 2º and 3º alcohols are passed over hot reduced copper at 300ºC, they give different products.
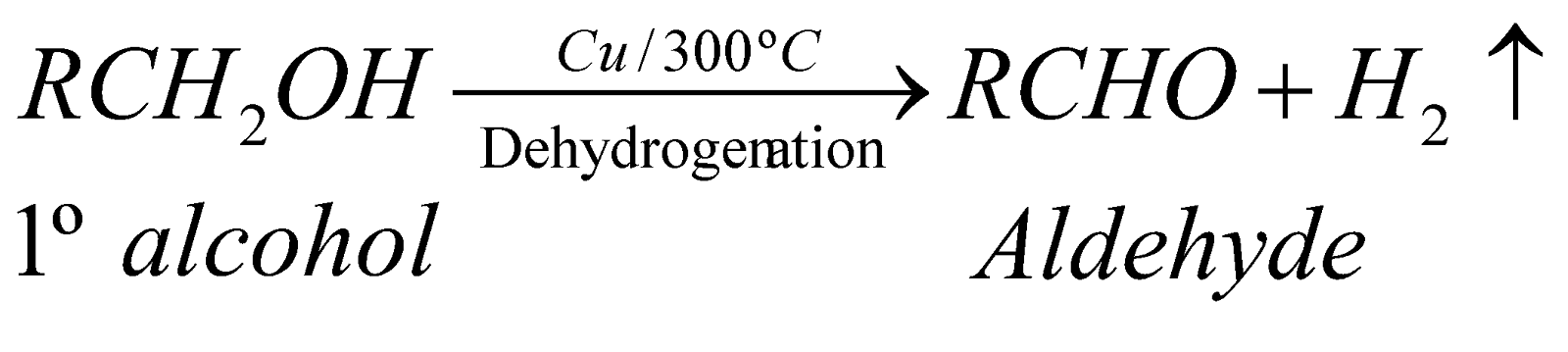
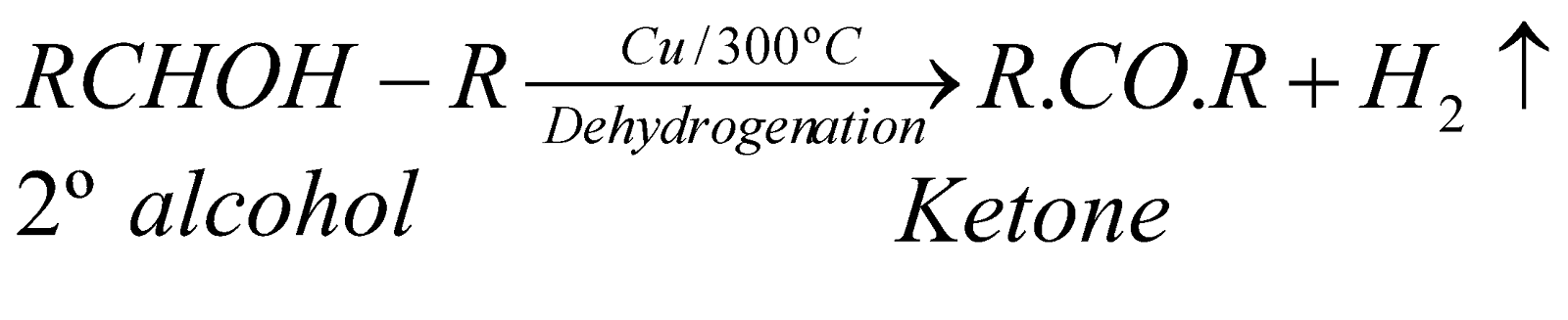
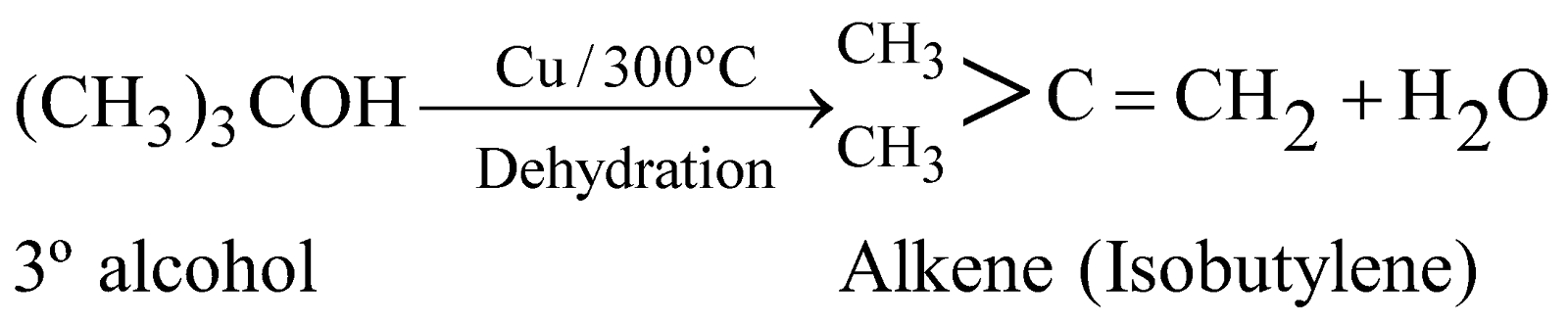
This property is also utilised for distinction between 1º, 2º and 3º alcohols
REACTION WITH HALOGENS
The reaction with halogens is oxidation and halogenation
REDUCTION OF ALCOHOLS
Normally an alcohol cannot be directly reduced to an alkane in one step

The –OH group is a poor leaving group. It is converted into other superior leaving groups e.g. tosylate group
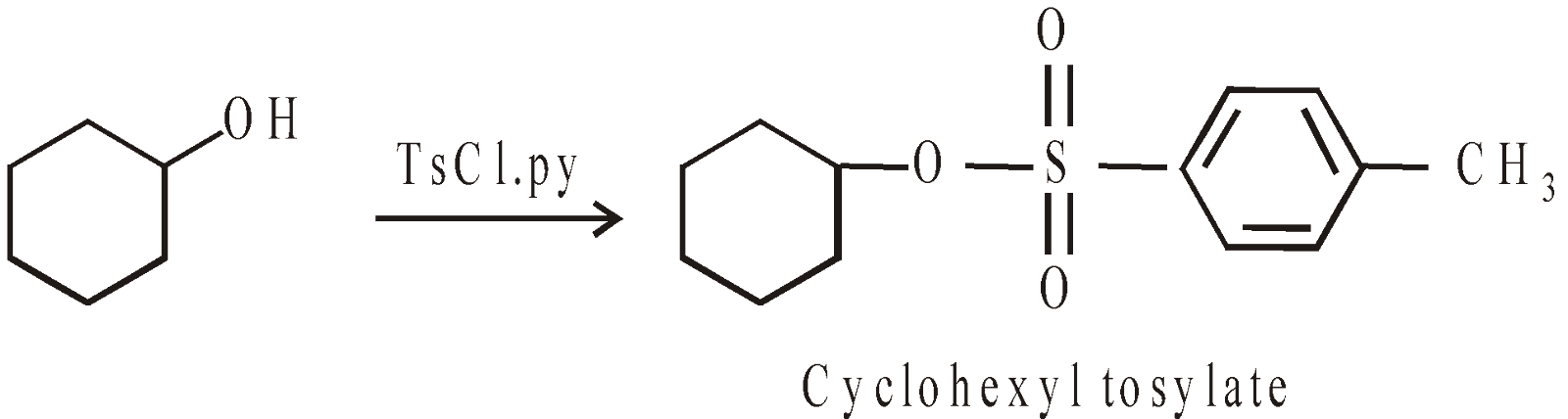
Tosylate groups undergo a variety of SN2 reactions, the common are
DISTINCTION BETWEEN 1º, 2º AND 3º ALCOHOLS
A distinction between them can be made by any of the following methods

3. Lucas test
Lucas reagent is [Conc. HCl + anhydrous ZnCl2]
3º alcohol + Lucas reagent – immediate turbidity
2º alcohol + Lucas reagent – turbidity after 5 minutes
1° alcohol + Lucas reagent – turbidity after 30 minutes
In fact alkyl chloride is formed which being insoluble in the medium form cloudiness (turbidity). Hence different alcohols react with Lucas reagent in the following order
3º alcohol > 2º alcohol > 1º alcohol
4. Victor Meyer’s test : The various steps involved are
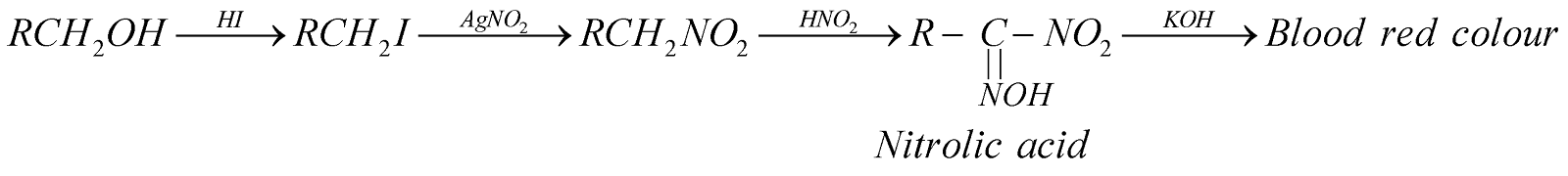
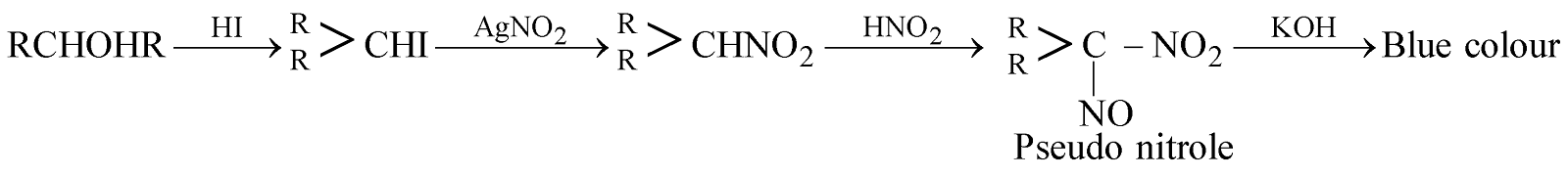
TESTS FOR ALCOHOLS
- Sodium metal test
- Phosphorus pentachloride test
Solution becomes warm with evolution of hydrogen chloride.
- Acylation
Evolution of hydrogen chloride
METHYL ALCOHOL (CARBINOL OR WOOD SPIRIT)
MANUFACTURE
- From water gas: (Patart process)
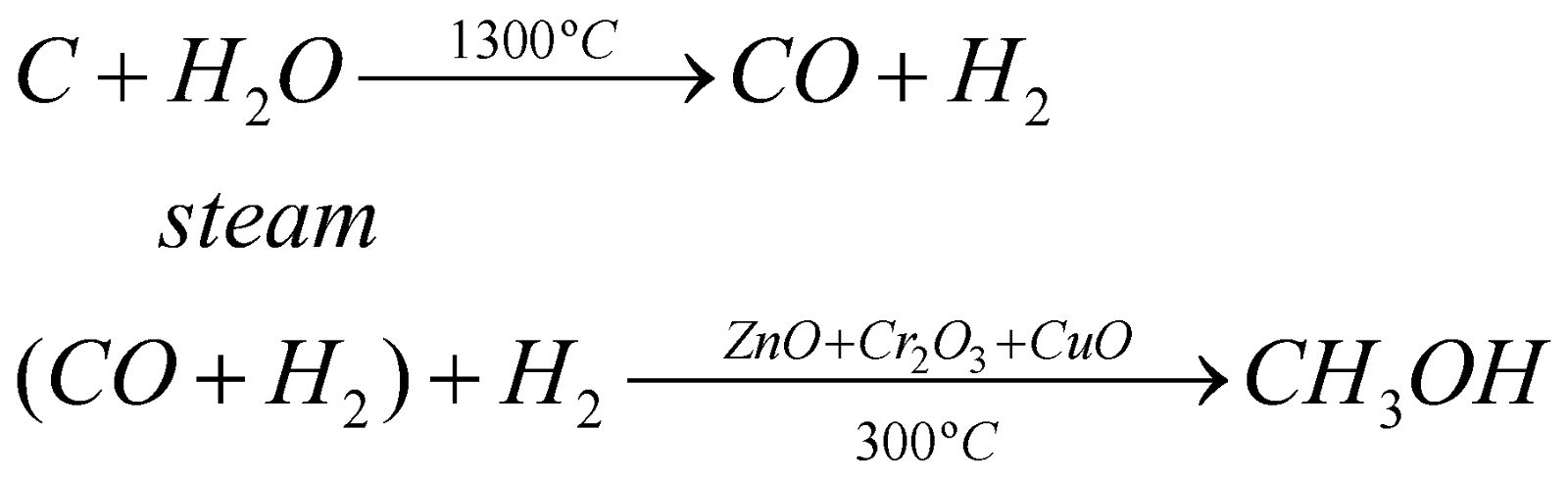
- From natural gas:
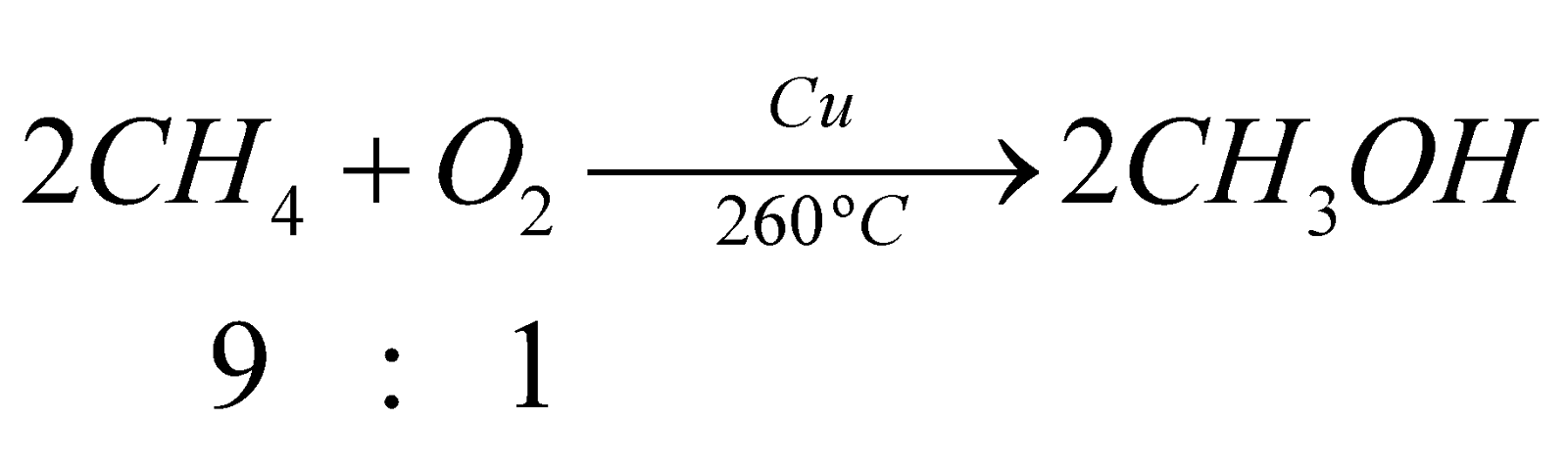
- From pyroligneous acid:
The pyroligneous acid is obtained by destructive distillation of wood and contains
Acetic acid 9-10%, methyl alcohol 2-2.50%, acetone 0.5%
PROPERTIES
Colourless liquid, extremely poisonous, inflamable
USES
- It is used as solvent for oils, paints and varnishes
- Manufacture of formaldehyde
- As an antifreeze
- To denature ethyl alcohol. (rectified spirit)
ETHYL ALCOHOL (GRAIN ALCOHOL)
MANUFACTURE
- From ethylene
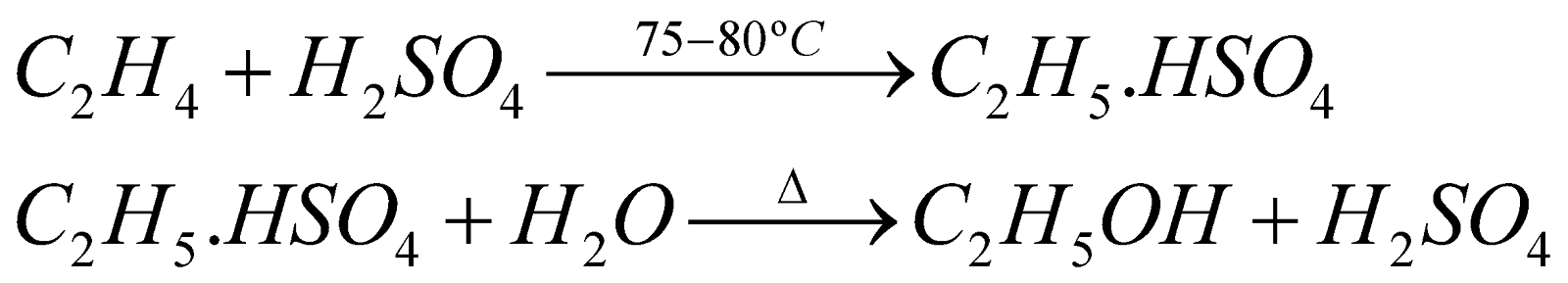
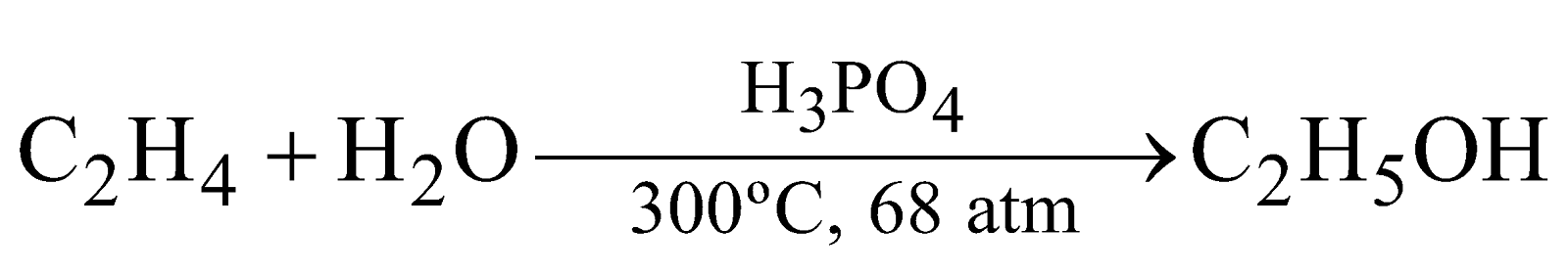
- Fermentation of carbohydrates. From molasses
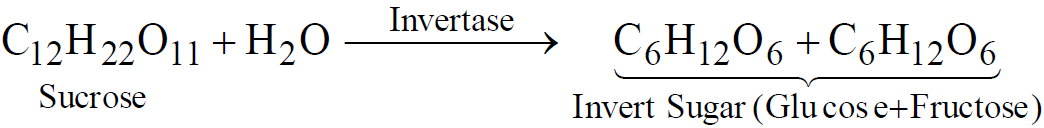
or
PROPERTIES
Colourless liquid, burns with blue flame, b.pt. 78.1ºC, Sp. gr. 0.789. It can not be dried over anhydrous calcium chloride due to formation of addition product. CaCl2.4C2H5OH. It gives iodoform test.
USES
- As a solvent for paints, oils, varnishes etc
- In the manufacture of alcoholic beverages
- Manufacture of acetic acid, chloroform, iodoform etc
- As an antifreeze
- With saturated calcium acetate solution forms a solid gel which burns like alcohol
SOME COMMERCIALLY IMPORTANT ALCOHOLS
RECTIFIED SPIRIT
It contains 95.5% ethyl alcohol and 4.50% water. It is an azeotrope (Constant boiling mixture) and boils at 74ºC.
ABSOLUTE ALCOHOL
Alcohol containing no water i.e. 100% C2H5OH is known as absolute alcohol. It is prepared as follows.
- Quick lime process :
Final traces of water are removed by adding anhydrous CuSO4 or metallic magnesium and again distillation, Mg forms Mg(OH)2.
- Azeotropic method: Rectified spirit + Excess of benzene → a temporary azeotrope is formed which is subjected to fractional distillation
Ist fraction distills at 64.8ºC consisting water 7.49%, alcohol 18.5%, benzene 74.1%
IInd fraction distills at 68.2ºC consisting of alcohol 32.4%, benzene 67.6%
IIIrd fraction distills at 78.2ºC and gives absolute alcohol
METHYLATED SPIRIT
The rectified spirit rendered poisonous by addition of 4-5% methyl alcohol, traces of pyridine and some copper sulphate is known as methylated spirit or denatured alcohol.
POWER ALCOHOL
Alcohol mixed with petrol and used in internal combustion engines is known as power alcohol. Mixing is done in presence of 1% benzene, or 1% ether or 1% tetralin.
ALCOHOLIC BEVERAGES
The liquors used for drinking purposes containing alcohol as the main intoxicating agent are known as the alcoholic beverages.
Undistilled: Prepared from fruit juices or grains
Name | Source | %alcohol |
Beer | Barley | 2-6 |
Cider | Apples | 3-6 |
Wine | Grapes | 8-10 |
Port and Sherry | Grapes | 14-20 |
Distilled: Prepared by distillation of fermented liquids
Name | Source | %alcohol |
Whiskey | Barley | 40-50 |
Brandy | Peeches, apples | 40-50 |
Rum | Molasses | 35-40 |
Gin | Maize | 40-45 |
PROOF-SPIRIT
An aqueous solution of alcohol containing 57.1% alcohol by volume or 49.3% alcohol by weight is called proof spirit.
- Over proof – A sample containing more percentage of alcohol than proof spirit is known as over proof. 20 O.P. means that 100 ml of this sample contains alcohol equivalent to 120 ml of proof spirit.
- Under proof – A sample containing lower percentage of alcohol than proof spirit is known as under proof. Thus 20 U.P. means that 100 ml of this sample contains alcohol equivalent to 80 ml of proof spirit.
ALCOHOLMETRY
The determination of the percentage of alcohol is known as alcoholometry. We simply determine the sp. gr. of the sample by means of hydrometer and find the exact percentage of alcohol by referring reference tables.
POLYHYDROXY ALCOHOLS
ETHYLENE GLYCOL (ETH 1,2-DIOL)
PREPARATION
- Hydroxylation of ethylene
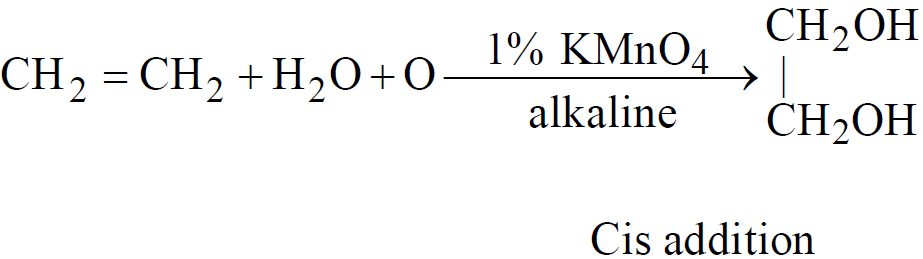
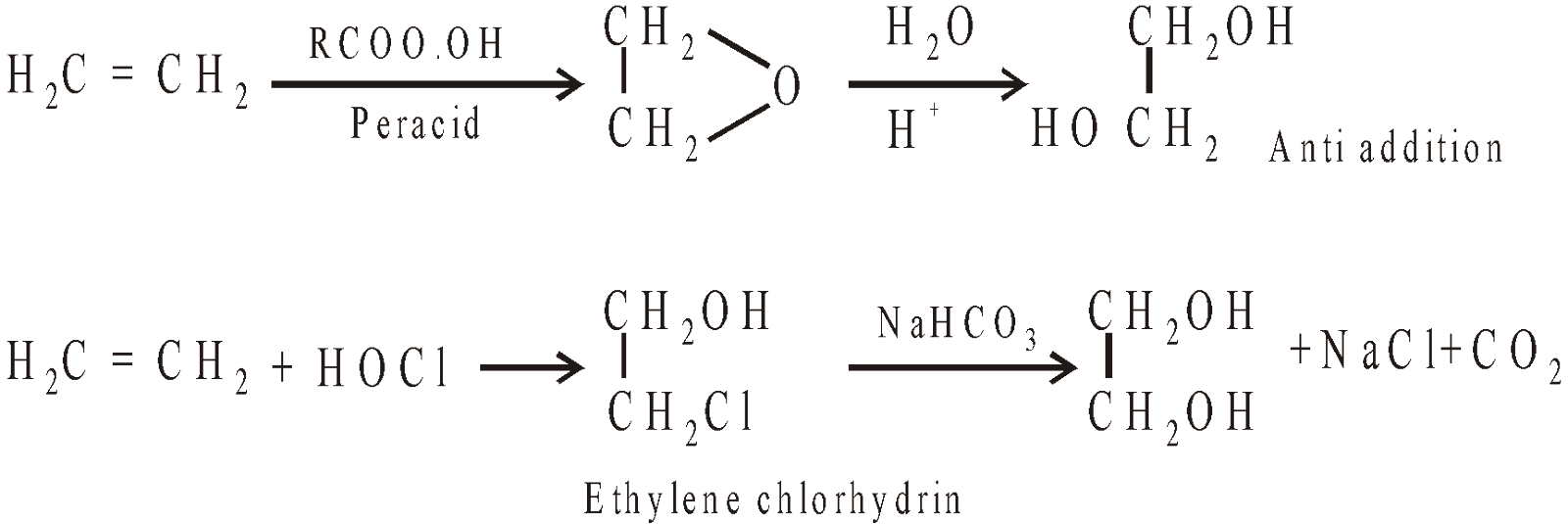
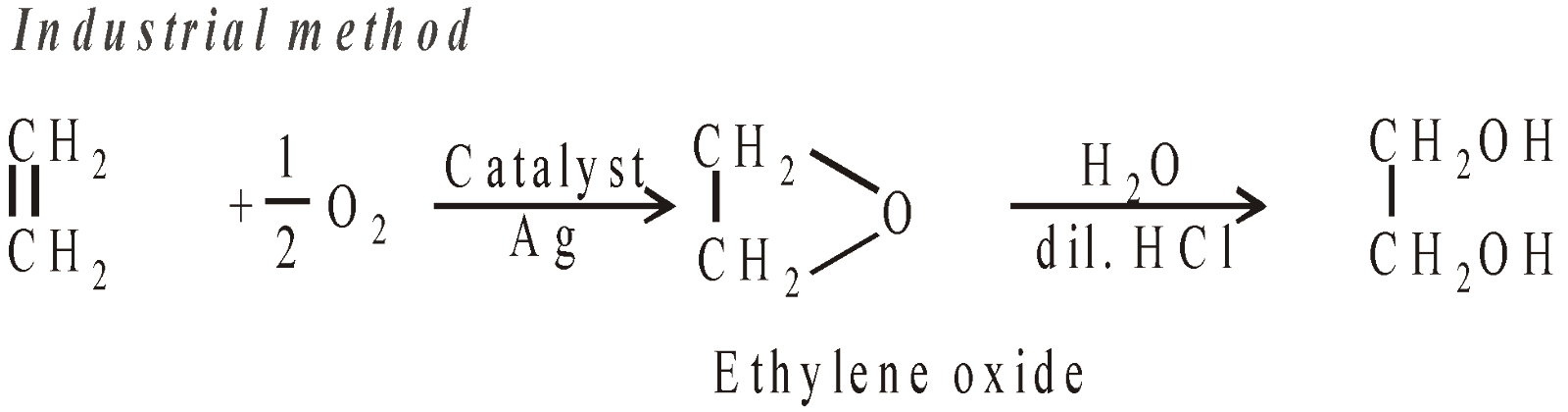
- From ethylene bromide
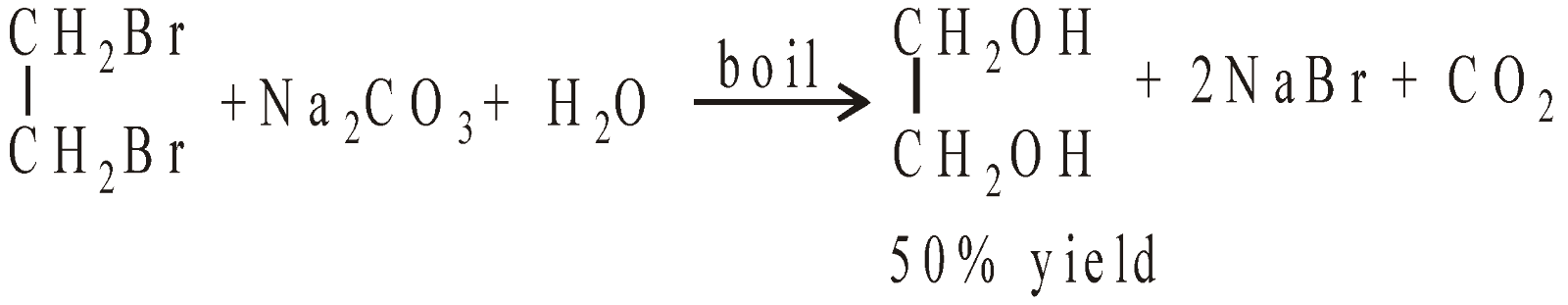
Some vinyl bromide is also formed

To get better yield ethylene bromide is heated with potassium acetate

PHYSICAL PROPERTIES
It is colourless viscous liquid bpt 197ºC mpt –11.5º, miscible with water in all proportions due to intermolecular hydrogen bonding.
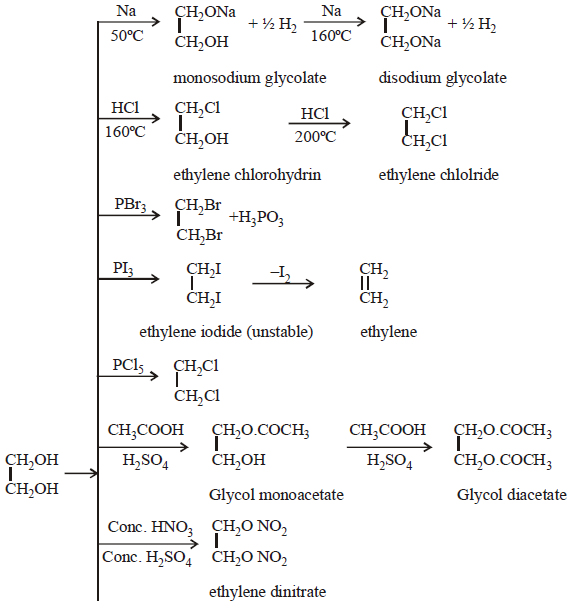
CHEMICAL PROPERTIES
These can be summarised as follows
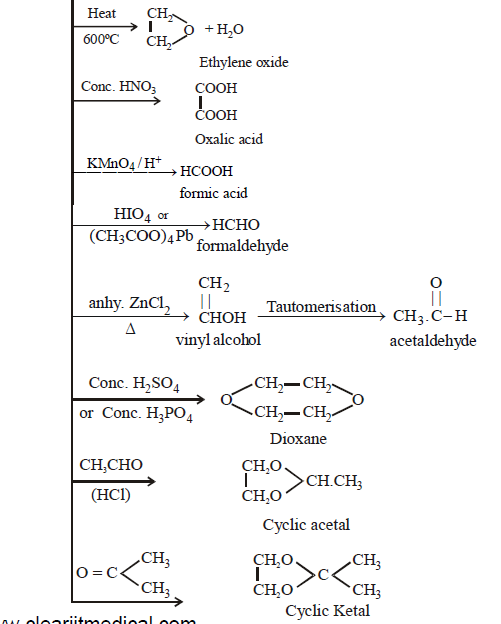
Oxidation products obtained from ethylene glycol
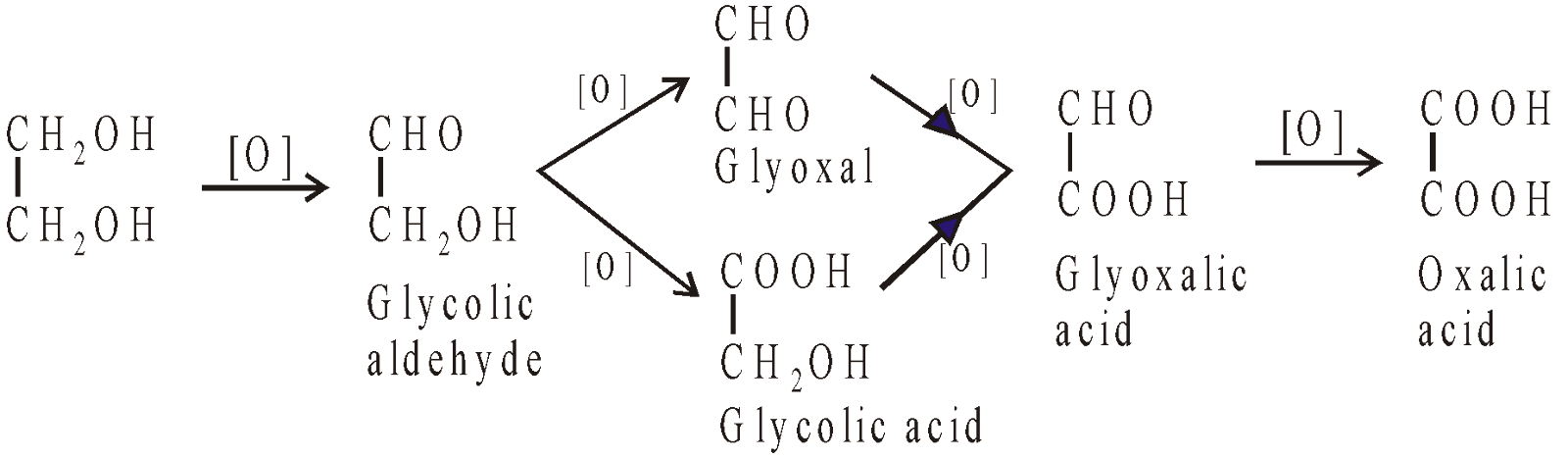
Pinacol Rearrangement : Reaction of Diols
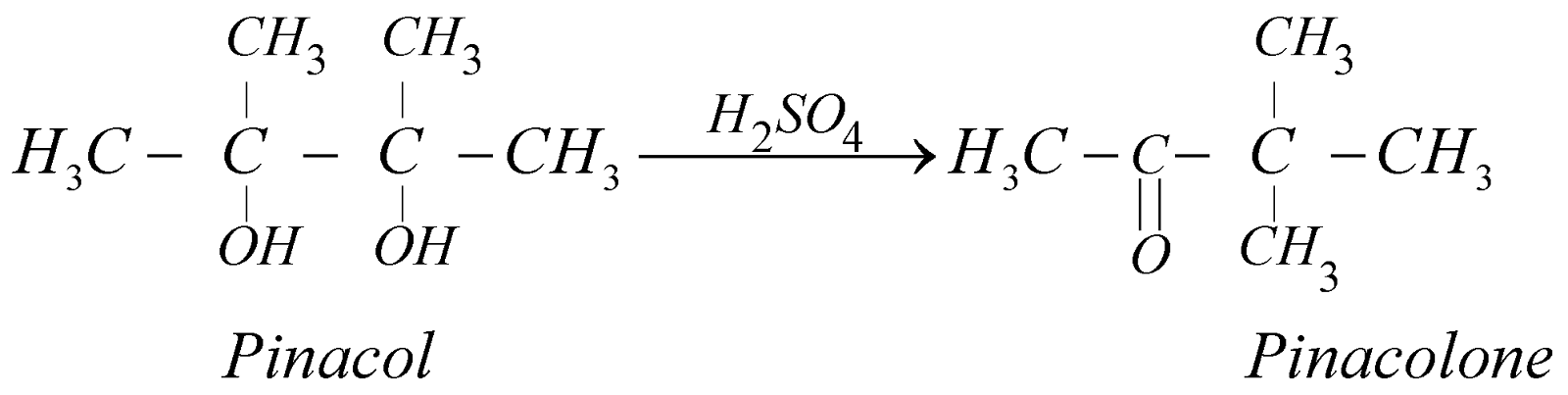
USES OF ETHYLENE GLYCOL
- As an antifreeze in car radiators
- In the manufacture of rayon
- As a cooling agent
GLYCEROL OR GLYCERINE (PROPAN-1,2,3-TRIOL)
MANUFACTURE
- It is obtained as a byproduct by hydrolysis of oils and fats in soap and candle industry
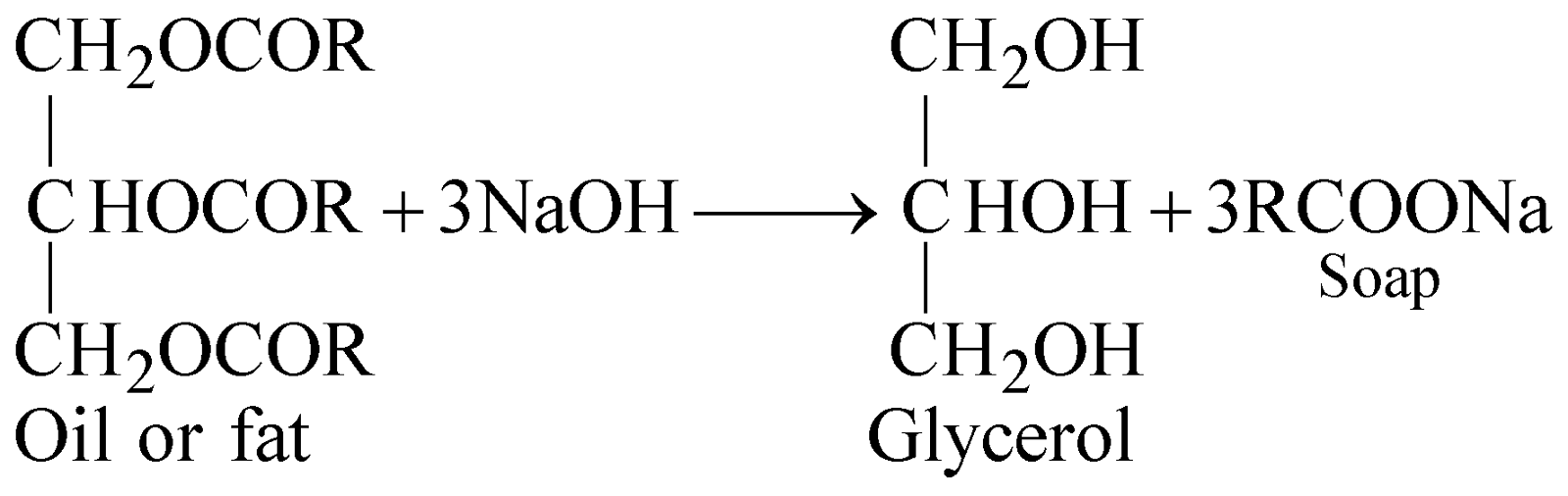
After salting out of soap by adding saturated solution of NaCl, the filtrate obtained is known as spent lye which is worked up for glycerol.
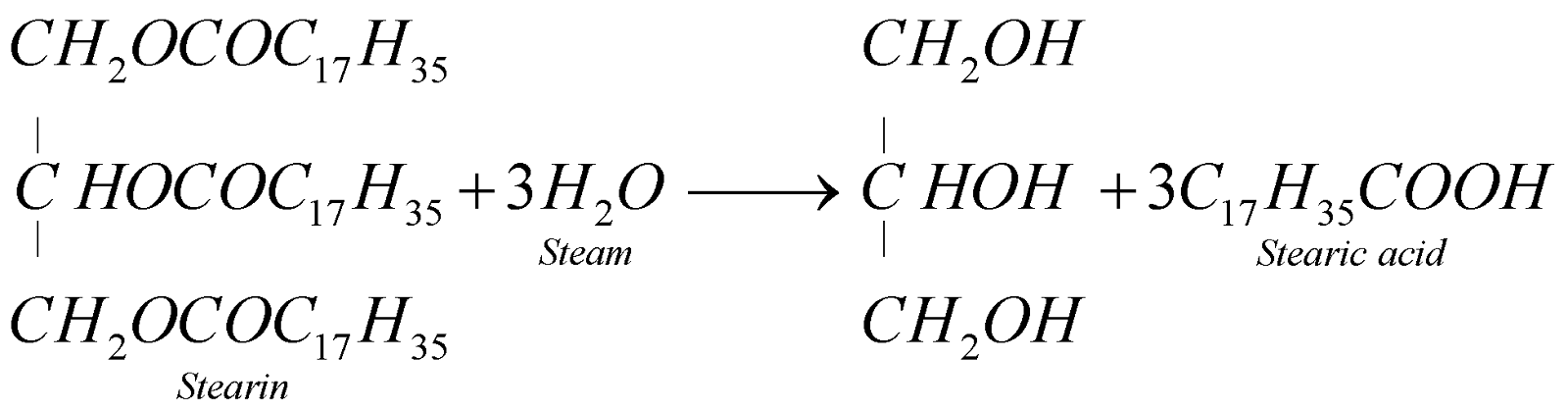
Stearic acid is removed by filtration. The filtrate, known as sweet lye, is worked up for glycerol. Stearic acid is used for the manufacture of candles.
- From propene
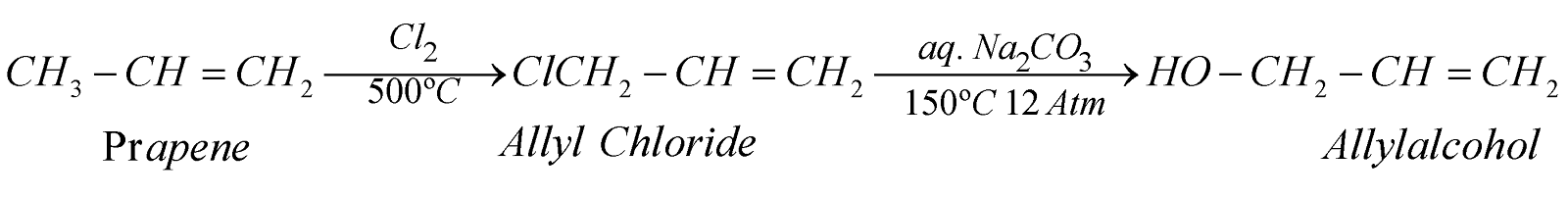
- By fermentation of sugars
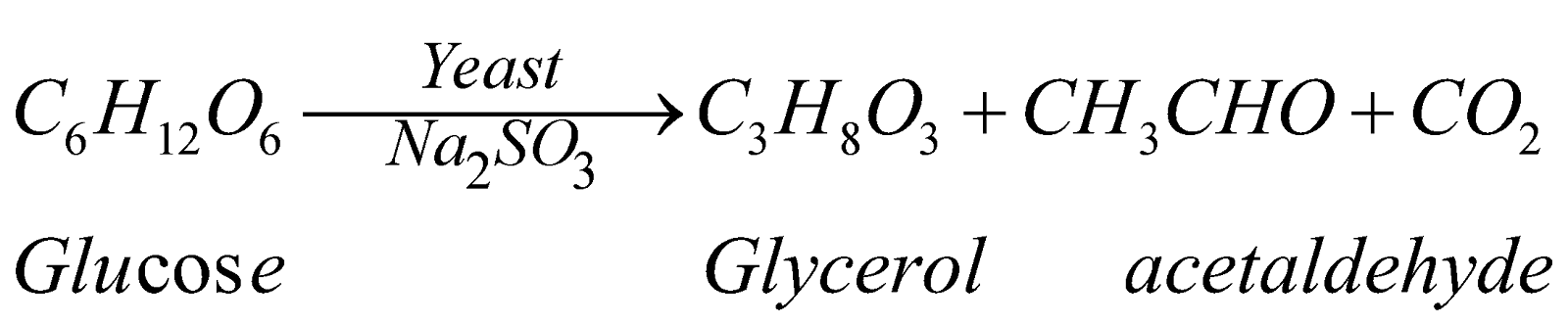
PHYSICAL PROPERTIES
It is colourless, odourless thick viscous liquid miscible with water in all proportions bpt 290ºC but at this temperature it is decomposed. Hence it is purified by distillation under reduced pressure. It shows intensive hydrogen bonding.
CHEMICAL PROPERTIES
It contains two primary and one secondary alcoholic group
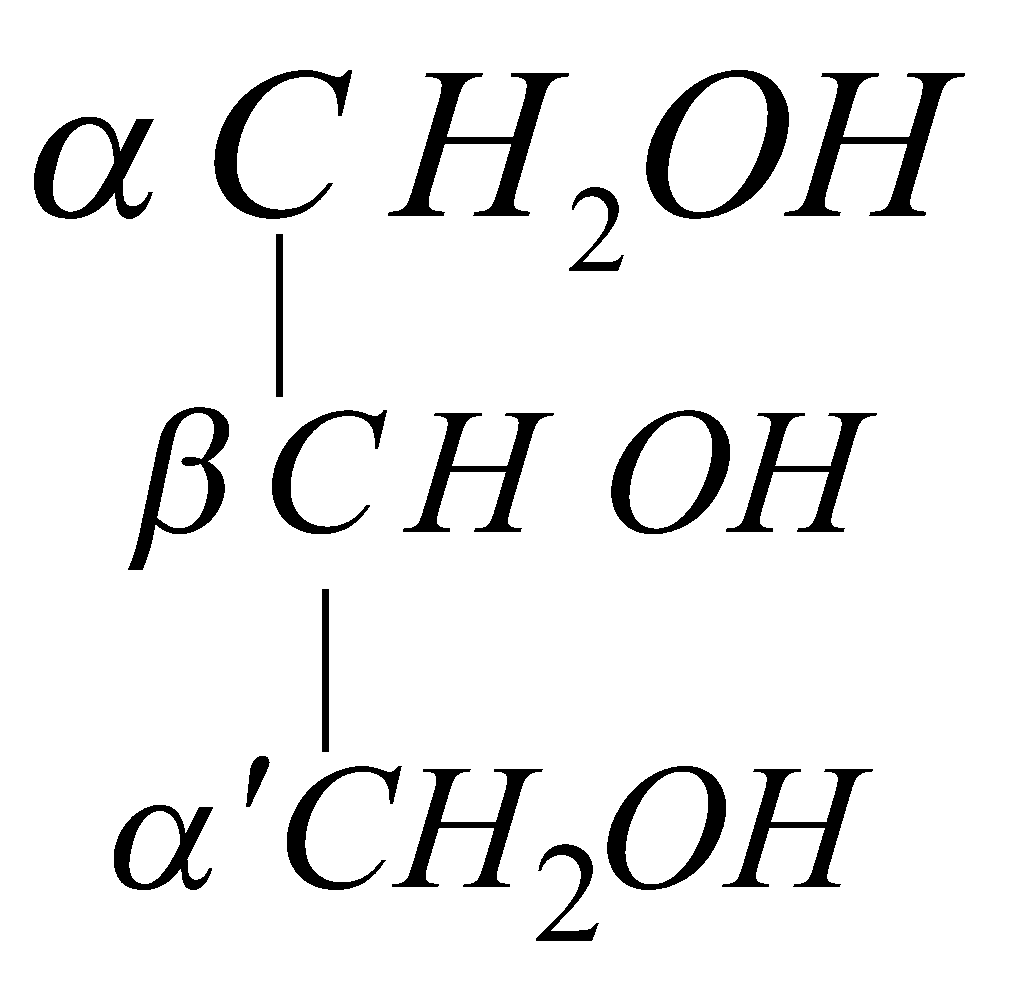
The primary alcoholic groups are more reactive than secondary alcoholic group.
- Reaction with sodium:
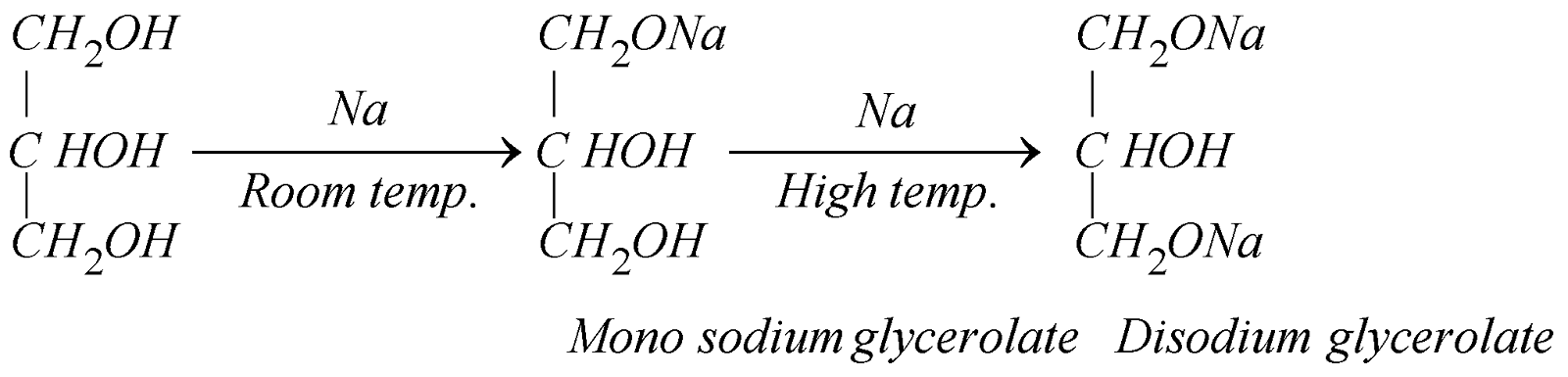
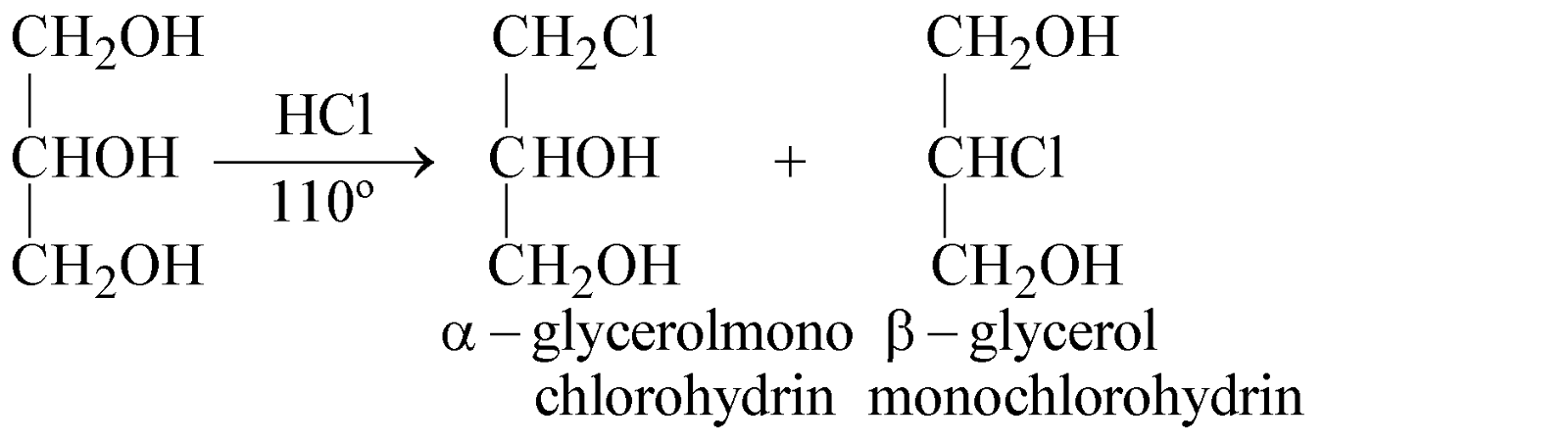
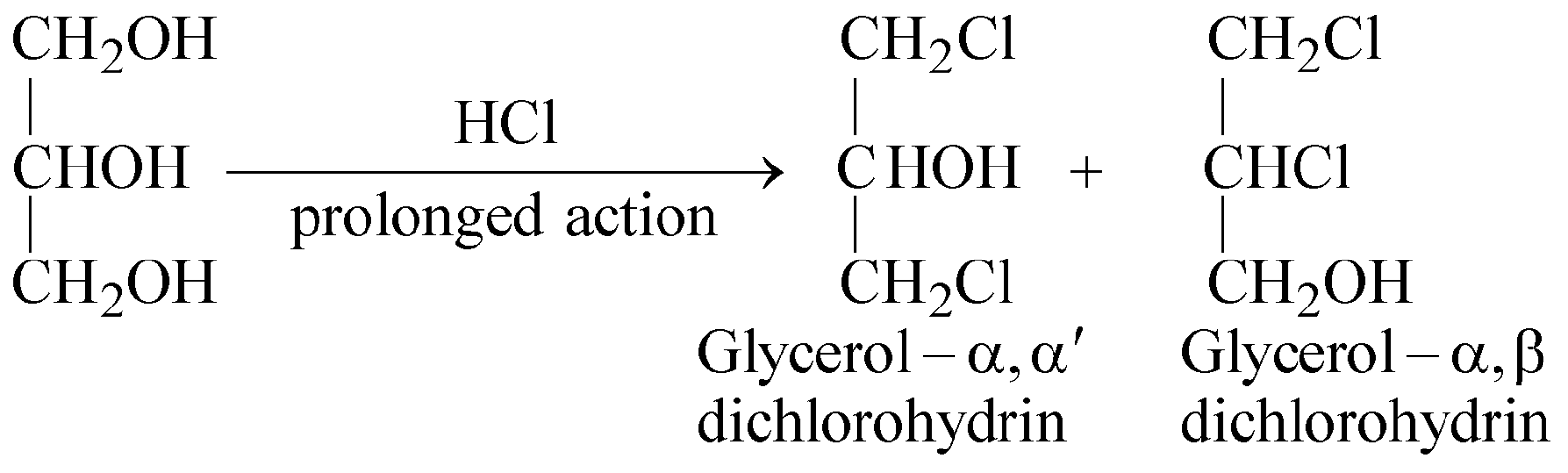
- Reaction with HCl
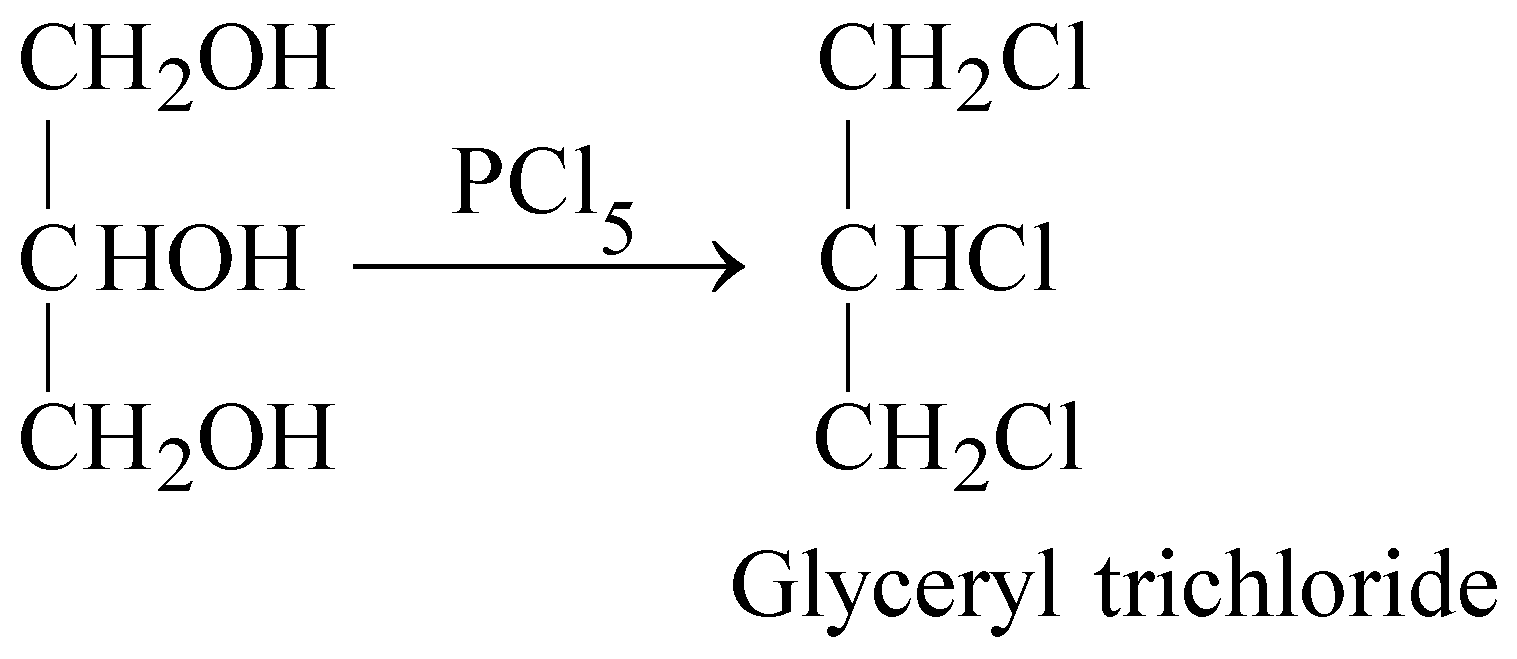
- Reaction with PCl5
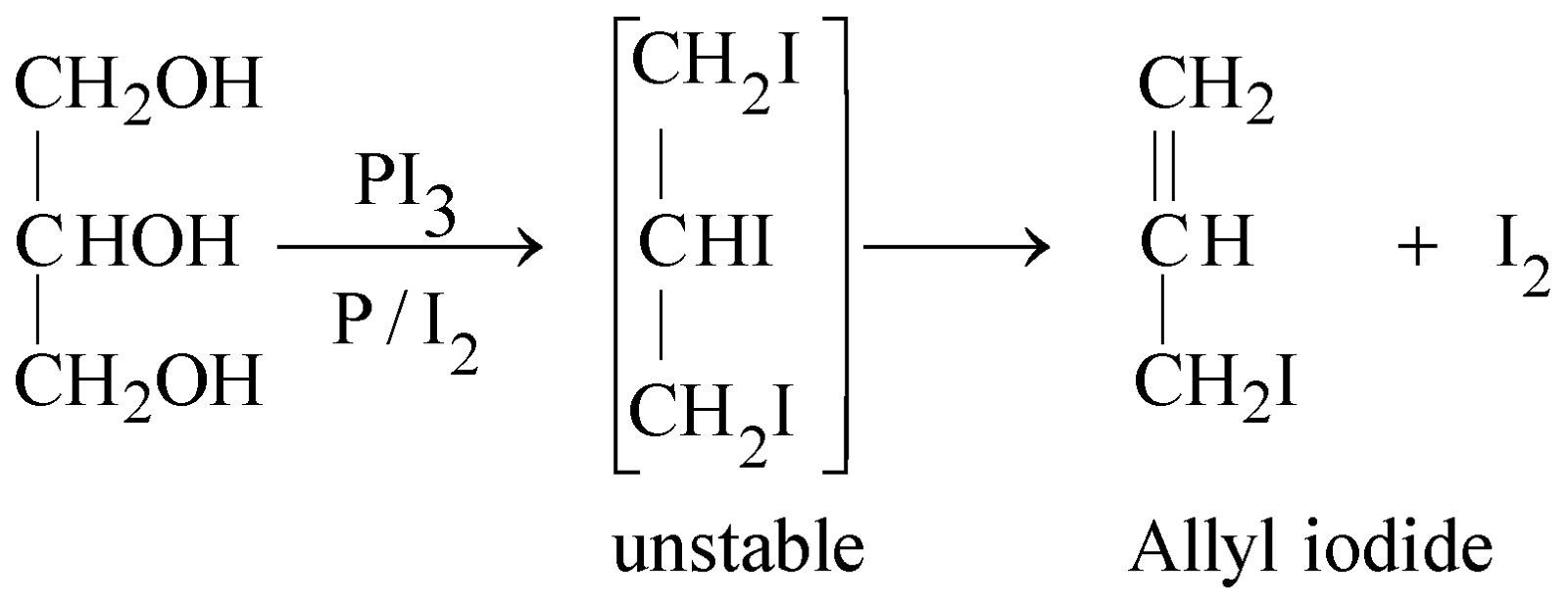
- Reaction with PI3
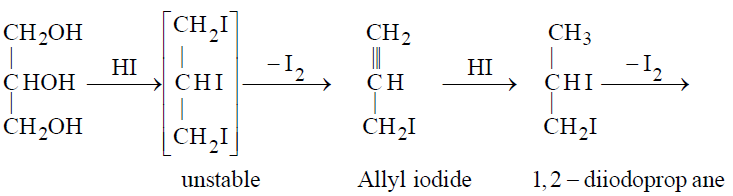
- Reaction with HI
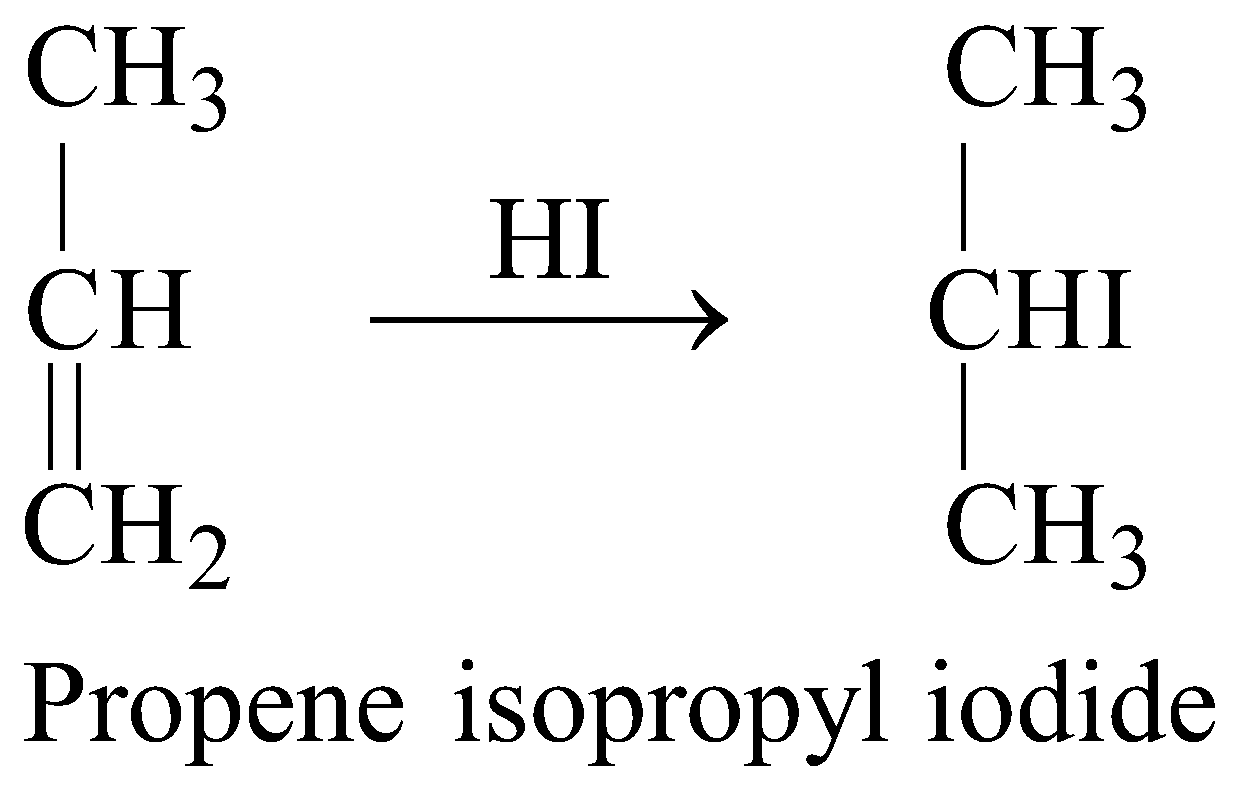
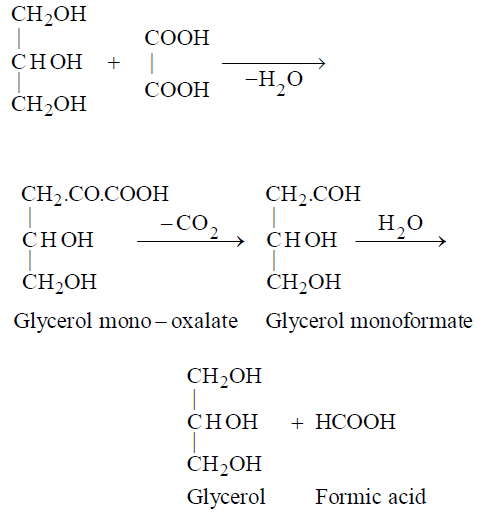
- Reaction with oxalic acid at 100ºC
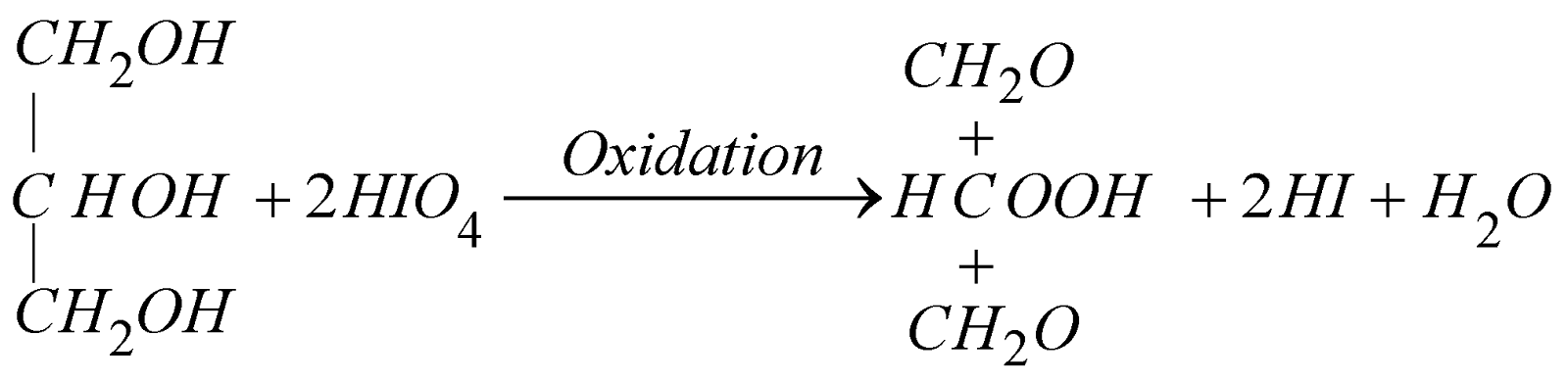
Lab method for the preparation of formic acid
At 260ºC allyl alcohol is the product
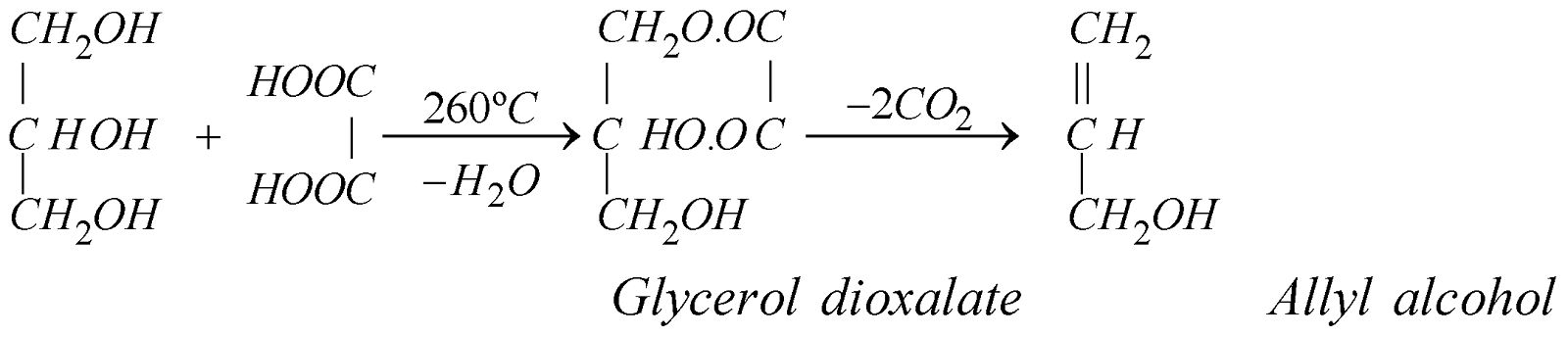
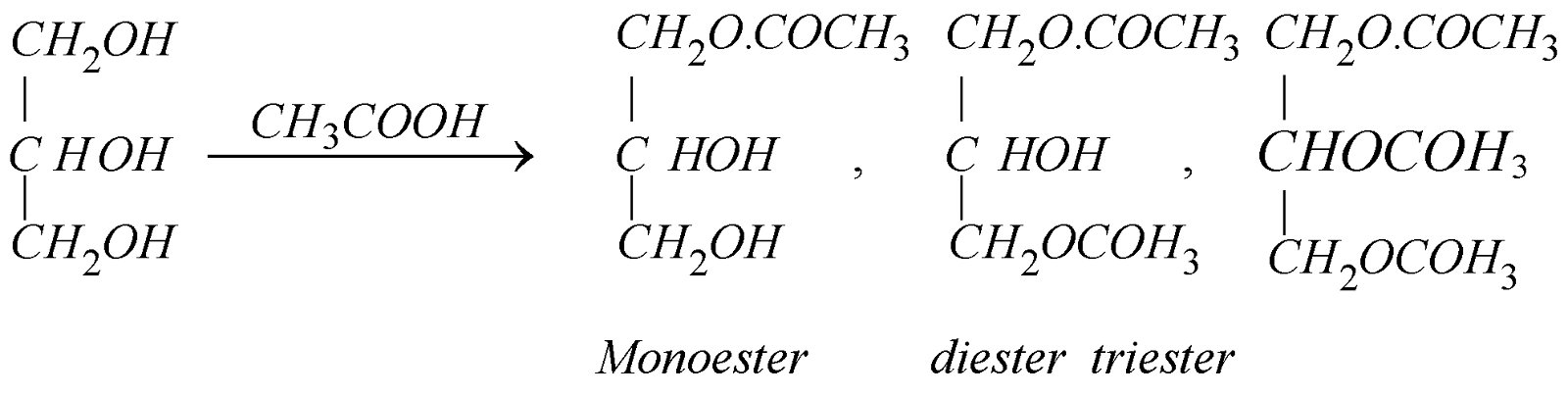
- Reaction with acetic acid, acetic anhydride or acetyl chloride
- Reaction with nitric acid
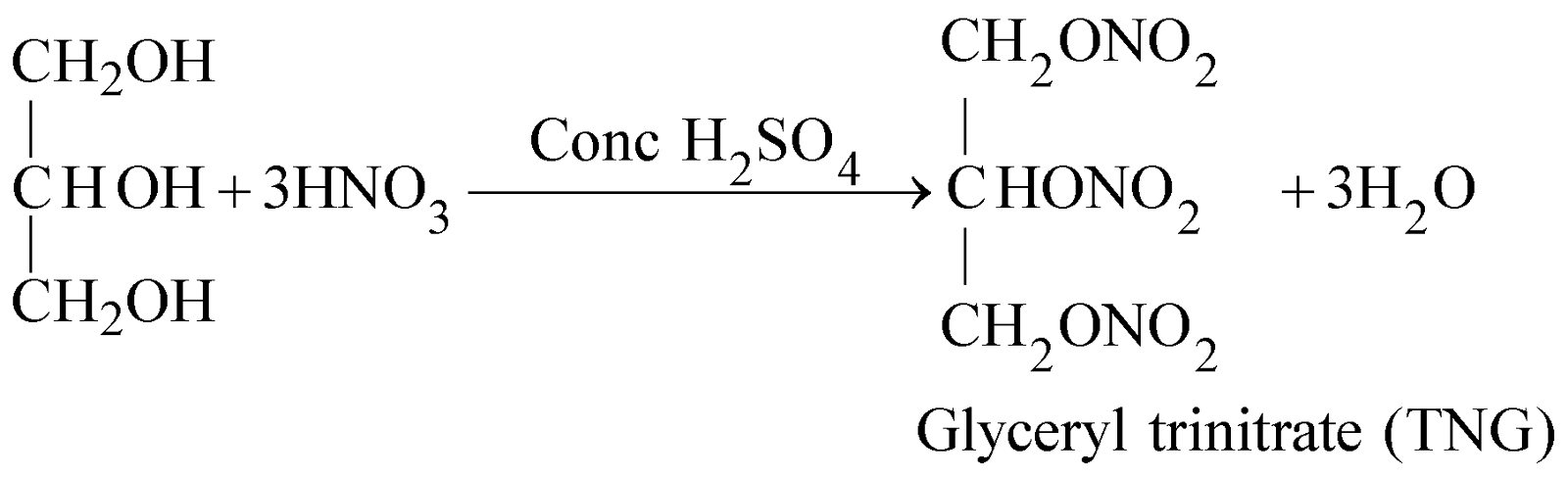
It is also known as Nobel oil after the name of its discoverer Alfred Nobel. It is dark coloured oily liquid, highly poisonous, explodes violently when heated giving huge volume of gases
Uses
- As an explosive (a) Dynamite – Starch + nitroglycerine (b) Blasting gelatin – nitroglycerine + gun cotton (cellulose nitrate) (c) Cordite-nitroglycerine + gun cotton + vaseline. It is smokeless explosive
- As medicine. In treatment of heart disease and asthma.
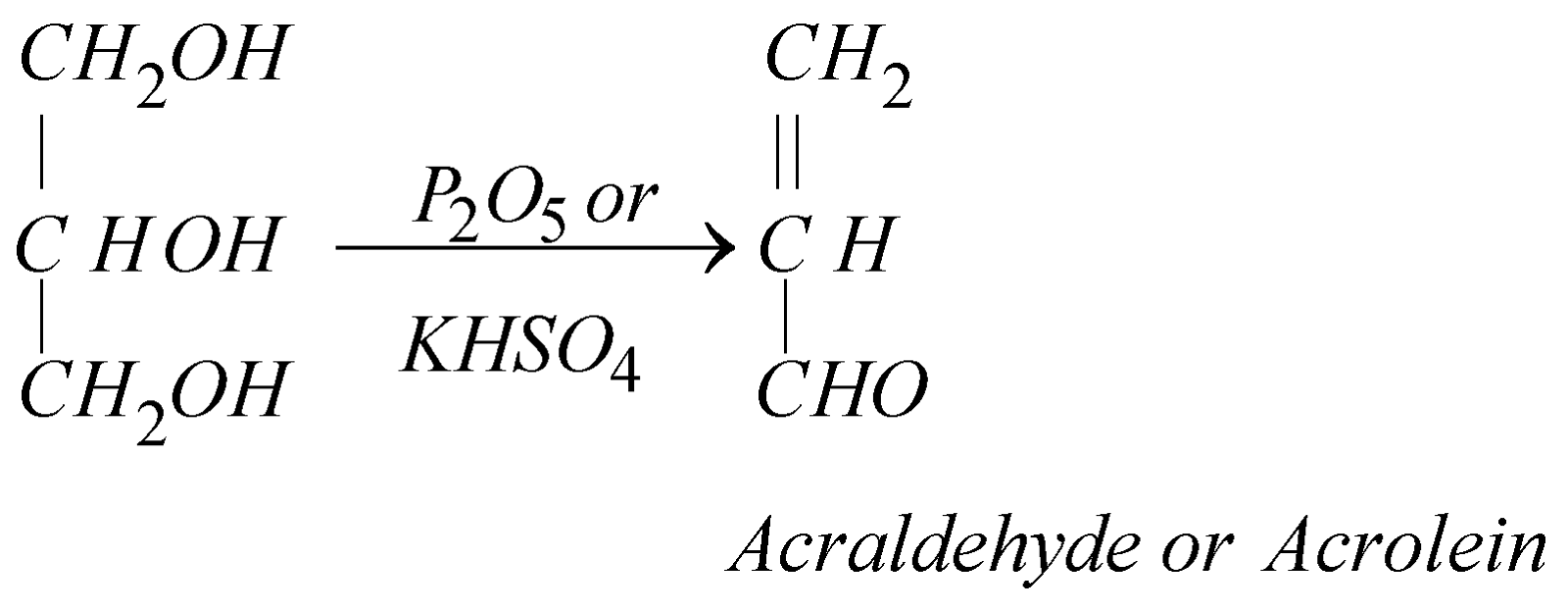
- Dehydration: It can be achieved by heating alone, with KHSO4 or P2O5 or Conc H2SO4
- Oxidation with periodic acid (HIO4)
- Oxidation: Glycerol can be oxidised to give different oxidation products under different conditions. The main products are
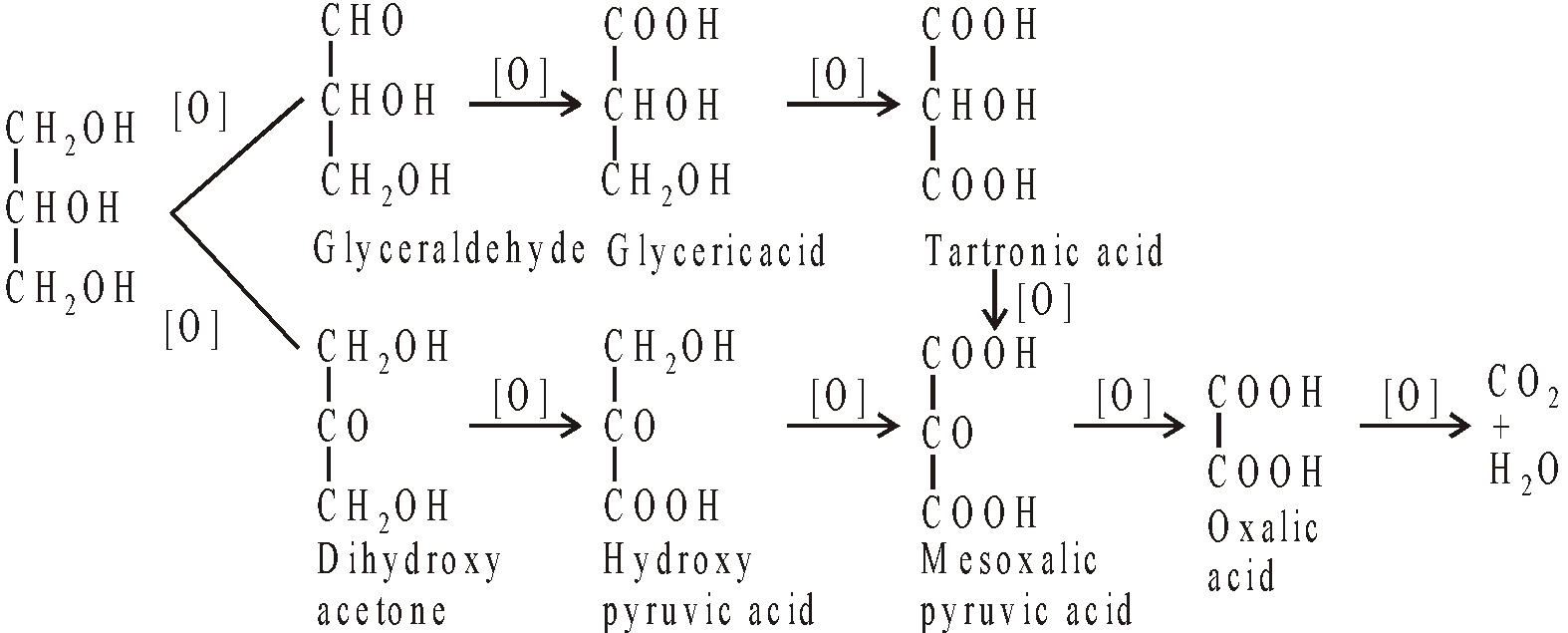
GLYCEROSE
It is a mixture of glyceraldehyde and dihydroxyacetone and is obtained by oxidation of glycerol with Fenton’s reagent (H2O2 + Fe SO4)
Dilute nitric acid gives glyceric acid as main product
Conc. nitric acid gives glyceric acid + tartronic acid
Bismuth nitrate gives mesoxalic acid
Uses
- In the manufacture of explosives
- In cosmetics
- For preserving tobacco
- As an antifreeze
- As lubricant in watches
AROMATIC HYDROXY COMPOUNDS (PHENOLS)
Aromatic hydroxy compounds are of two types
- Phenols : In phenols the hydroxyl group is directly attached to aromatic nucleus.
- Aromatic alcohols : In aromatic alcohols the hydroxyl group is present in the side chain e.g. C6H5.CH2OH benzyl alcohol.
Phenols are classified as-
- Monohydric phenols
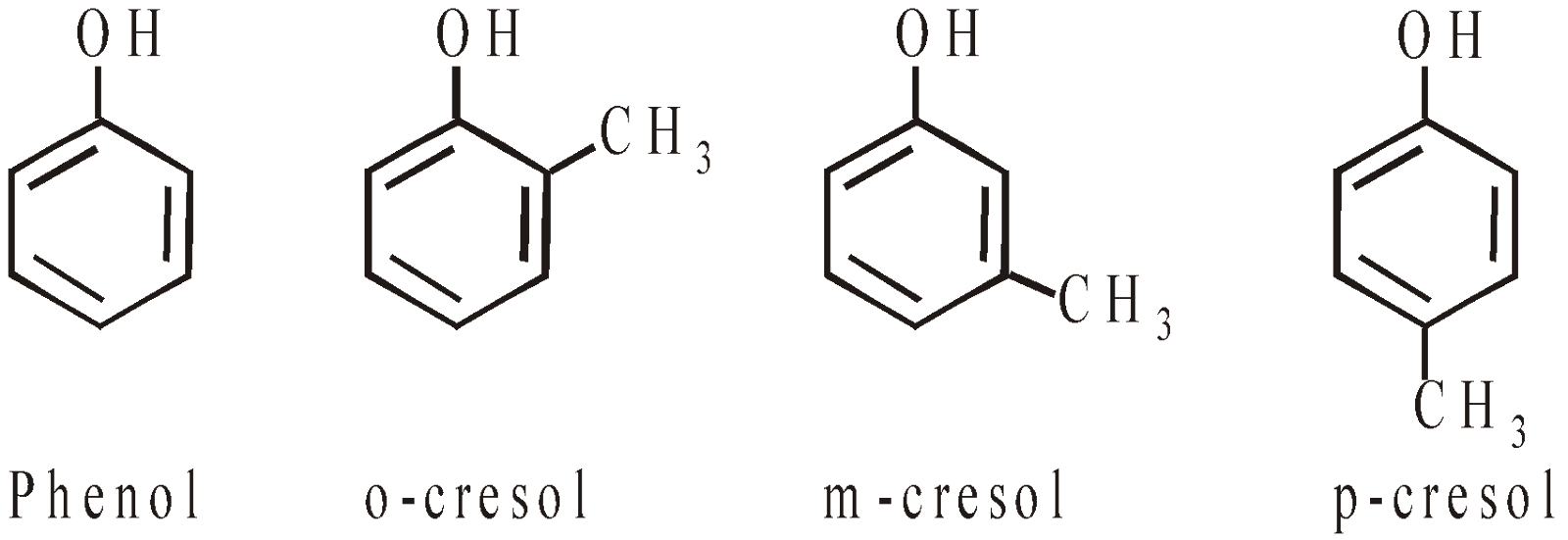
- Dihydric phenols
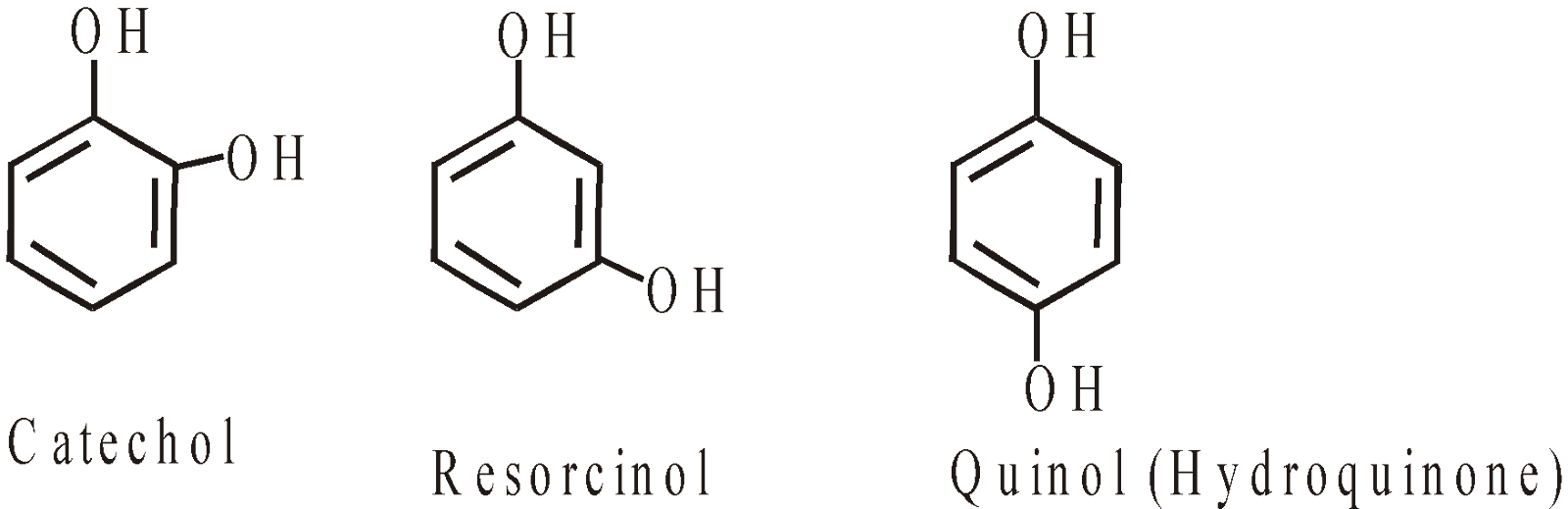
- Trihydric phenols
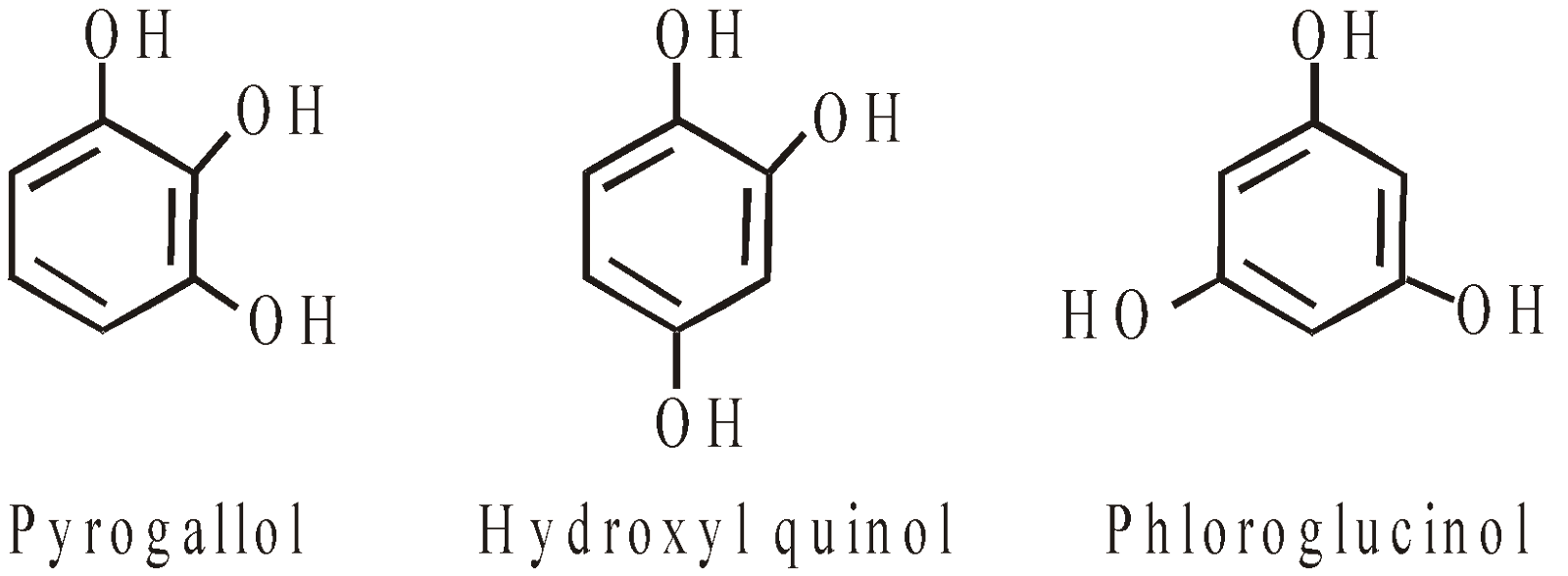
Phenol (Carbolic acid)
GENERAL METHODS OF PREPARATION OF PHENOLS
- Hydrolysis of diazonium salts (particularly sulphates)
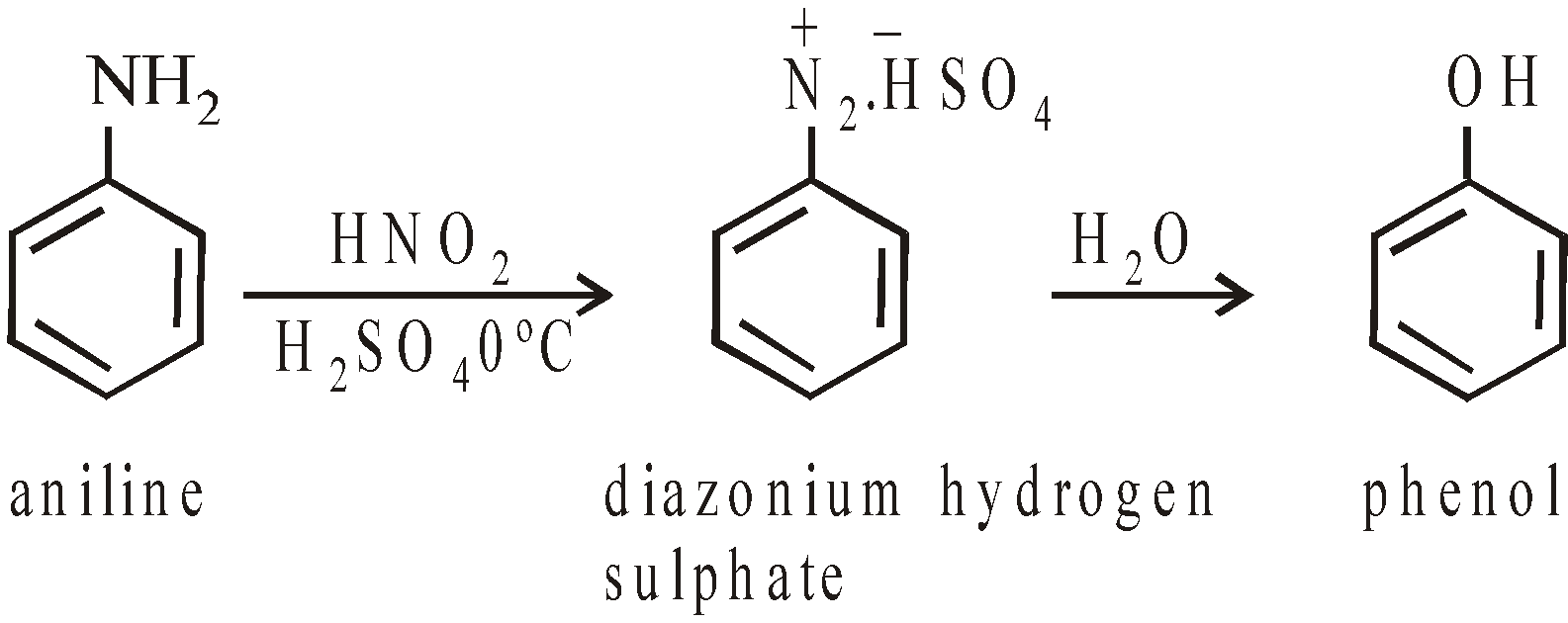
- From Sulphonic acids
Alkali Salts of aryl sulphonic acids are fused with caustic alkalies to get phenates, which are decomposed by acids
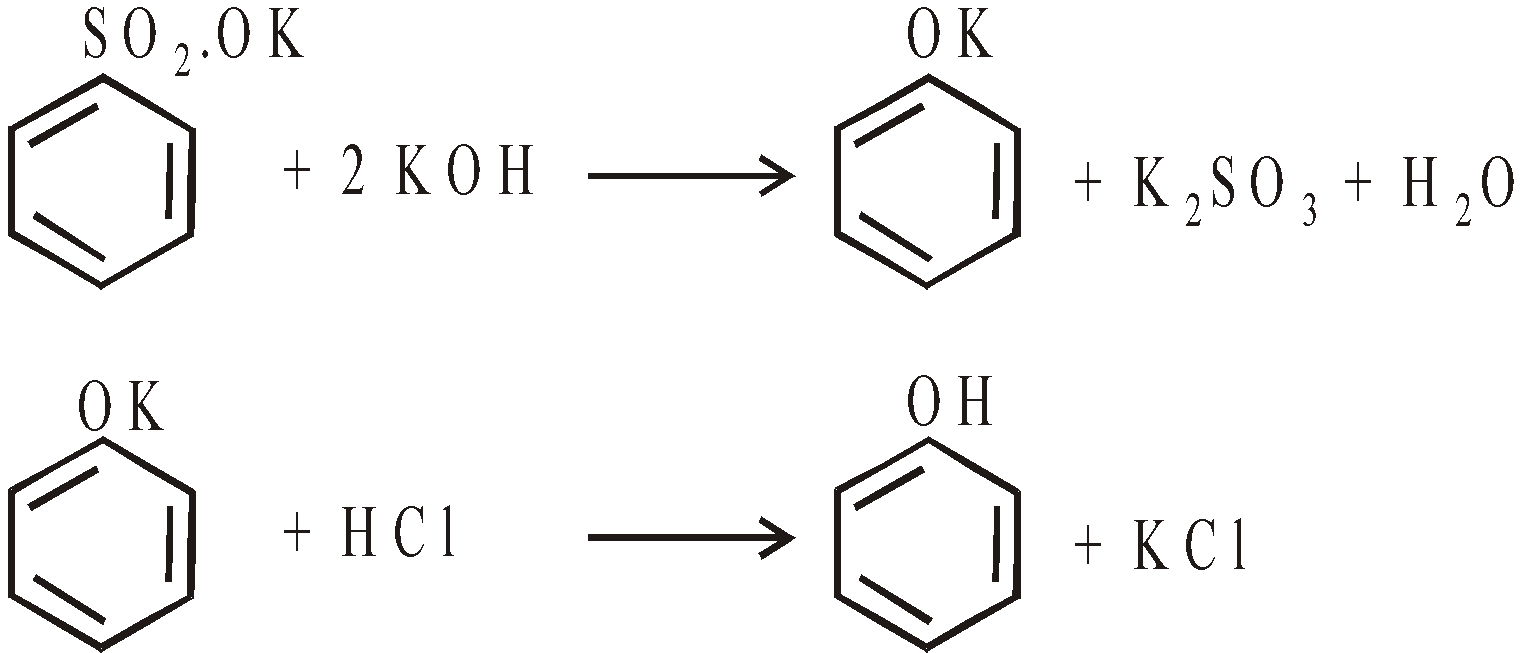
- From phenolic acids
Sodium salts of phenolic acids distilled with soda lime
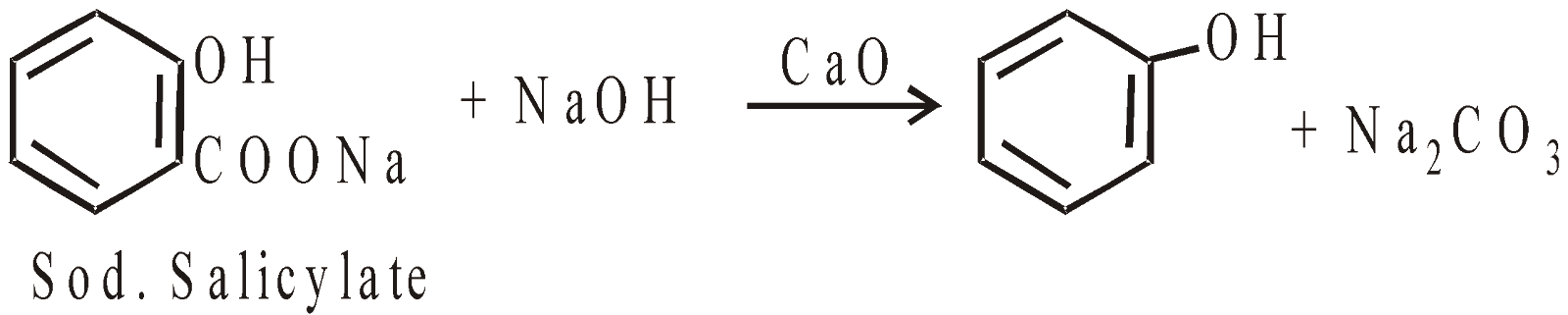
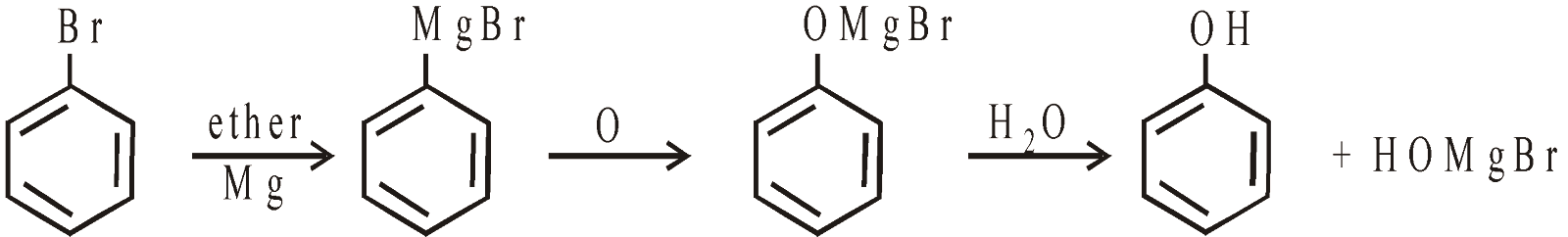
- From Grignard’s reagents
- From aryl halides
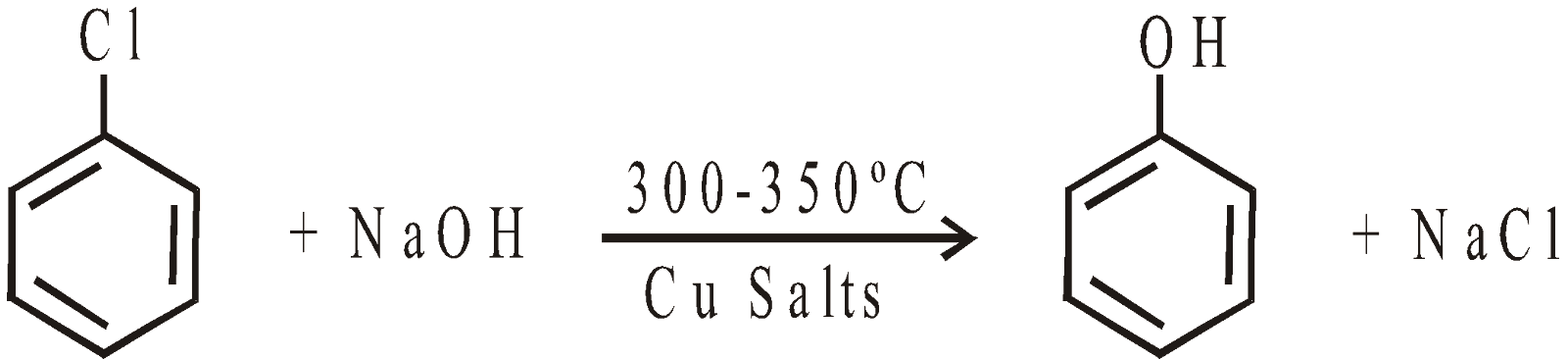
MANUFACTURE OF PHENOL
- From middle oil fraction of coaltar : The middle oil and heavy oil fraction contain phenols and naphthalene. On cooling naphthalene separates out and removed by filter pressing. The oil left is treated with aqueous NaOH when phenols form phenoxides. To liberate phenols CO2 is then passed
Phenols separate out as oily layer and separated from aqueous layer containing Na2CO3 and fractionally distilled.
- Fraction distilling at 180ºC – phenol
- Fraction distilling at 190-203ºC – cresols (o, m and p)
- Fraction distilling at 211-225ºC – xylenols
- Cumene method : Recent and best method
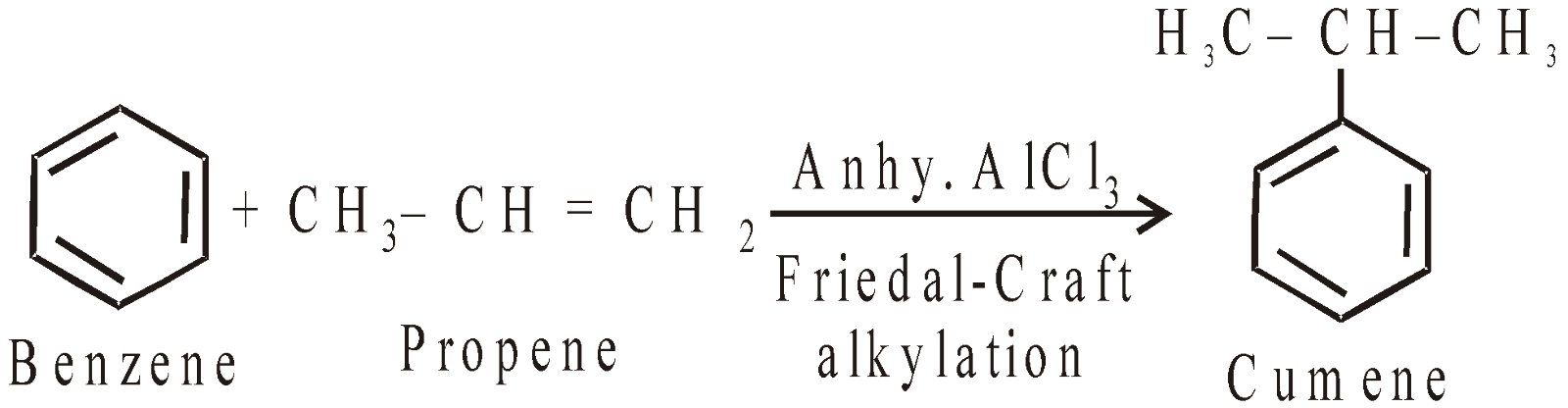
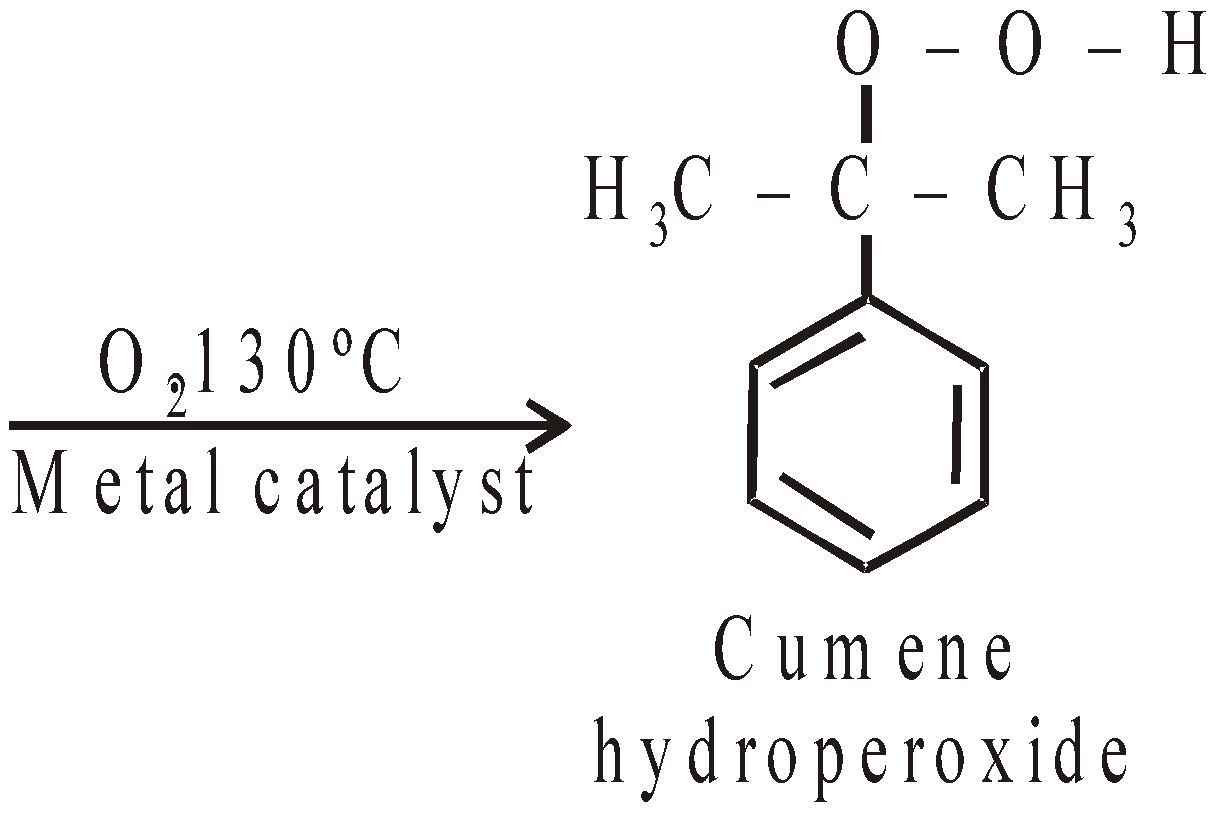
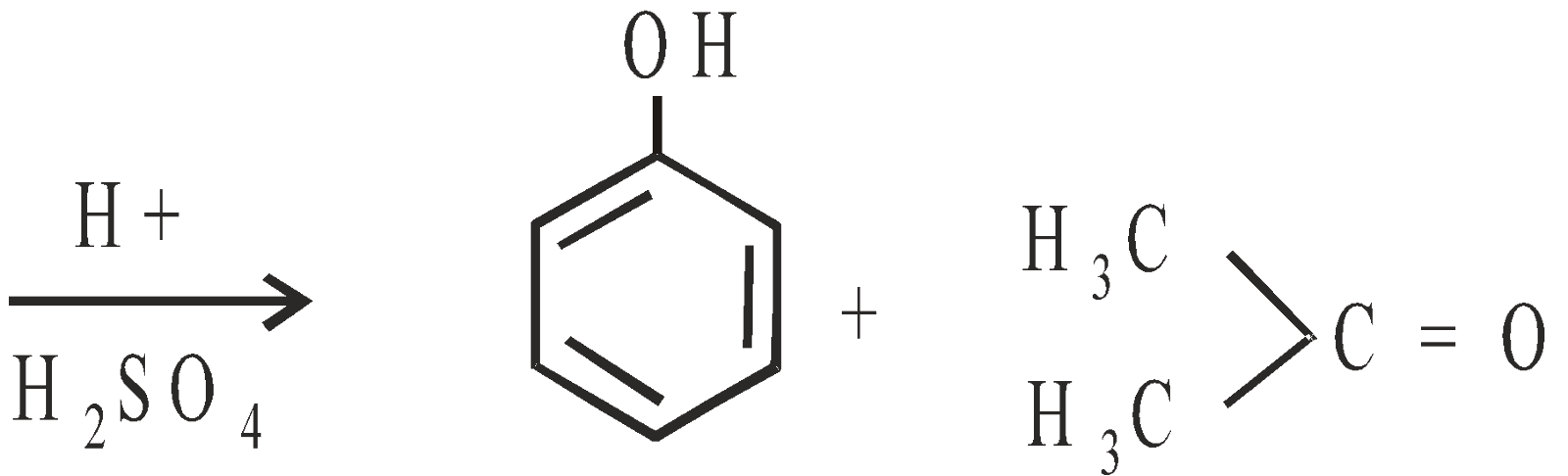
Phenol Acetone
- Raschig process : Developed in Germany
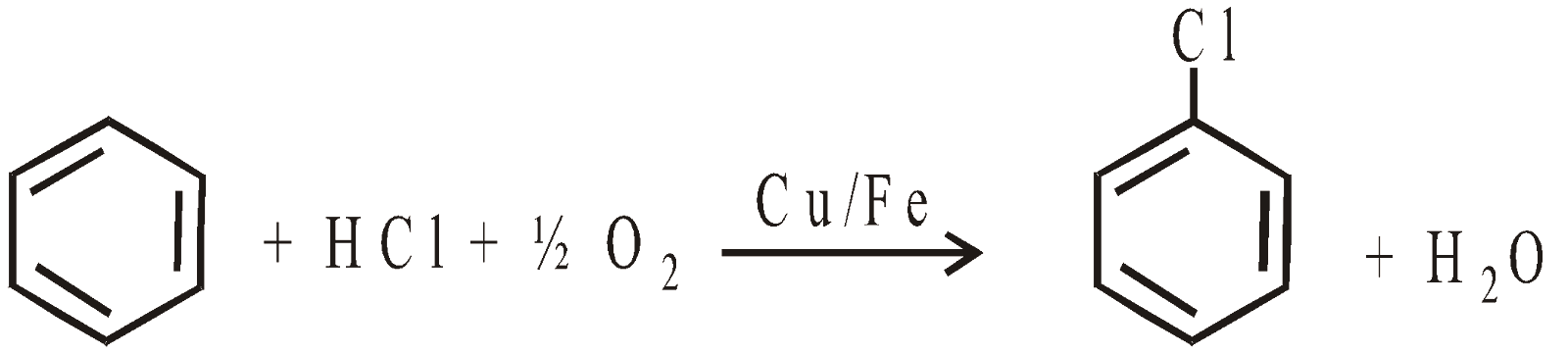
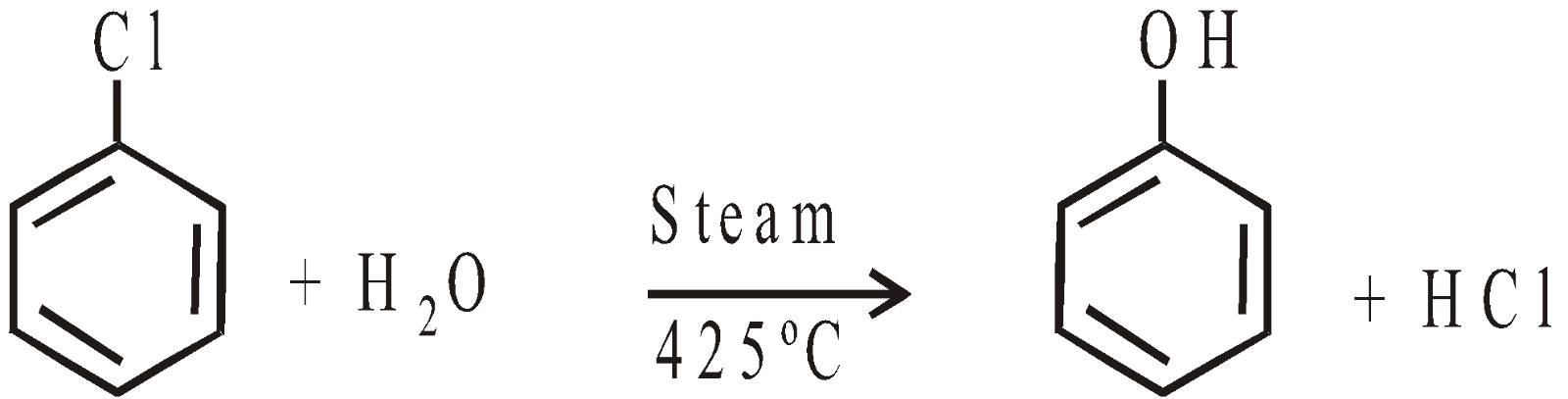
- From Chlorobenzene “Dow Process”
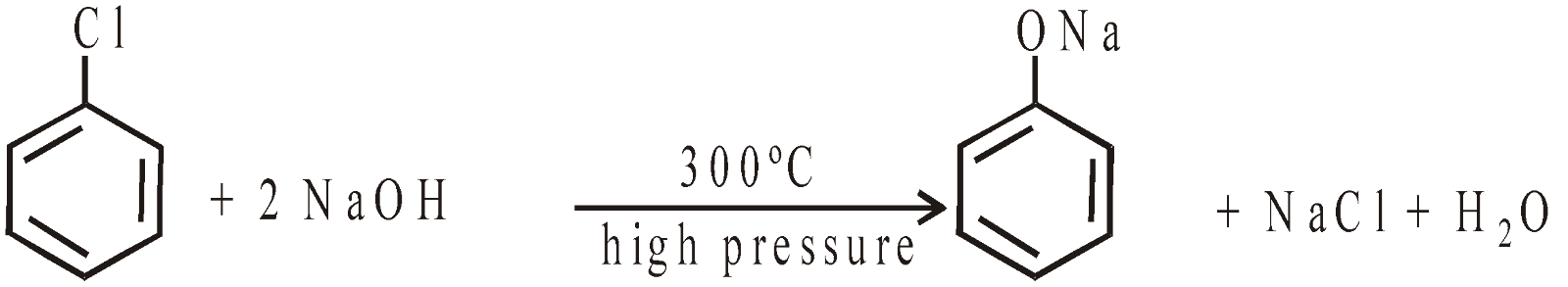
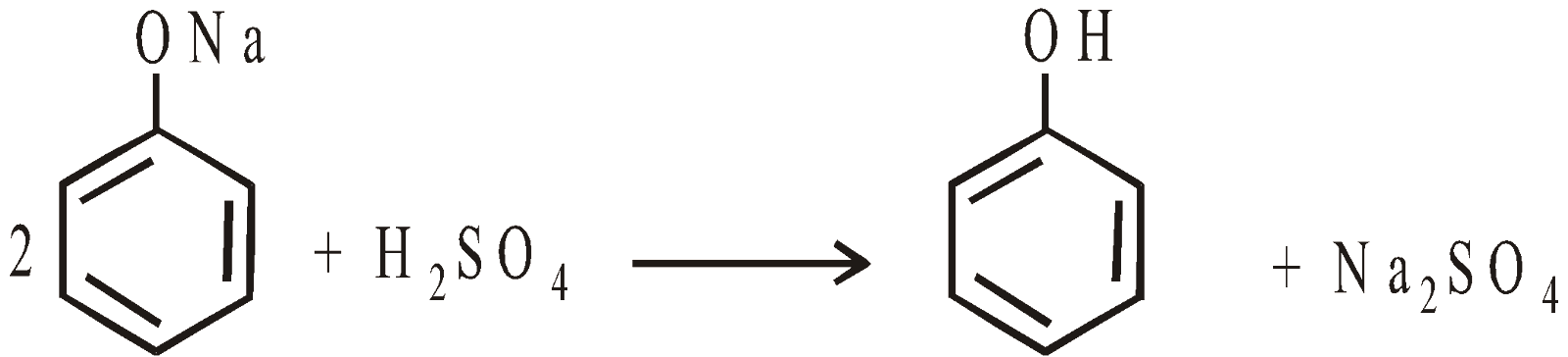
- Oxidation of benzene

PHYSICAL PROPERTIES
Phenol is a colourless crystalline solid mpt. 42ºC and bpt 183ºC. It is deliquescent, becomes pinkish on exposure to air due to oxidation, sparingly soluble in cold water.
CHEMICAL PROPERTIES
ACIDIC NATURE
Phenol is acidic in nature due to greater resonance stabilisation of phenoxide ion than phenol itself
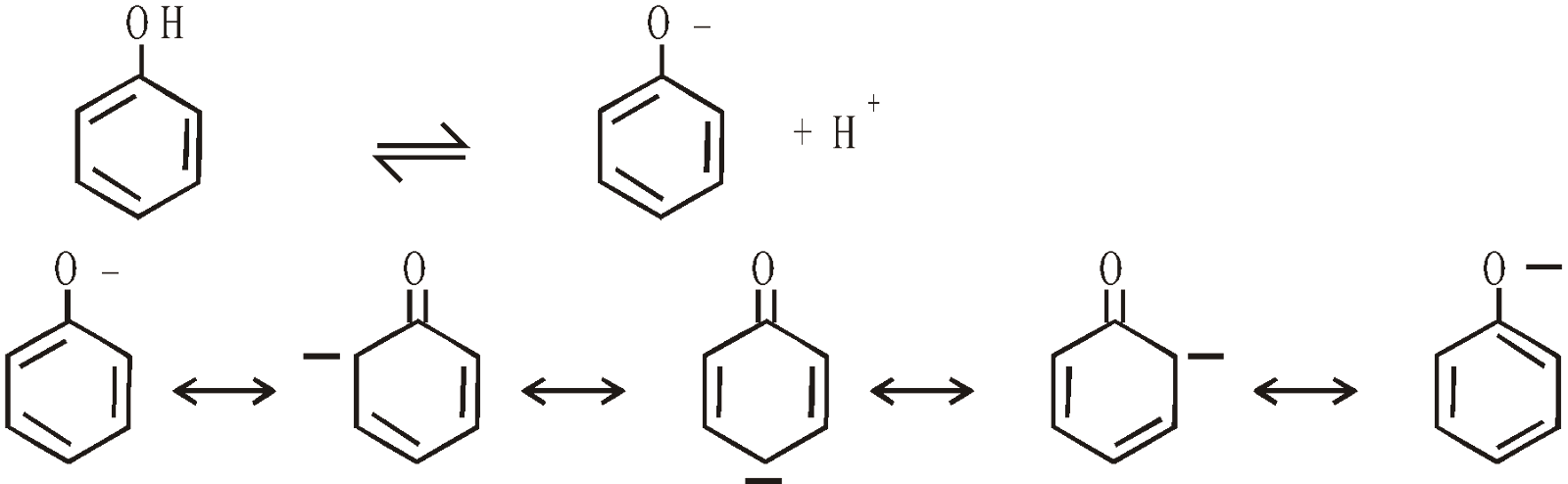
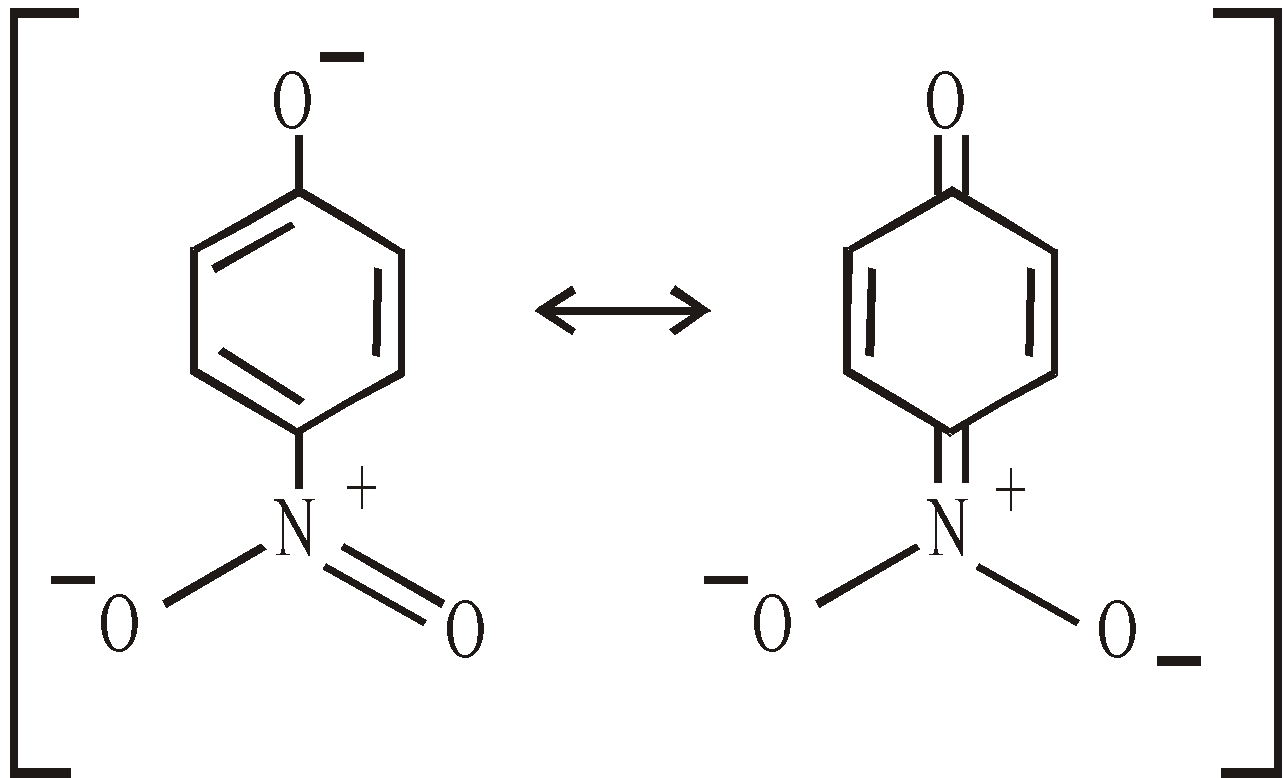
The presence of electrons withdrawing groups in the nucleus increase the acidity. This is due to inductive effect and mesomeric effect.The latter effect is operative when the group is in the o- or p- but not in m-position. The resultant anion is more stabilised through delocalisation of electrons.
Compound pka
The presence of electron donating groups in the nucleus decreases the acid character of phenol though the effect is small
Compound pka
The smaller the value of pka the more is the acid character.
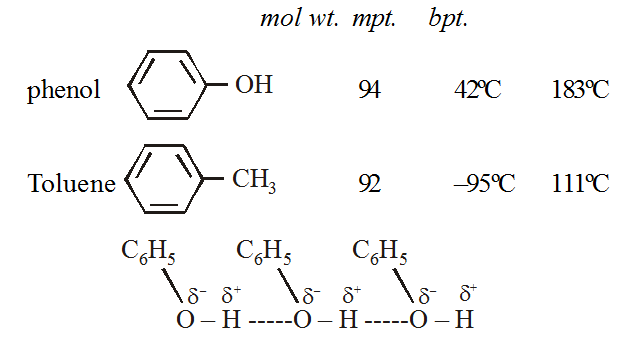
MELTING AND BOILING POINTS
Phenols form the intermolecular hydrogen bonding and their mpts and bpts are much higher than hydrocarbons of comparable molecular weights
REACTIONS OF PHENOLS RESEMBLING ALCOHOLS
ACTION OF Na METAL

FORMATION OF ETHERS
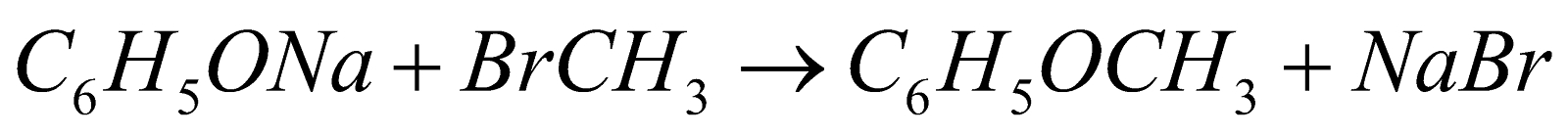
Claisen’s rearrangment : When o-allyl ether of phenols are heated to 100-250ºC without solvent or catalyst, the allyl group migrates to ortho position with inversion of configuration
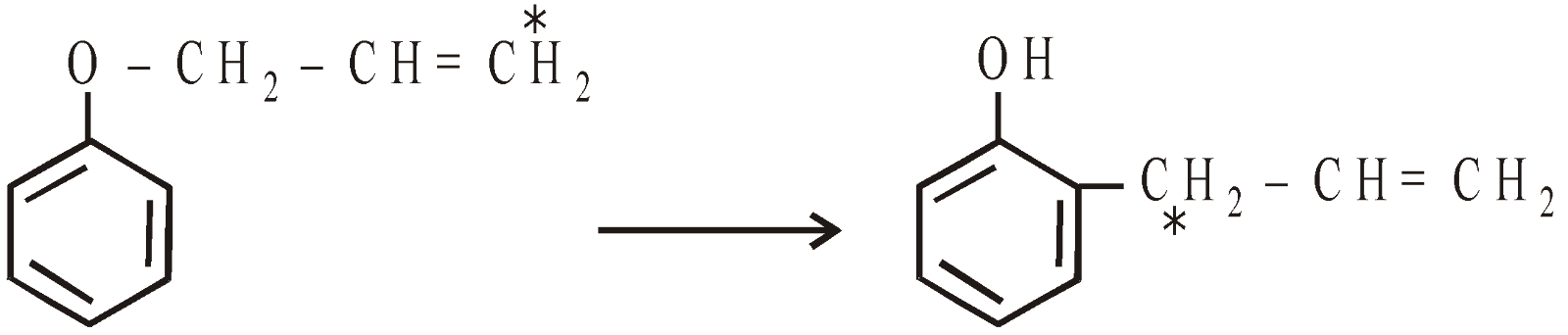
If both the ortho positions are occupied the allyl group migrates to para position without inversion of configuration
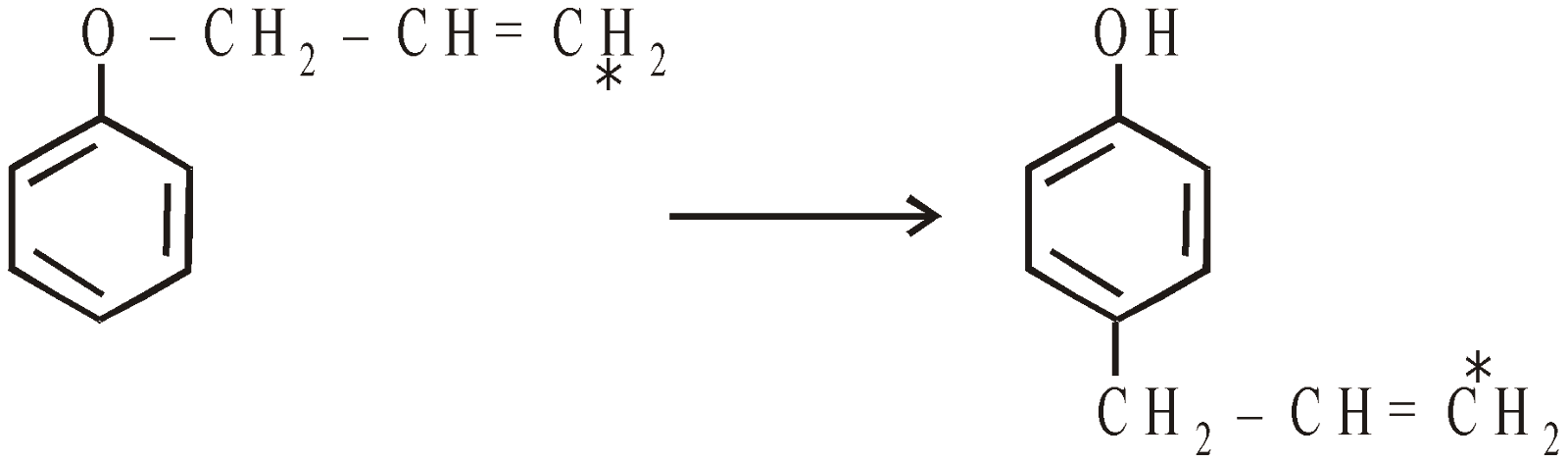
ACYLATION – with acid chloride or acid anhydride
FRIES REARRANGEMENT
The phenyl ester when heated with anhy. AlCl3 or ZnCl2 the acetyl group migrates to ortho and para positions to form hydroxy ketones
The phenyl ester when heated with anhy. AlCl3 or ZnCl2 the acetyl group migrates to ortho and para positions to form hydroxy ketones
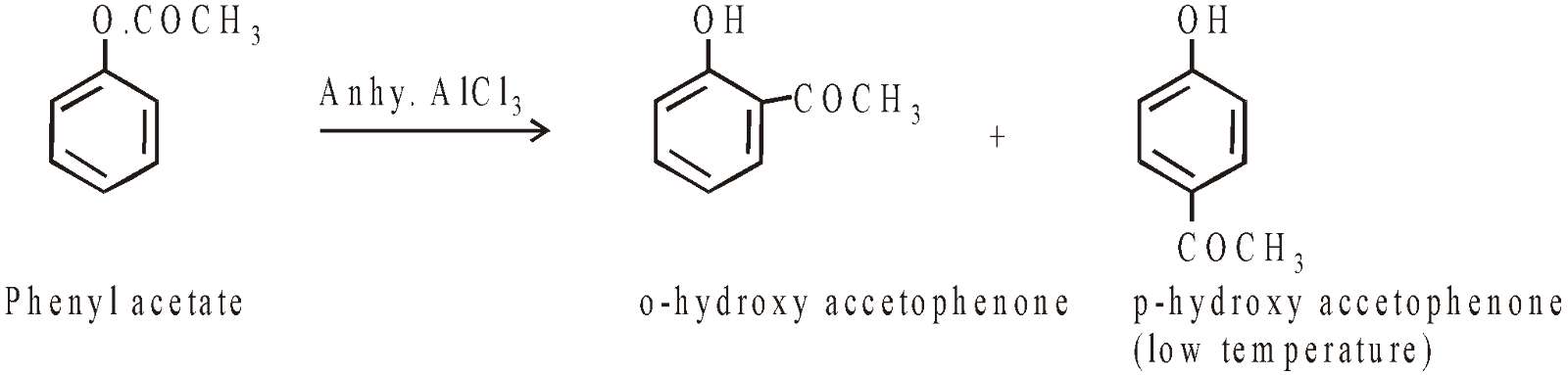
BUCHERER REACTION
ACTION OF PCl5
ACTION OF P2S5
REACTIONS GIVEN BY PHENOLS ONLY
ACTION OF NaOH
ACTION OF AQUEOUS FeCl3
A violet coloured coordination complex is formed
A violet coloured coordination complex is formed
DISTILLATION WITH Zn DUST
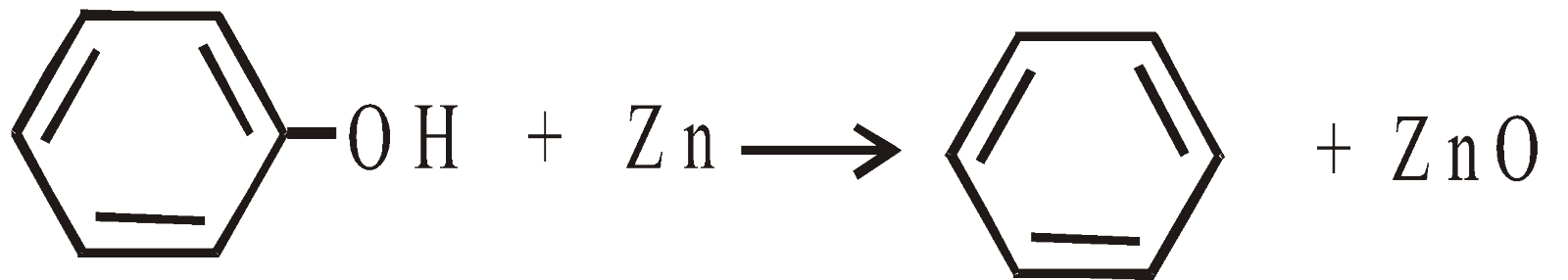
REDUCTION WITH H2/Ni
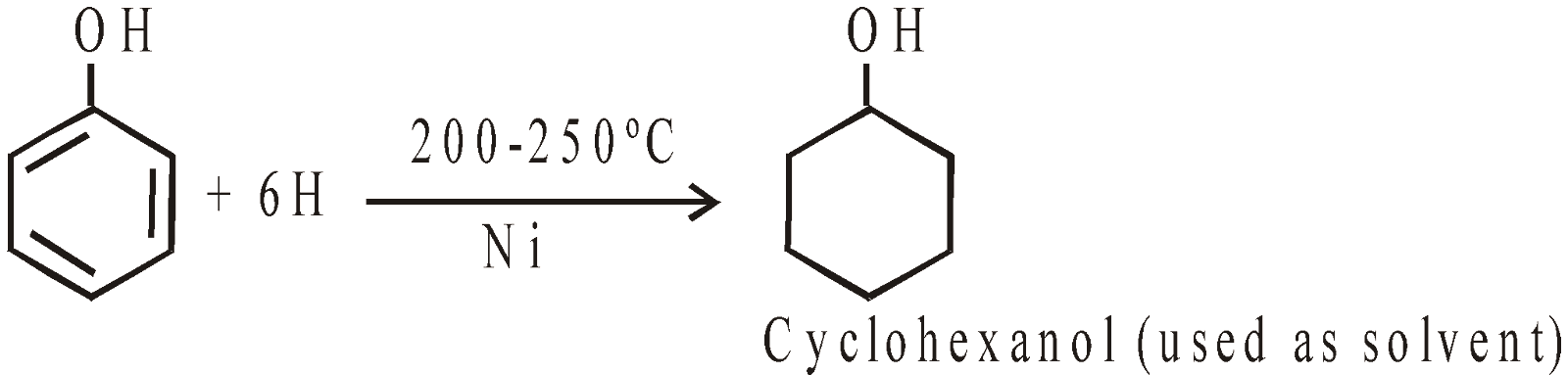
COUPLING REACTION
with diazonium salts
with diazonium salts
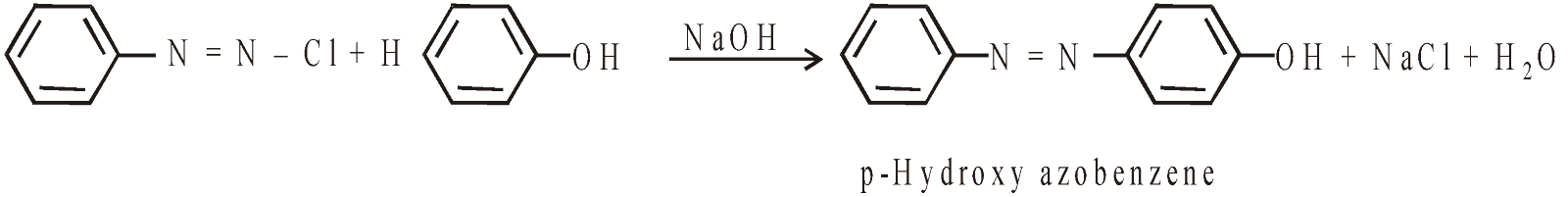
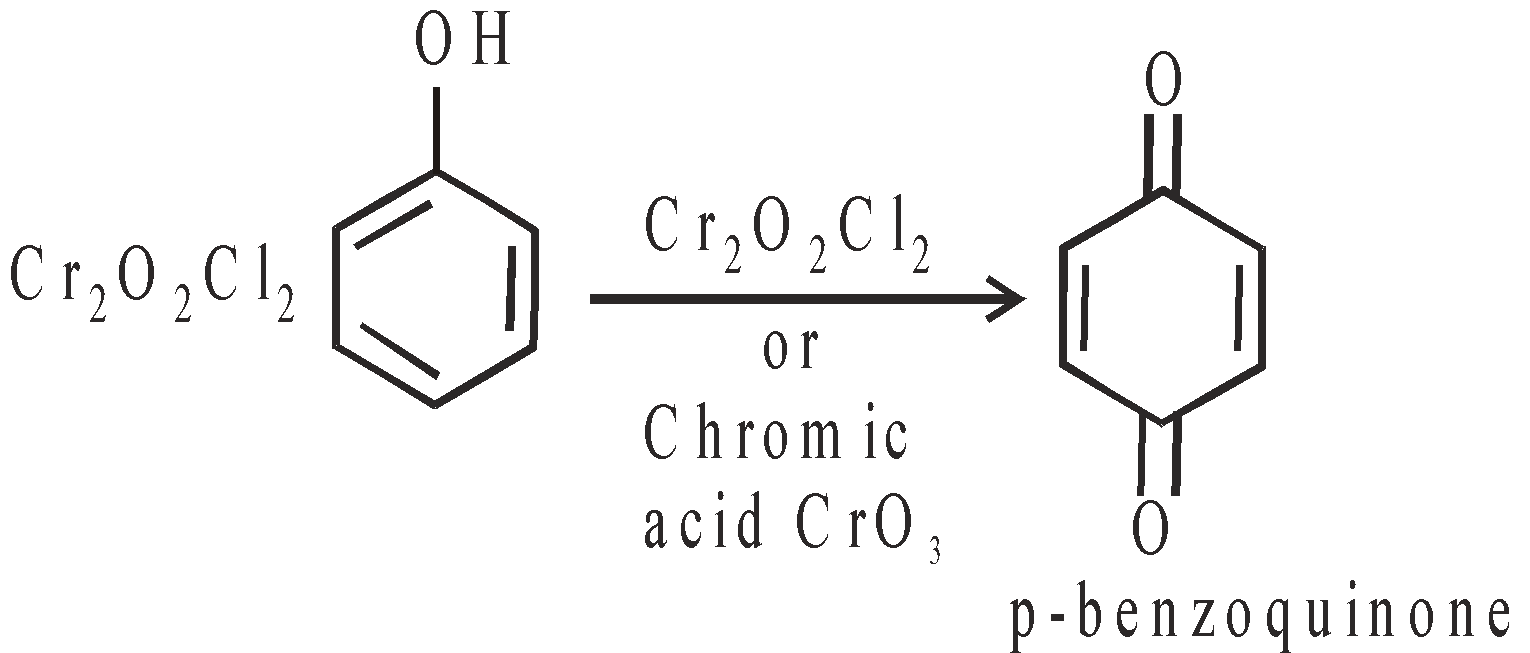
OXIDATION
Different products are obtained under different conditions.
- With Cr2O2Cl2
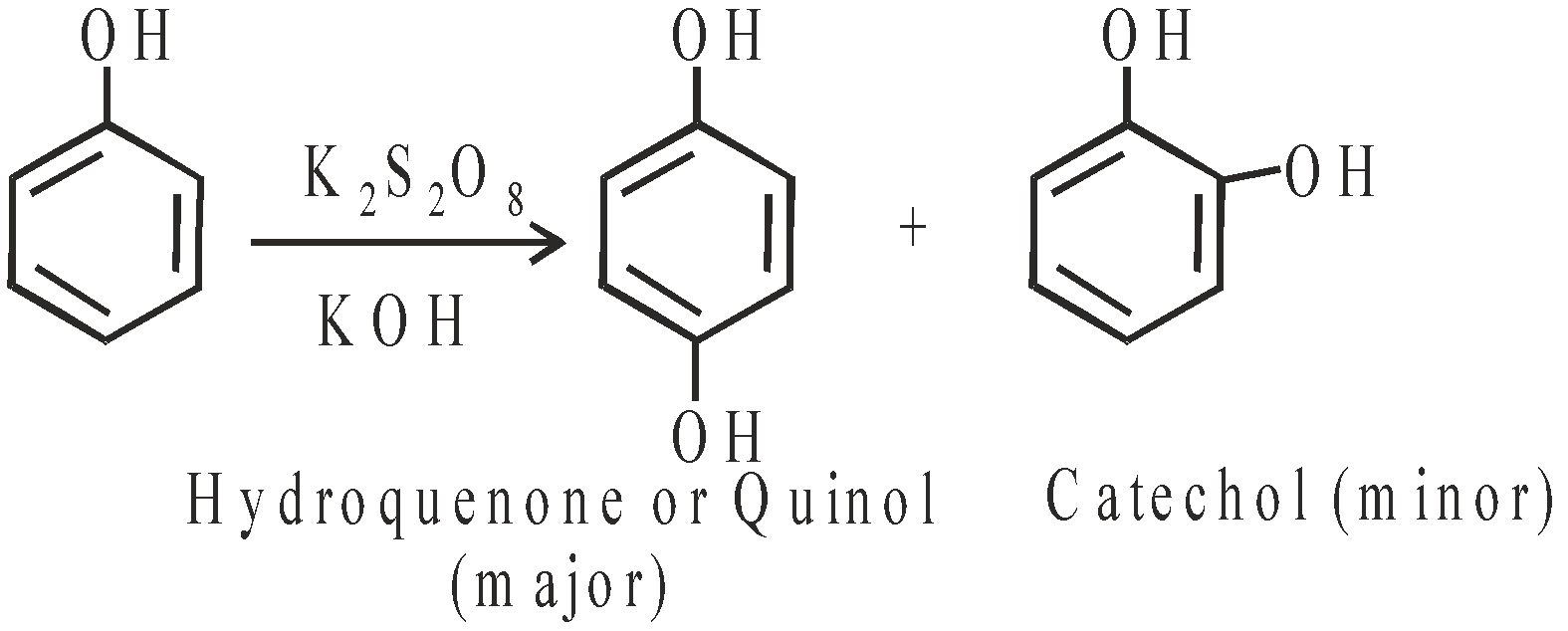
- Elbs persulfate oxidation
- With potassium permanganate : Meso Tartaric acid is obtained
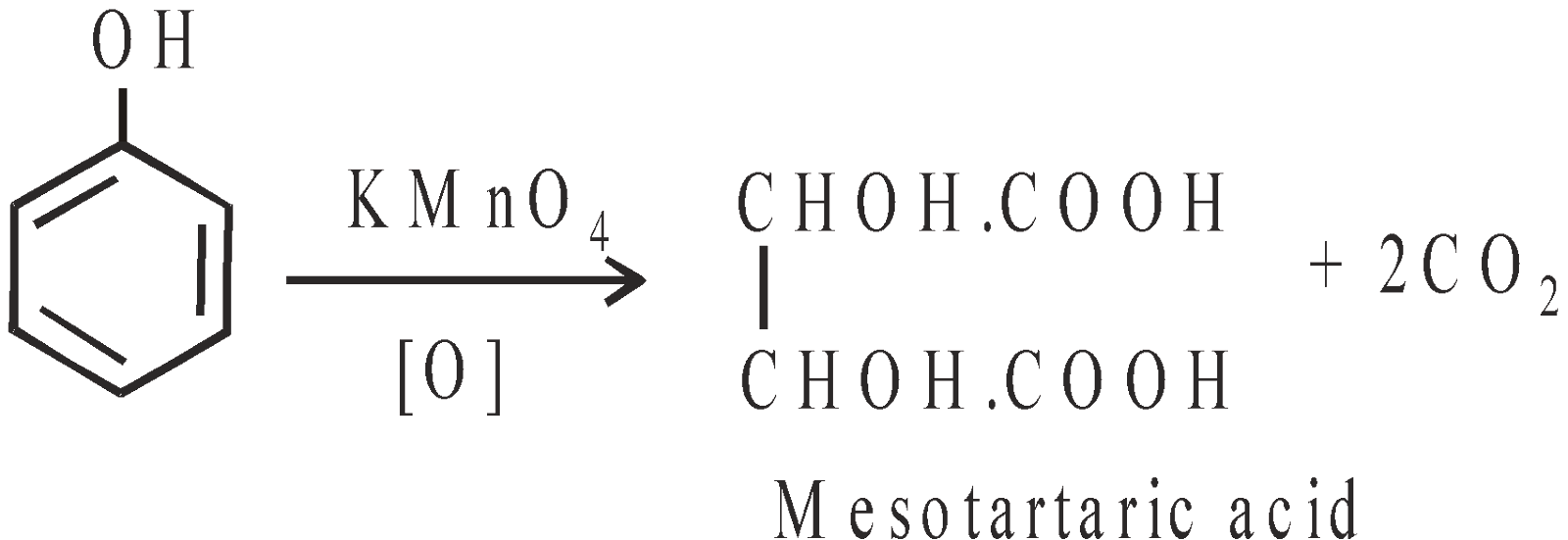
- With a mixture of KClO3 and Conc. HCl : Chloranil called tetrachloro quinone is obtained
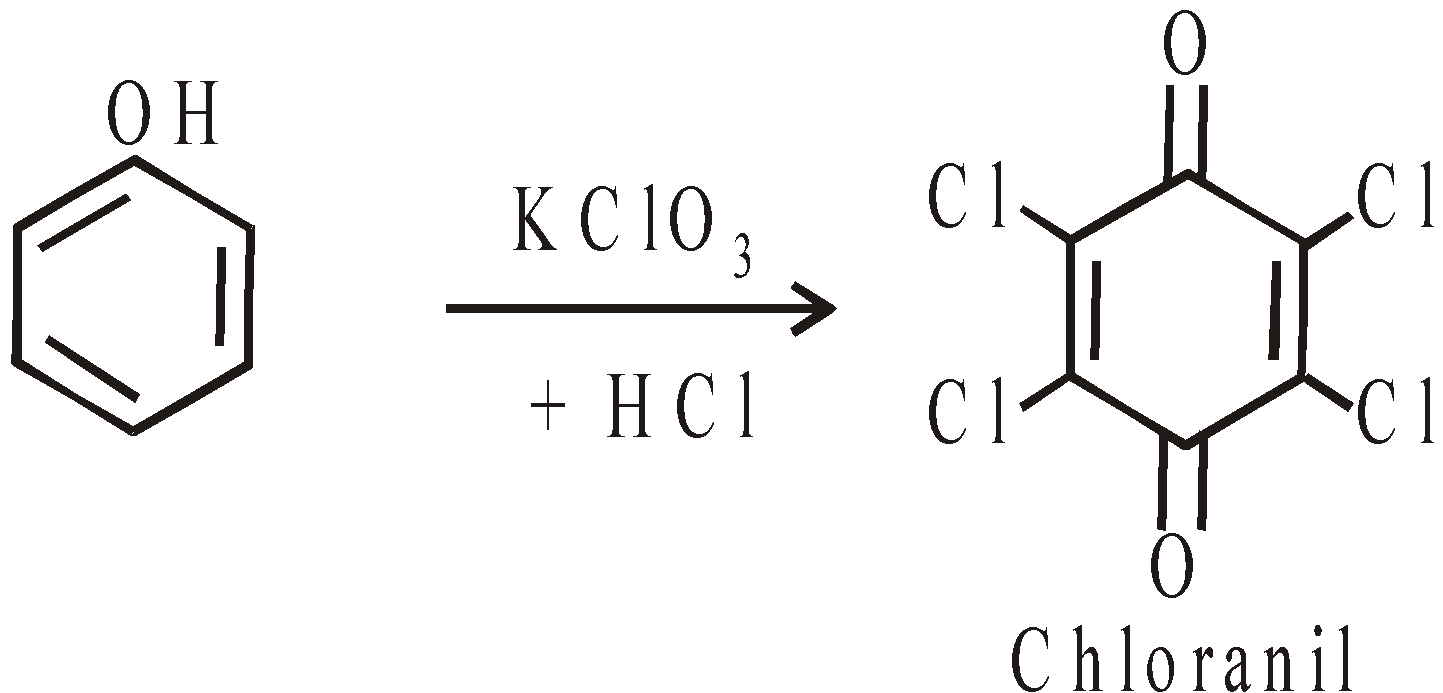
- Atmospheric oxidation : It turns pink when exposed to air and light due to slow oxidation to quinone. Quinone forms a brilliant red addition product with phenol known as diphenoquinone, through hydrogen bonding.
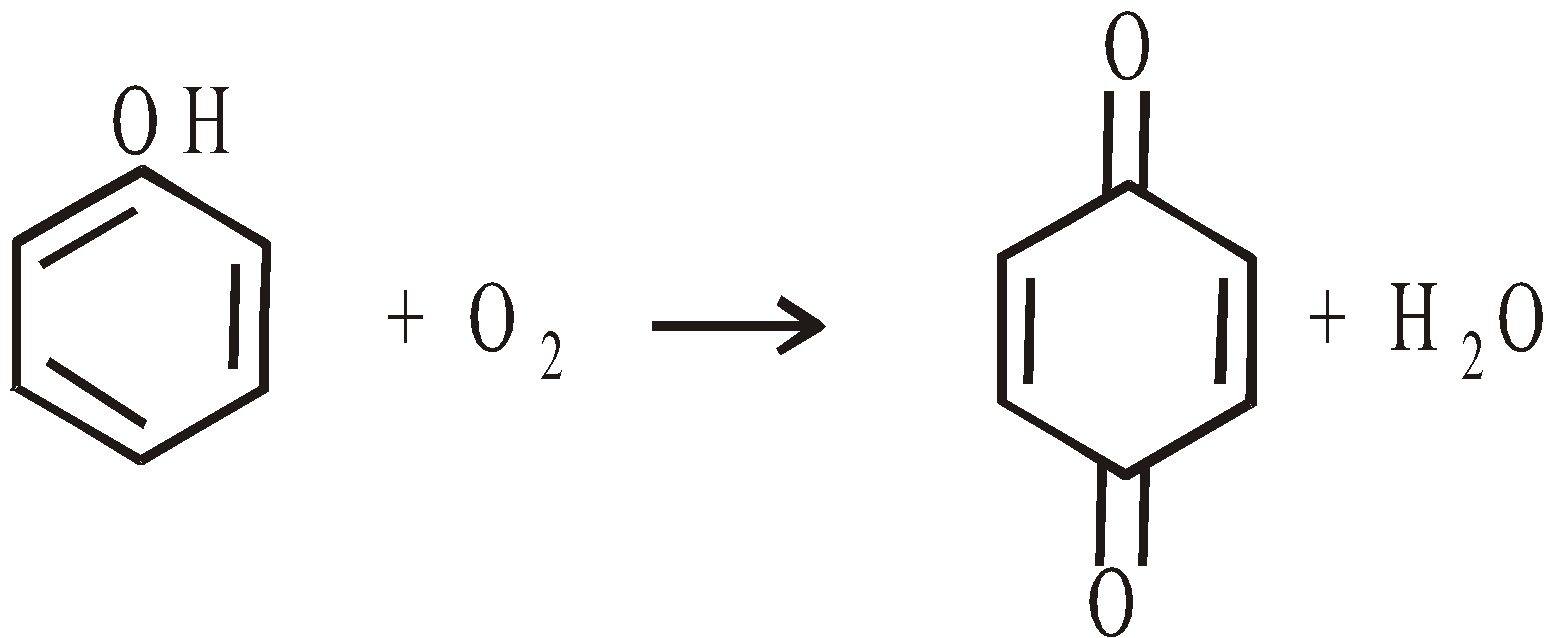
ELECTROPHILIC SUBSTITUTION REACTIONS
The OH group is o, p-directing in nature with activation of benzene nucleus
The OH group is o, p-directing in nature with activation of benzene nucleus
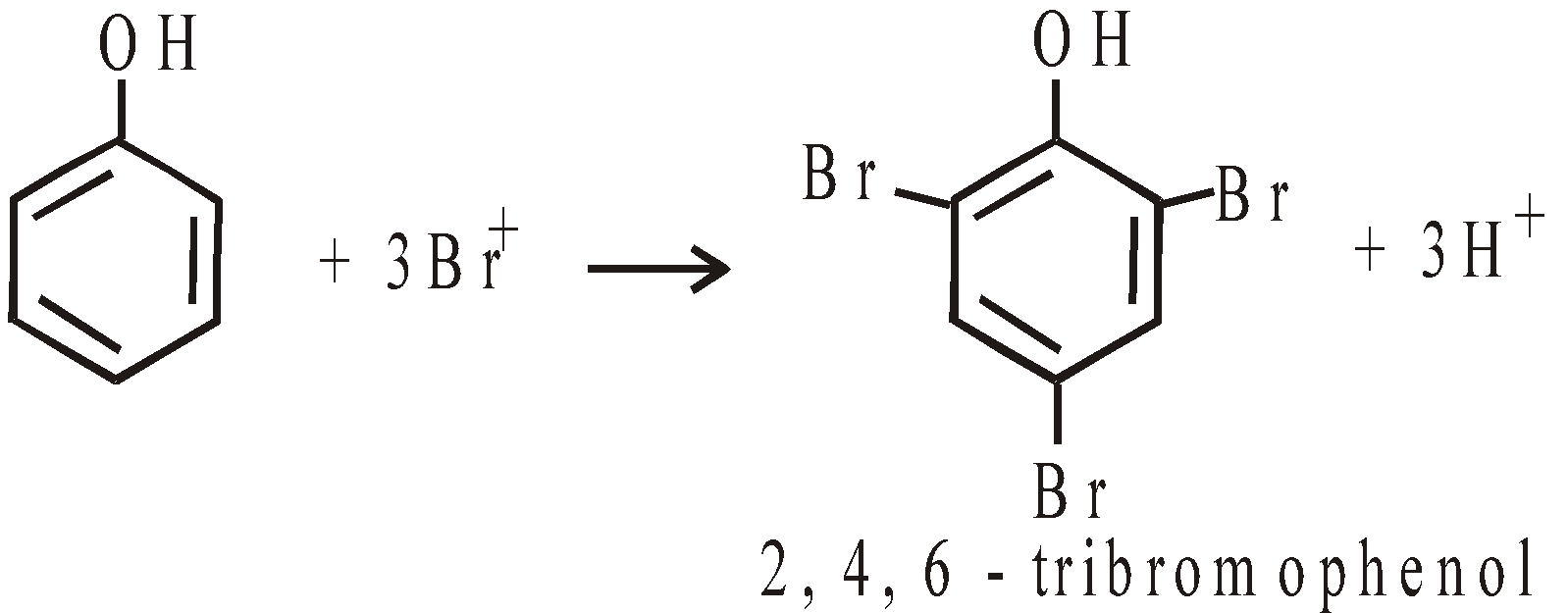
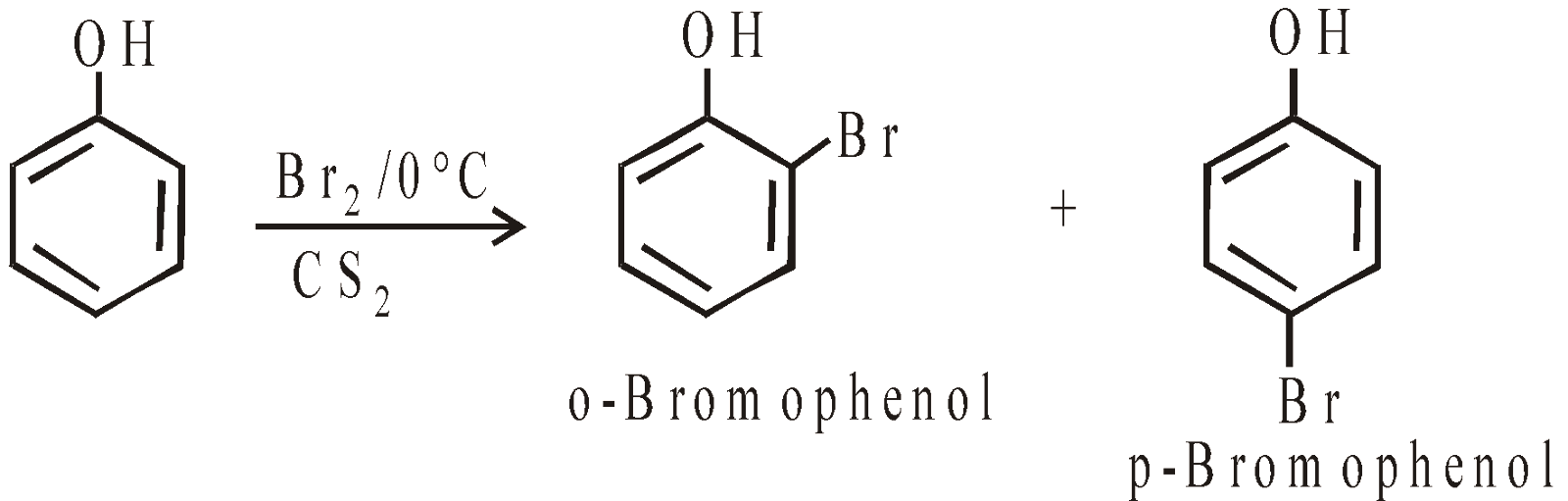
- Halogenation with excess of bromine solution it gives 2, 4, 6 – tribromophenol (curdy precipitate)
With calculated amount of Br2 in CS2 or CHCl3 gives ortho and para product
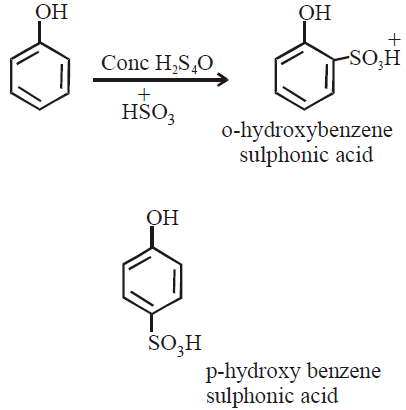
- Sulphonation with Conc H2SO4
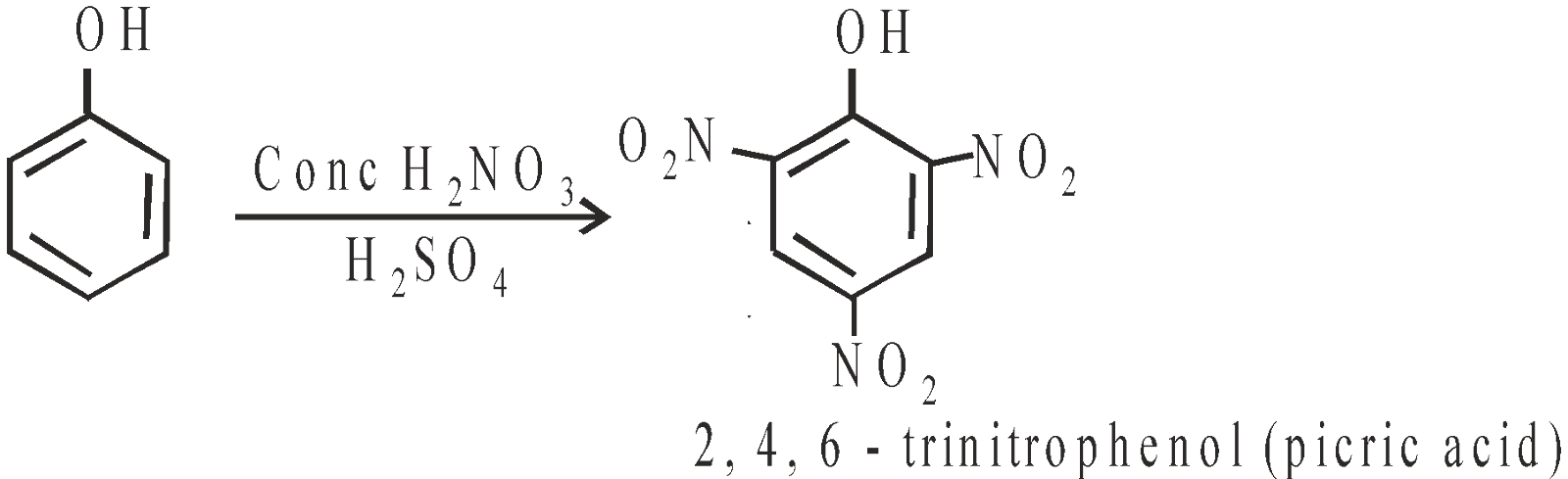
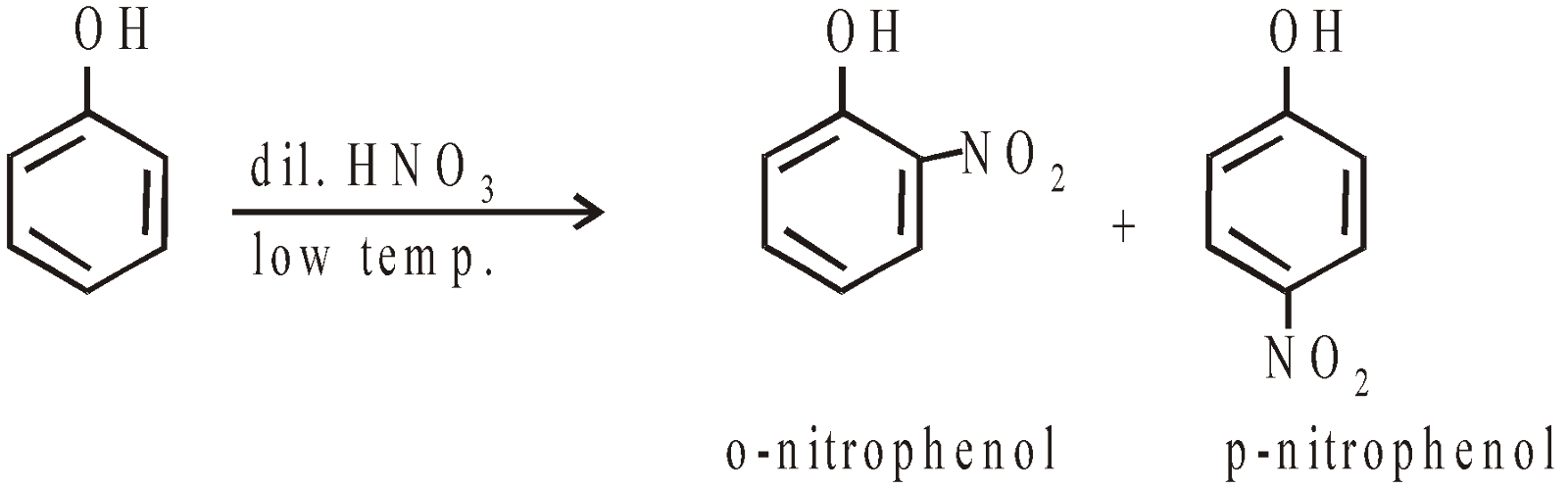
- Nitration with Conc HNO3 + Conc H2SO4
- Nitrosation
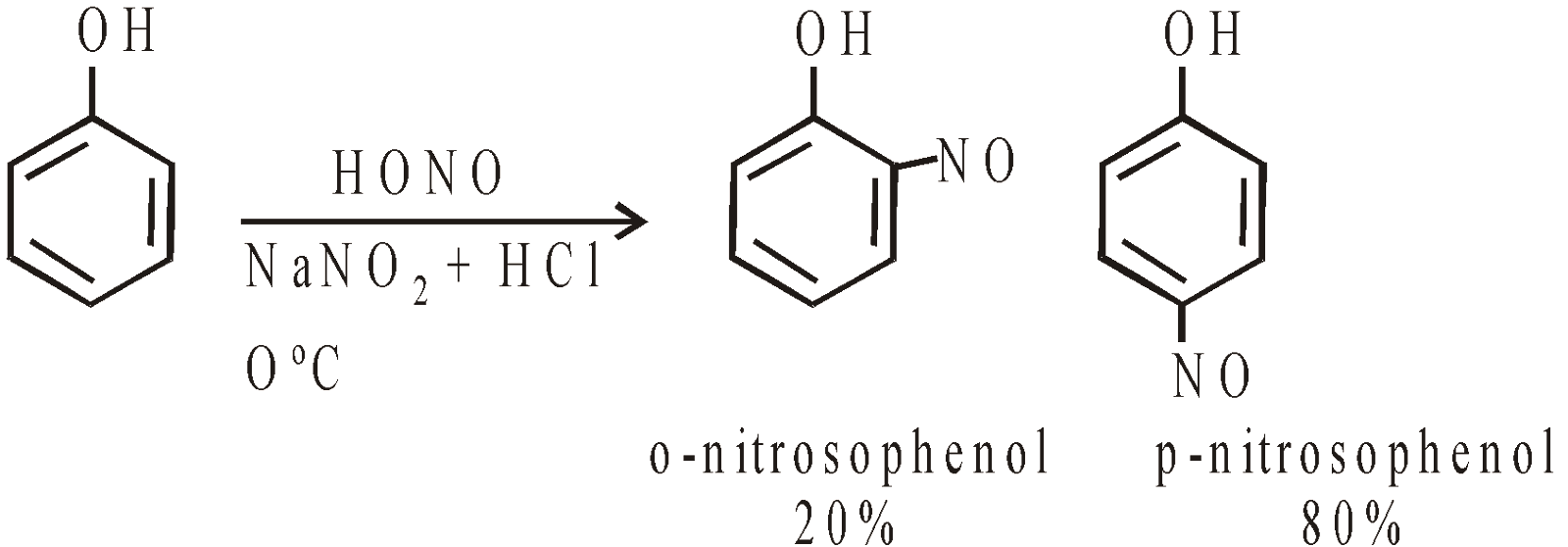
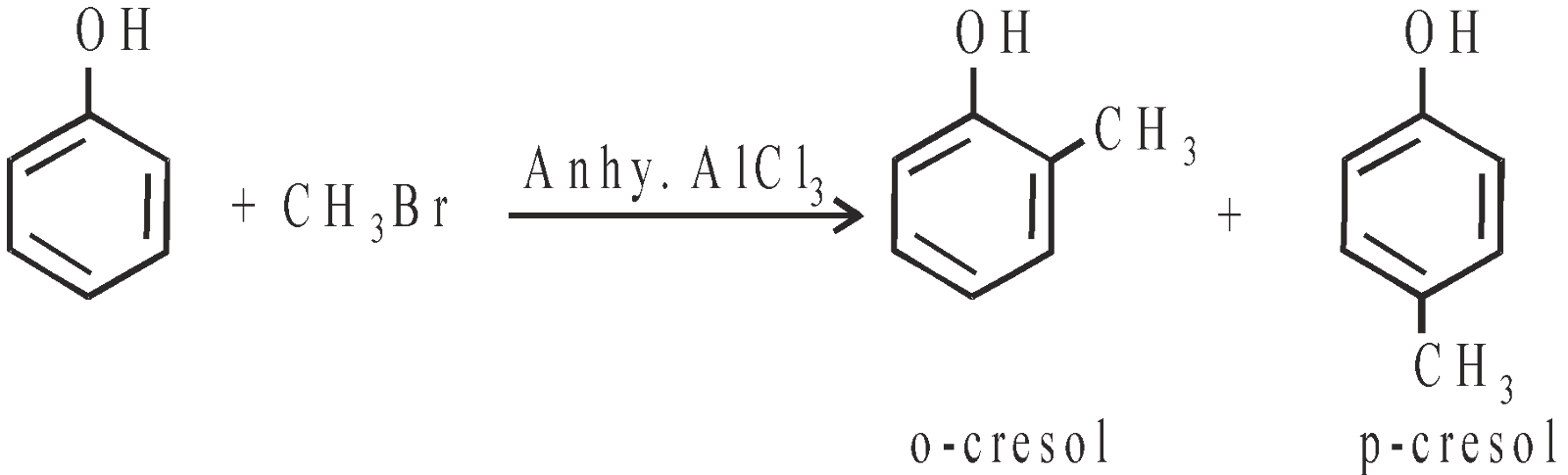
- Friedel-crafts alkylation and acylation
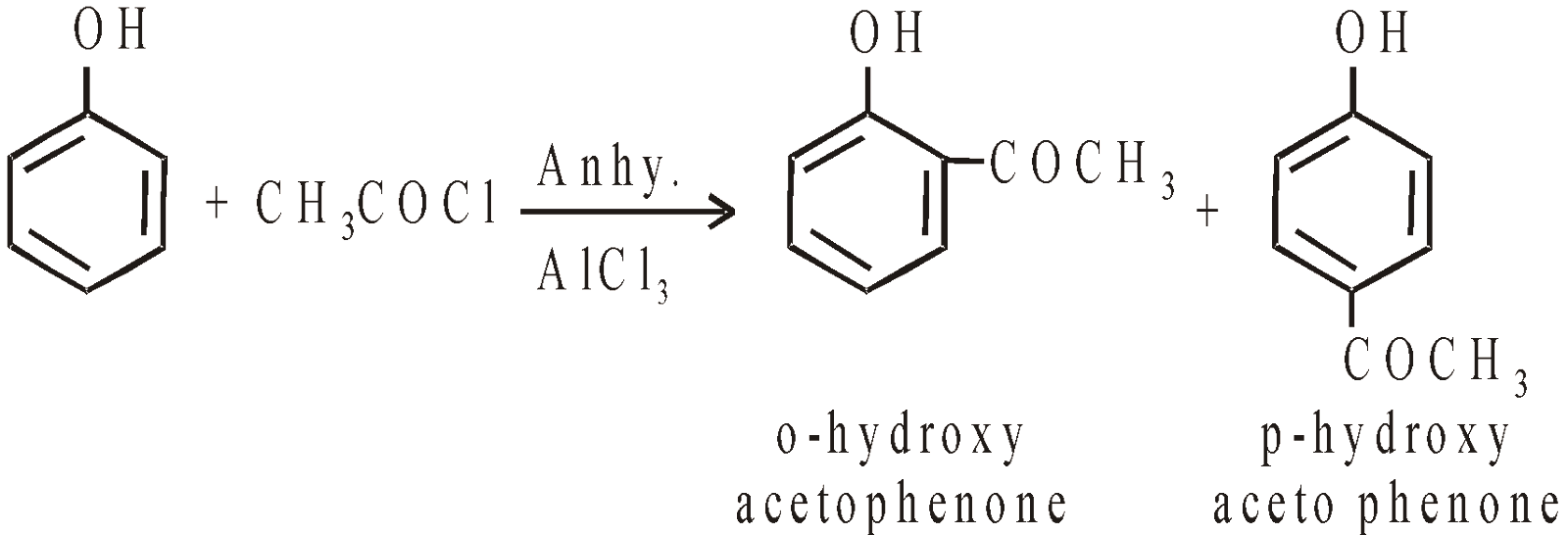
- Mercuration
Phenol undergoes electrophilic substitution when refluxed with mercuric acetate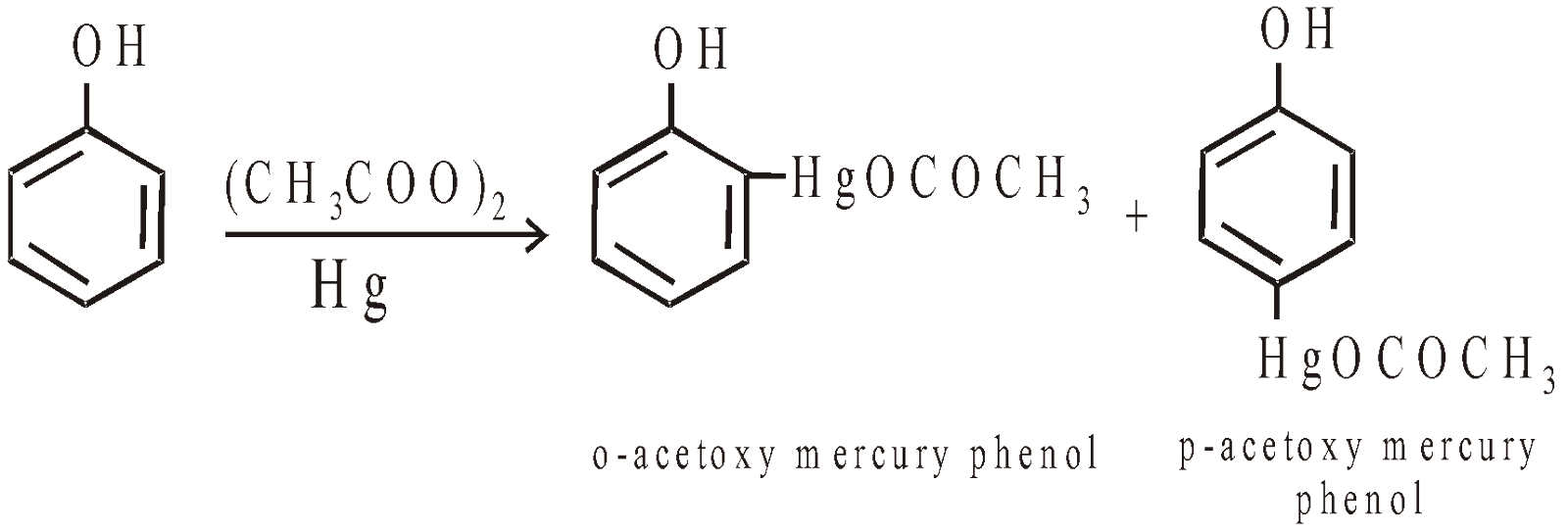
MISCELLANEOUS REACTIONS
Kolbe’s reaction or Kolbe’s Schmidt reaction
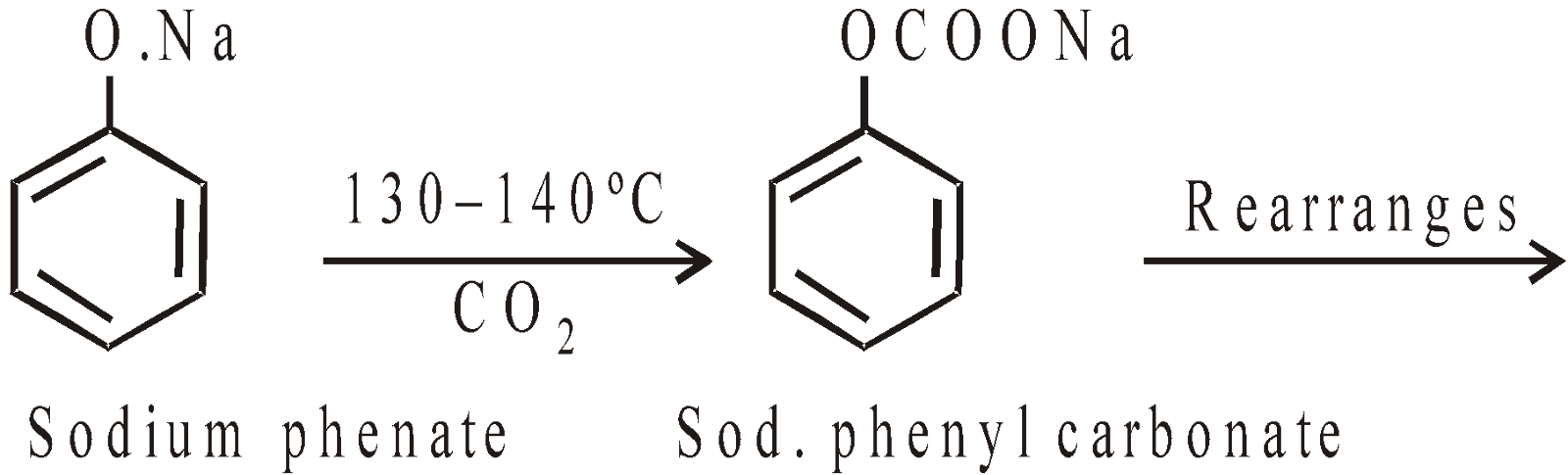
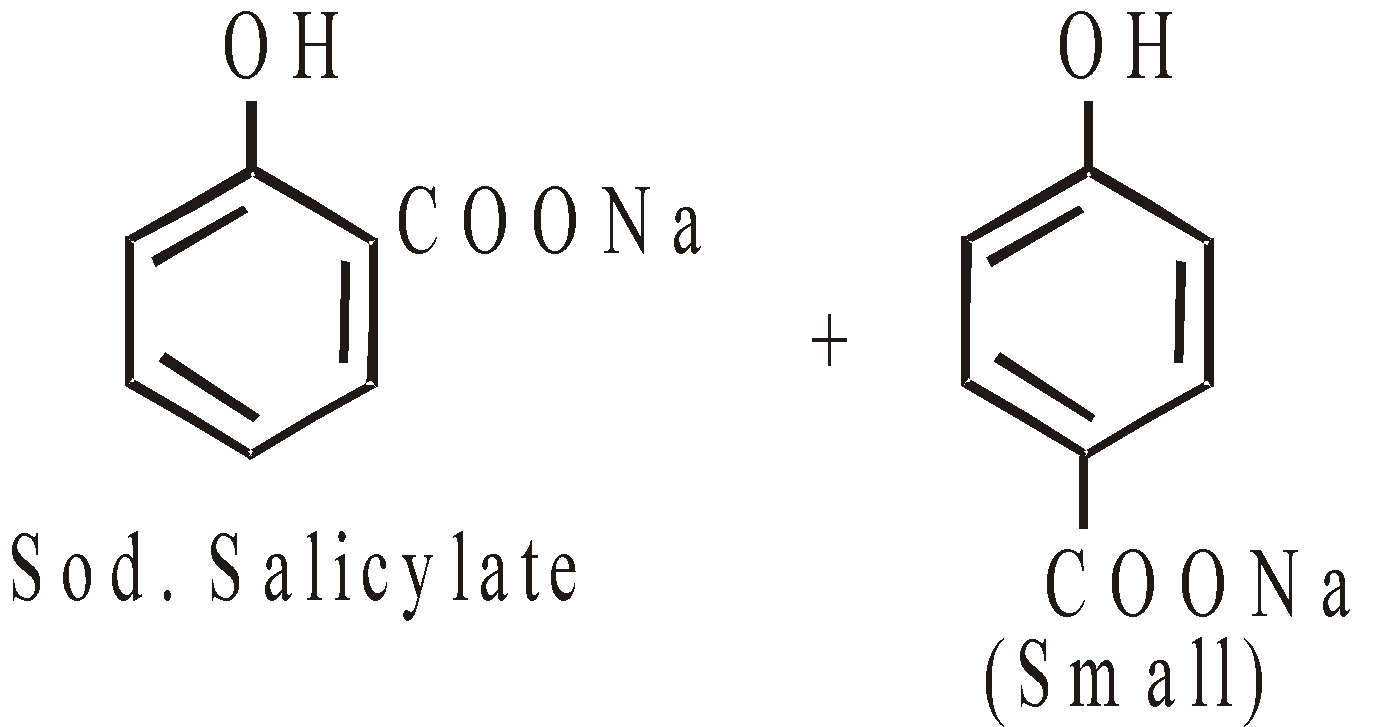
The p-isomer is the main product if temperature rises above 160°C or when potassium phenate is used in place of sodium salt.
Reimer-Tiemann Reaction
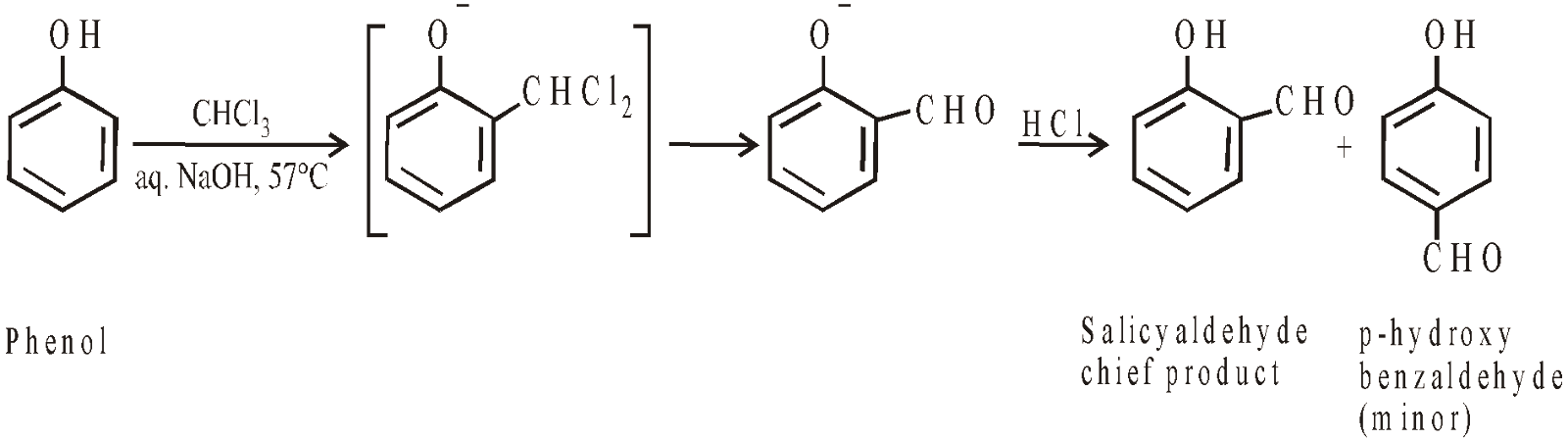
Reimer-Tiemann reaction is electrophilic substitution reaction and electrophile is dichlorocarbene. Similarly with carbon tetrachloride and alkali o- and p-hydroxybenzoic acid are obtained
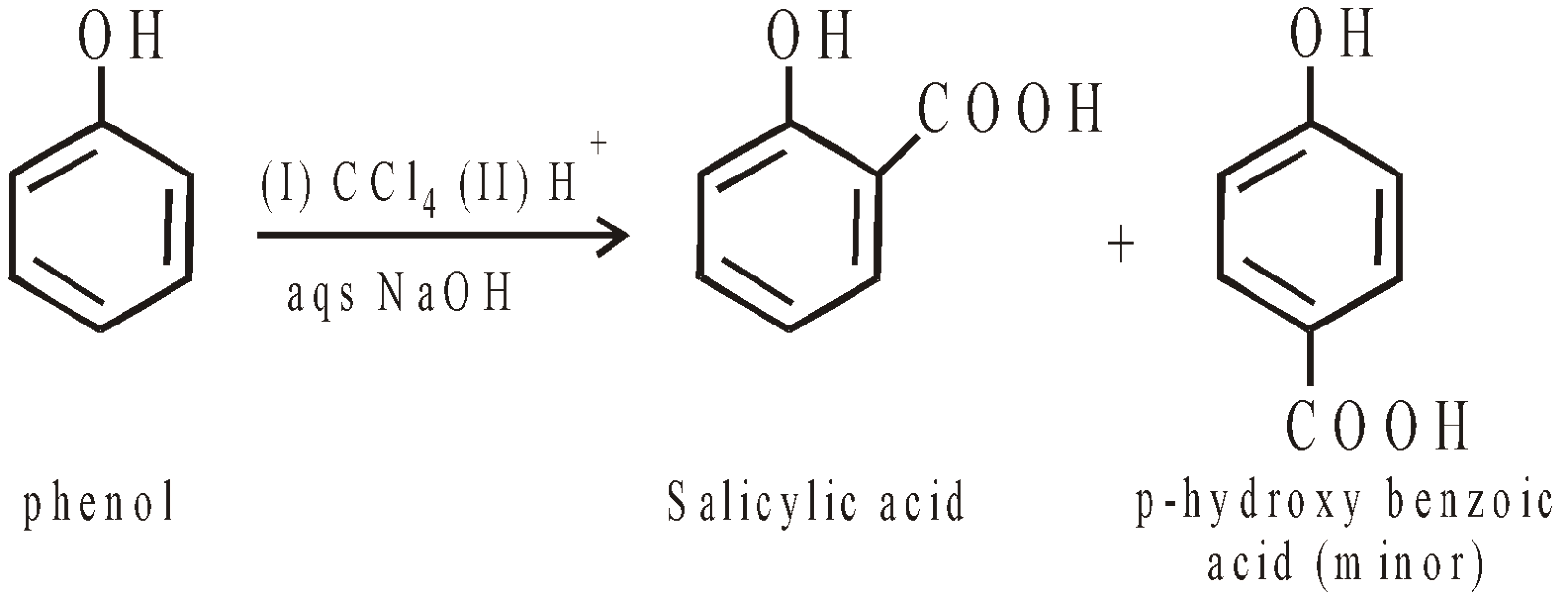
Liebermann’s Nitroso Reaction
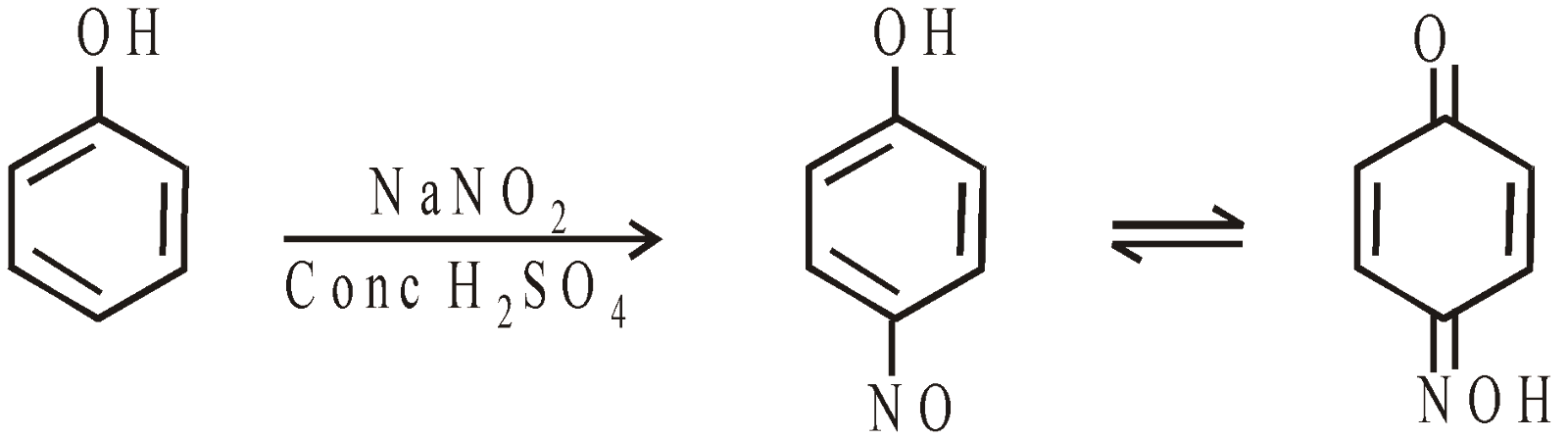
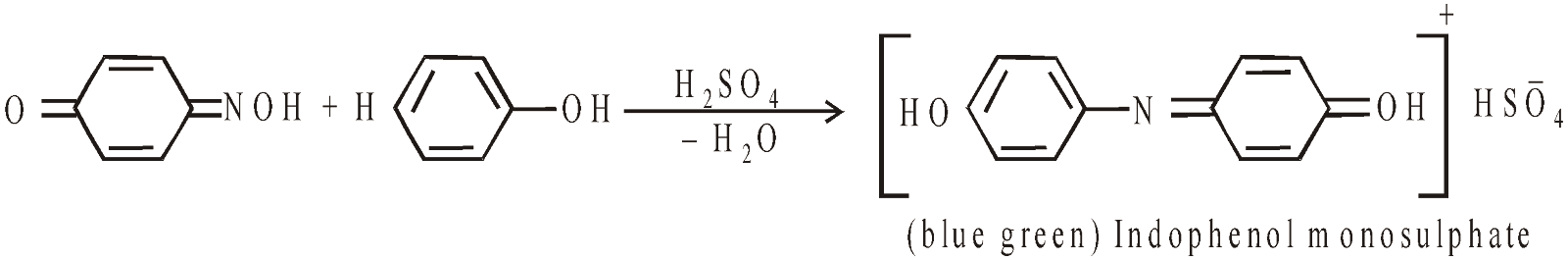
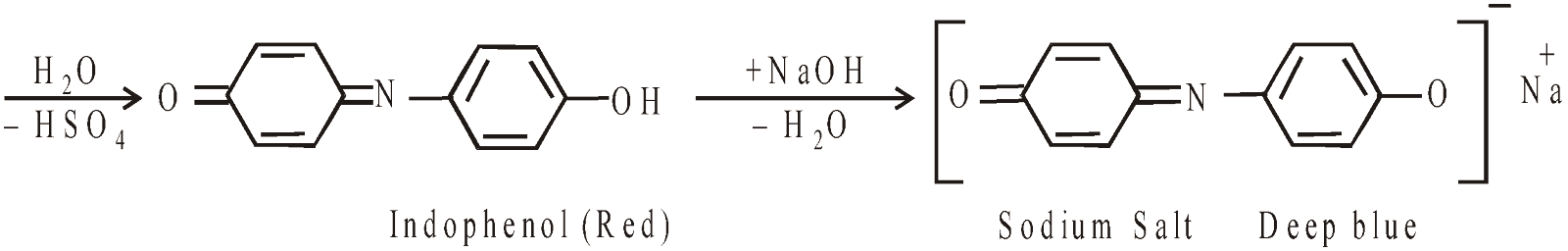
Formation of phenolphthalein
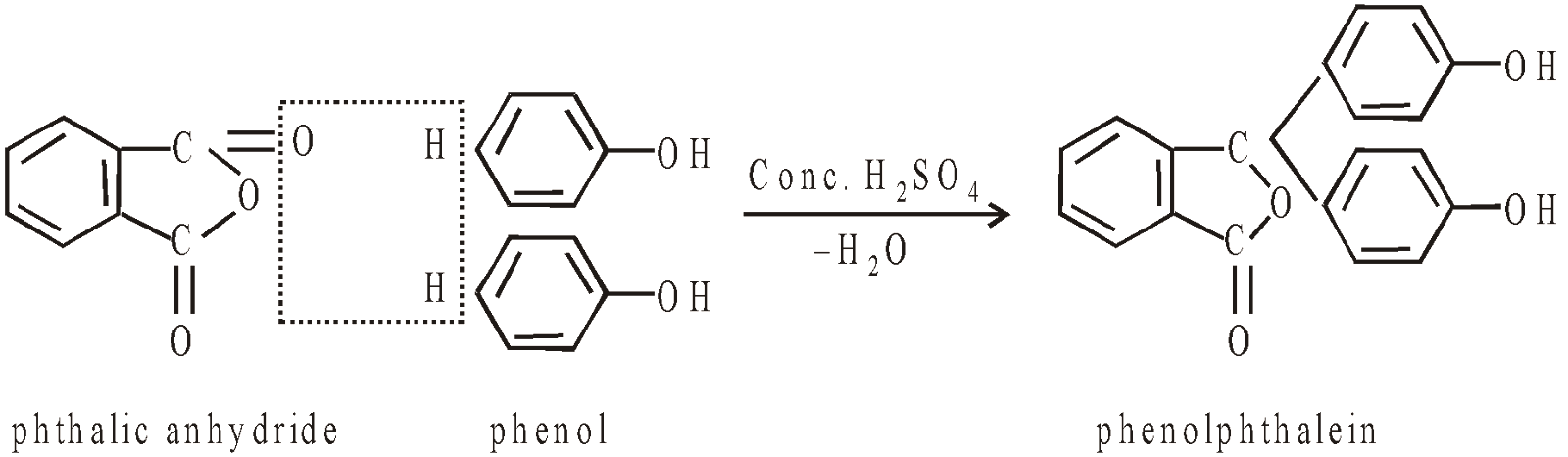
It is used as an internal indicator in acid base titration. It gives pink colour in alkaline medium and colourless in acid medium. Its pH range is 8-9.8.
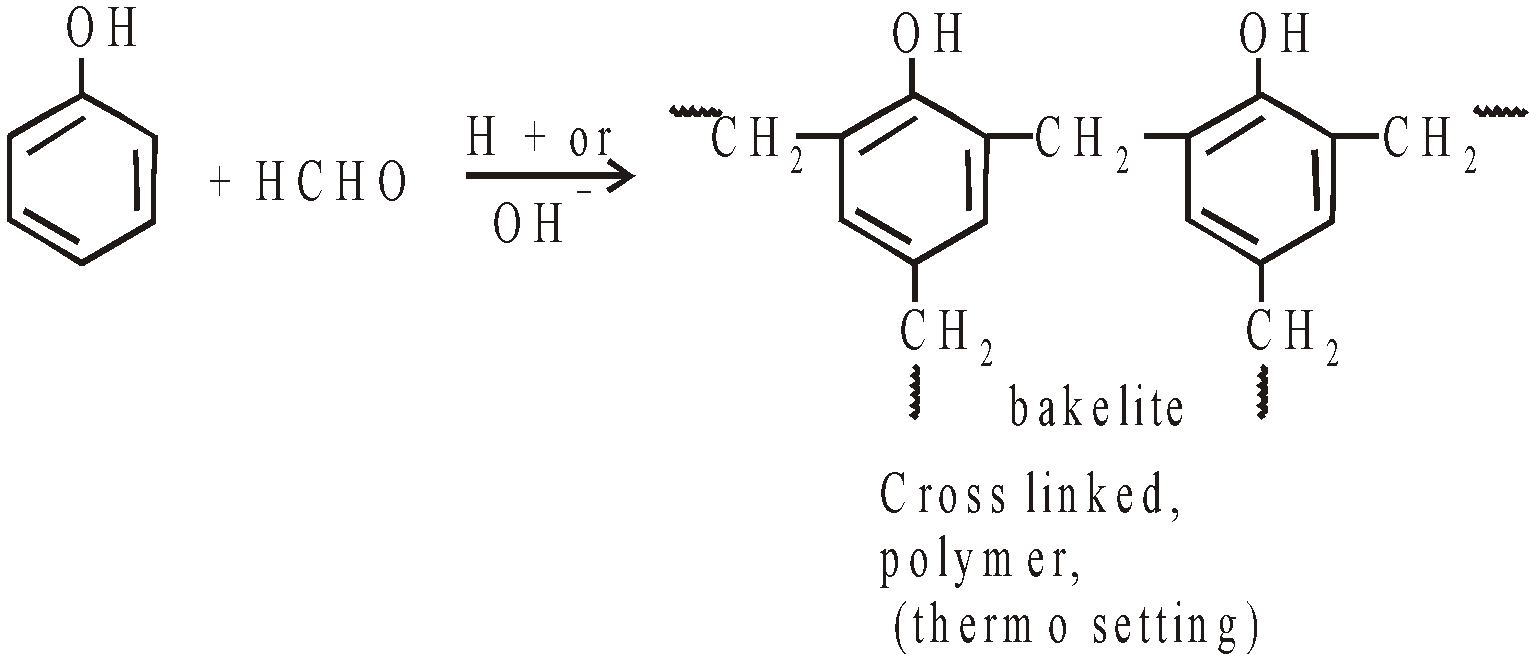
Condensation with HCHO (Lederer-Manasse reaction)
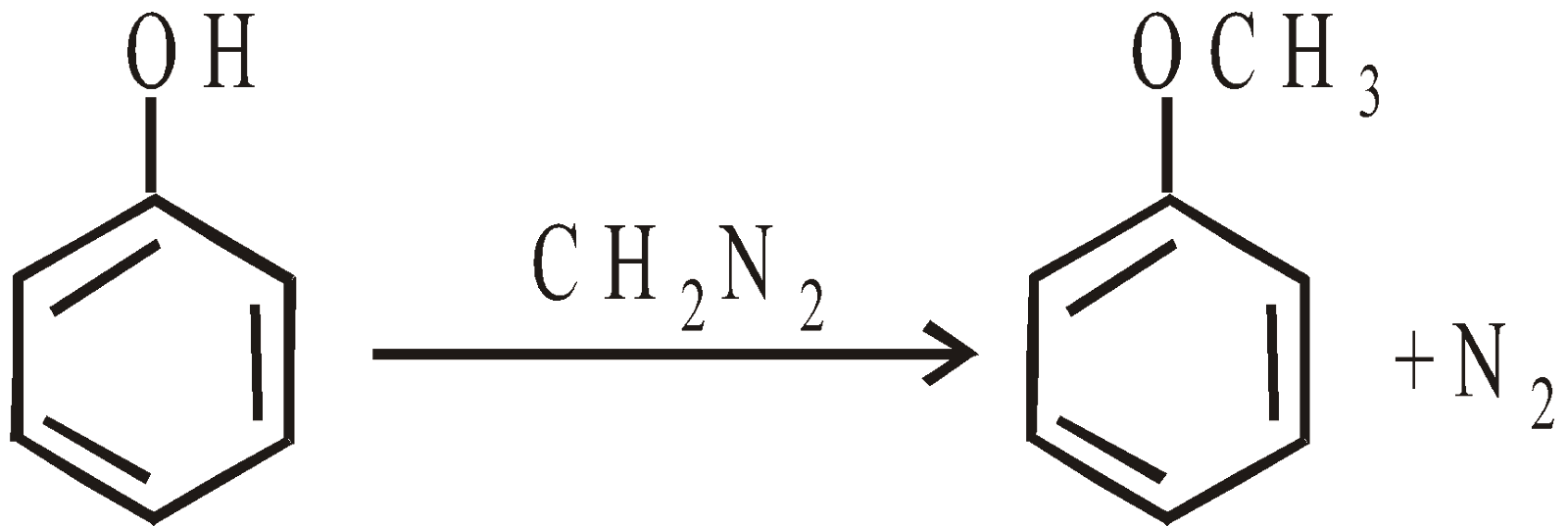
With diazomethane (CH2N2)

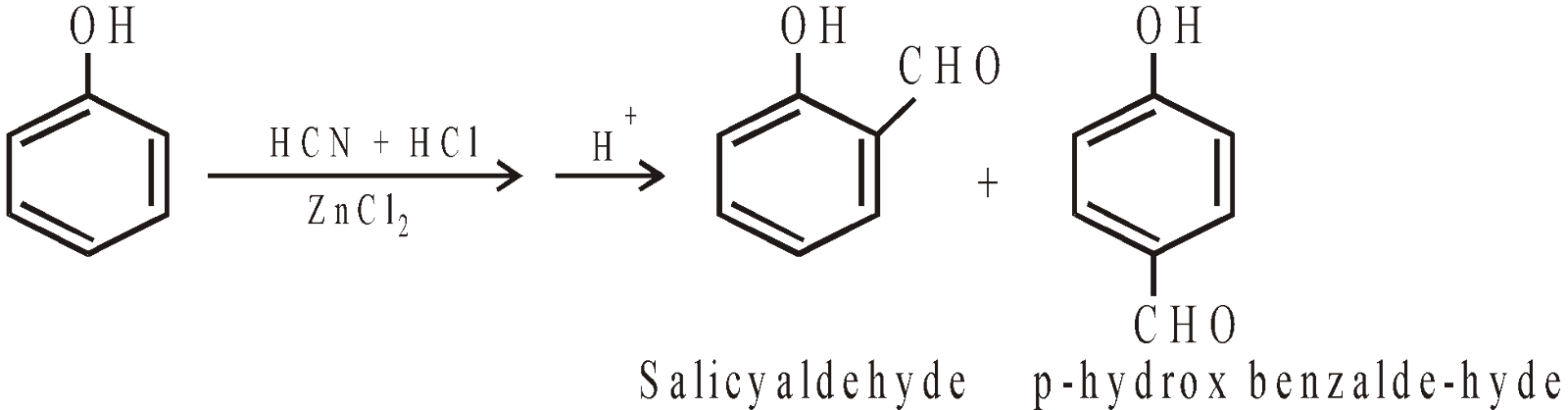
Gatterman Reaction
Schotten Baumann Reaction
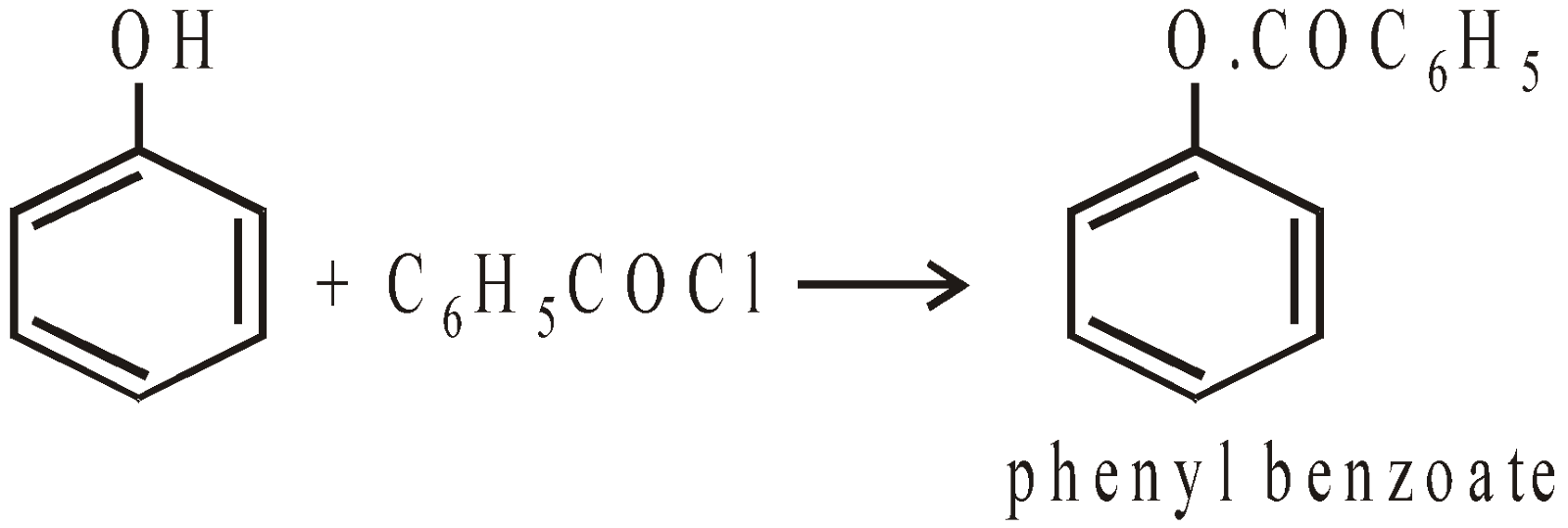
TEST
- Ferric chloride test: phenol + 1% FeCl3 solution – violet colour
- Bromine water: phenol + bromine water – curdy precipitate
- Phenolphthalein test : (phenol + phthalic anhydride + conc. H2SO4) + NaOH heat pink colour
- It gives liebermann’s nitroso reaction
USES
- As an antiseptic
- Manufacture of bakelite
- Manufacture of salol, aspirin, phenacetin etc.
ETHERS
The compounds having the general formula R–O–R are known as ethers
Simple ethers : when alkyl or aryl groups are the same R–O–R
Mixed ethers : when alkyl or aryl groups are different R–O–R’
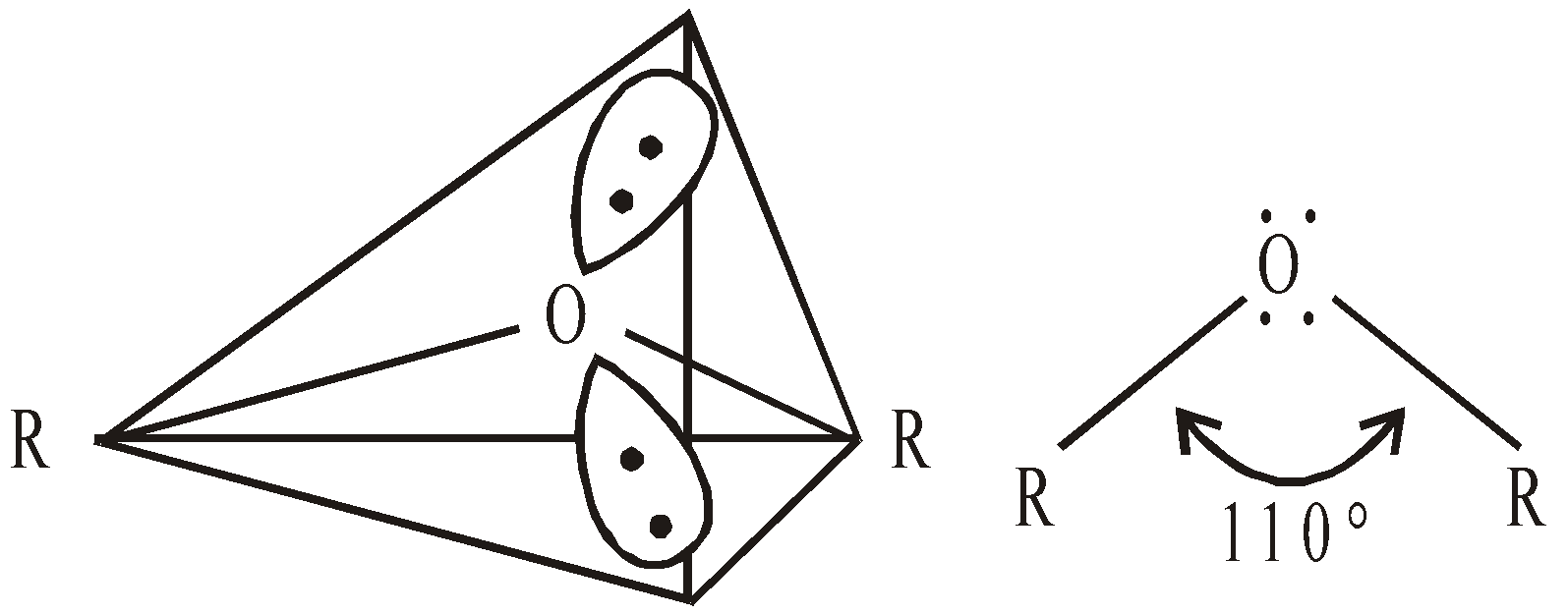
STRUCTURE
The hybridisation of O atom in ethers is sp3 (tetrahedral) and shape is V.
NOMENCLATURE
Common System : They are named as dialkyl ether when the two alkyl groups are the same eg.
diethyl ether dimethyl ether
If alkyl groups are different, they are named alphabetically e.g. 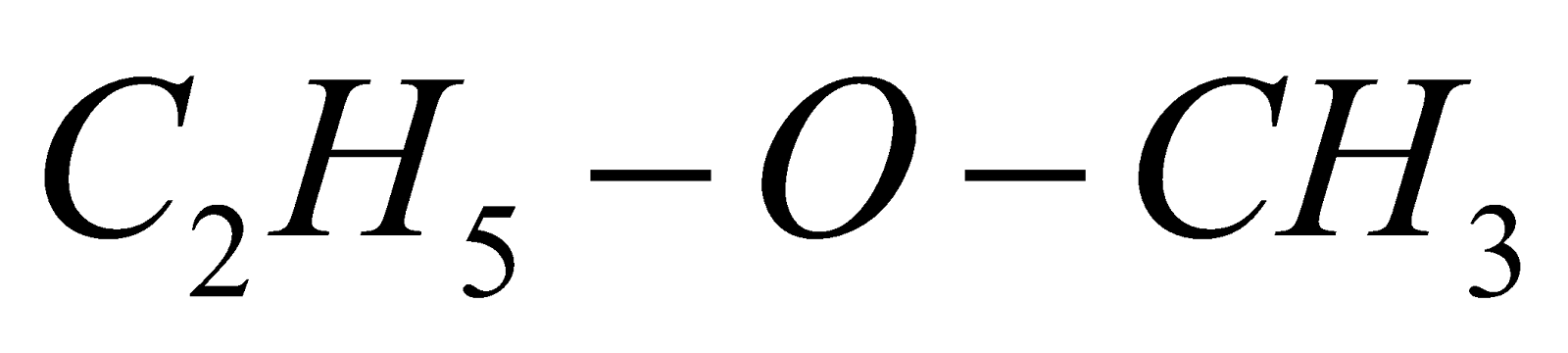 ethyl methyl ether
ethyl methyl ether
IUPAC System : They are named as Alkoxy alkane. The smaller group is made alkoxy and larger the alkane e.g.
Methoxymethane Methoxyethane
1-methoxy propane
ISOMERISM
Ethers exhibit two types of isomerism
Functional Isomerism
They are isomeric with monohydric alcohols e.g.
They are isomeric with monohydric alcohols e.g.
methoxy methane ethyl alcohol
Metamerism
ethoxy ethane
1-methoxy propane
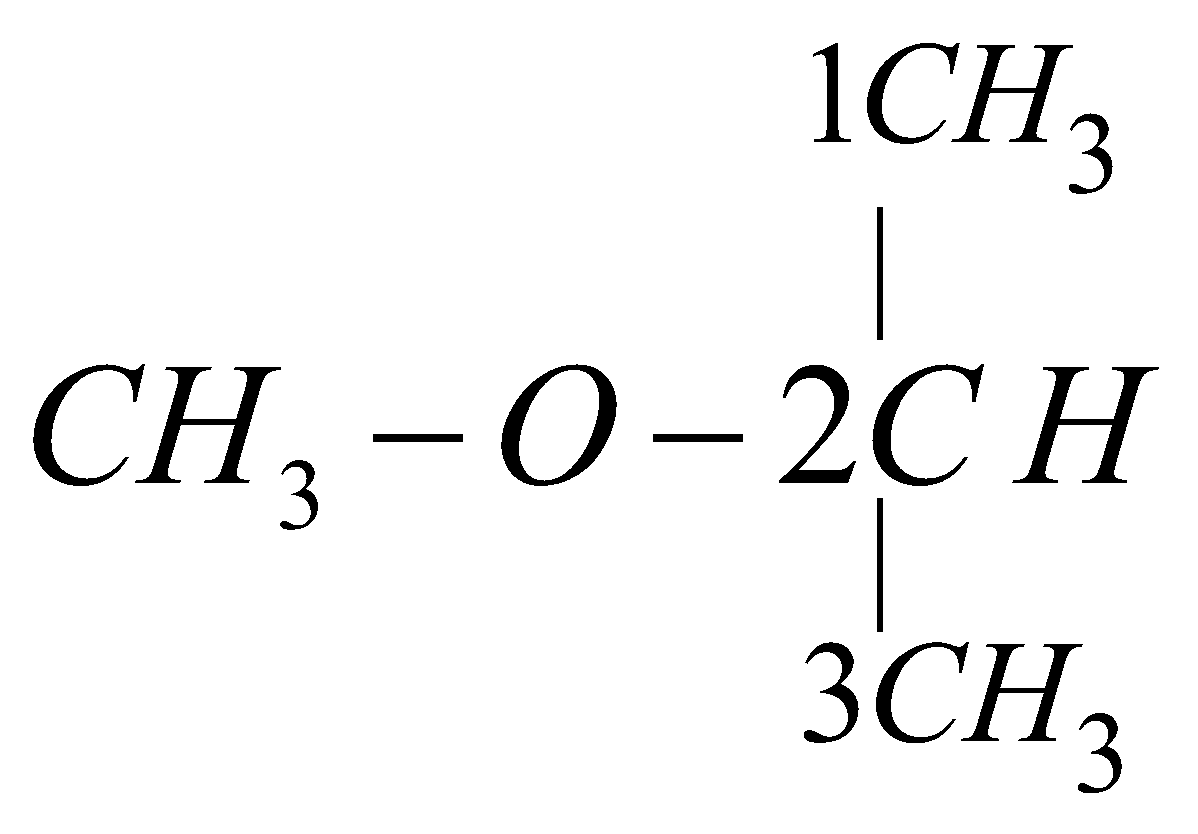
2-methoxy propane
GENERAL METHODS OF PREPARATION
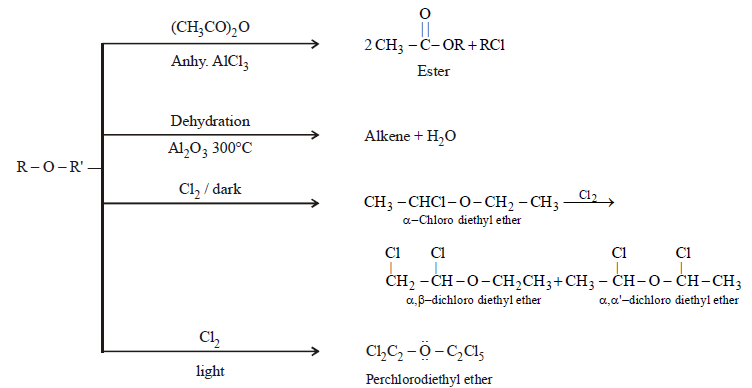
- By Dehydration of alcohols
- Catalytic dehydration of alcohols
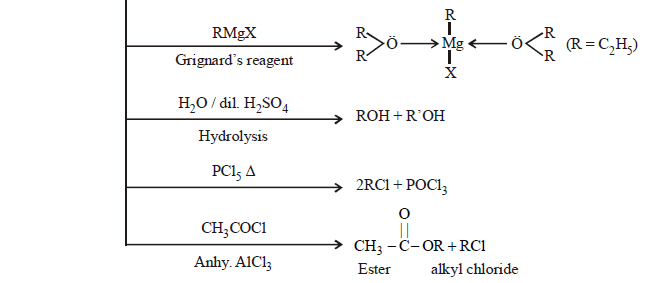
- Williamson’s Synthesis:
The reaction involves SN2 attack of an alkoxide ion on primary alkyl halide
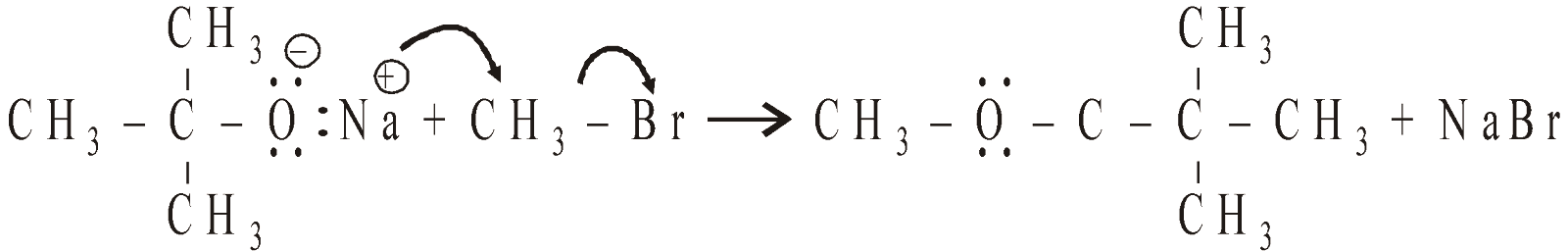
The alkyl halide should be primary. The secondary and tertiary alkyl halides undergo elimination reaction to give alkenes.
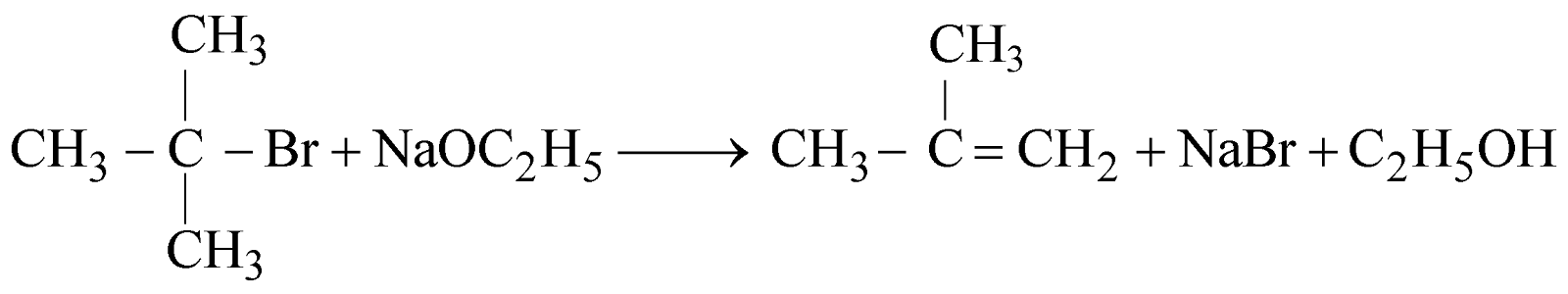
- Heating alkyl halide with silver oxide
- From alcohol by the action of diazomethane
- From Phenol
- From Grignard’s reagent: Synthesis of higher ether
- Vinyl ethers:
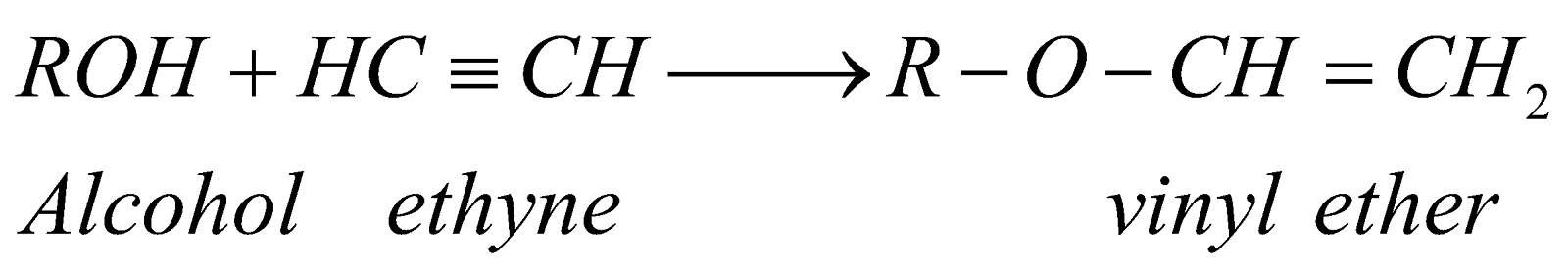
PHYSICAL PROPERTIES
Dimethyl ether and ethyl methyl ether are gases. Others are highly volatile, inflammable liquids. Slightly soluble in water due to H-bond formation.
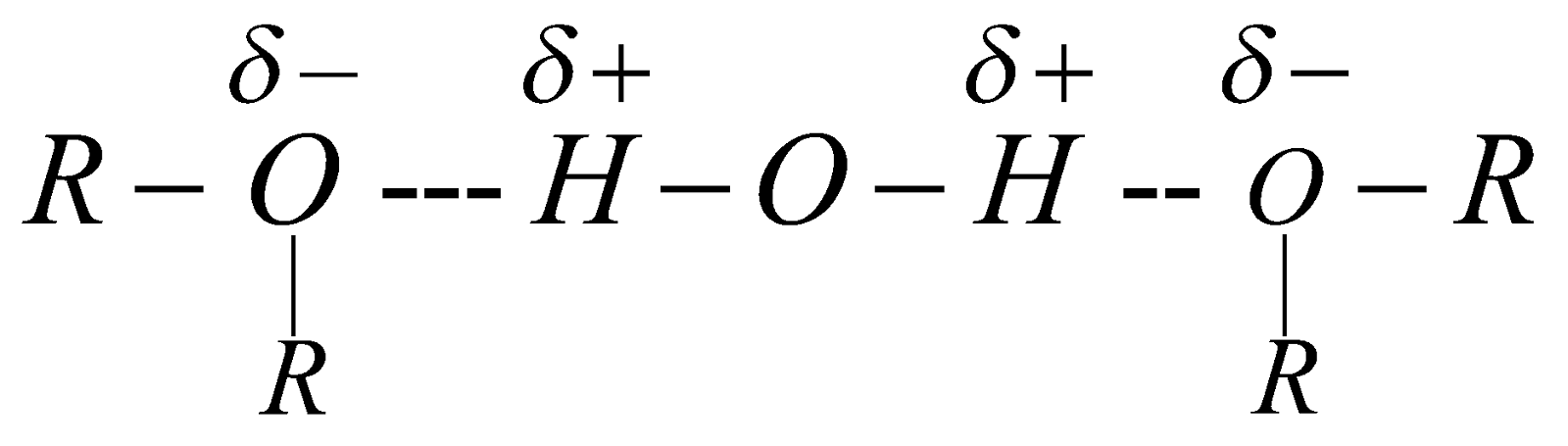
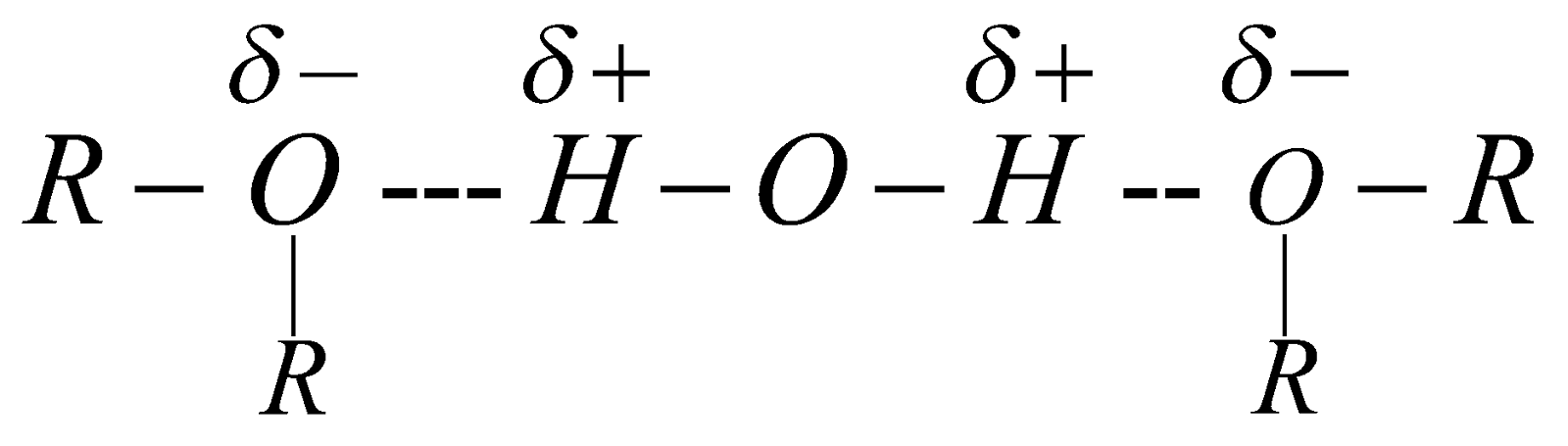
They are polar in nature the value of dipole moment is 1.18D.
CHEMICAL PROPERTIES
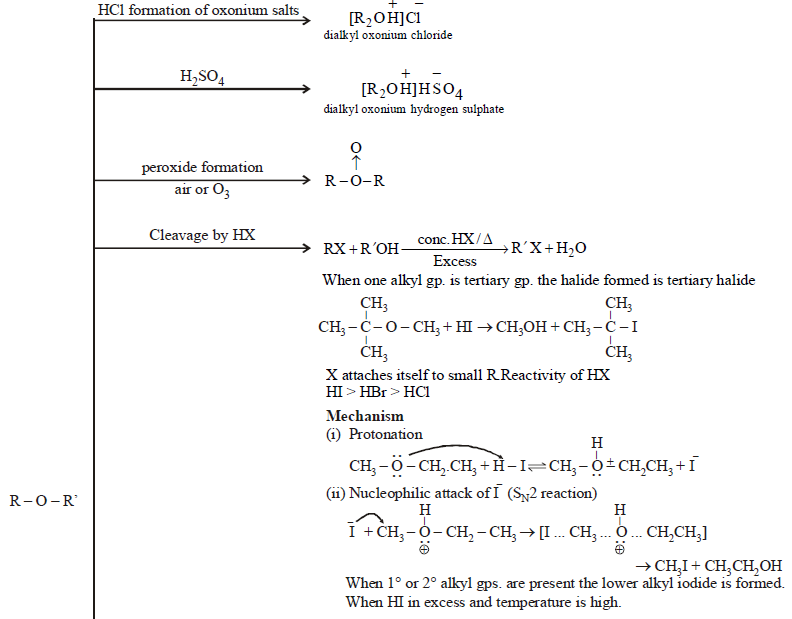
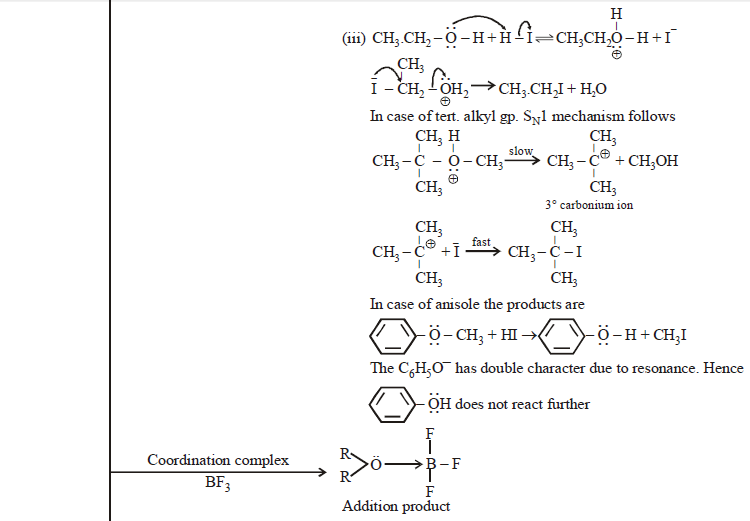
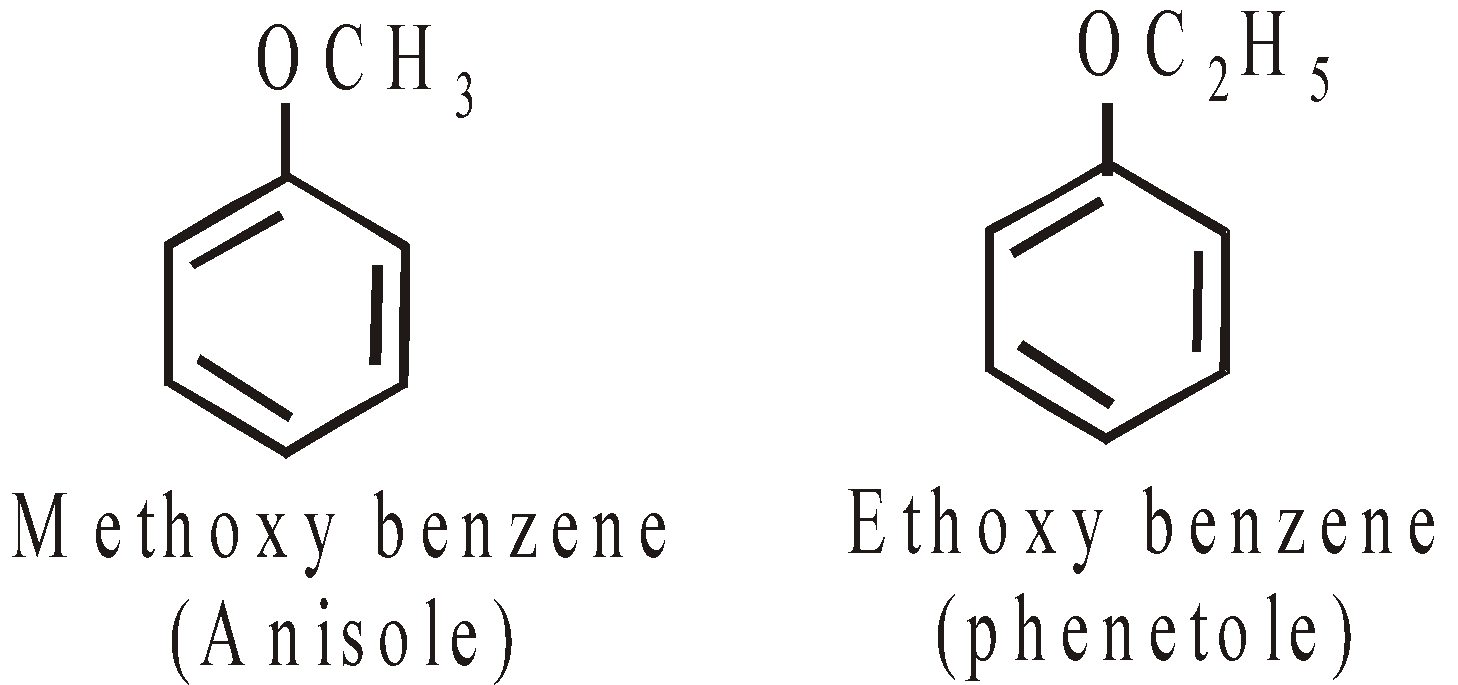
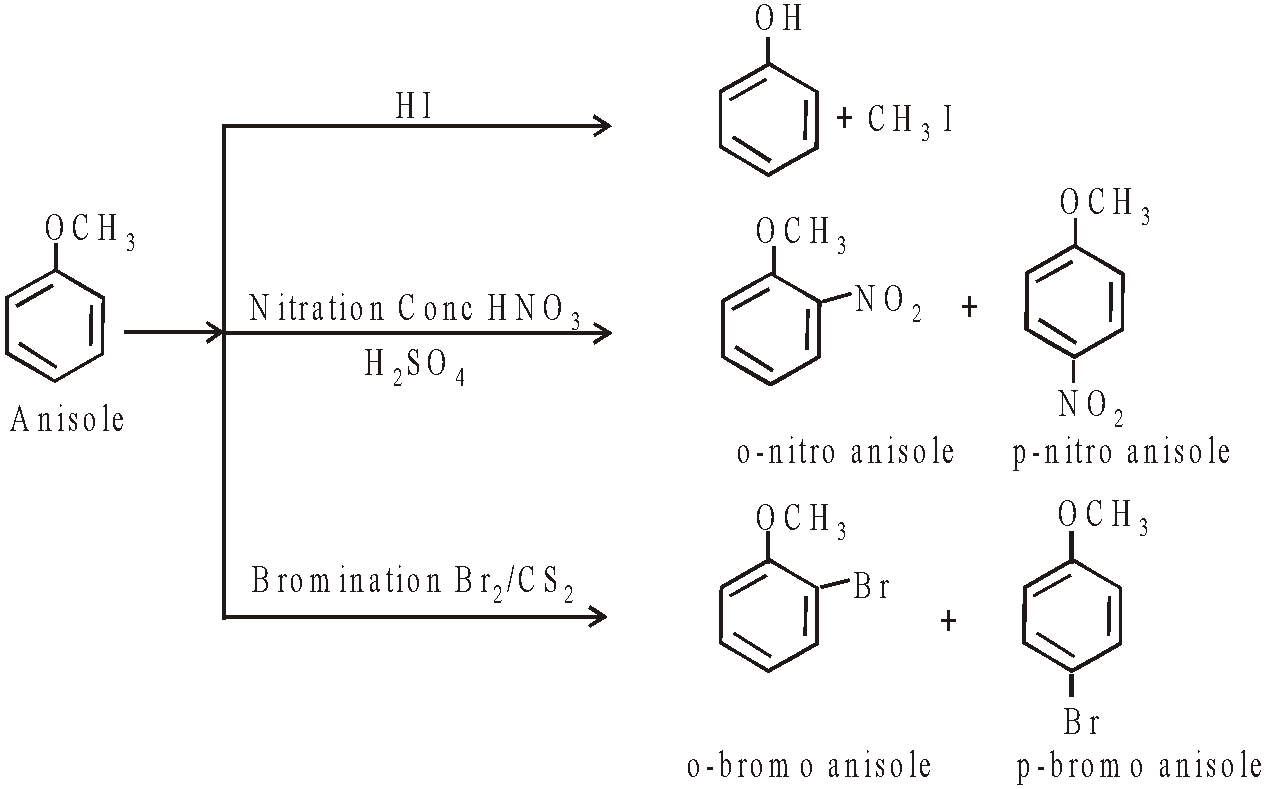
AROMATIC ETHER: ANISOLE
PREPARATION
PROPERTIES
- Detection of peroxides in ether
Ether + aqueous solution of ferrous ammonium sulphate or potassium thiocyanate – Red colour.
- Diisopropyl ether is used in petrol as anti knock compound.
- Zeisel method
- AgI is estimated, therefore alkoxy group is estimated.
- Petrol mixes with alcohol in 1% diethyl ether.
- Mixture of chloroform and ether is general anaesthetic.
- Epoxides
Epoxides are cyclic ethers. Ethereal oxygen forms a part of three membered ring 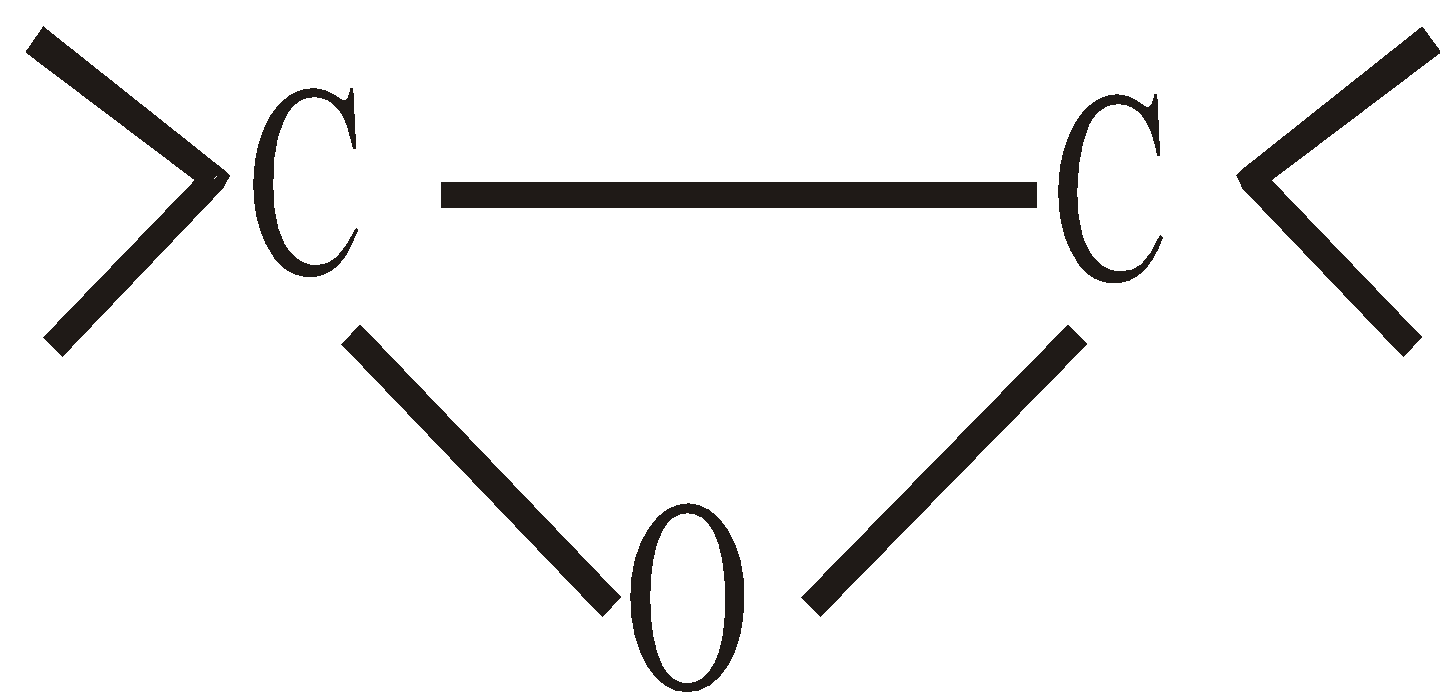
- Crown ethers
They are cyclic polyethers
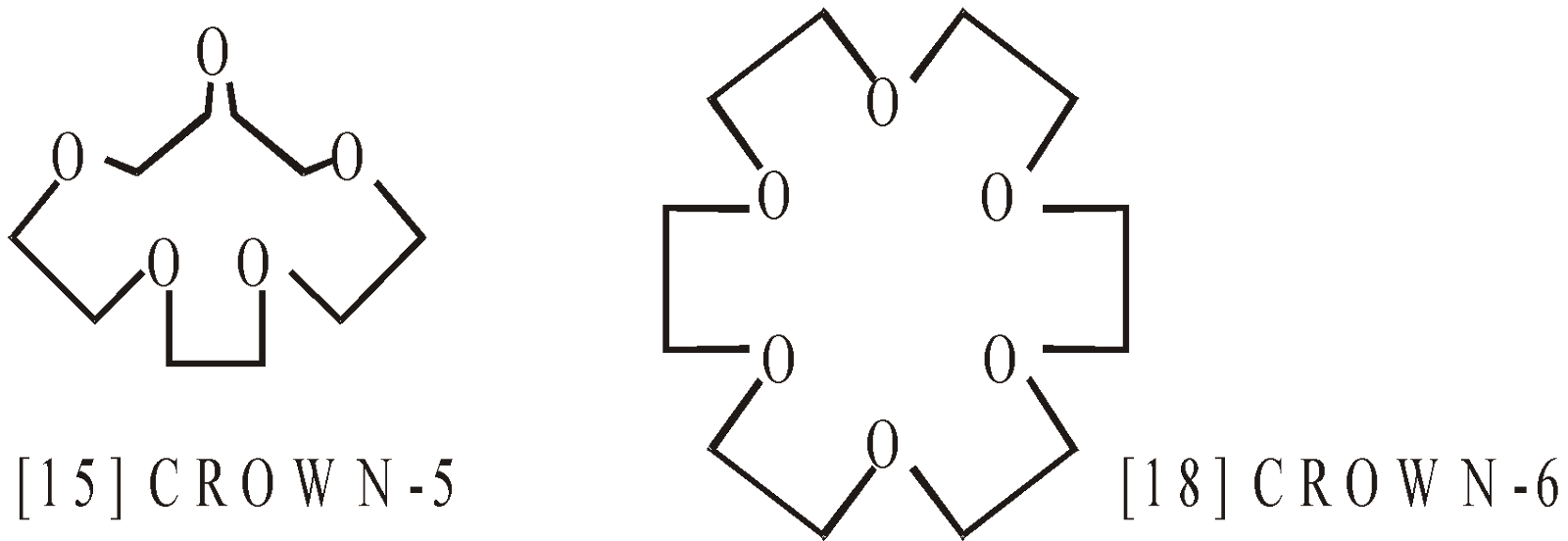
The first number indicates ring size and second number indicates the number of oxygen atoms
- Thio ethers
R–S–R – in place of –O– there is –S– linkage. Also known as sulphides.
