| Biomolecules NEET and AIIMS Special |
| Biomolecules Refresher Course |
| Biomolecules : Master File |
| Biomolecules : Quick Revision Notes |
| Biomolecules : Concepts File |
| Biomolecules : Brain Map |
| Biomolecules Reference Book |
About this unit
Carbohydrates- Classification (aldoses and ketoses), monosaccharide (glucose and fructose), D.L. configuration, oligosaccharides (sucrose, lactose, maltose), polysaccharides (starch, cellulose, glycogen): importance.Proteins- Elementary idea of – amino acids, peptide bond, polypeptides, proteins, primary structure, secondary structure, tertiary structure and quaternary structure (qualitative idea only), denaturation of proteins; enzymes.Hormones- Elementary idea (excluding structure).Vitamins- Classification and function.Nucleic Acids: DNA and RNA
BIOMOLECULES
CARBOHYDRATES
Polyhydroxy aldehydes (Aldoses) or Ketones (Ketoses) or compounds which on hydrolysis give these and contain at least one asymmetric carbon atom are known as carbohydrates. They are also called saccharides (Latin Saccharum = sugar) due to sweet taste of simpler members, the sugars.
The carbonyl group does not occur in the free form but forms intramolecular hemiacetal or acetal linkages with the -OH groups.
Carbohydrates are of three types.
- Monosaccharides
- Oligosaccharides
- Polysaccharides
MONOSACCHARIDES
These cannot be hydrolysed to simpler molecules and further subdivided into tetroses, pentoses or hexoses depending upon the number of carbon atoms.
Aldotetroses: Erythrose, Threose CH2OH.(CHOH)2 CHO
Aldopentoses: Arabinose, xylose, Ribose, lyxose CH2OH(CH OH)3 CHO
Aldohexoses: Glucose, Mannose, Allose, Galactose etc. CH2OH(CH OH)4 CHO
Ketotetrose: Erythrulose CH2OH.CO.CHOH.CH2OH
Ketopentoses: Ribulose, xylulose CH2OH.CO(CHOH)2 CH2OH
Ketohexoses: Fructose, sorbose CH2OH.CO(CHOH)3.CH2OH
OLIGOSACCHARIDES
(Greek oligos, few)
On hydrolysis they generally give two to nine monosaccharides (same or different) and further classified as Disaccharides eg. sucrose, maltose, lactose C12H22O11 give two monosaccharides
The bond formed between two monosaccharides is called a glycosidic bond and normally it is (1, 4) bond.
Sucrose is most abundant in plants and known as cane sugar or table sugar.
Invert-sugar: Equimolar mixture of glucose and fructose obtained by hydrolysis of sucrose.
Trisaccharides: Raffinose (C18H32O16)
Tetrasaccharides: Stachyose C24H42O21
POLYSACCHARIDES
These are polymers of monosaccharides. eg. Starch, cellulose, glycogen, inulin, pectin etc.
STARCH (C6H10O5)N
A polymer of 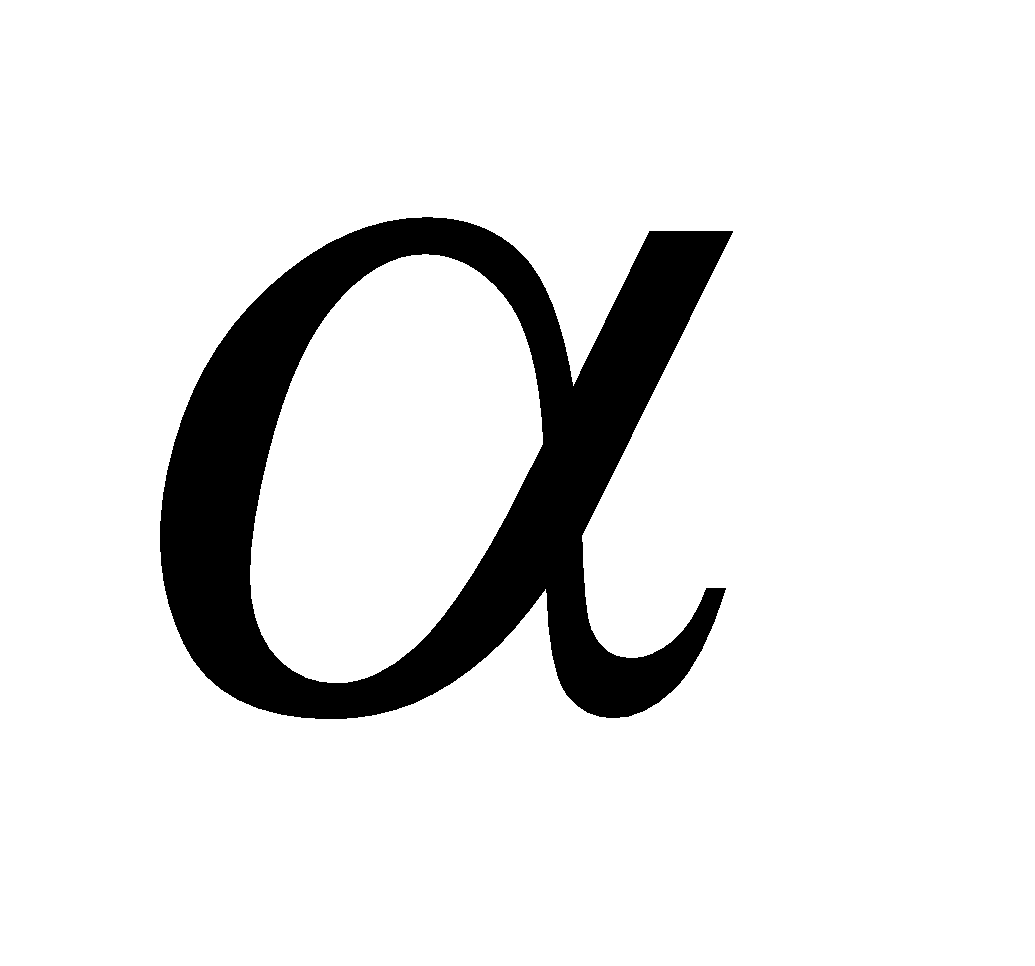 – glucose and major reserve food in plants, turns blue with iodine. It is a mixture of two components (i) amylose (20%), an unbranched polymer water soluble (ii) amylopectin (80%), a branched polymer water insoluble. Sources of starch are potatoes, wheat; rice, maize, bananas etc.
– glucose and major reserve food in plants, turns blue with iodine. It is a mixture of two components (i) amylose (20%), an unbranched polymer water soluble (ii) amylopectin (80%), a branched polymer water insoluble. Sources of starch are potatoes, wheat; rice, maize, bananas etc.
CELLULOSE (C6H10O5)N
It is the most abundant and structural polysaccharide of plants. It is important food sources of some animals. It is polymer of D(+)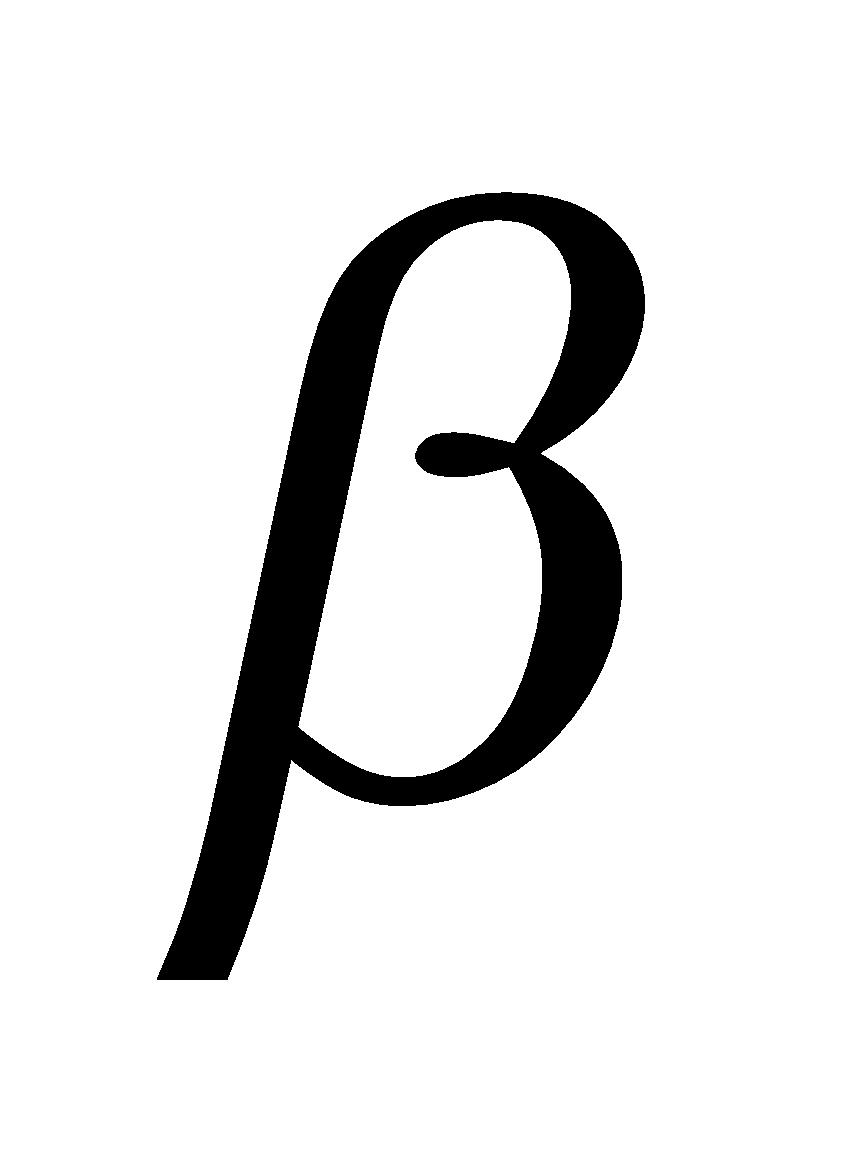 – glucose.
– glucose.
The chief sources of cellulose are wood (contains 50% cellulose rest being lignin, resins etc.) and cotton (contains 90% cellulose rest being fats and waxes).
Useful materials obtained from cellulose
- Mercerized cotton : Cellulose treated with conc. sodium hydroxide solution acquire silky lustre.
- Gun cotton or cordite : It is completely nitrated cellulose (cellulose nitrate), highly explosive in nature and used in the manufacture of smokeless gunpowder blasting gelatin.
- Pyroxylin : It is partially nitrated cellulose. When dissolved in ether and alcohol it forms a transparent film called collodion which is used as covering for cuts and skin abrasions, manufacture of cellulose paints and lacquers.
- Cellulose acetate : It is used for making Acetate Rayon and motion picture films.
- Cellulose Xanthate : It is obtained by treating cellulose with sodium hydroxide and carbon disulphide and is the basic material for VISCOSE rayon.
- Celluloid : It is obtained by warming a mixture of camphor and pyroxylin in alcohol. It is used for making toys and photographic films.
- Cellophane : Cellulose xanthate film softened with glycerol.
Homo polysaccharides – They give one kind of monosaccharides on hydrolysis e.g. starch, cellulose.
Hetero polysaccharides – They give two or more than two kind of monosaccharides e.g. Insulin.
Reducing carbohydrates – Carbohydrates reducing Fehling reagent or Tollen’s reagent are termed reducing carbohydrates. Examples – All monosaccharides and most of the disaccharides (except sucrose).
Sugars and non sugars – The monosaccharides and oligosaccharides having sweet taste are collectively known as sugars. Polysaccharides, insoluble in water are non sugars
D and L Configurations
Glyceraldehyde is optically active and has the following Fischer projections
Glyceraldehyde is optically active and has the following Fischer projections
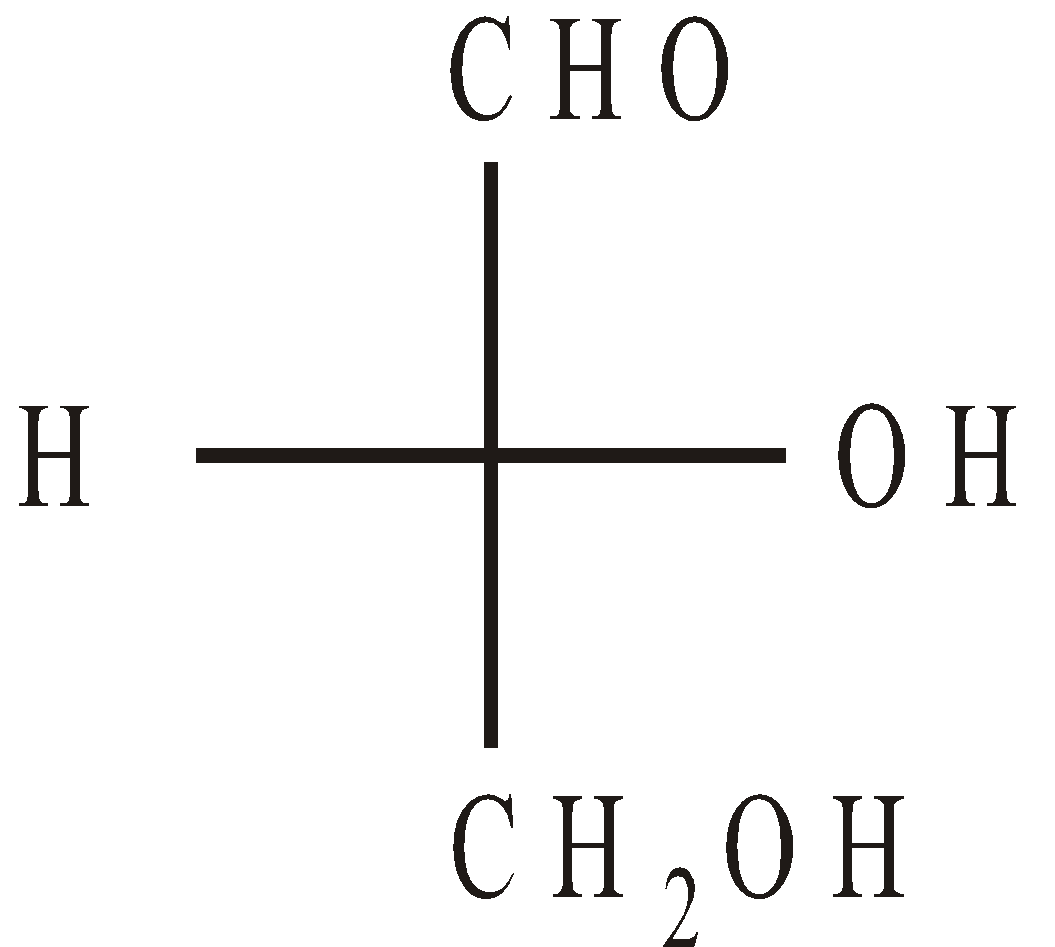 D (+) Glyceraldehyde
D (+) Glyceraldehyde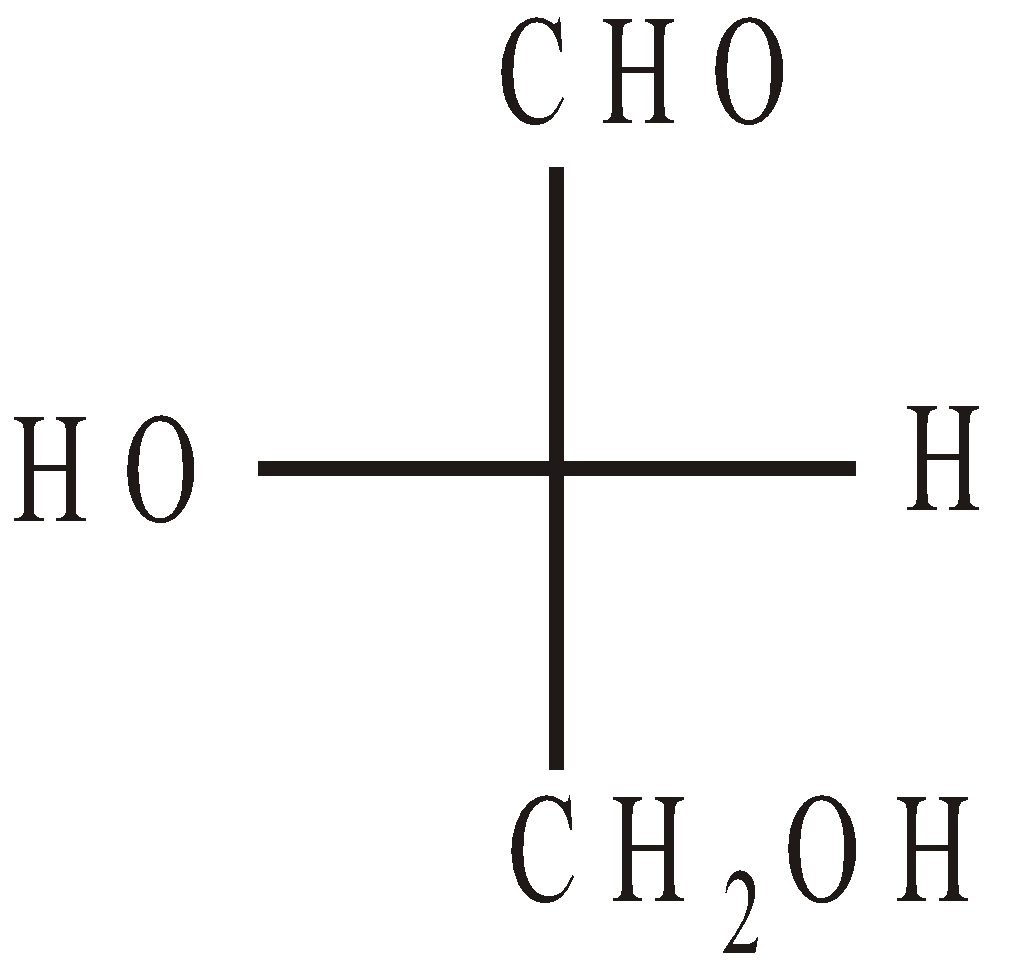 L (–) Glyceraldehyde
L (–) GlyceraldehydeSugars having the same configuration as D – glyceraldehyde at the most distant asymmetric carbon atom from the carbonyl group are designated as D – sugars and opposite to it L – sugars.
All naturally occurring monosaccharides belong to D – series.
GLUCOSE – DEXTROSE, GRAPE SUGAR, CORN SUGAR, BLOOD SUGAR C6H12O6
Manufacture
By hydrolysis of starch with hot dil. mineral acids
By hydrolysis of starch with hot dil. mineral acids
Structure
Glucose has pyranose structure i.e. ring structure consisting of 5C atoms and 10 hydrogen atoms.
Glucose has pyranose structure i.e. ring structure consisting of 5C atoms and 10 hydrogen atoms.
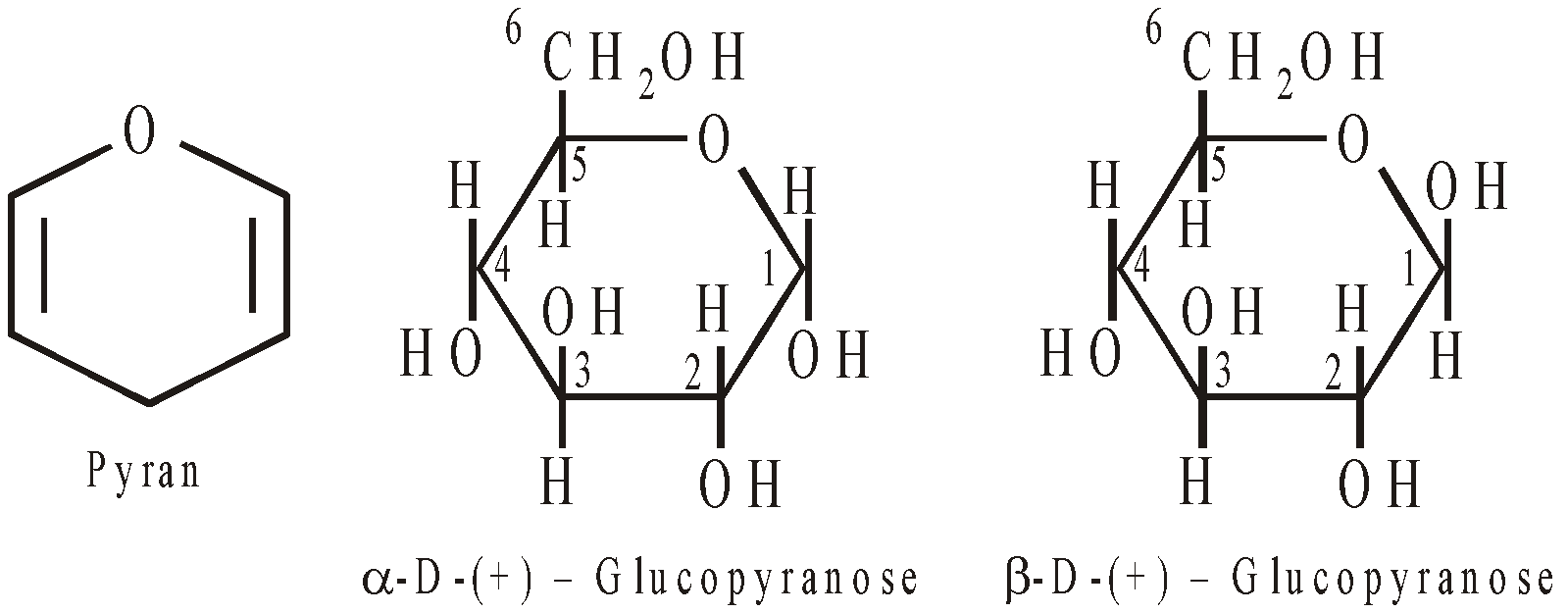
Glucose having (i) configuration about C1 is the 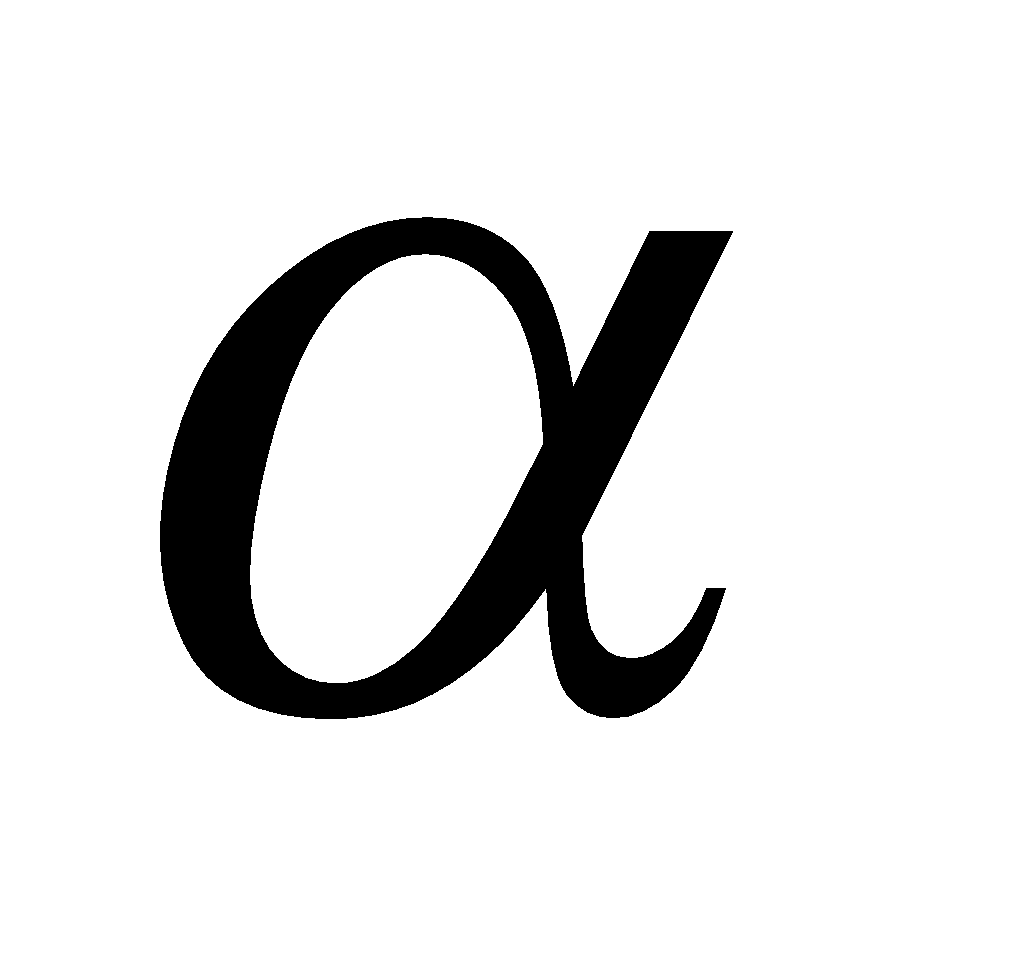 – glucose and having (ii) configuration about C1 is
– glucose and having (ii) configuration about C1 is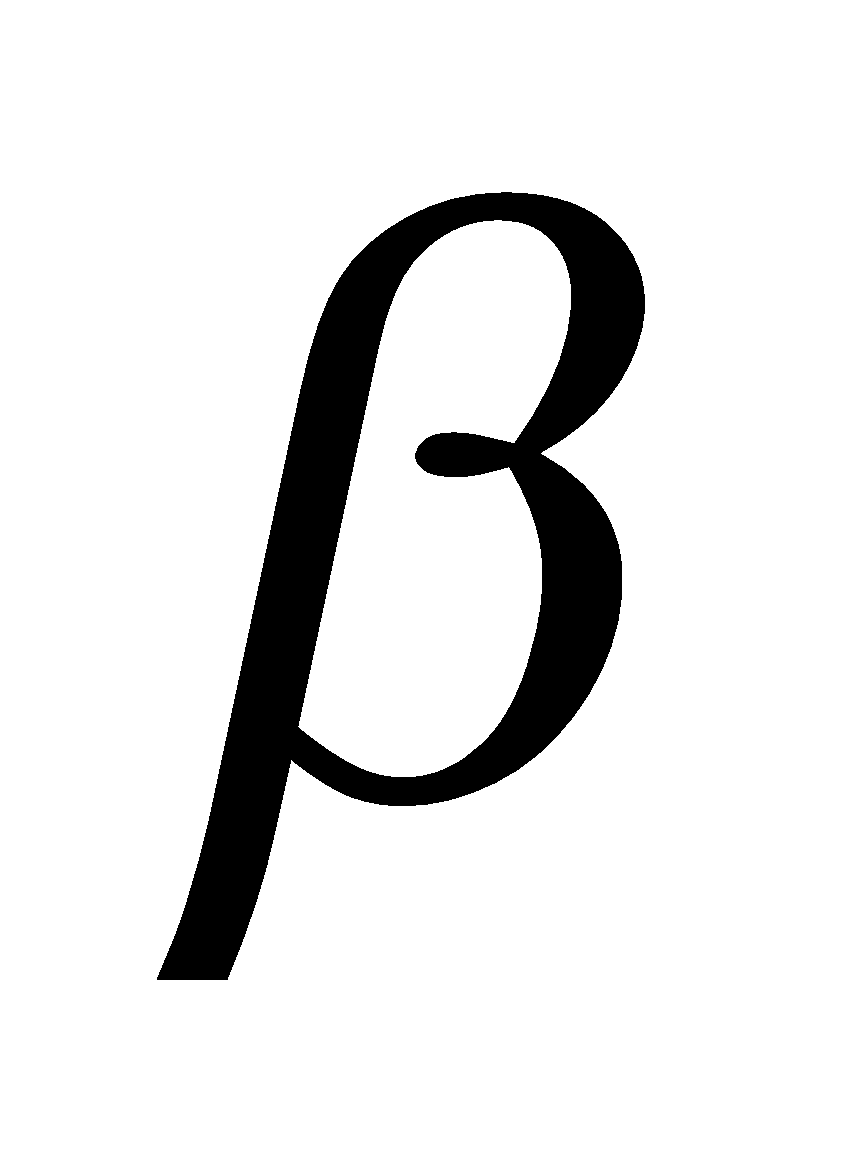 -glucose. The carbon C1 is known as anomeric carbon and
-glucose. The carbon C1 is known as anomeric carbon and  and
and 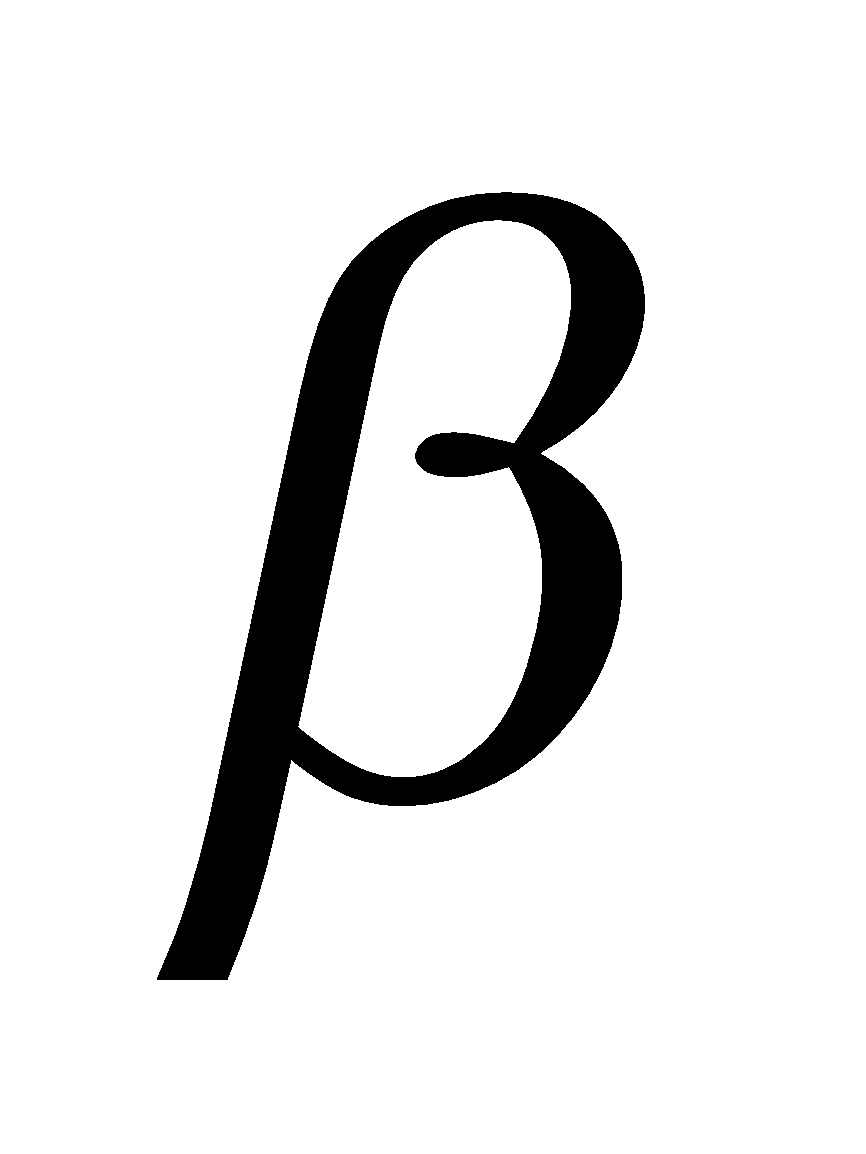 forms as anomers. Both the forms are optically active,
forms as anomers. Both the forms are optically active,  -D- glucose has specific rotation + 111.5º and
-D- glucose has specific rotation + 111.5º and  -D-glucose has specific rotation + 19.5º.
-D-glucose has specific rotation + 19.5º.
Mutarotation – When either of the two forms of glucose is dissolved in water there is change in rotation till the equilibrium value of + 52.5º. This is known as mutarotation
FRUCTOSE, LAEVULOSE, FRUIT SUGAR C6H12O6
Manufacture
By hydrolysis of inulin
By hydrolysis of inulin
Structure
Fructose has furanose structure i.e. ring structure consisting of 4C atoms and 1O atom.
Fructose has furanose structure i.e. ring structure consisting of 4C atoms and 1O atom.
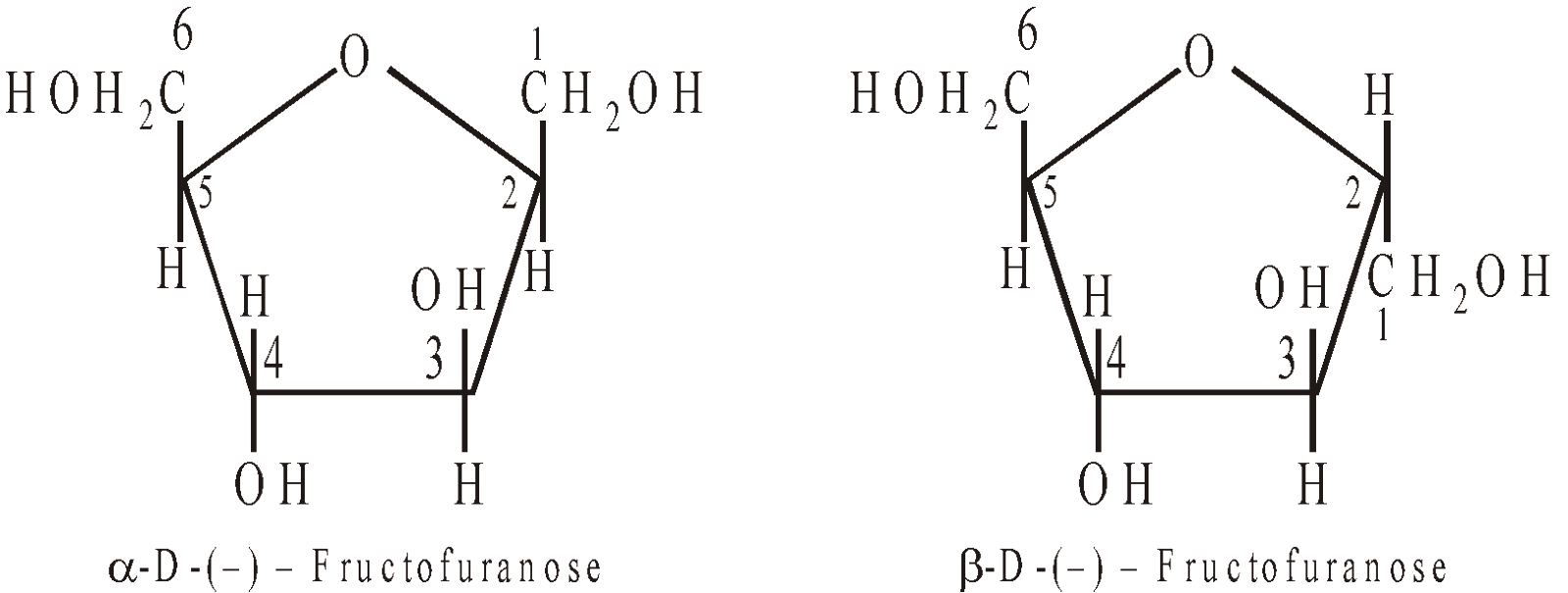
EPIMERS
Monosaccharides differing in configuration at a carbon other than anomeric carbon are called epimers e.g. glucose and galactose differ in configuration at C4 hence called C4 epimers.
OSAZONES
Monosaccharides and reducing disaccharides react with excess of phenyl hydrazine to form crystalline substances of the structure
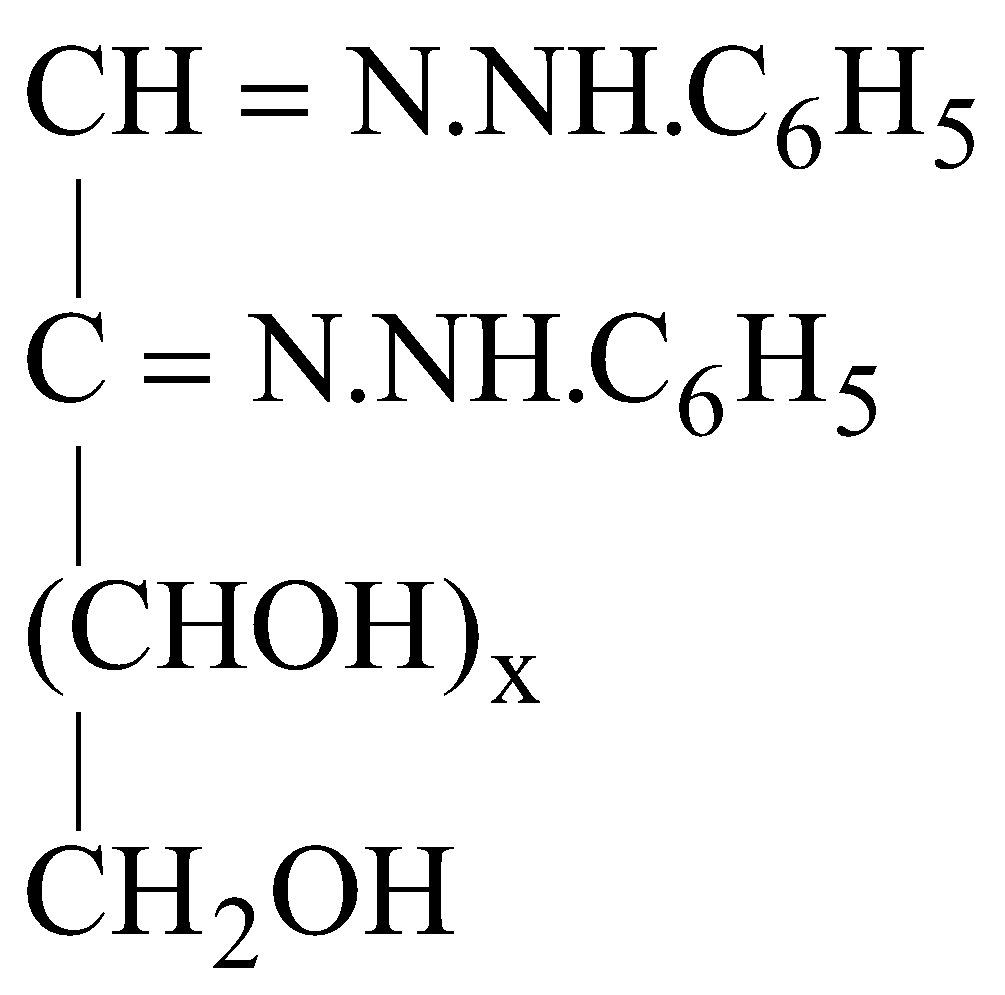
Known as osazones. Glucose and Fructose give same osazone
DISACCHARIDES
These on hydrolysis with dil. acids or enzymes yield two molecules of same or different monosaccharides. The latter are joined together by an oxide linkage formed by the loss of water and is called glycosidic linkage.
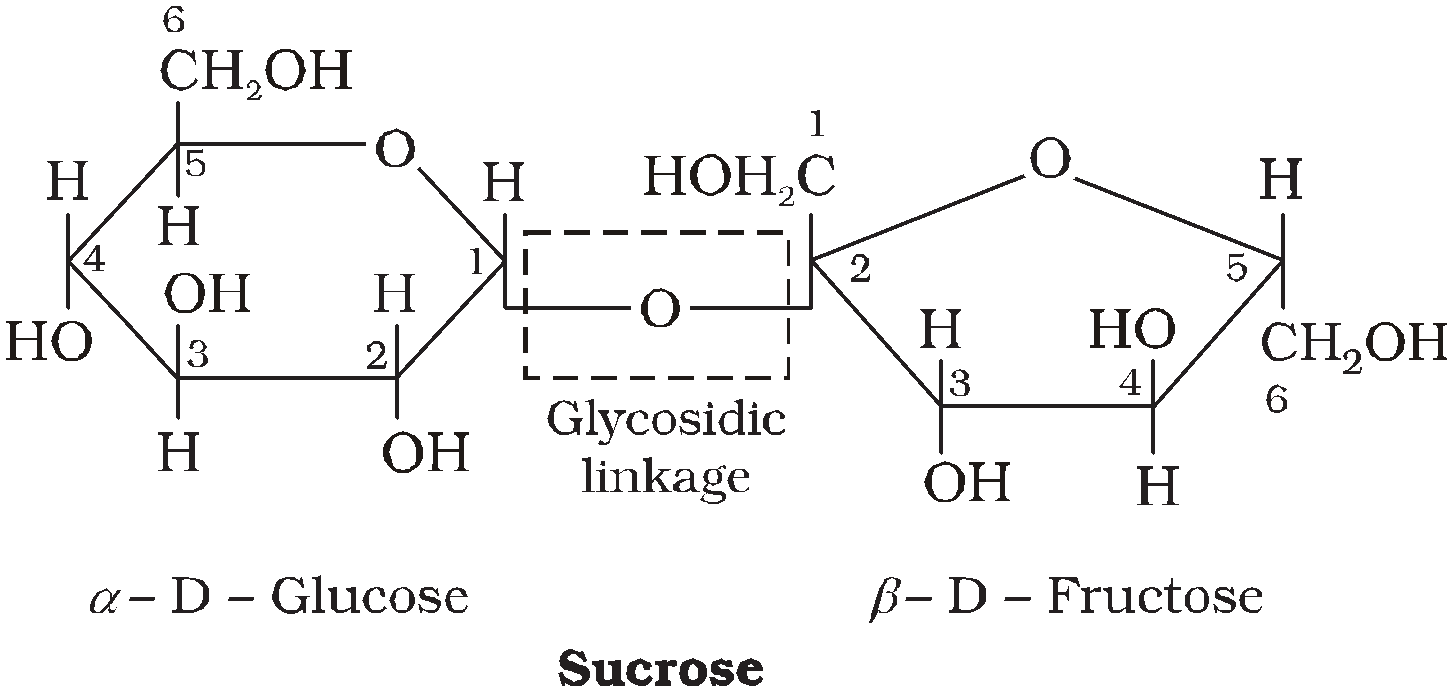
SUCROSE C12H22O11
It is non reducing sugar. It has glycosidic linkage between C1 of a-glucose and C2 of b-fructose. It is dextrorotatory but on hydrolysis give laevorotatory inert sugar. Fructose (– 92.4) and glucose (+ 52.5)
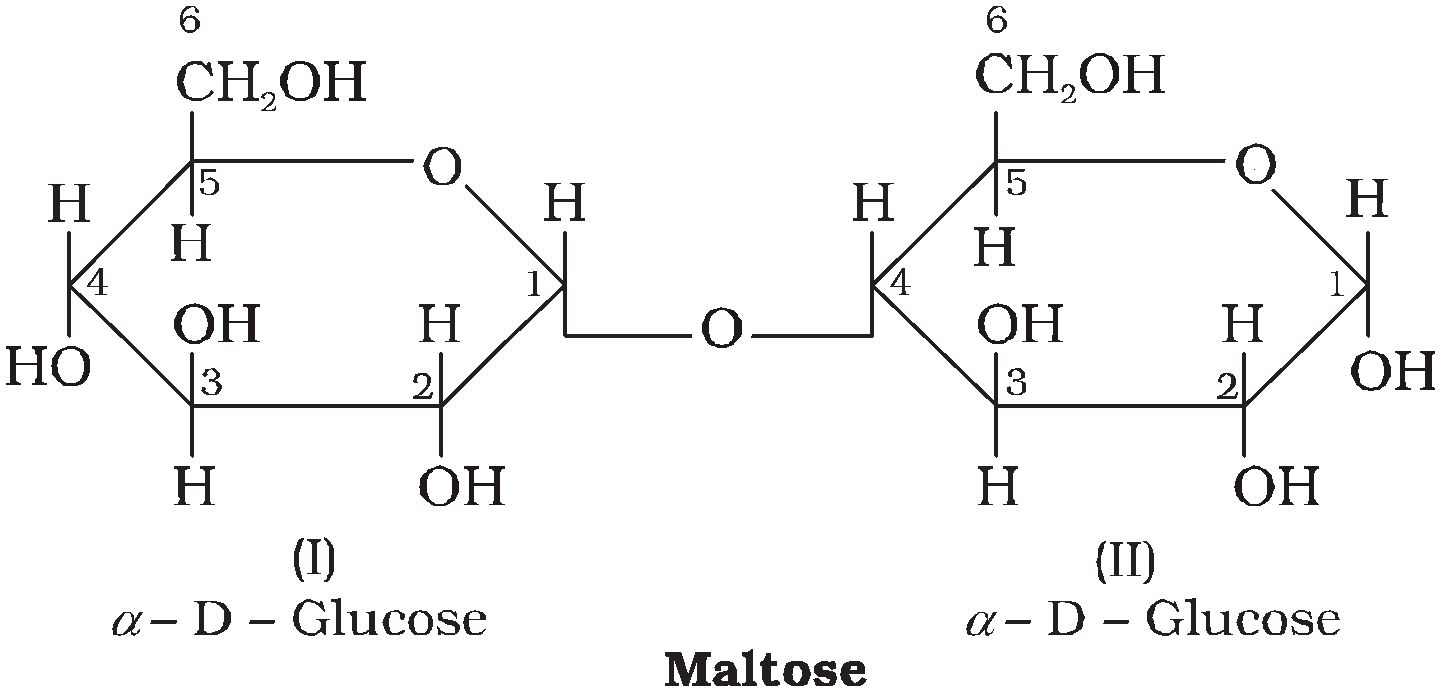
MALTOSE C12H22O11
It is reducing sugar. On hydrolysis it gives two  -D-glucose units in which C1 of one glucose is linked to C4 of another glucose.
-D-glucose units in which C1 of one glucose is linked to C4 of another glucose.
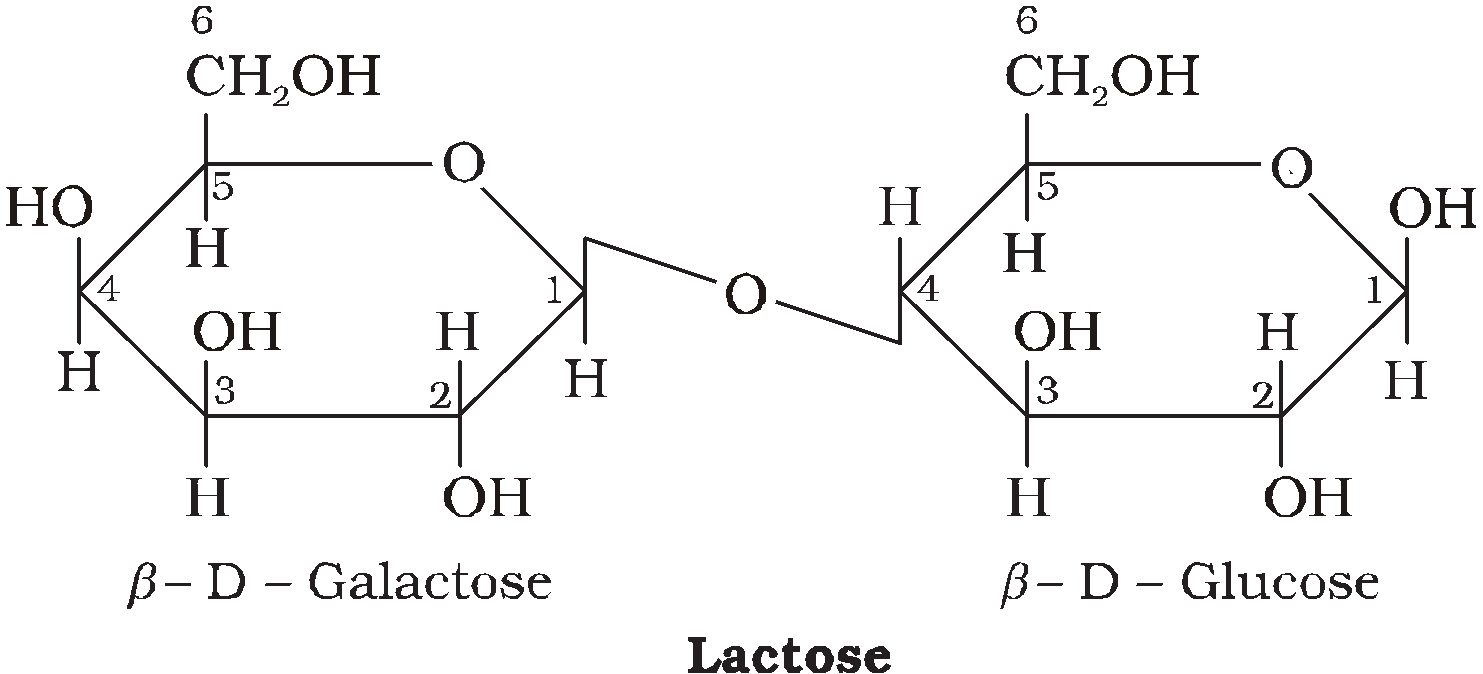
LACTOSE C12H22O11
It commonly known as milk sugar due to presence in milk. The linkage is between C1 of 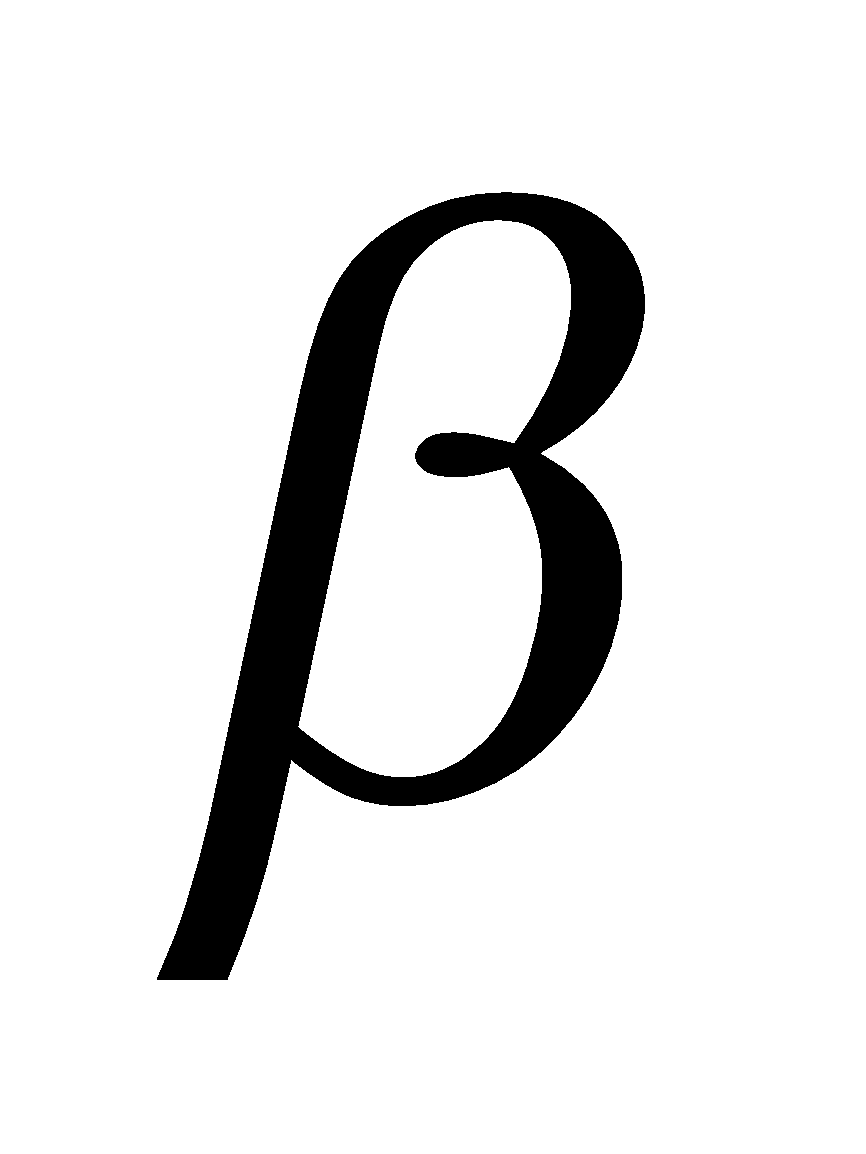 -D-galactose and C4 of
-D-galactose and C4 of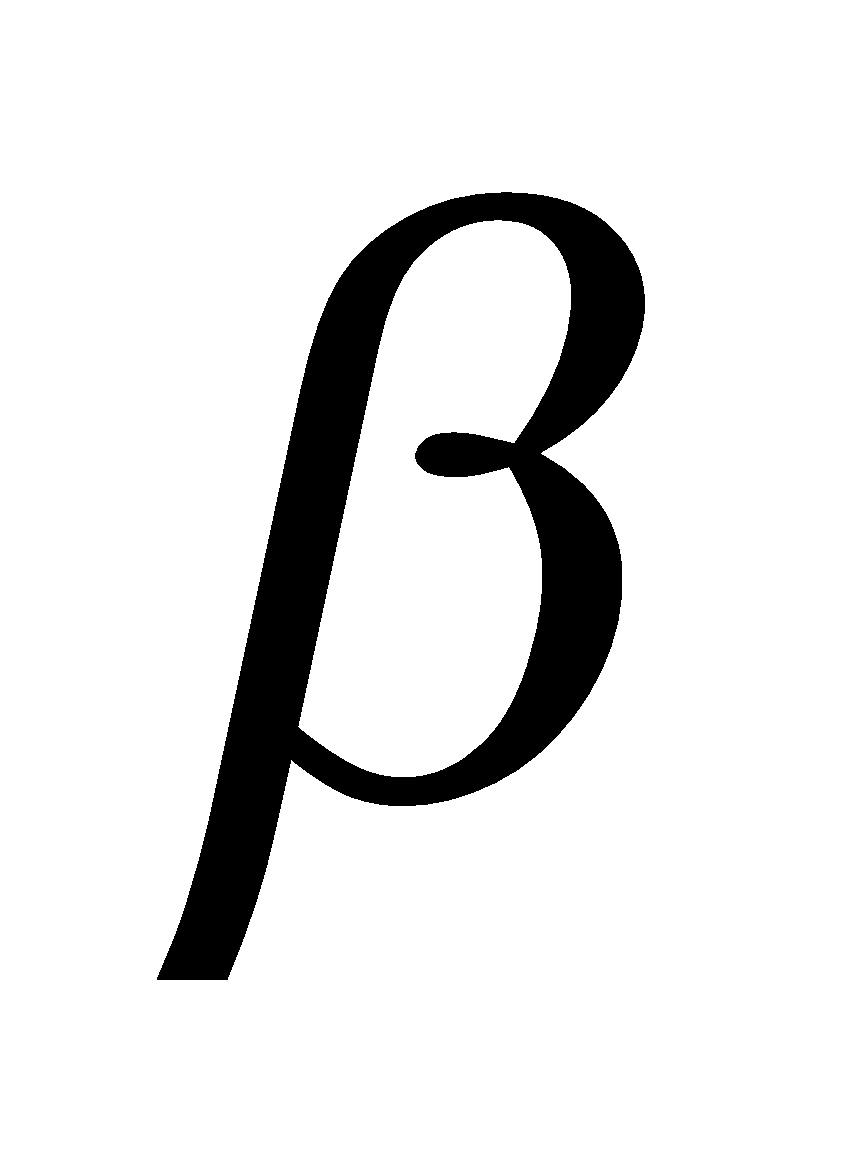 -D-glucose. It is also reducing sugar.
-D-glucose. It is also reducing sugar.
AMINO ACIDS
The compounds containing amino group (–NH2) and carboxylic group (–COOH).
GENERAL FORMULA OF AMINO ACIDS
R = H, alkyl or aryl group.
Except glycine (H2N.CH2COOH) others are optically active in nature.
CLASSIFICATION OF AMINO ACIDS
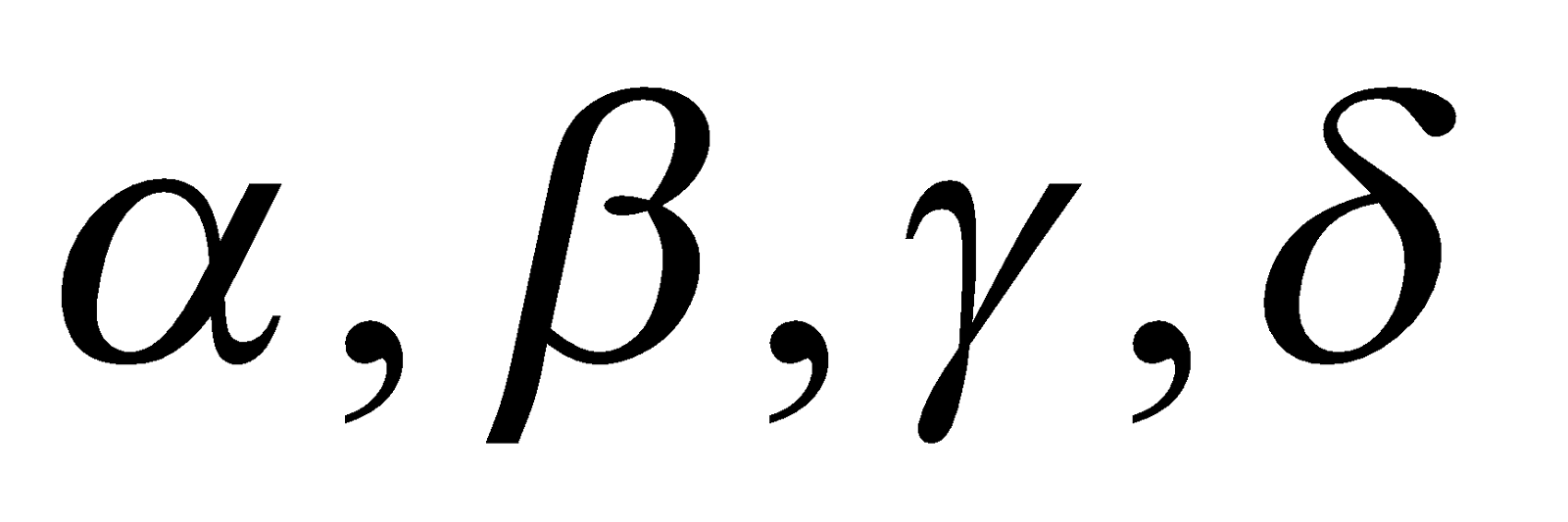 amino acids depending upon the position of – NH2 w.r.t. – COOH group.
amino acids depending upon the position of – NH2 w.r.t. – COOH group.- Neutral – Having one –NH2 and one –COOH
e.g. NH2.CH2.COOH (Glycine)
- Acidic – Having one –NH2 and two –COOH
e.g.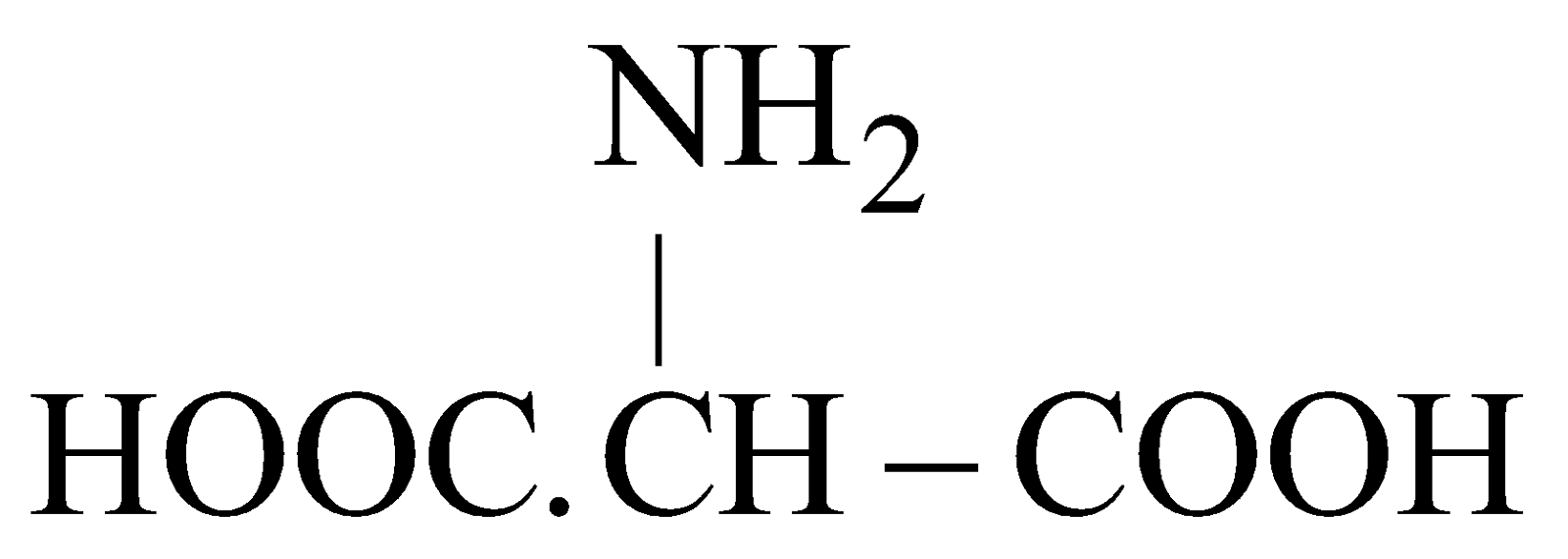 (Aspartic acid)
(Aspartic acid) - Basic – Having two or more –NH2 and one COOH
e.g.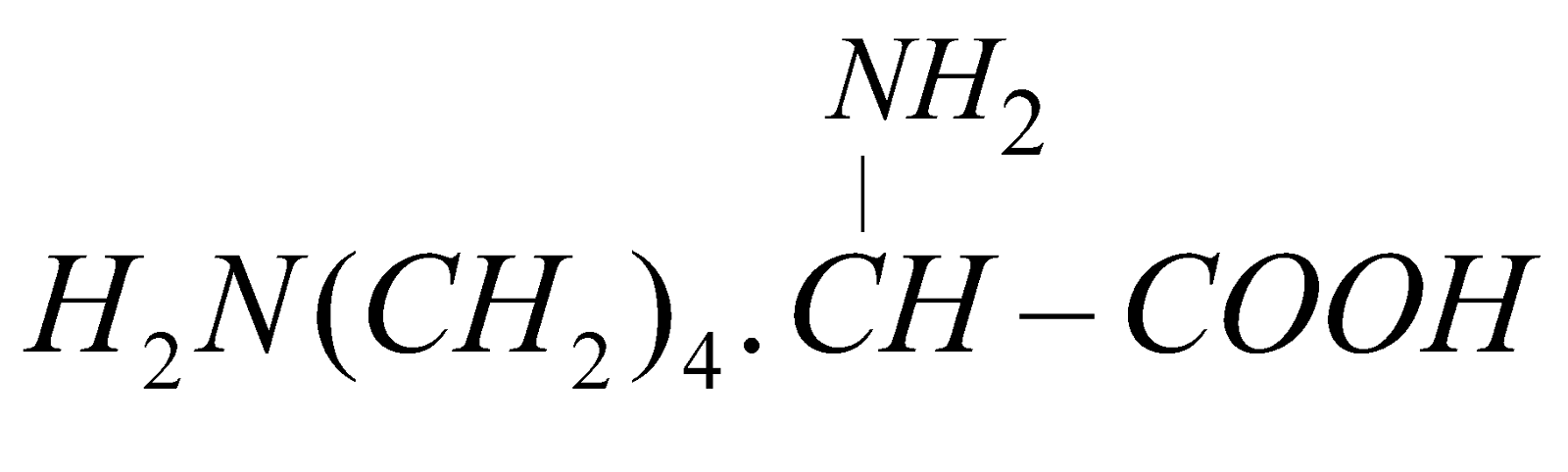 (Lysine)
(Lysine)
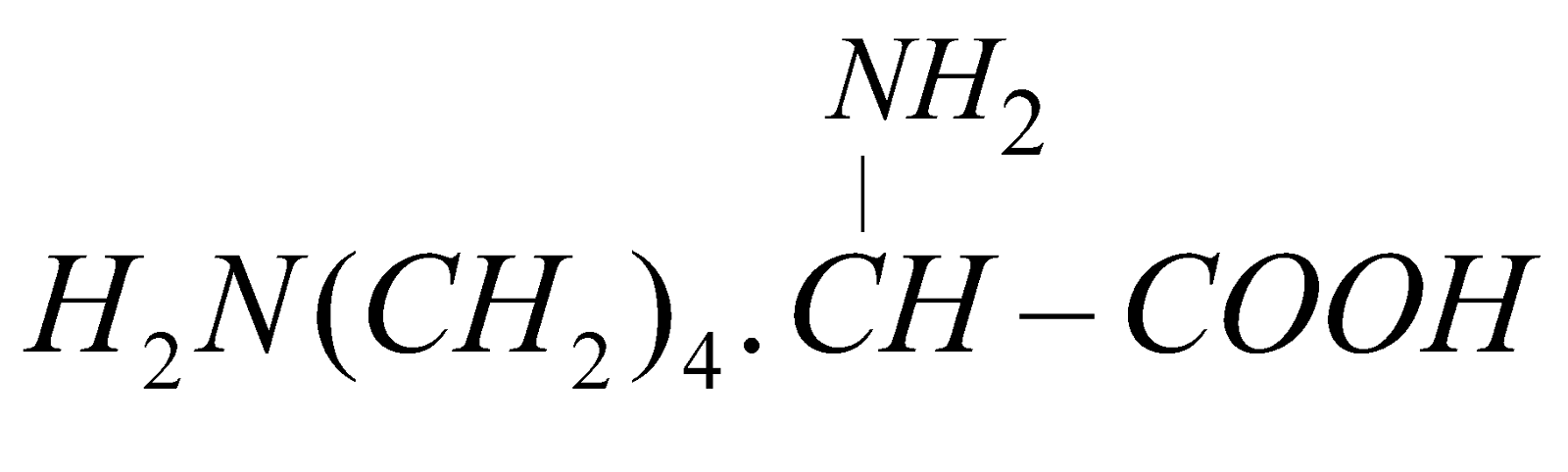 (Lysine)
(Lysine)ESSENTIAL AND NON ESSENTIAL AMINO ACIDS
Human body can synthesise ten amino acids called non essential amino acids. The remaining ten amino acids required for protein synthesis are called essential amino acids.
- Phenylalanine
- Histidine
- Tryptophane
- Valine
- Methionine
- Theonine
- Arginine
- Leucine
- Isoleucine
- Lysine
NOMENCLATURE
They are known by their common names and abbreviated by first three letter of their common names eg. glycine is gly and alanine is ala.
CONFIGURATION OF α-AMINO ACIDS
Naturally occuring α-amino acids are L-amino acids. D-Amino acids occur in some antibiotics and bacterial cell walls.
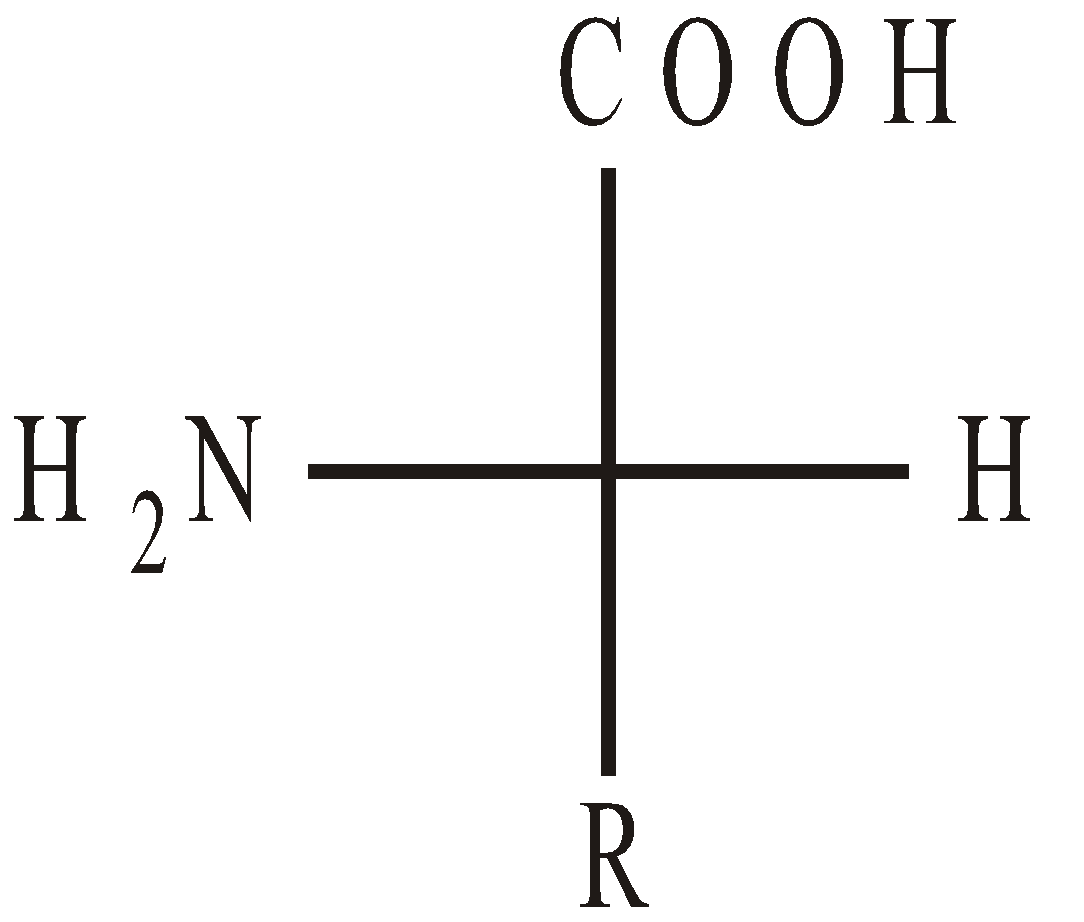
L-Amino acid
(NH2 on L.H.S.)
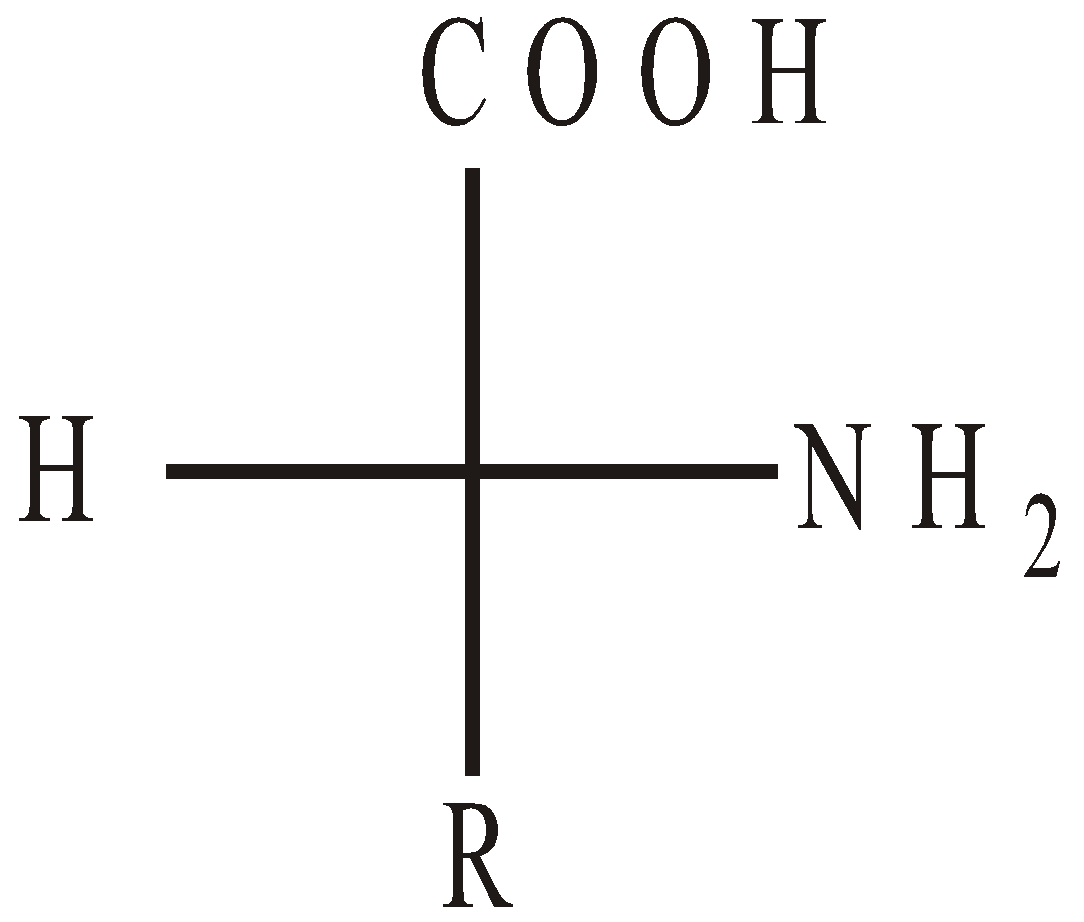
D-Amino acid
(NH2 on R.H.S.)
STRUCTURE
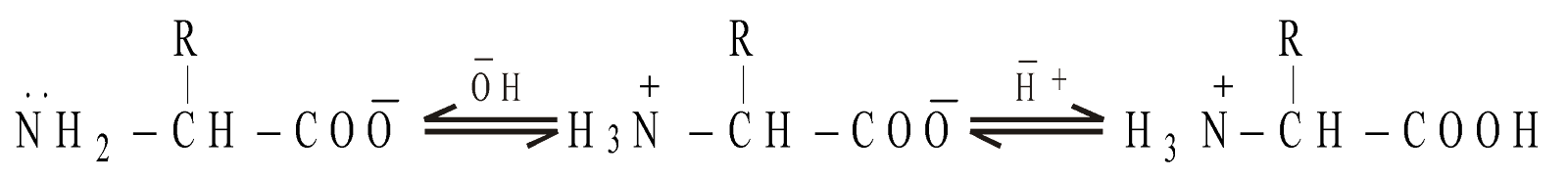
As anion (high pH) Zwitterion, isoelectric point As cation (low pH)
PEPTIDES
Peptides are condensation products of two or more amino acids
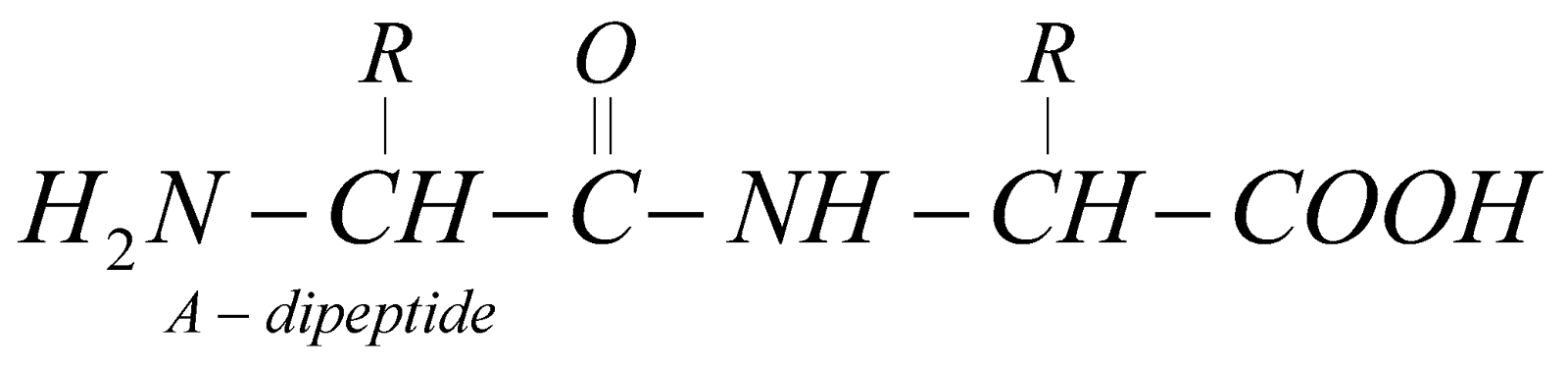
Polypeptides – Condensation products of many amino acids.
PROTEINS
They are linear polymers of – amino acid (Berzelius 1830)
STRUCTURE OF PROTEINS
- Primary structure – It simply reveals the sequence of amino acids.
- Secondary structure – – helix structure maintained by hydrogen bonds or – pleated sheet structure when size of R groups is small.
- Tertiary structure – The folding and super imposition of polypeptide chains forms a compact globular shape, termed as tertiary structure. It is stabilised by covalent, ionic, hydrogen and disulphide bridge.
- Quaternary structure – The precise arrangement constitutes the quaternary structure.
CLASSIFICATION ON THE BASIS OF HYDROLYSIS PRODUCTS
- Simple proteins – Which yield only α-amino acids upon hydrolysis.
- Conjugated proteins – Which yields α-amino acids and non protein called prosthetic group. (Gr prosthesis, an addition).
Protein Prosthetic group
Nucleoproteins Nucleic acid
Phospho proteins Phosphoric acid
Glycoproteins Carbohydrates
Metalloproteins Metals
Chromoproteins Pigment
Lipo proteins Lipids
- Derived proteins – They are obtained by partial hydrolysis of simple or conjugated proteins
Proteins  Proteoses
Proteoses  Peptones
Peptones  Polypeptides
Polypeptides
CLASSIFICATION ACCORDING TO FUNCTIONS
- Structural proteins : Fibrous proteins
- Enzymes : Serve as catalyst pepsin, trypsin etc.
- Hormones : Insulin
- Contractile Proteins : Found in muscles eg myosin, actin.
- Anti bodies : Gamma globulins present in blood.
- Blood protein : Albumins, haemoglobin and fibrinogen.
DENATURATION OF PROTEIN
The process that changes the three dimensional structure of native protein. It can be caused by change in pH, addition of electrolyte, heating or addition of solvent like water, alcohol or acetone.
TESTS
- Biuret – Protein solution + NaOH + dil. CuSO4
 Pink or violet colour
Pink or violet colour - Ninhydrin – Protein solution + Ninhydrin blue colour
- Hopkin’s cole – Protein solution + glyoxylic acid + conc. H2SO4
 Blue – violet
Blue – violet - Million’s – Protein solution + Millon’s reagent Pink colour.
Millon’s reagent – Solution of mercuric nitrate and nitrite in nitric acid containing traces of nitrous acid.
- Iodine reaction – Protein solution + iodine in potassium iodide solution
 yellow colour
yellow colour - Xanthoproteic test – Protein solution + conc HNO3
 yellow colour
yellow colour  orange colour
orange colour
ENZYMES
Enzymes – A group of complex proteinoid organic compounds, elaborated by living organisms which catalyse specific organic reactions are called enzymes.
Coenzymes – Non proteinous components required for the activity of certain enzymes are known as coenzymes. Coenzymes include metal ions like Mn2+, Mg2+, K+, Na+, Zn++, Co++ etc. heterocyclic ring systems (pyrrole, purine, pyridine etc), a sugar residue, phosphoric acid residue or vitamins like thiamine, riboflavin, niacin etc.
In such cases the protein part of enzymes is called apoenzyme
Endoenzyme – acts in the same cell in which it is synthesised.
Exoenzyme – acts outside the cell in which it is synthesised.
Nomenclature – They are usually named by adding the suffix – ase to the root name of the substrate eg urease, maltase.
Hydrolytic enzymes – Catalyse hydrolysis and are mostly simple proteins.
Oxidative enzymes – catalyse oxidation reduction reaction and are mostly conjugated proteins.
CHARACTERISTIC FEATURES OF ENZYMES
- Rate of reaction – They increase the rate of reaction upto 10 million times.
- Specific nature – Urease catalyse the hydrolysis of urea and not methyl urea.
- Optimum temperature – It is about 20 °C- 40º C.
- pH of medium – It is about 7 but for Pepsin (1.8-2.2) Trypsin (7.5 – 8.3)
- Concentration – Dilute solutions
- Amount – Very small amount can accelerate the reaction.
- Exergonic reaction – Enzyme catalyse the exergonic reactions.
Enzyme inhibitors – The compounds which inhibit the enzyme action. With the help of such compounds the reaction can be controlled.
MECHANISM OF ENZYME ACTION
Enzyme + Substrate  [Enzyme substrate]
[Enzyme substrate]  Product + Enzyme
Product + Enzyme
APPLICATIONS
- Treatment of diseases – The congenital disease phenylketonuria caused by phenylalanine – hydroxylase can be cured by diet of low phenylalanine content. Albinism is caused by deficiency of tyrosinase. Enzyme streptokinase is used for blood clotting to prevent heart disease.
- In industry – Tanning of leather, fermentation process.
NUCLEIC ACIDS
Nucleotides – Nucleotides consist of 5 – carbon sugar (pentose) + nitrogenous base + 1-3 phosphate groups.
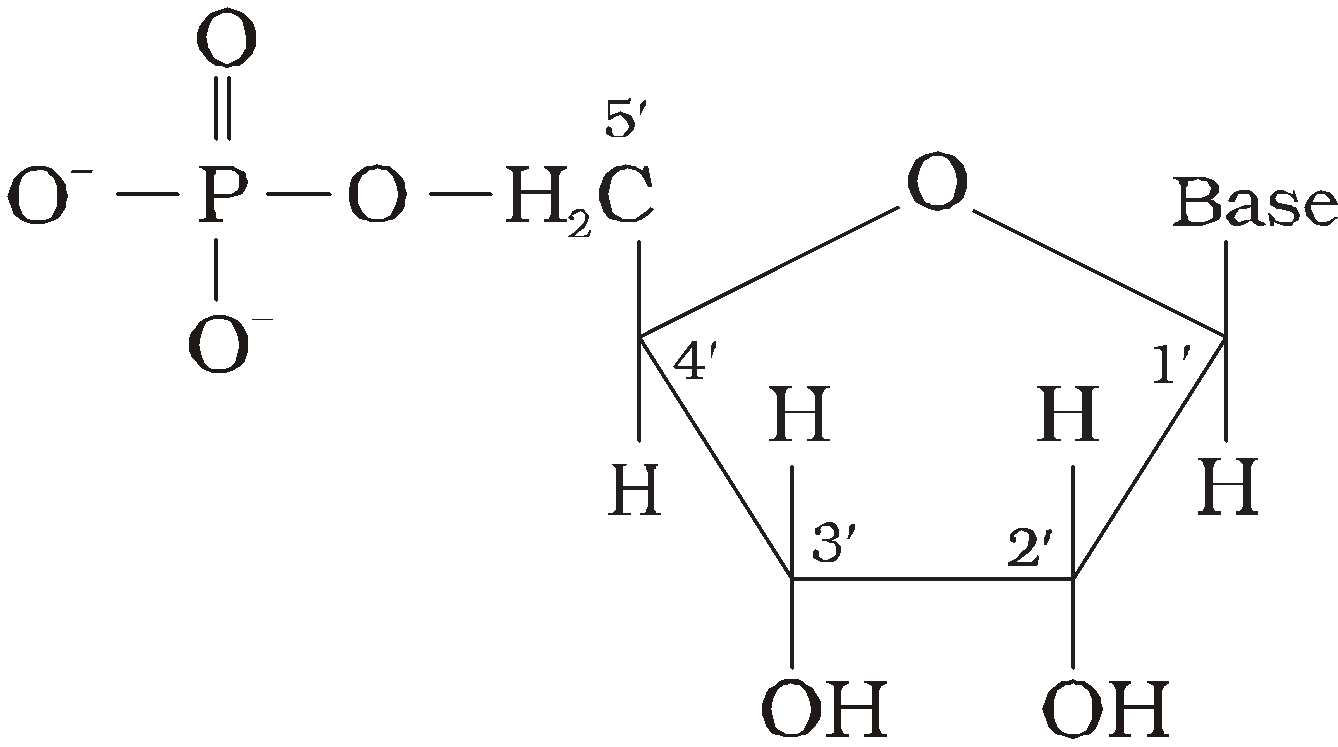
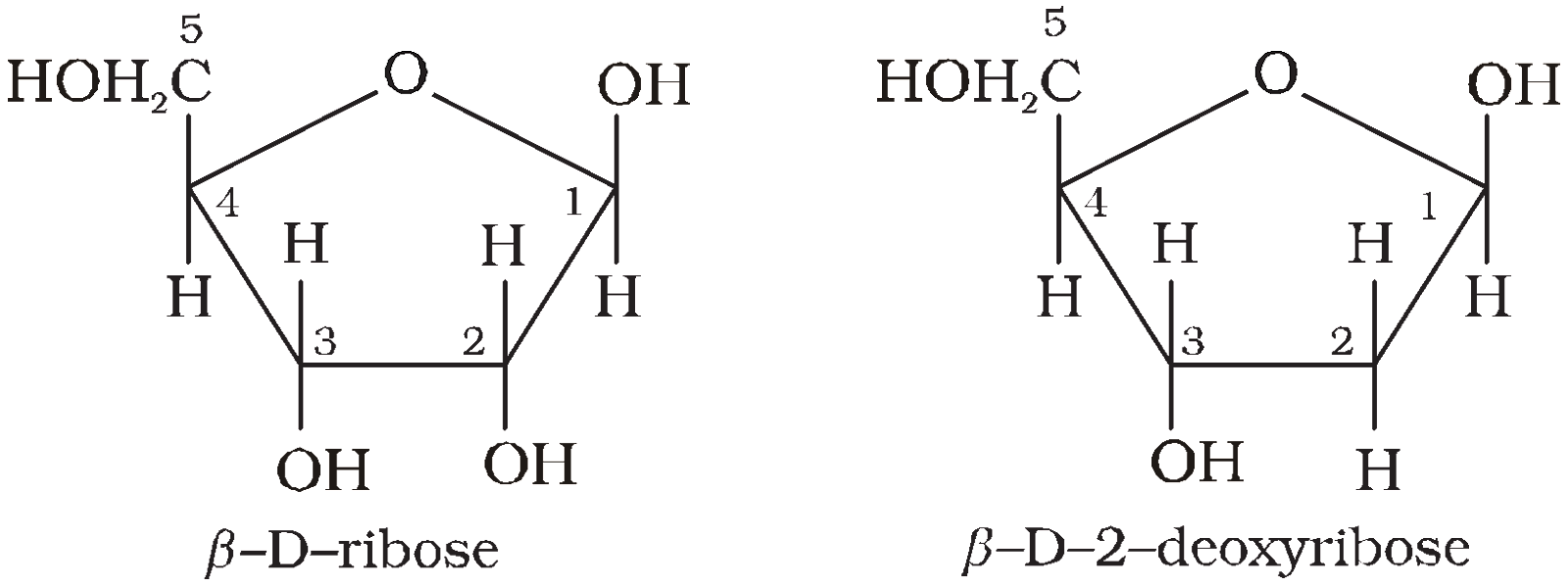
Pentose – is either Ribose or deoxyRibose (not having oxygen at C2).
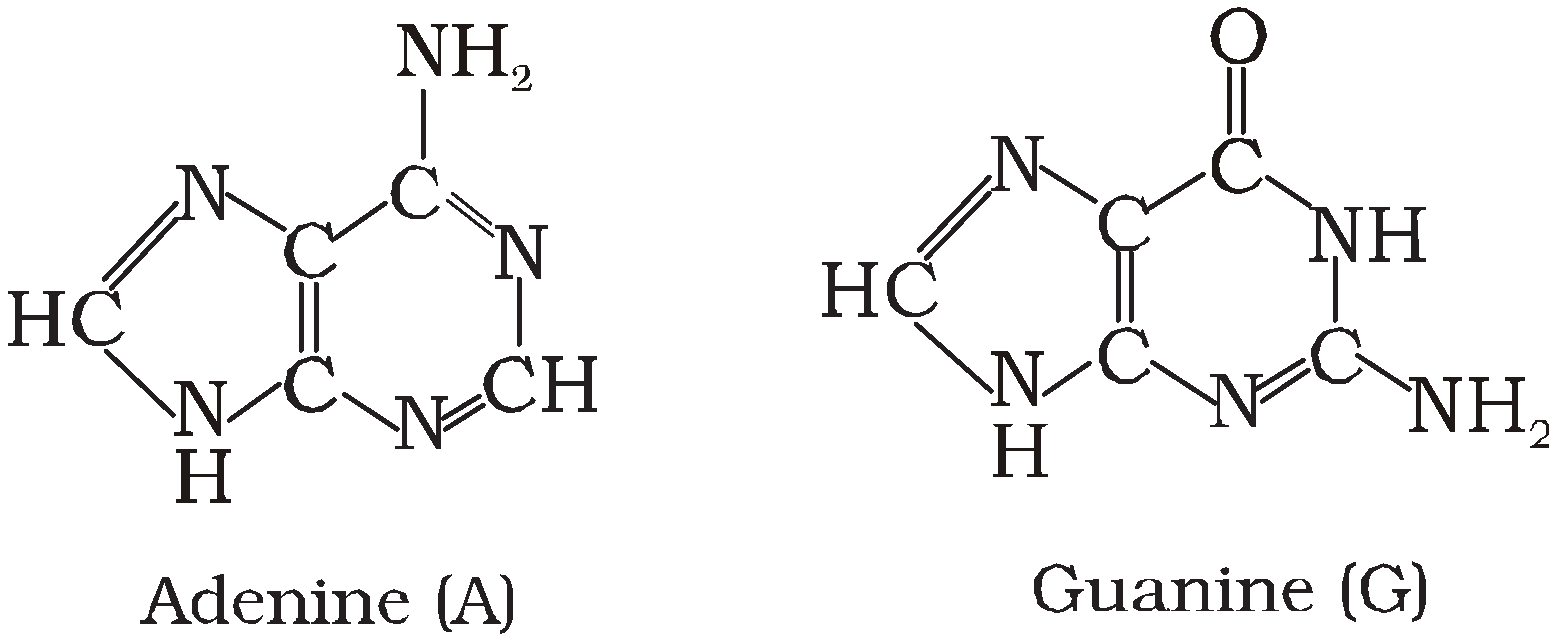
NITROGENOUS BASE
Derived from purines having two rings in their structure
Examples : Adenine (A) and Guanine (G).
Examples : Adenine (A) and Guanine (G).
Derived from Pyrimidines having one ring in their structure
Examples : Thymine (T), Uracil(U) and Cytosine (C).
Examples : Thymine (T), Uracil(U) and Cytosine (C).
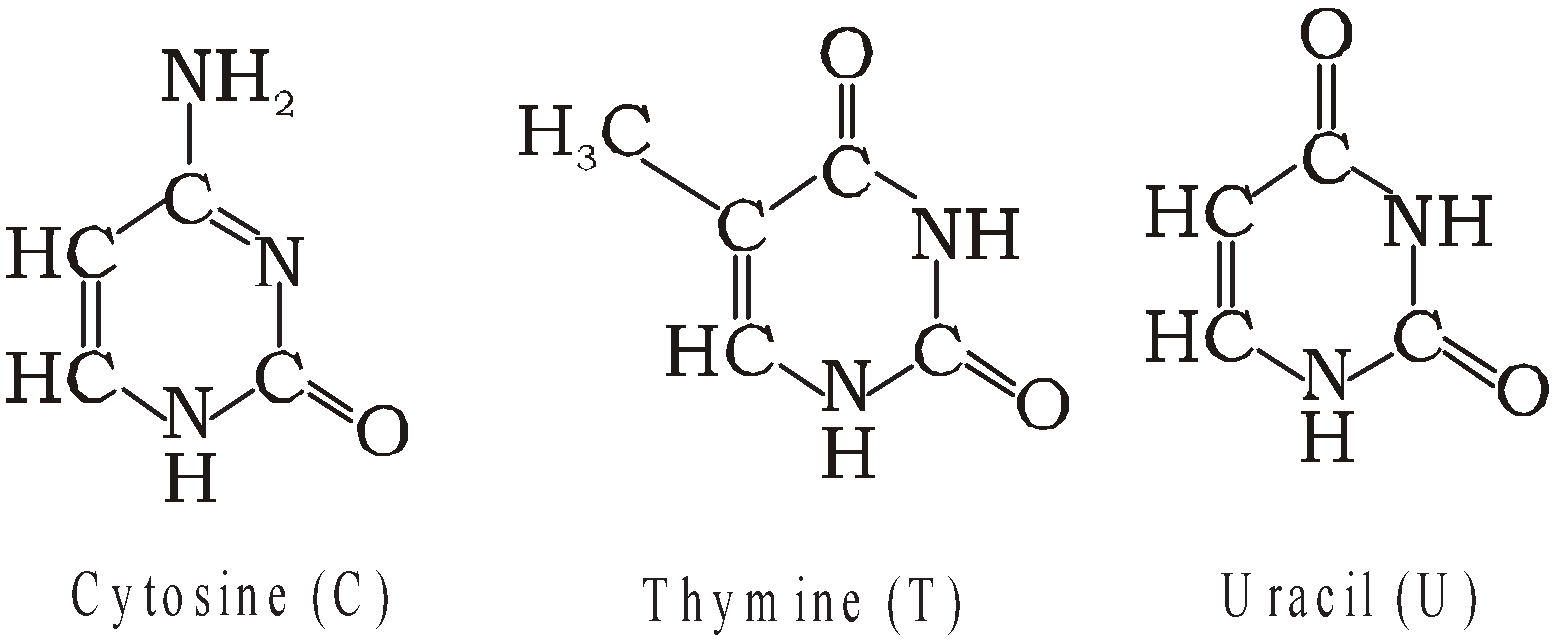
Ribonucleotide – Phosphate unit + Ribose + One base unit from AGC or U.
Deoxy Nucleotide – Phosphate unit + Deoxyribose + One base from AGC or T.
Nucleoside – Ribose + one base unit from AGCT or U
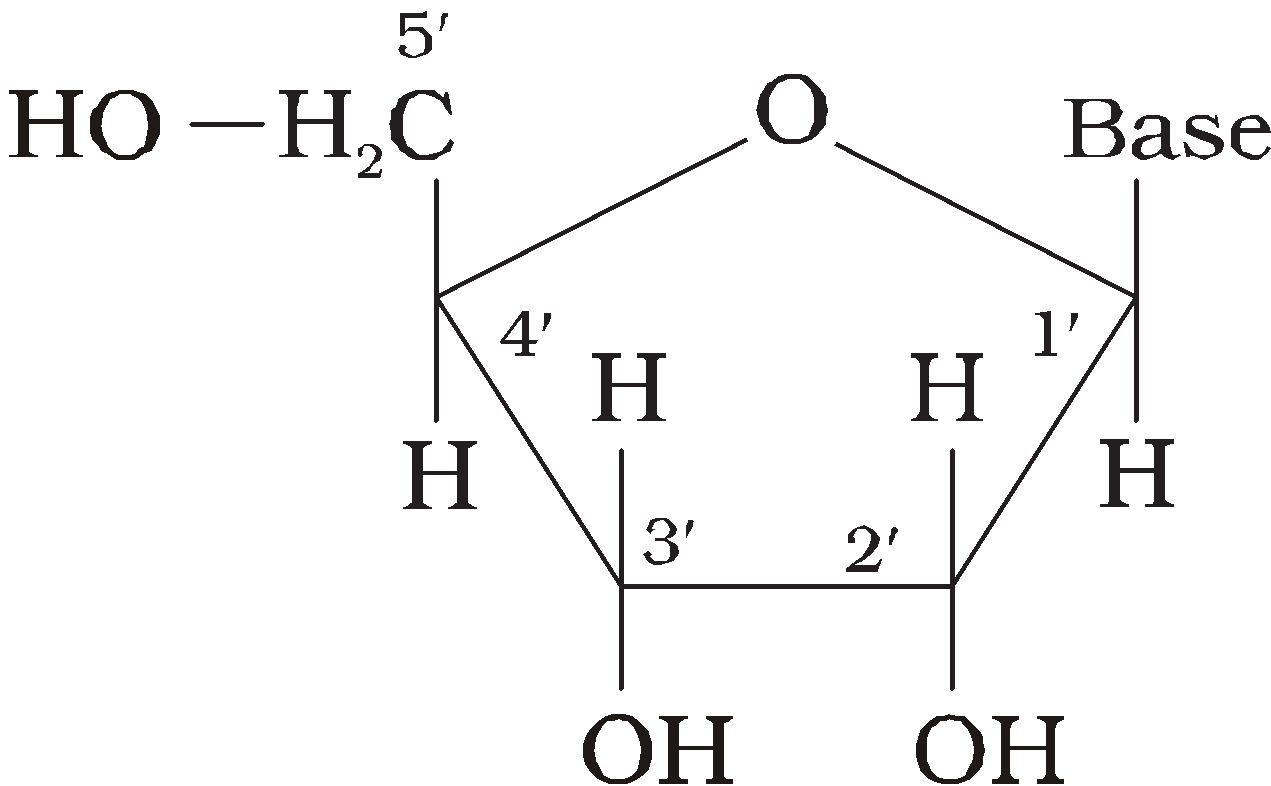
NUCLEIC ACID
It is polynucleotide, present in the nucleus of the living cells or bacterial cells having no nucleus and in viruses having no cells.
DNA – Deoxyribonucleic acid
DNA + H2O  Phosphoric acid + Deoxy ribose + AGCT
Phosphoric acid + Deoxy ribose + AGCT
RNA – Ribonucleic acid
RNA + H2O Phosphoric acid + Ribose + AGCU
Phosphoric acid + Ribose + AGCU
STRUCTURE OF DNA
It consists of two polynucleotide chains, each chain forms a right handed helical spiral with ten bases in one turn of the spiral. The two chains coil to double helix and run in opposite direction held together by hydrogen bonding.
DNA exists in five forms A,B,C,D and E.
Wang/ Rich (1979) reported DNA with left handed double helical configuration.
It was named as Z – DNA.
STRUCTURE OF RNA
It is usually a single strand of ribonucleotides and take up right handed helical conformation. Ribonucleotide consists of three different molecules – phosphate, ribose sugar and nitrogenous base. The latter may be purine (adenine or guanine) or pyrimidine (cytosine or uracil). Upto 12,000 nucleotides constitute as RNA.
It can pair with complementary strands of DNA or RNA according to standard base pairing rules -G pairs with C, A pairs with U or T. The paired strands in RNA – RNA or RNA – DNA are anti parallel as in DNA.
Messenger RNA (m-RNA). It is produced in the nucleus and carries information for the synthesis of proteins.
Ribosomal RNA (r – RNA) – Its role yet not known.
Transfer RNA (Soluble or Adoptive RNA) (S.RNA, t – RNA) – It is found in cytoplasm. Its function is to collect amino acids from cytoplasm for protein synthesis.
FUNCTION OF NUCLEIC ACIDS
- To direct the synthesis of proteins.
- To transfer the genetic information (hereditary characters).
Replication – A molecule of DNA can exactly duplicate of itself. The process is called replication.
Template – It means pattern. In the process of replication of DNA the parent strand serves as template.
Gene – The portion of DNA carrying information about a specific protein is called gene.
Genetic Code – The relation between the amino acid and the nucleotide triplet is called genetic code.
Codons – The nucleotide bases in RNA function in groups of three (triplet) in coding amino acids. These base triplets are called codons.
Mutation – Error in genetic code is called mutation.
LIPIDS
Lipids (Greek lipos = fat). The constituents of animals and plants soluble in organic solvents (ether, chloroform, carbon tetrachloride, hexane, benzene etc.) but insoluble in water are called lipids.
CLASSIFICATION OF LIPIDS
SIMPLE LIPIDS
- Fats and oils on hydrolysis give long chain fatty acids + glycerol
- Waxes – Long chain fatty acids + long chain alcohols.
Vegetable and animal oils and fats have similar chemical structure and are triesters of glycerol called glycerides. Simple glycerides contain one type of fatty acids. Mixed glycerides contain two or three types of fatty acids.
COMPLEX LIPIDS –
- Phospholipids
Phosphate + glycerol + fatty acids + a nitrogen containing base
General formula
General formula
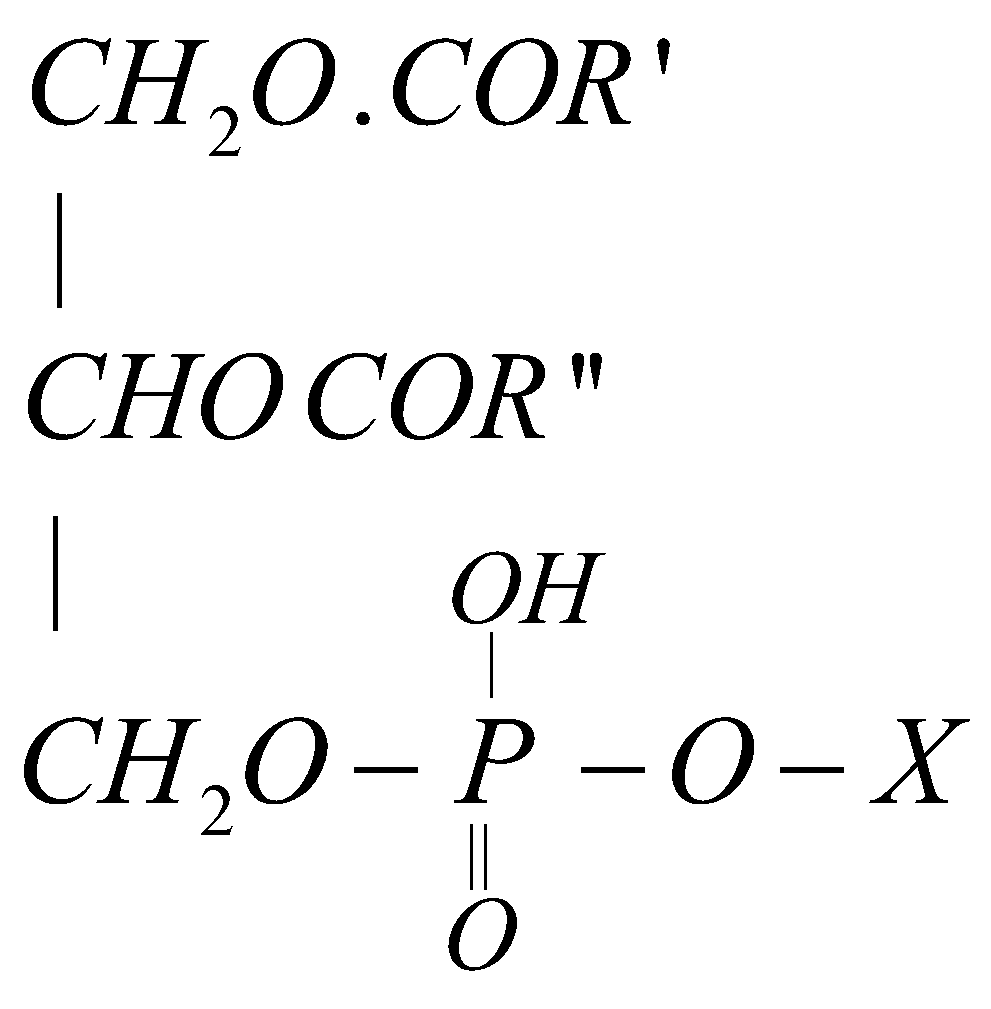
X =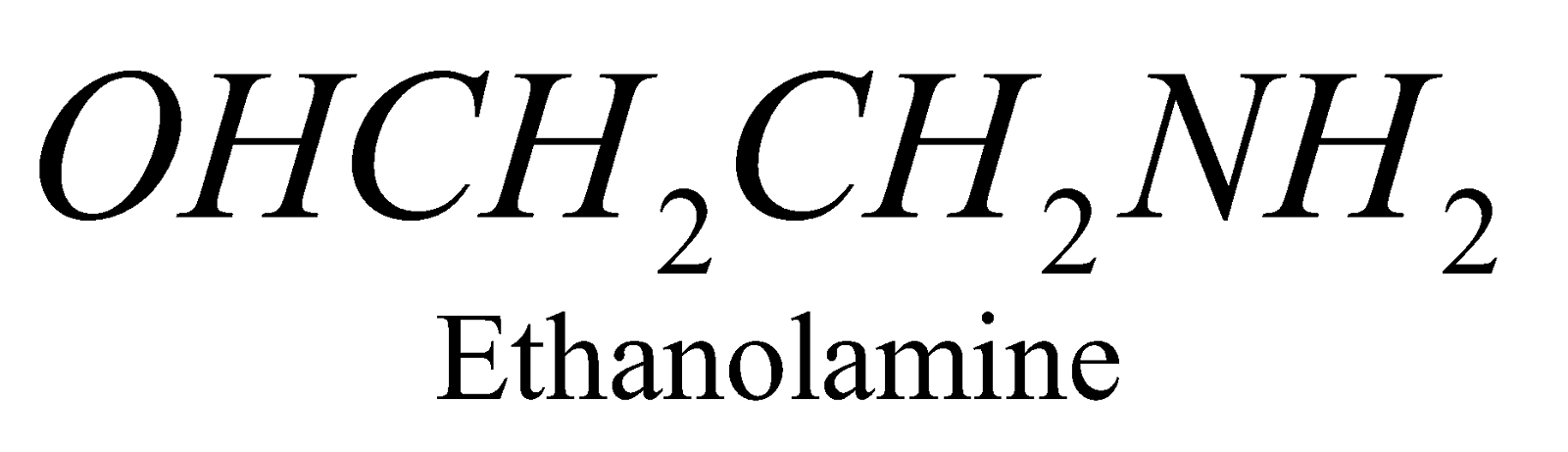 ,
,
When X = choline the phospholipid is called Lecithine. The latter is found in brain tissues, lever and egg yolk and an important component of nerve tissue membrane.
Function of Phospholipids
- As emulsifying agents since they carry hydrophilic polar groups and hydrophobic non polar groups.
- They absorb fatty acids from the intestine and transport to blood cell.
- Glycolipids
They contain one or more simple sugars and are important components of cell membranes and chloroplast membranes.
Common saturated fatty acids
CH3 – (CH2)n COOH
When n = 4 caproic acid; n = 6 caprylic acid; n = 8 capric acid, n = 10 lauric acid n = 12 myristic acid; n = 14 Palmitic acid, n = 16 stearic acid
Common unsaturated acids
C17H33COOH oleic acid; C17H33COOH Linoleic acid
Difference between oils and fats – Oils are liquids at ordinary temperature (below 20ºC) and contain lower fatty acids or unsaturated fatty acids
Fats are solids or semi solids above 20ºC and contain higher saturated fatty acids. Both oils and fats act as “energy reservoirs” for the cells.
Plant Waxes
Carnauba wax C25H51.COO C31H63 myricyl car otate. Ester of myricyl alcohol C31H63OH and cerotic acid C25H51.COOH
They protect the plant from dehydration and invasion of harmful organism
Animal waxes
Beeswax C13H27COOC26H53 cetyl myristate, C15H31COO C31H63 myricyl palmitate, spermaceti wax cetyl palmitate C15H31COO C16H33
Bee’s wax is used in the manufacture of polishes, candles and water proof coating.
Steroids and Terpenes – Menthol, Camphor are common plant terpenes. Carotenoids and pigments are also terpenes.
Essential oils – The volatile, sweet smelling liquids obtained from flowers, leaves, stems etc. Example terpenes or esters of lower fatty acids eg clove oil, rose oil, Lemon oil.
Drying oils – The oils which are converted into tough, transparent mass when exposed to air by oxidation – polymerisation process are called drying oils. eg. Linseed oil, perilla, poppy seed oils. Cotton seed oil and til oil are semi drying oils.
CHARACTERIZATION OF FATS
- Acid value – The number of milligrams of KOH required to neutralise the free acid present in 1 gm of oil or fat.
- Saponification value – The number of milligrams of KOH required to saponify 1 gm of oil or fat. or The number of milligrams of KOH required to neutralise the free acid resulting from the hydrolysis of 1 gm of an oil or fat.
- Iodine value – The number of gms of iodine absorbed by 100 gms of oil or fat.
- Reichert-Meissl Value (R/M value) – The number of cc of N/10 KOH required to neutralise the distillate of 5 gm of hydrolysed fat.
VITAMINS
The organic compounds that can not be produced by the body but are necessary for life, growth and health known as Accessory dietary factors or vitamins. The vitamins are neither structural building units nor supplier of energy. They simply transform energy into action.
CLASSIFICATION
Vitamins are classified into two groups
- Water soluble vitamins : Vitamin B, complex and vitamins C
- Fat soluble vitamins : Vitamins A, D, E and K
VITAMIN A
Its chemical name is Retinol. It has following structure

Sources: Milk, liver, butter, egg yolk, carrot spinach, cod liver and shark lever.
It is essential for growth and for vision. A lack of vitamin A results in, night blindness, loss of weight, hardening of cornea (Xerophthalmia), drying up and scale formation (Keratinization ) of epithelial tissues.
VITAMIN B1
Thiamine (aneurin)
Sources : Polished rice, yeast, egg, in all cells as its pyrophosphate ester, certain vegetables.
Its deficiency causes a disease beriberi. It also affects nervous system and the symptoms are weakness, headache loss of appetite.
VITAMIN B2
Riboflavin (lactoflavin)
It is bright yellow powder showing a green fluorescence.
It is bright yellow powder showing a green fluorescence.
Sources : Yeast, green vegetables, milk, meat, liver, kidney. Its deficiency causes lips, mouth and tongue are usually sore, eyes burn.
VITAMIN B6
(Pyridoxine, Adermin)
Sources : meat, fish, egg, yolk and whole cereals.
It causes nervous disturbances and convulsions.
VITAMIN B12
It contains the element cobalt.
Sources :- liver, meat, egg and rain water
Lack of  causes pernicious anaemia
causes pernicious anaemia
VITAMIN C
(Ascorbic Acid)
Sources : Fresh fruits, orange, lemons, grapes, green vegetables and potatoes. During cooking the large proportion of it is destroyed and process is accelerated by traces of copper.
It helps in proper healing of cuts and abrasions. Its deficiency causes scurvy. Which is characterised by weakness, tendency for bleeding in the muscles.
VITAMIN D
It consists about ten compounds which are designated as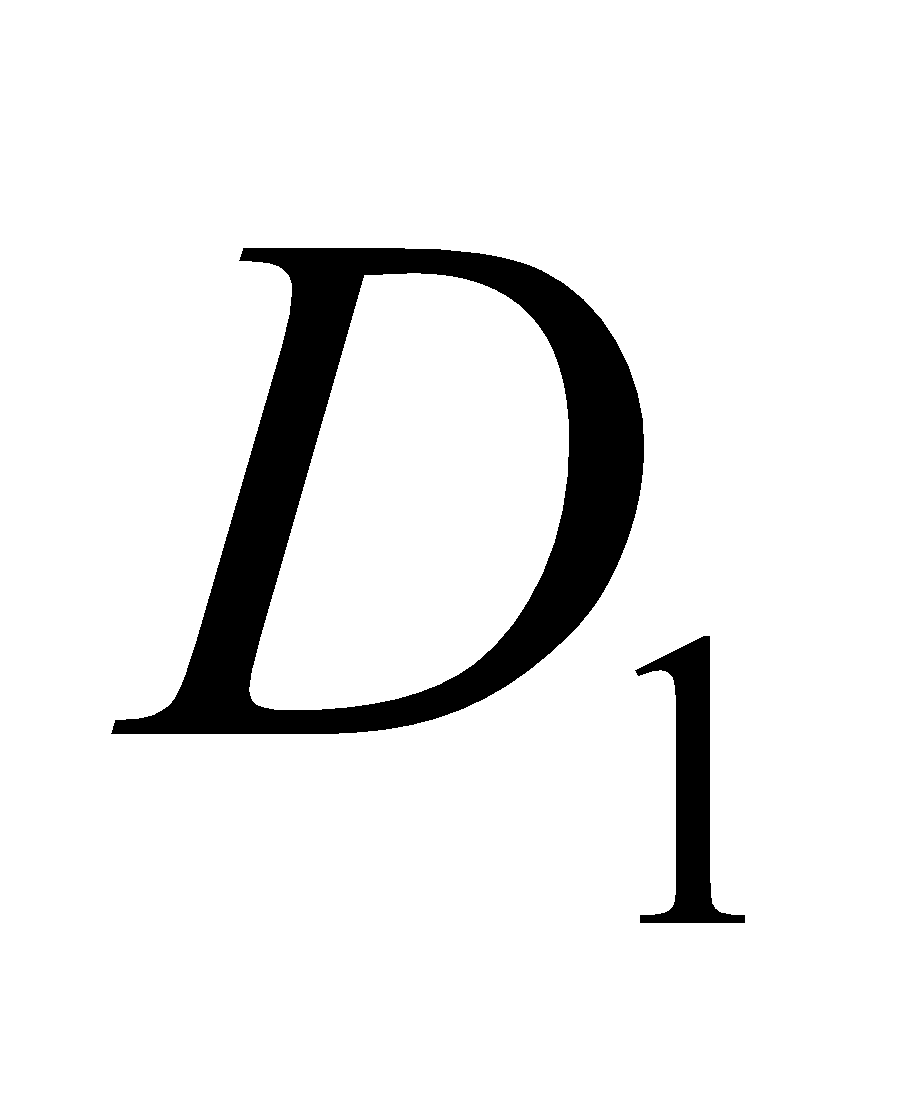 ,
, 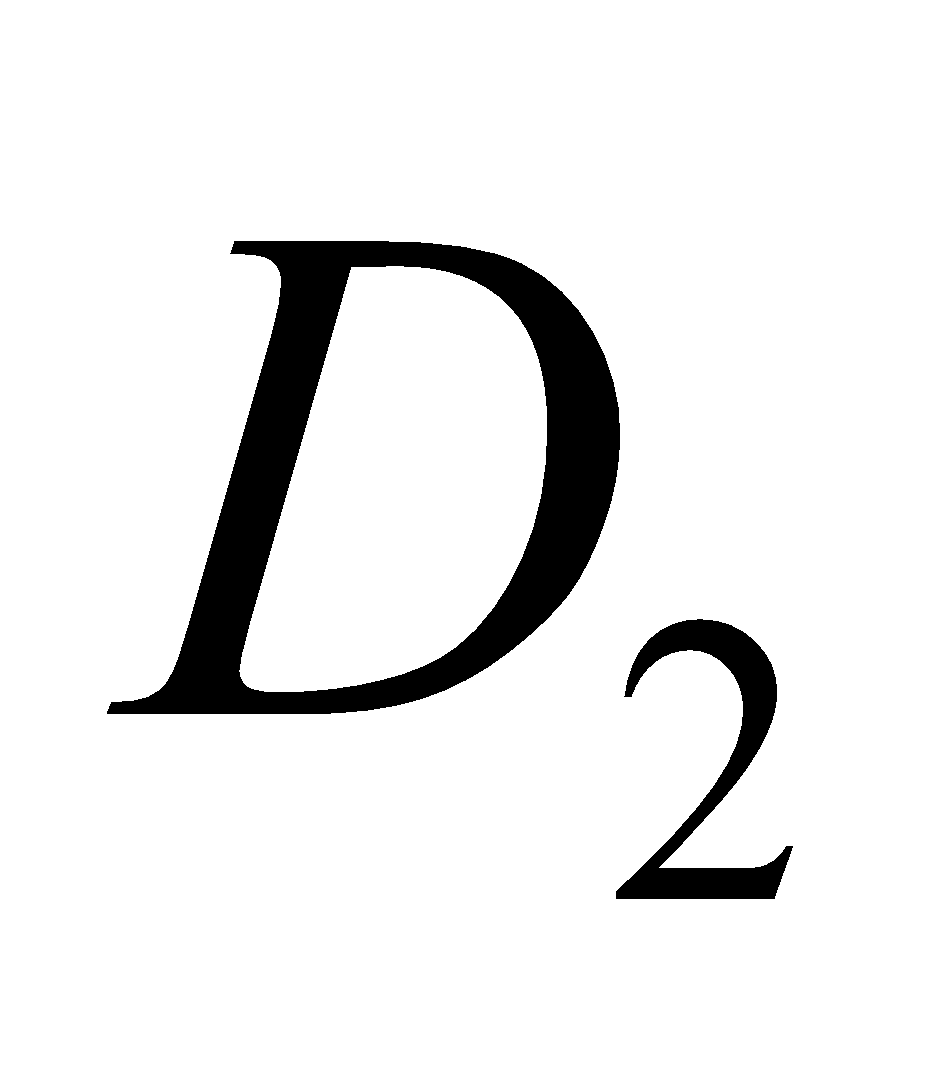 ,
, 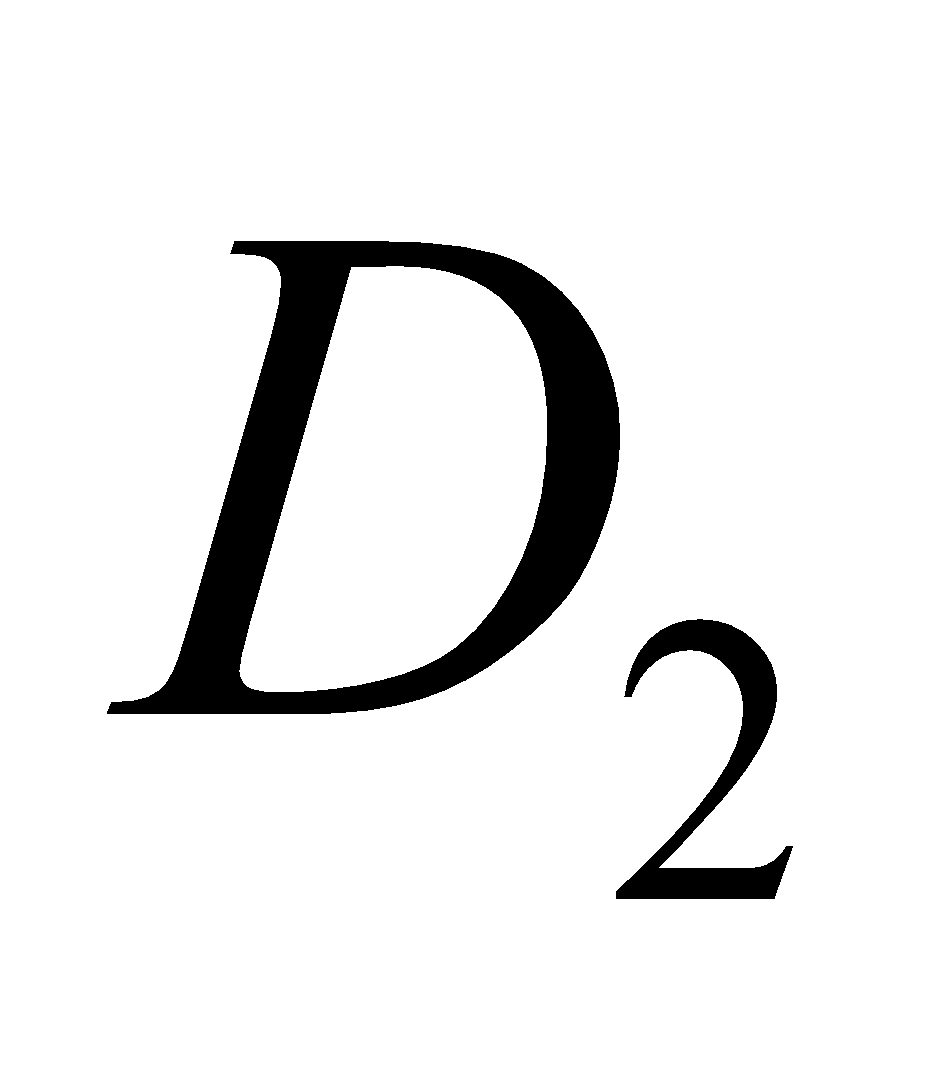 etc. The
etc. The 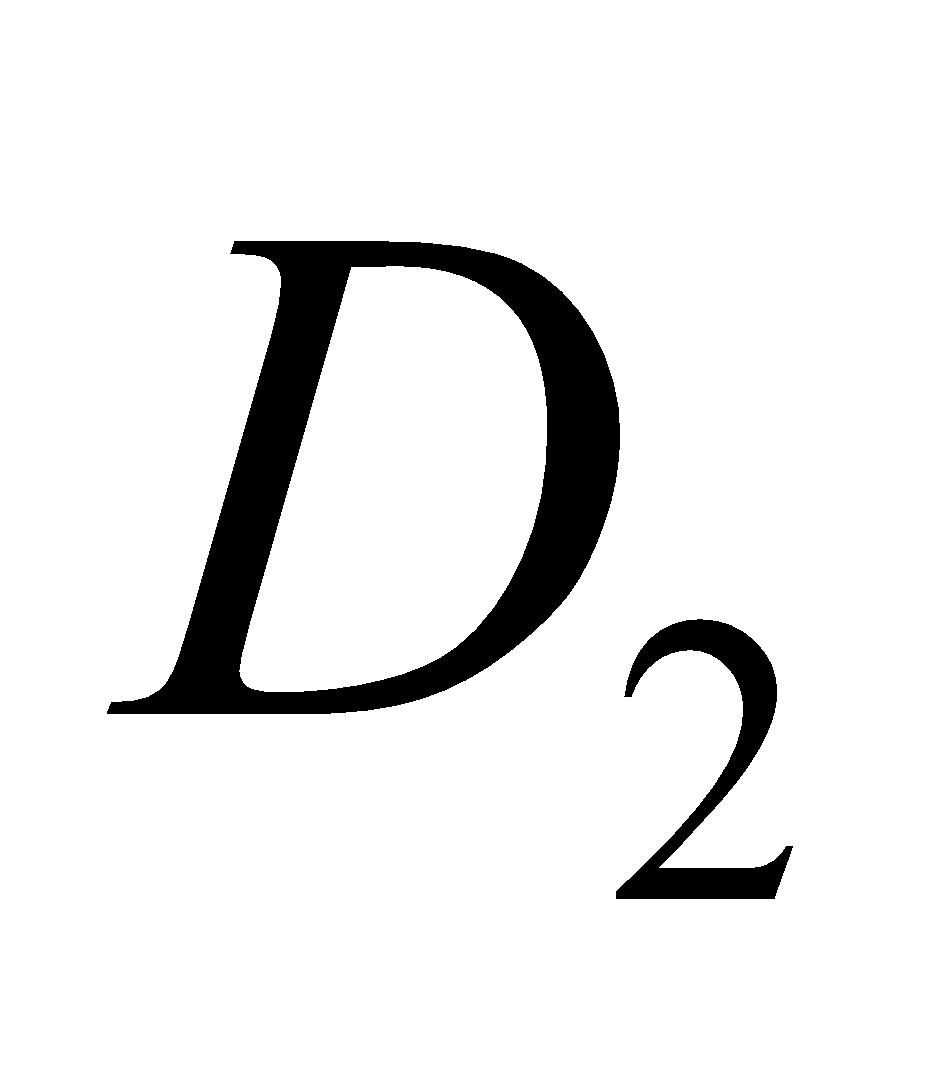 is calciferol, found in egg, milk, cod liver oil. It is formed by U. V. irradiation of ergosterol.
is calciferol, found in egg, milk, cod liver oil. It is formed by U. V. irradiation of ergosterol.
Vitamin D regulates absorption of calcium and phosphate from intestine and promotes the formation of bones. A lack of vitamin D causes rickets (bones become soft; common in children) and osteomalacia modified form of rickets in adults, during pregnancy. Vitamin 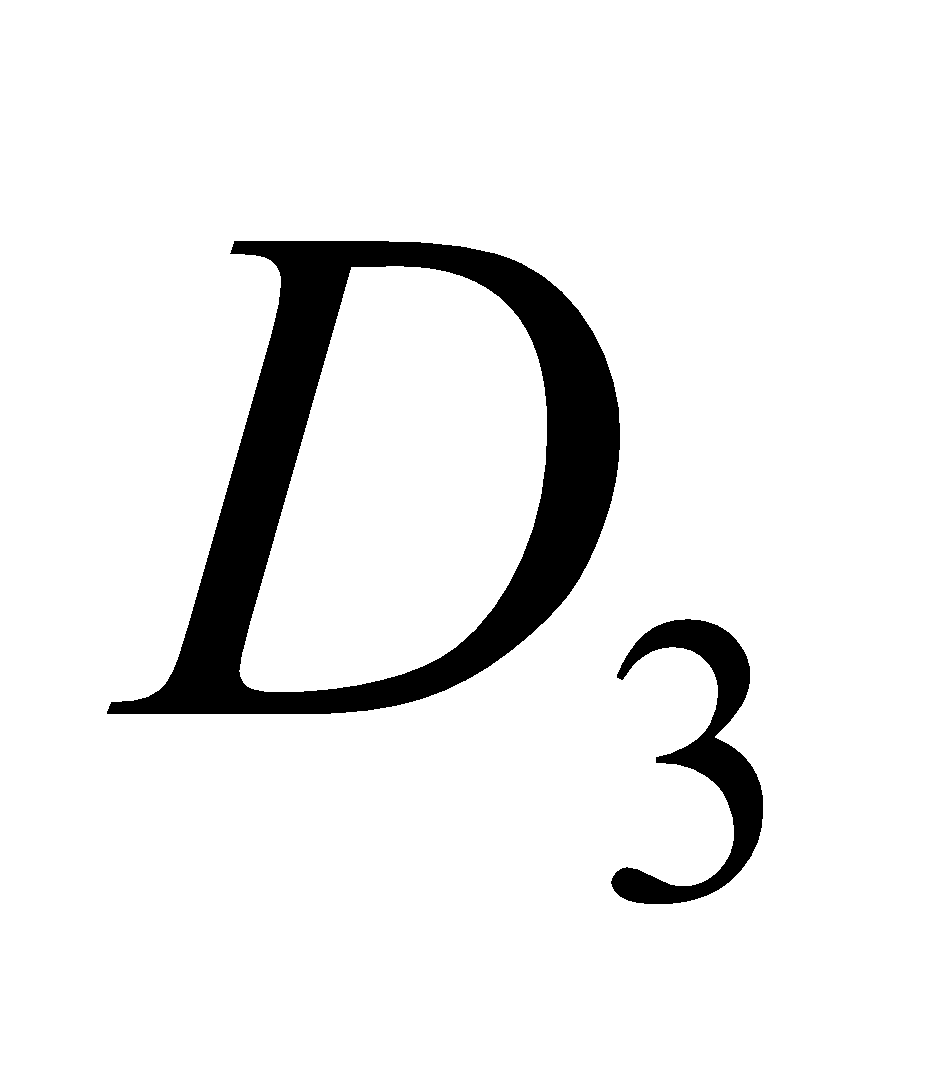 is cholecalciferol.
is cholecalciferol.
VITAMIN E
The three closely related compounds comprising vitamin E are  ,
, 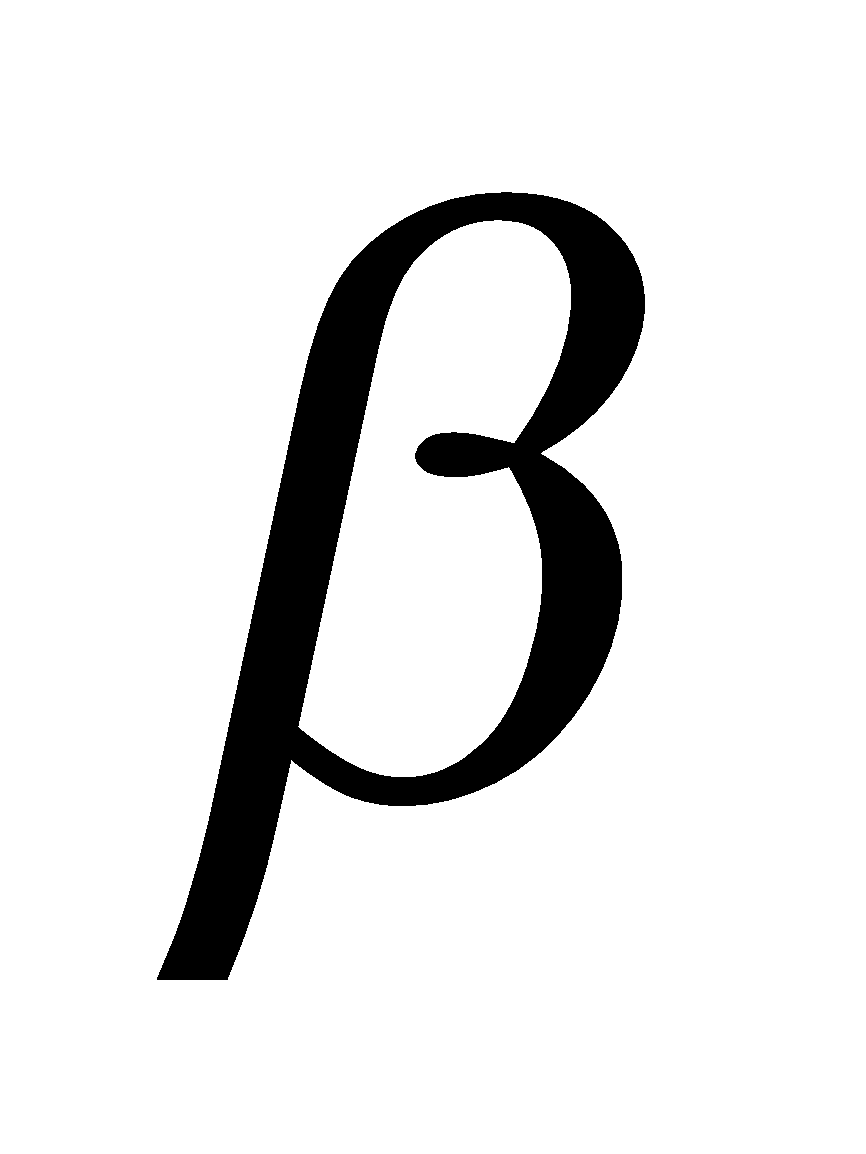 and
and 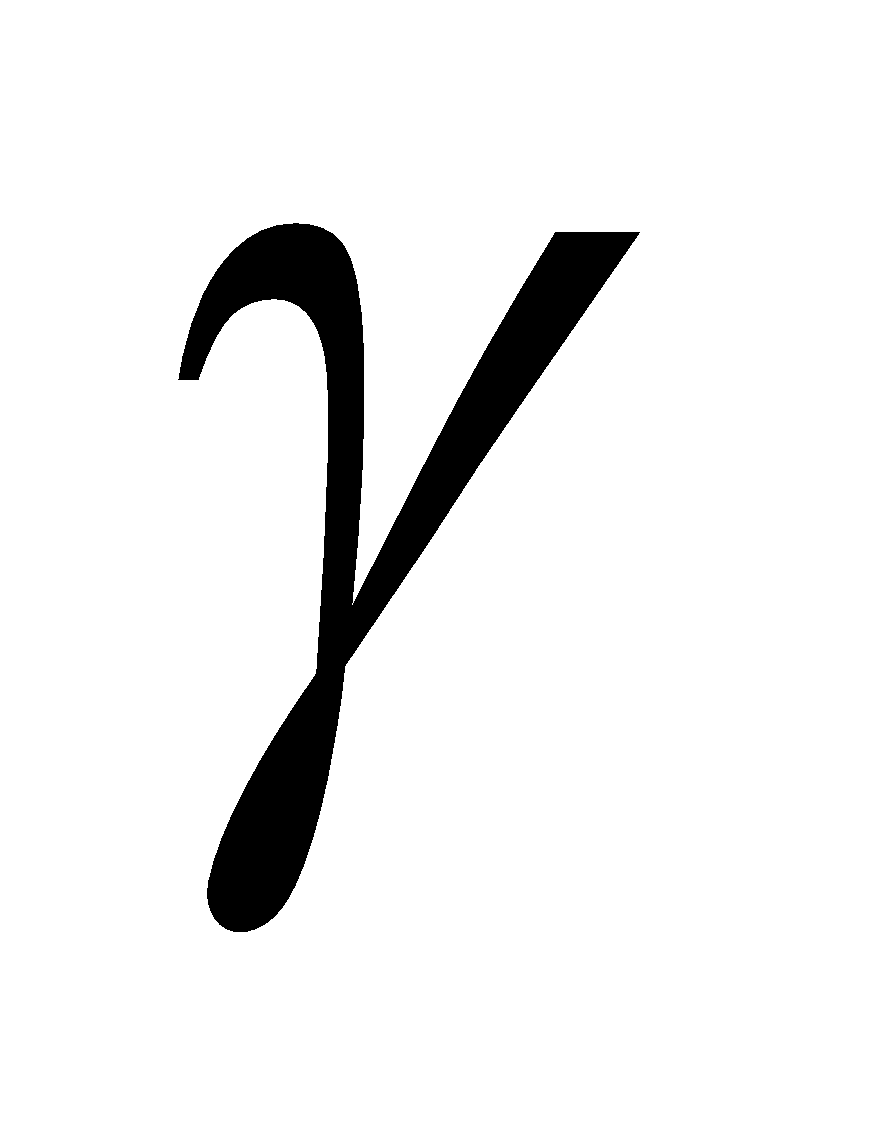 – tocopherol.
– tocopherol.
Sources of  ,
, : wheat germ, seed germ oils, cotton seed oil, green leaves.
: wheat germ, seed germ oils, cotton seed oil, green leaves.
Sources of 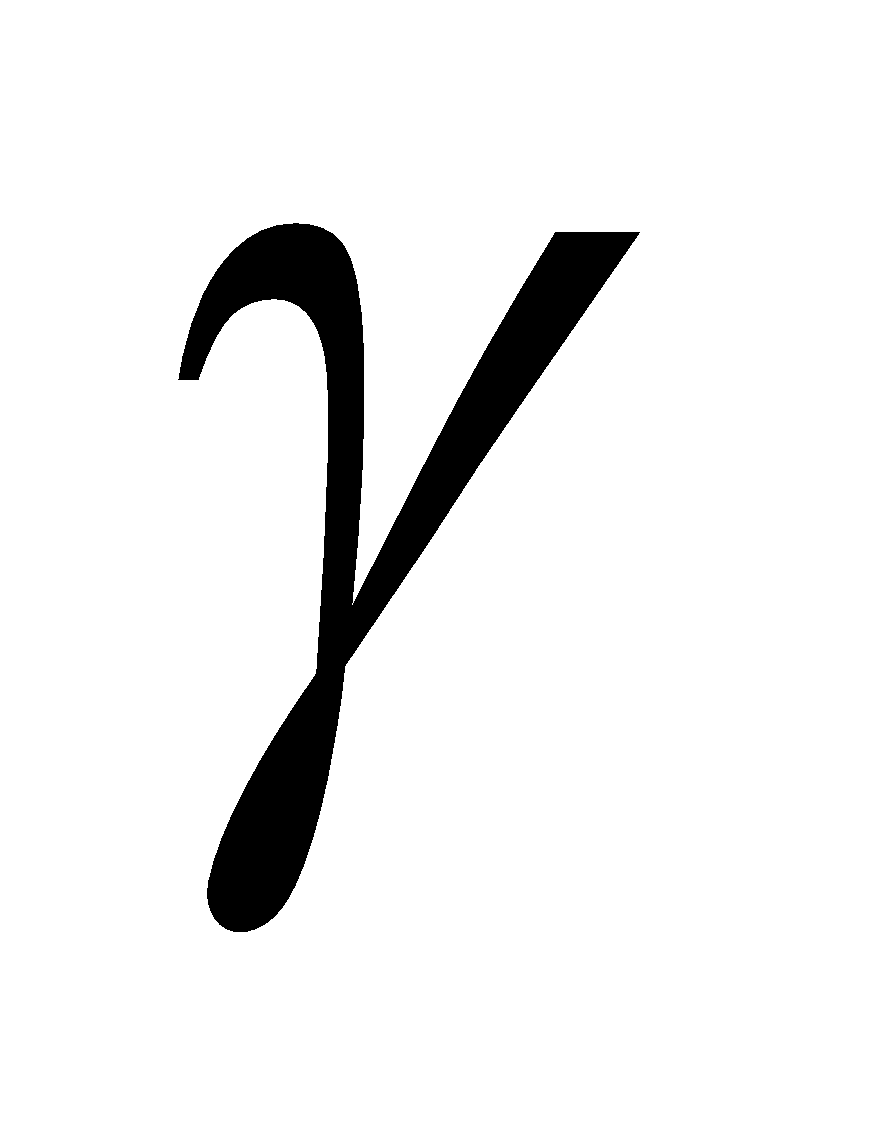 :- cotton seed oil.
:- cotton seed oil.
The most potent is  – tocopherol. Its deficiency causes loss of reproductive sterility. Hence it is also known as “anti- sterility” vitamin.
– tocopherol. Its deficiency causes loss of reproductive sterility. Hence it is also known as “anti- sterility” vitamin.
VITAMIN K
It is also known as coagulation or anti – haemorrhagic vitamin. It consists in compounds 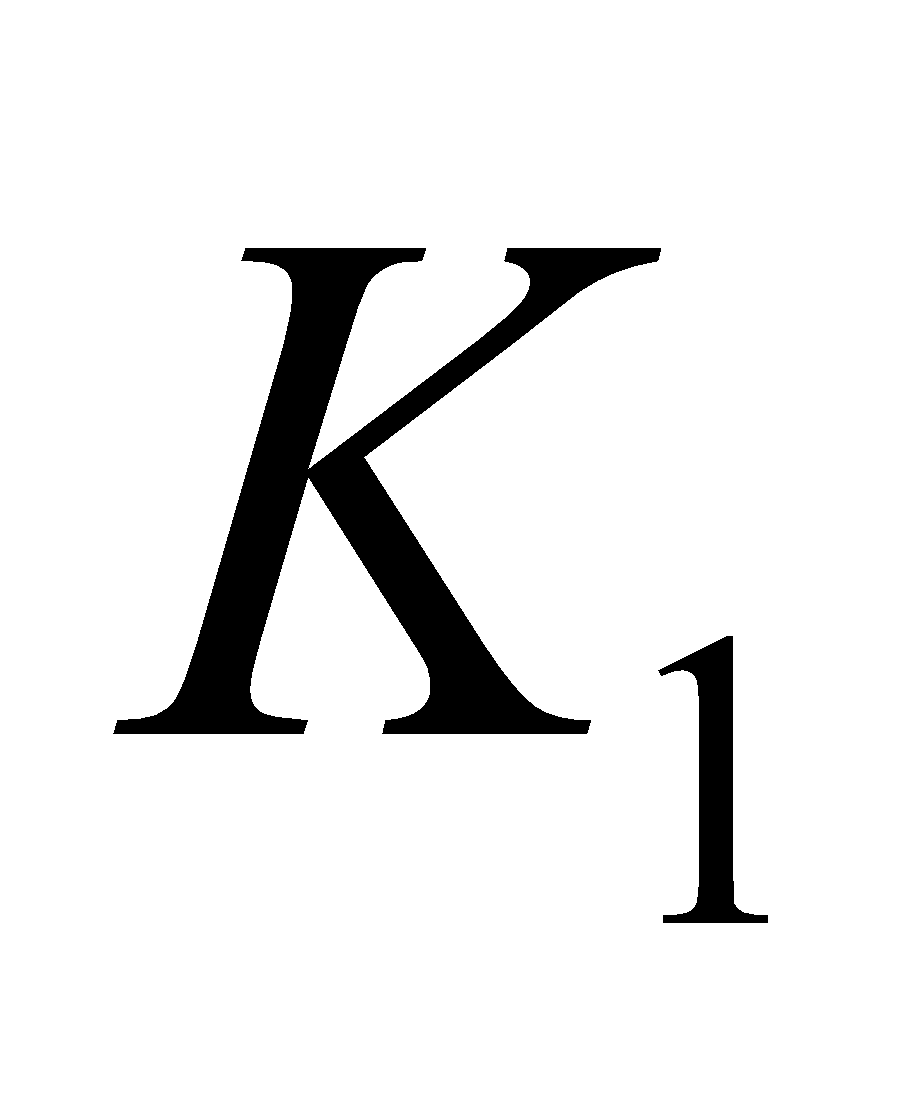 (phylloquinone) and
(phylloquinone) and 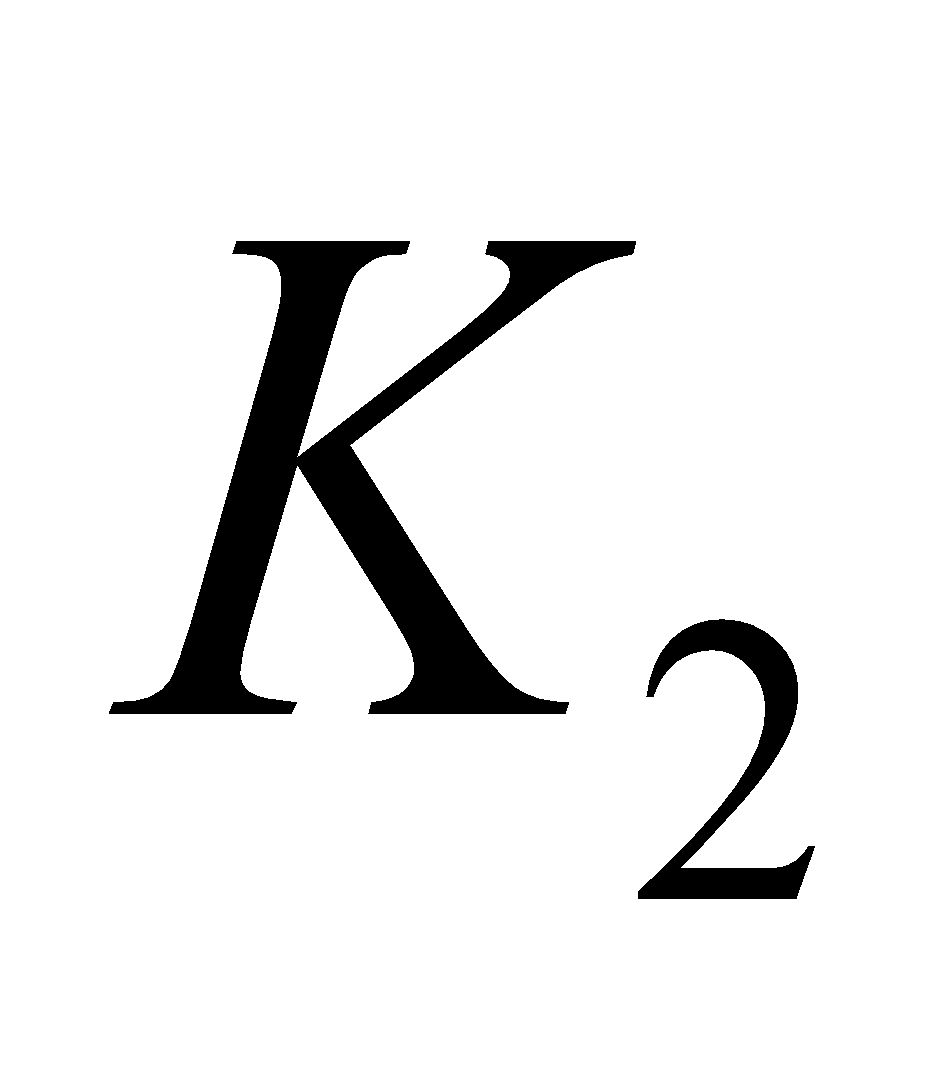 (menaquinone – 6 (
(menaquinone – 6 (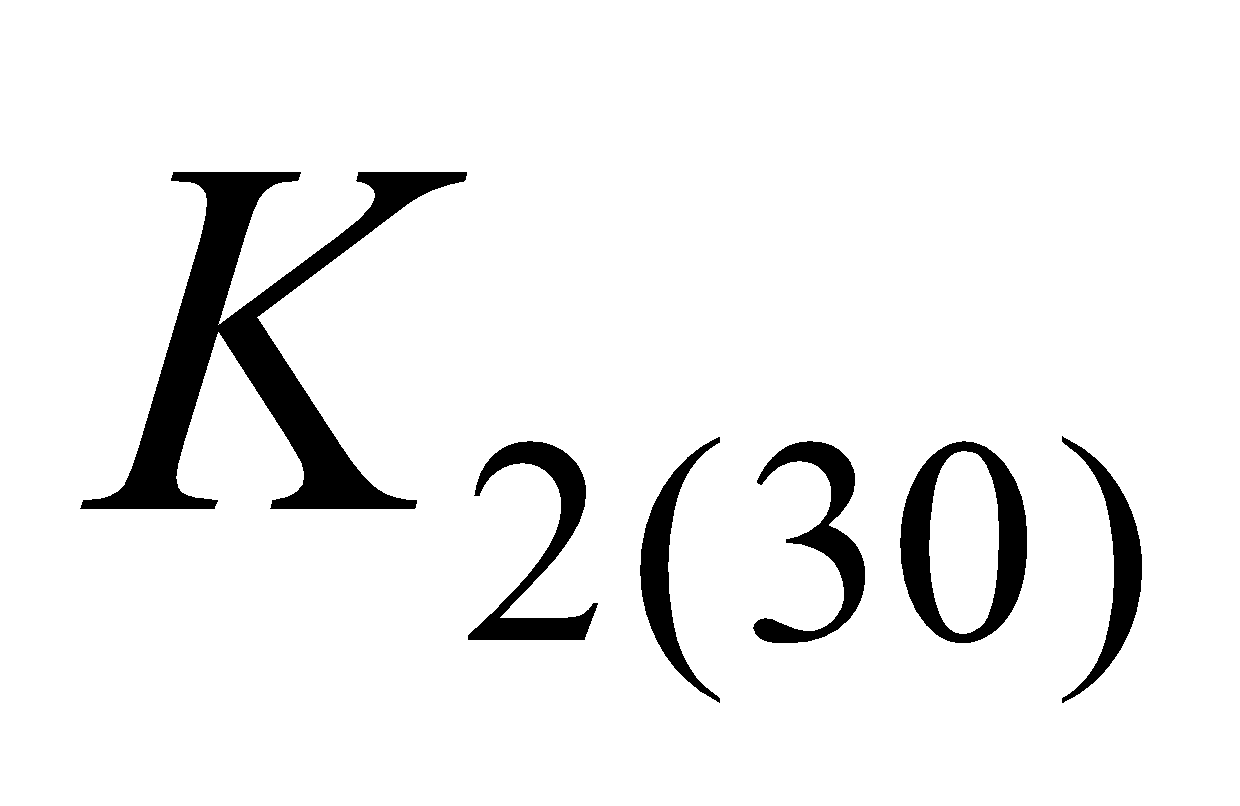 ), menaquinone – 7 (
), menaquinone – 7 (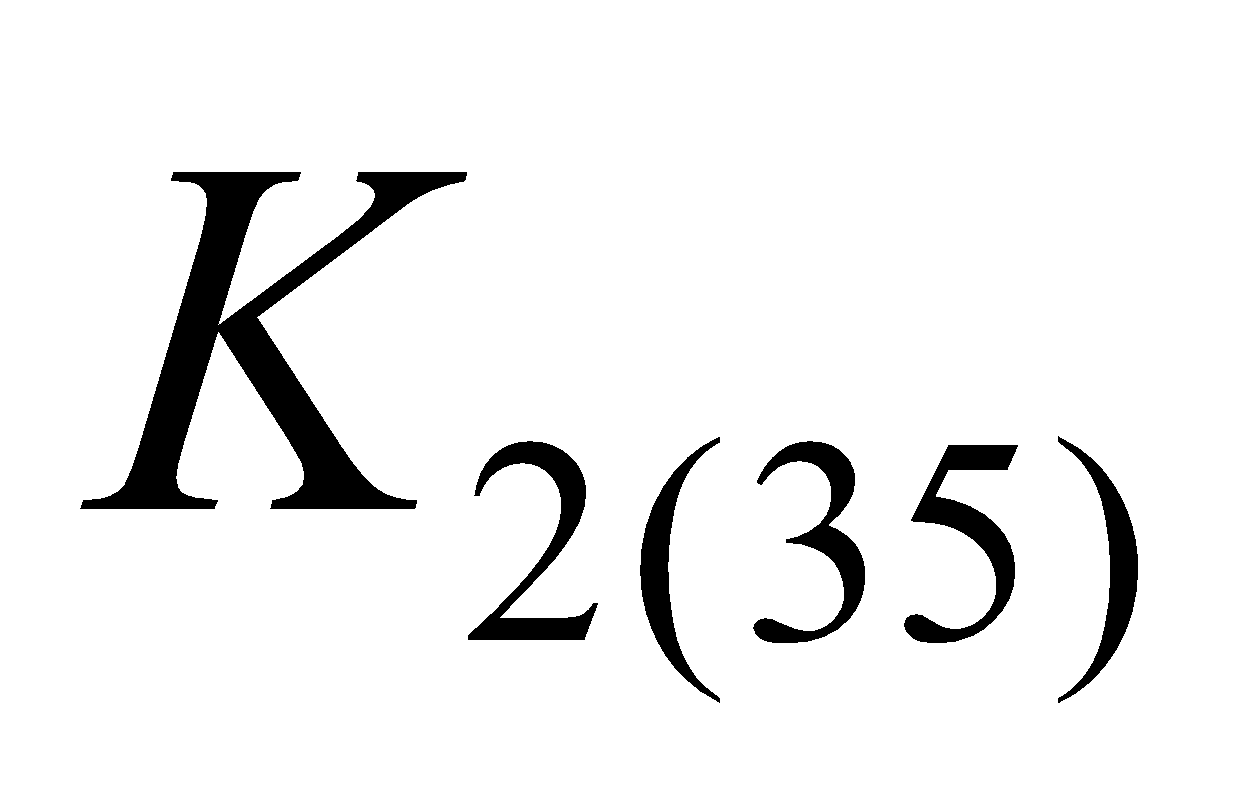 ) menaquinone – 9 (
) menaquinone – 9 (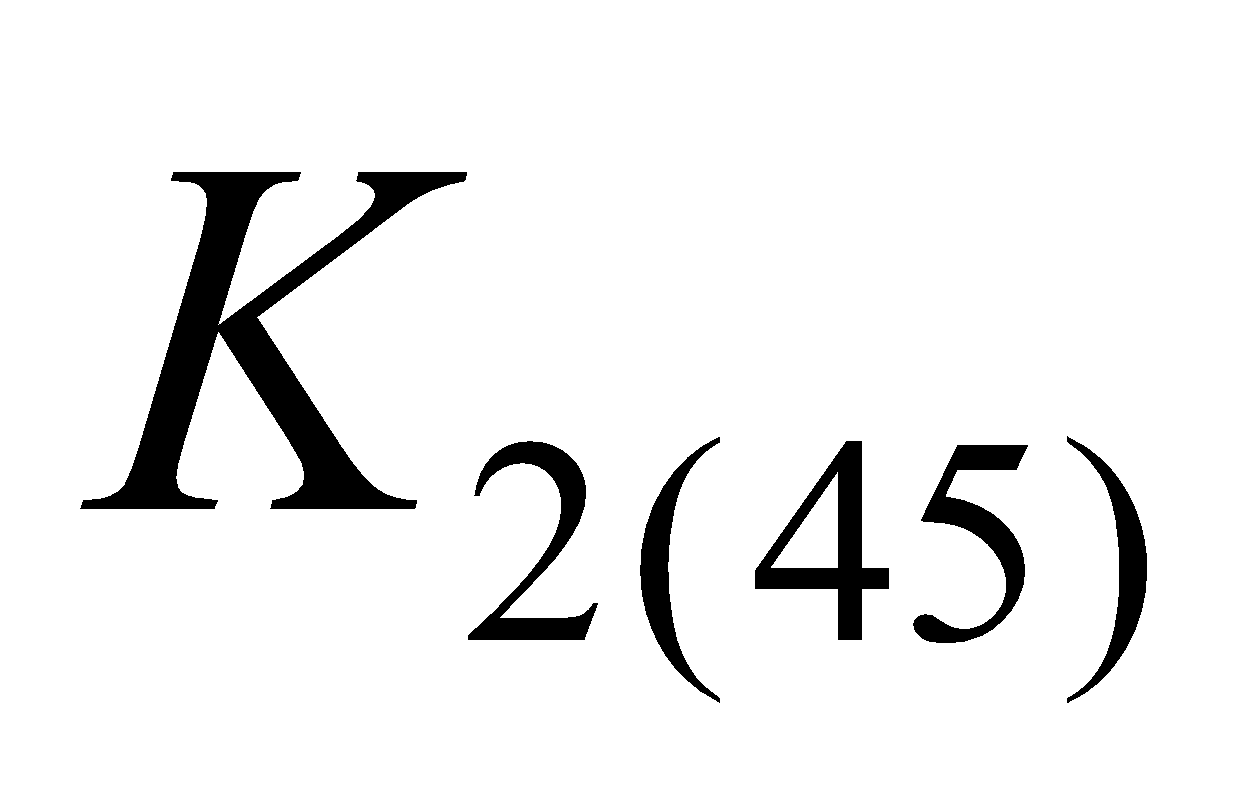 ) indicating the number of isoprene units.
) indicating the number of isoprene units.
Sources : green plants, fish, egg yolk and liver.
HORMONES
The chemical substances secreted by ductless glands such as thyroid, adrenals are called Hormones. They directly pass into the bloodstream and regulate the function of other organs of the body. They are also called chemical messengers. The glands which produce hormones are called endocrine glands.
CLASSIFICATION OF HORMONES
On the basis of their chemical constitution the hormones have been classified into three types
- Steroid hormones e.g. Cortisone, Androgens
- Peptide hormones e.g. Oxytocin, Insulin
- Amino acid hormones e.g. Epinephrine, Thyroxine
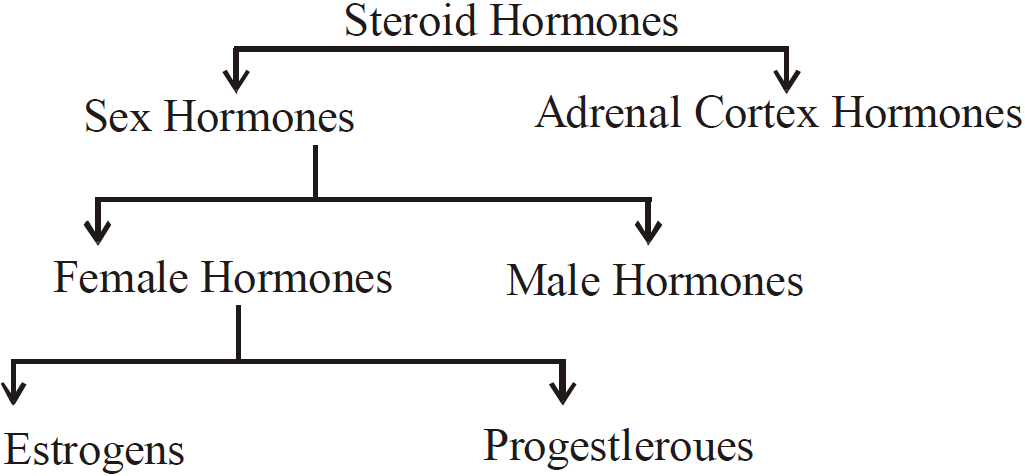
CLASSIFICATION OF STEROID OF HORMONES
The steroid hormones have been divided into the types as follows
TYPES OF HORMONES AND THEIR FUNCTIONS
STEROID HORMONES
- Estrogens and Progesterones – from ovary/uterus. Maintains pregnancy and develops sex organs of female characteristics
- Testosterone/Androgens – from Testis. Develops and maintains male sex organs.
- Cortisone and related hormones – from Adrenal cortex. Regulates metabolism of fats, proteins, carbohydrates, water mineral acids and employed in the treatment of inflammatory diseases.
AMINO ACID HORMONES
- Thyroxine – from thyroid. Stimulates oxidative metabolism and regulates general growth.
- Adrenalino/epinephrine – from Adrenal medulla. Releases glucose from glycogen and fatty acids from fats. It also increases blood pressure and pulse rate.
PEPTIDE HORMONES
- Insulin – from Pancreas. Decreases blood glucose level.
- Glucagon – from Pancreas. Increases blood glucose level
- Oxytocin – from Pituitary. Causes contraction of some smooth muscles.
- Vasopressin – from Pituitary. Inhibits excretion of water from body by way of urine.
