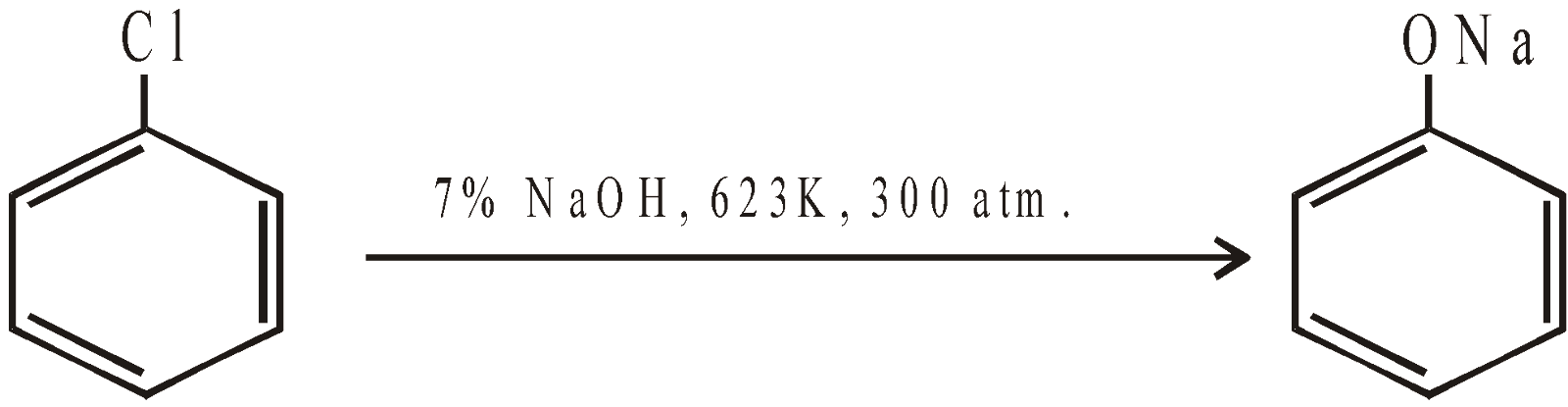| Haloalkanes and Haloarenes NEET and AIIMS Special |
| Haloalkanes and Haloarenes Refresher Course |
| Haloalkanes and Haloarenes : Master File |
| Haloalkanes and Haloarenes : Quick Revision Notes |
| Haloalkanes and Haloarenes : Concepts File |
| Haloalkanes and Haloarenes : Brain Map |
| Haloalkanes and Haloarenes Reference Book |
About this unit
Haloalkanes: Nomenclature, nature of C –X bond, physical and chemical properties, mechanism of substitution reactions. Optical rotation.Haloarenes: Nature of C-X bond, substitution reactions (directive influence of halogen for monosubstituted compounds only).Uses and environment effects of – dichloromethane, trichloromethane, tetrachloromethane, iodoform, freons, DDT.
HALOALKANES & HALOARENES
Halogen derivatives of alkanes, alkenes, alkynes and arenes are known as alkyl halides (haloalkanes), alkenyl halides (haloalkenes), alkynyl halides (haloalkynes) and aryl halides (halobenzenes), respectively. On the basis of the number of halogen atom, they are further classified as mono-, di-, tri- poly- and per-halohydrocarbons.
The word per-halohydrocarbon means all the hydrogen atoms of the compound are replaced by corresponding number of halogen atoms.
Besides the nature and number of halogen atoms, alkyl halides may be classified into primary (1°), secondary (2°) and tertiary (3°) according to the nature of the carbon atom bearing halogen.
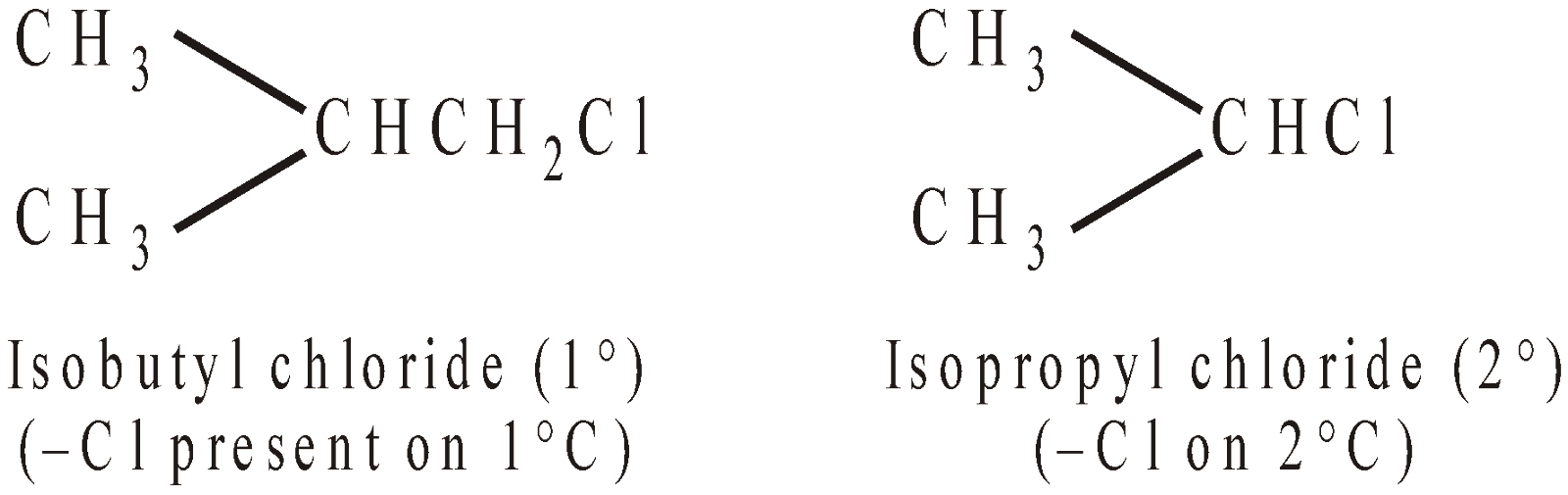
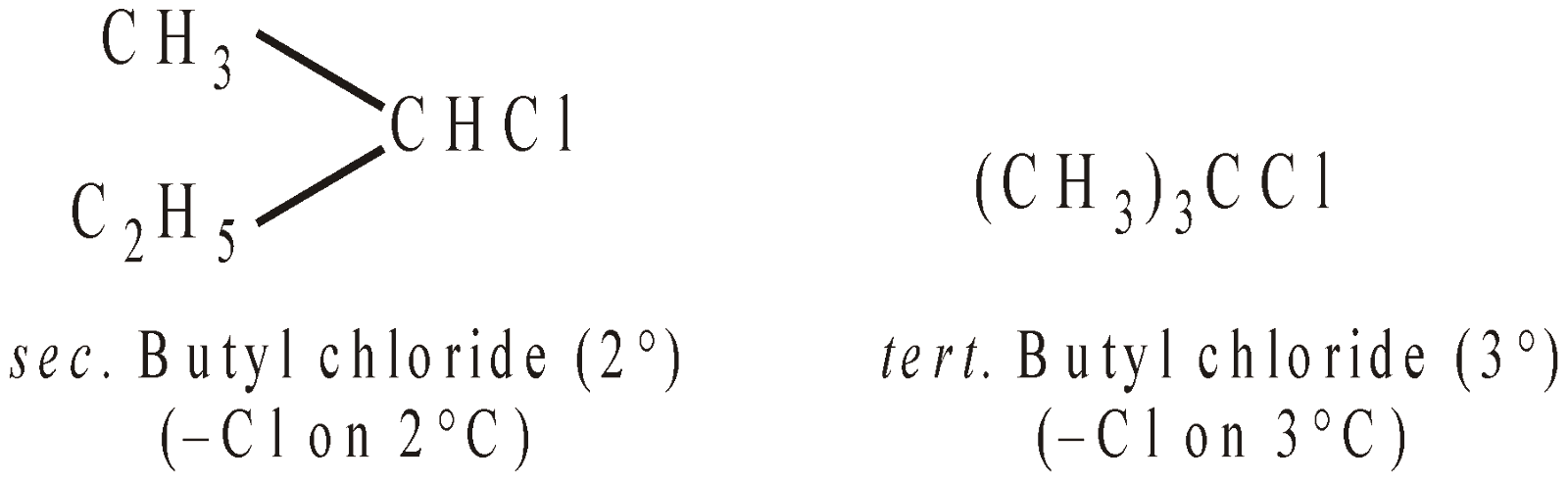
Alkyl halides may show chain, position and optical isomerism; alkenyl halides may show geometrical; while aryl halides may show position isomerism.
PREPARATION
- From alcohols with (X = I, Br, Cl) or SOCl2
- Order of reactivity among HX : HI > HBr > HCl >> HF
This is in accordance with the bond length, longer the H – X bond, weaker it will be.
- Order of reactivity among ROH : 3° > 2° > 1° > CH3OH
- tert-Alcohols react with HX by SN1 pathway, while primary alcohols react via SN2, secondary may follow either or both of the paths (SN1 and SN2 are discussed later).
- Mixture of conc. HCl and anhydrous ZnCl2 is used for differentiating three types of alcohols (3° > 2° > 1°) under the name of Lucas reagent.
- Primary and secondary alcohols can best be converted to the corresponding chlorides (by SOCl2), bromides (by PBr3) and iodides, by PI3 (P + I2).
- SOBr2 is less stable and SOI2 does not exist, PBr5 and PI5 are highly unstable hence not used.
- Alkyl halides are generally not prepared by direct halogenation of alkanes because the reaction generally gives a mixture of several compounds. However, compounds containing only one type of hydrogen atom can be converted into mono-halogeno products in good yield by taking excess of the concerned hydrocarbon; examples of such compounds are CH4, CH3CH3, (CH3)4C, C6H5CH3 etc.
- Since allylic hydrogen atoms are much more reactive than the vinylic hydrogen, former are easily replaced by halogen.
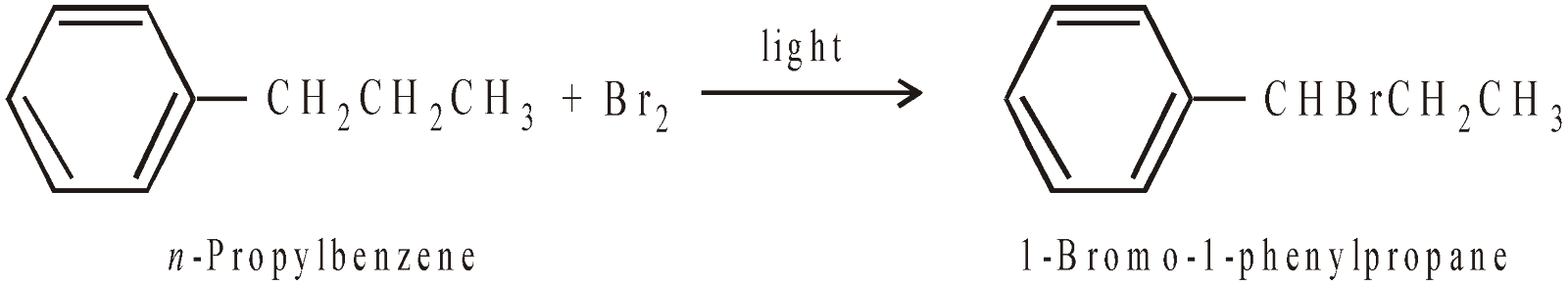
- Benzylic hydrogens (hydrogen present on C attached directly to benzene) are more reactive, hence easily replaced than 1°, 2° or 3° hydrogen.
- Halogenation of hydrocarbons in presence of light, heat and absence of halogen carrier takes place through free-radical mechanism.
- More reactivity of benzylic and allylic hydrogens is due to stability of the corresponding free radical due to resonance.
- Bromination at allylic and benzylic positions may best be carried by N-bromosuccinimide, NBS (Wohl-Ziegler Reaction).
- Addition of hydrogen halides to alkenes is an example of electrophilic addition involving carbocations as intermediates (ionic mechanism).
Markownikoff’s rule
When an unsymmetrical alkene or alkyne reacts with unsymmetrical reagent, then negative part of reagent attach with that carbon atom which contains lesser number of hydrogen atom during the addition. For example:
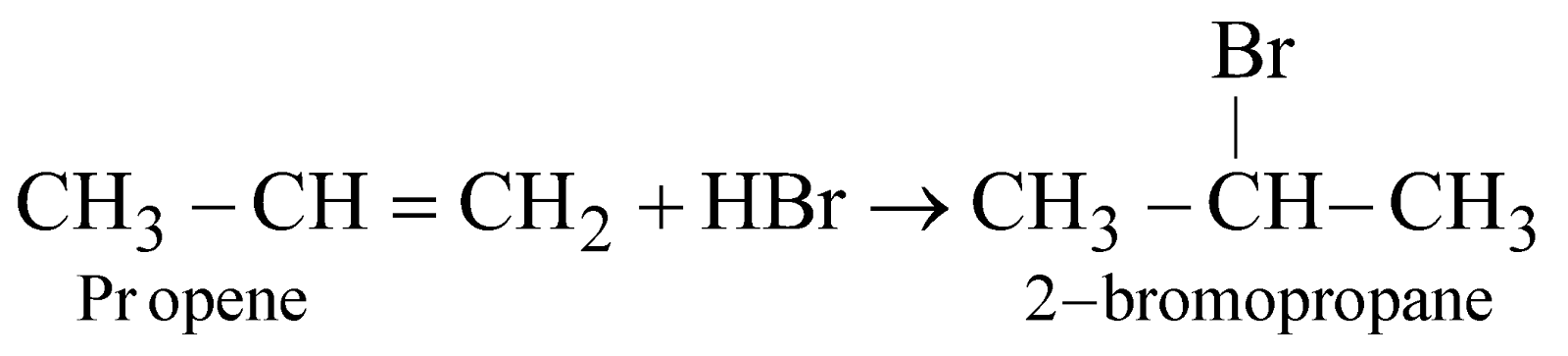
Markownikoff’s addition
Addition of HBr (not HCl, HI and HF) on alkenes in presence of peroxides takes place in
anti-Markownikoff’s way (Peroxide effect). Here addition takes place via free-radical mechanism.
anti-Markownikoff’s way (Peroxide effect). Here addition takes place via free-radical mechanism.
- Halide exchange method (anti-Markownikoff’s addition) is considered to be best for alkyl fluorides and alkyl iodides. For alkyl fluorides
Alkyl fluorides have lowest boiling point of all the alkyl halides, hence can be removed by distillation.
Swarts reaction
Alkyl chloride/bromide is heated in presence of a metallic fluoride such as AgF, Hg2F2, CoF2 or SbF3
CH3–Br + AgF  CH3F + AgBr
CH3F + AgBr
Finkelstein reaction
For alkyl iodides
For alkyl iodides
- Hunsdiecker method (suitable for bromides)
- Reaction involves free radical mechanism.
- Reaction gives an alkyl halide having one carbon atom less.
- Yield of alkyl bromides follows the order : 1° > 2° > 3°.
- Yield is very low in case of chlorides, while iodine in such cases react differently.
PHYSICAL PROPERTIES
- Alkyl halides, although polar, are insoluble in water due to inability to form hydrogen bonds with water molecules.
- Density
RI > RBr > RCl (For the same R– or Ar– group)
CH3I > C2H5I > C3H7I (For the same halogen)
Methyl iodide is densest halide because contribution of alkyl part is minimum.
- Boiling points
RI > RBr > RCl > RF (When R is same)
CH3CH2CH2Cl > CH3CH2Cl > CH3Cl (For the same halide)
CH3CH2CH2CH2Cl > CH3CH2CHClCH3 > (CH3)2 CHCH2Cl > (CH3)3 CCl
(For isomeric halides, b.p. decreases with the increase in branching)
(For isomeric halides, b.p. decreases with the increase in branching)
- Dipole moment
Except fluoride, dipole moment decreases with the decrease in electronegativity from Cl to I. Fluorides, although having highest electronegativity have lower dipole moment than chloride due to the very small size of F and hence very small C–F bond length which outweighs the effect of electronegativity (recall that m = d × e). Thus the order for dipole moment is CH3Cl > CH3F > CH3Br > CH3I. - Stability order : R – F > R – Cl > R – Br > R – I (Similar to C–X bond length)
CHEMICAL PROPERTIES
NUCLEOPHILIC SUBSTITUTION
- Alkyl halides undergo nucleophilic substitution very easily.
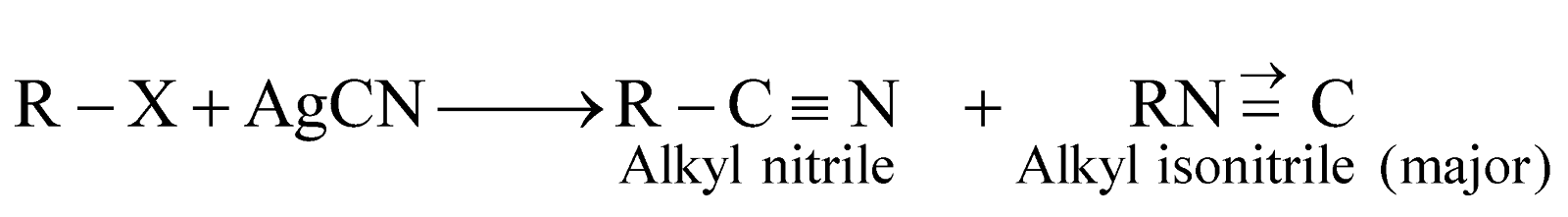
Nucleophilic substitution reactions occur either by SN1 or SN2 mechanism.
- SN1 (Unimolecular nucleophilic substitution) : Although it is a two step process, the rate of reaction depends only upon the first (slow) step which involves ionization of the alkyl halide to form carbocation. Hence rate of reaction depends only upon the concentration of the alkyl halides, r = k[RX] and is independent of the concentration of the nucleophile which adds on the carbocation in the second (fast) step.
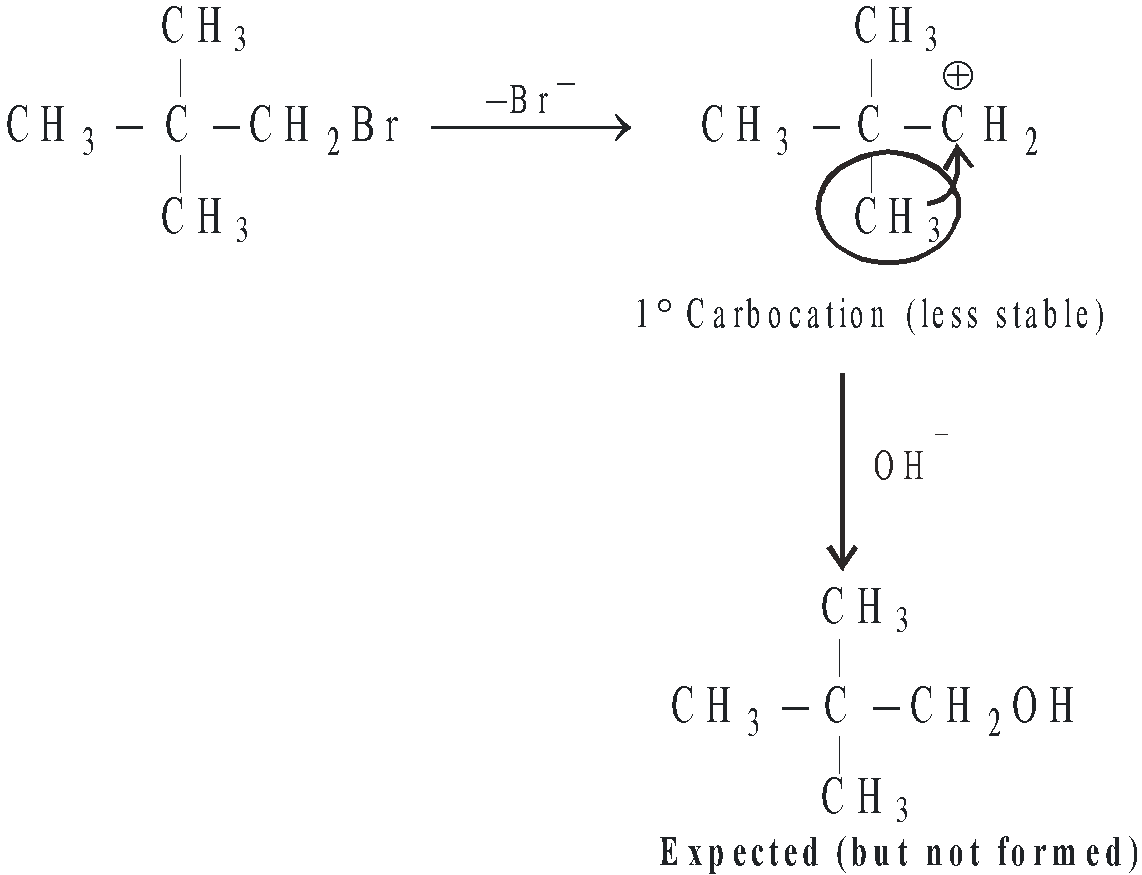
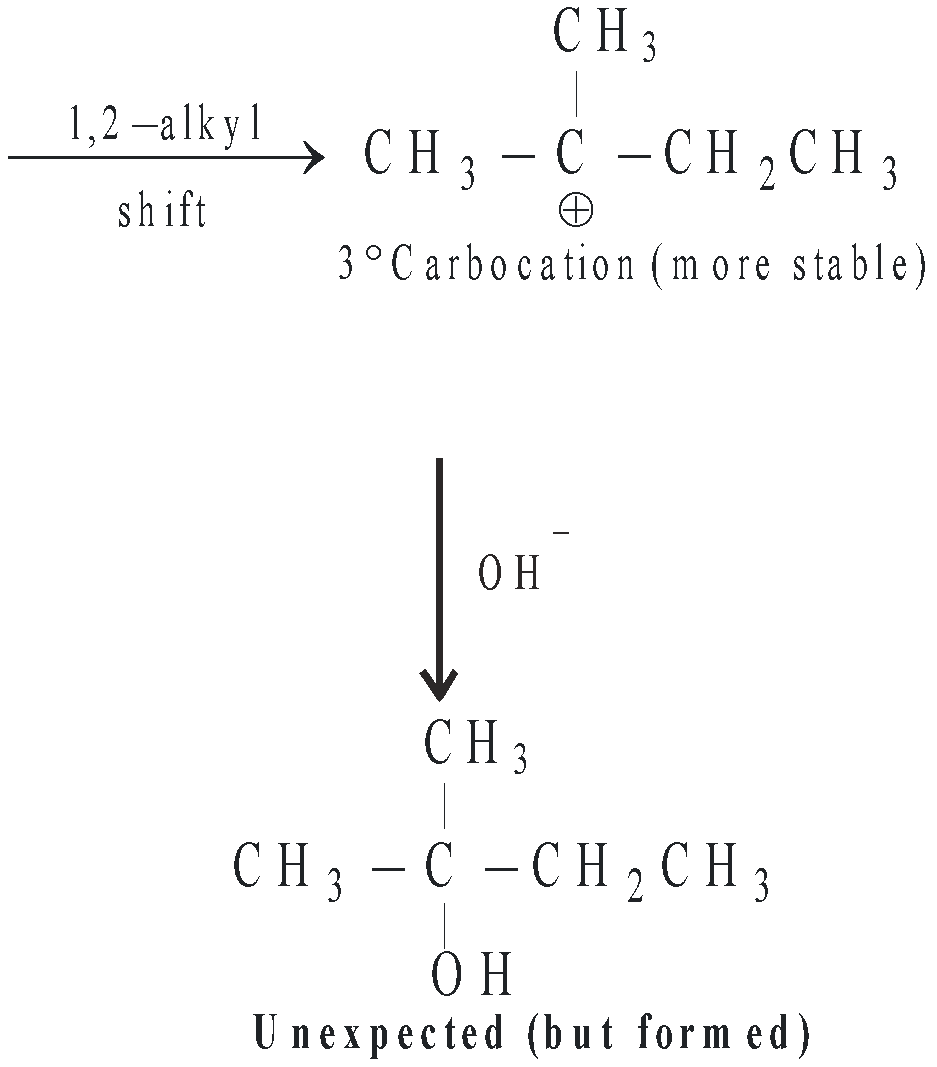
- In SN1 mechanism, carbocations are formed as intermediate, hence more the stability of the intermediate carbocation, greater are chances for their formation and hence more reactive will be the parent alkyl halide for SN1 reaction. Hence the order of reactivity of alkyl halides toward SN1 reaction follows the order : 3° > 2° > 1°
- When the intermediate carbocation is capable of undergoing rearrangement, lesser stable carbocation (1° < 2° < 3°) rearranges to the more stable carbocation and hence under such conditions unexpected product is formed.
- If the alkyl halide is optically active, the product formed in reaction is always a racemic mixture. This is due to the formation of carbocations as intermediates which, being planar (sp2 hybridised) can be equally attacked by the nucleophile on either side of the face forming two enantiomers.
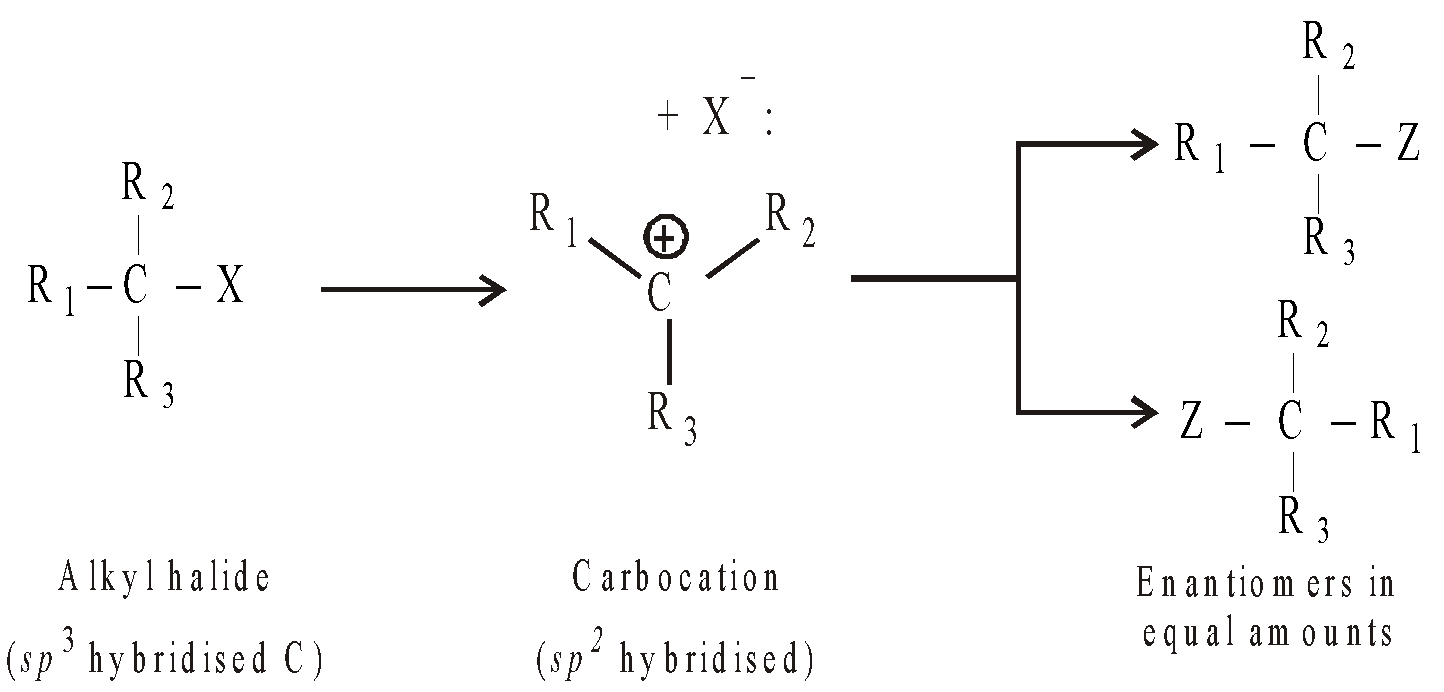
However, remember that the departing halide shields to some extent, the frontal attack of the carbocation, racemization is only partial and the inverted configuration predominates.
- SN2 (Bimolecular Nucleophilic Substitution) : The rate of SN2 reactions depends on the concentration of alkyl halide as well as nucleophile, i.e. r = k[RX][Nu]. This implies that both the reactants are involved in the rate-determining step, i.e. the reaction occurs in one step only or it is a concerted reaction. Concerted reactions occur through a transition state (an imaginary state in which both the reactant molecules are partially linked to each other).
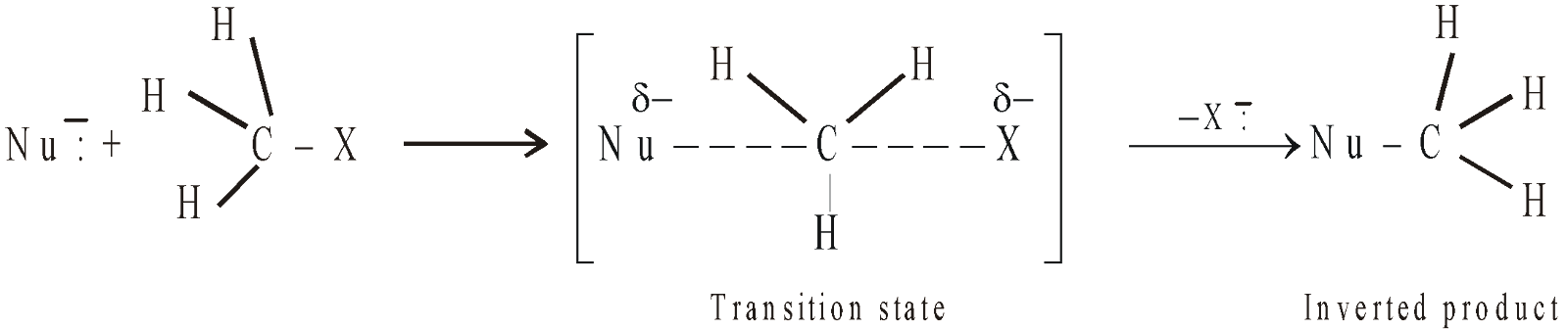
Remember that the nucleophile attacks from the back side of the halide ion, bulkier the alkyl group present on the carbon bearing halogen lesser will be its tendency to undergo SN2 reaction. Thus the reactivity of alkyl halides towards SN2 mechanism is
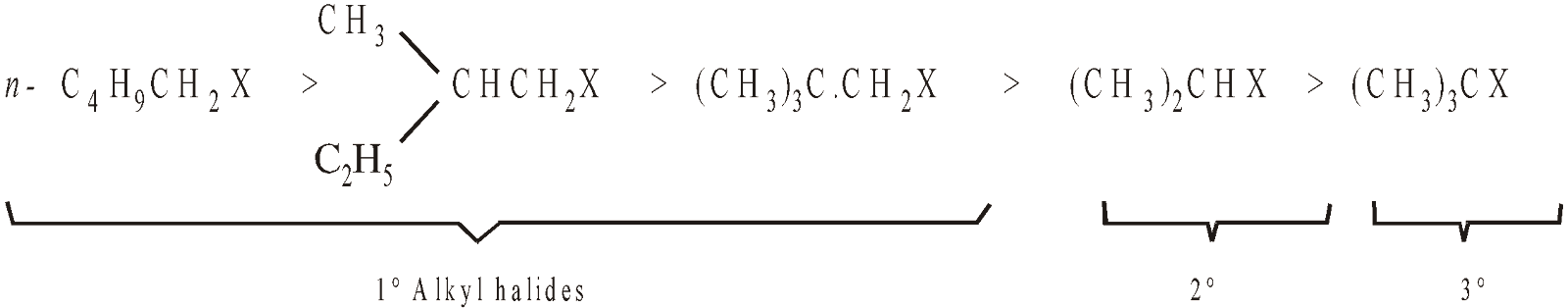
Since the nucleophile attacks from the back side and the halide ion leaves from the front side, the product obtained will have an inverted configuration [Walden inversion]. This implies that if the alkyl halide is optically active, the product will also be optically active, although the sign of rotation may be same or different. All primary alkyl halides undergo substitution via SN2 pathway.
Remember
- Polar solvents favour SN1 reactions while non-polar solvents favour SN2 reactions.
- Low concentration of nucleophile favours SN1 reactions, while high concentration favours SN2.
- Rate of reaction in SN1 mechanism is independent of the nature of the attacking nucleophile because it is not involved in rate-determining step; while rate of SN2 reactions depends upon the strength of the nucleophile. Strength of some common nucleophiles is
- Primary alkyl halides undergo SN2 reactions, 3° halides SN1, while 2° halides may undergo SN2 and/or SN1 mechanism.
ELIMINATION REACTIONS
- Alkyl halides lose a molecule of hydrogen halide (dehydrohalogenation) when heated with alcoholic potash. Dehydrohalogenation is a b-elimination reaction in which halogen and hydrogen atoms are lost from the two adjacent carbon atoms.
- Dehydrohalogenation is governed by Saytzeff rule according to which more highly substituted alkene is the major product, i.e. hydrogen atom is lost from the carbon atom carrying minimum number of hydrogen atoms (poor becomes poorer).
- Ease of dehydrohalogenation among halides is : 3° > 2° > 1°
- Elimination reactions dominate over substitution when strong Bronsted base
e.g.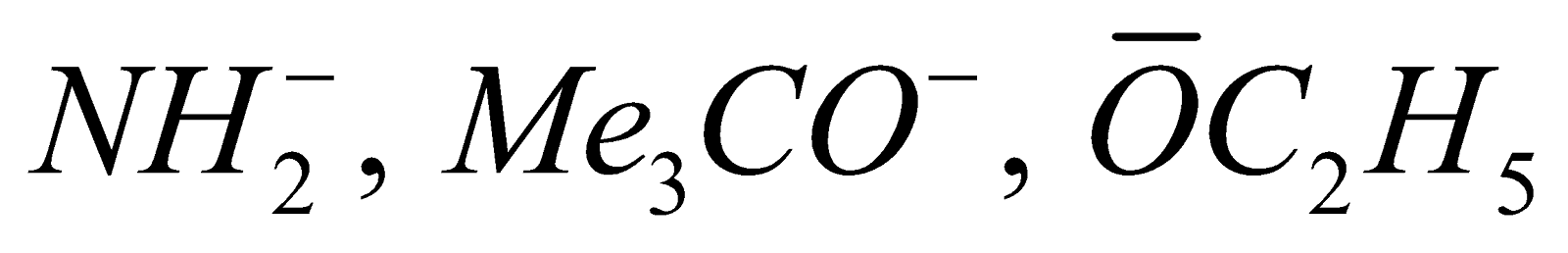 etc.) is used and alkyl halide is 3° or 2°.
etc.) is used and alkyl halide is 3° or 2°.
Remember
- 1° Halides undergo SN2 reactions except when a hindered strong base like is used.
- 2° Halides undergo SN2 reactions with weak base like I–, CN–, RCOO– etc., and elimination reaction with strong base like RO–.
- 3° Halides undergo SN1 reaction in absence of a strong base and only solvent acts as a base/nucleophile (solvolysis), however in presence of strong base (–OR) elimination reaction predominates.
REACTION WITH METALS
Tetraethyl lead, commonly known as TEL, is used as an anti-knocking agent in petrol.
- Solvent used must be perfectly anhydrous because even a trace of water or alcohol reacts with metals to form insoluble hydroxide or alkoxide that coat the surface of the metal. Moreover, water or alcohol may react with the product (organometallic compound) to form hydrocarbon.
- Alkyl lithiums react with copper halides to form higher alkanes (Corey-House synthesis)
- Among alkyl halides, order of reactivity is I > Br > Cl > F
- The reactivity of a metal toward an alkyl halide depends upon its reduction potential; more easily a metal is reduced, more reactive it is, e.g. Mg > Zn
REDUCTION
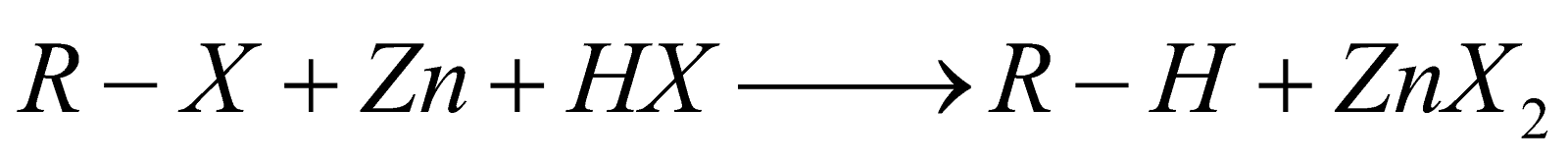
- In this reaction, zinc atoms transfer electrons to the carbon atom of the alkyl halide. Zinc is a good reducing agent because it has two electrons in an orbital far from the nucleus, which are readily donated to an electron acceptor.
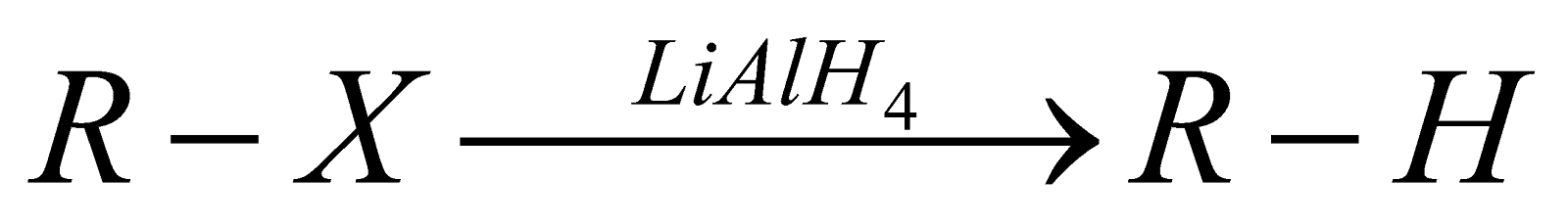
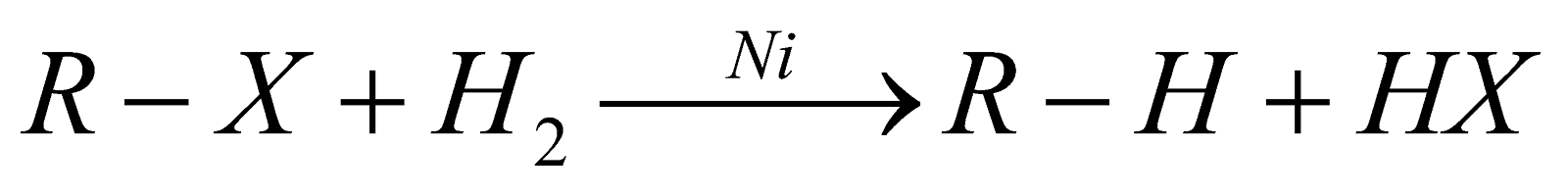
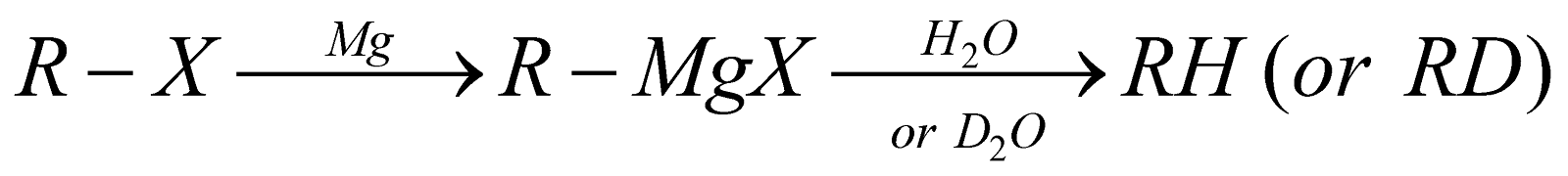
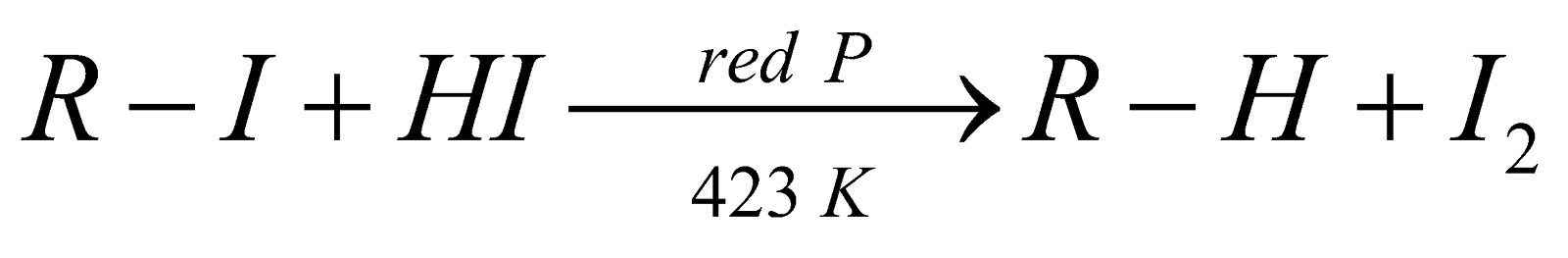
HALOGENATION
ISOMERISATION
ARYL SUBSTITUTED ALKYL HALIDES
- Aryl halides are the compounds having halogen atom directly attached to the aromatic ring. They are represented by the general formula Ar–X where Ar may be phenyl, substituted phenyl or other aromatic system, e.g. naphthyl.
All compounds containing aromatic ring and a halogen atom should not be considered as aryl halides, e.g. benzyl chloride, C6H5CH2Cl because chlorine is not directly attached to the ring. Such compounds resemble alkyl halides in structure as well as in properties, hence grouped as aryl substituted alkyl halides. - The carbon-halogen bonds of aryl halides are both shorter and stronger (due to possibility of resonance) than the carbon-halogen bonds of R–X and in this respect as well as in their chemical behaviour, they resemble vinyl halides (CH2 = CHX) more than alkyl halides.
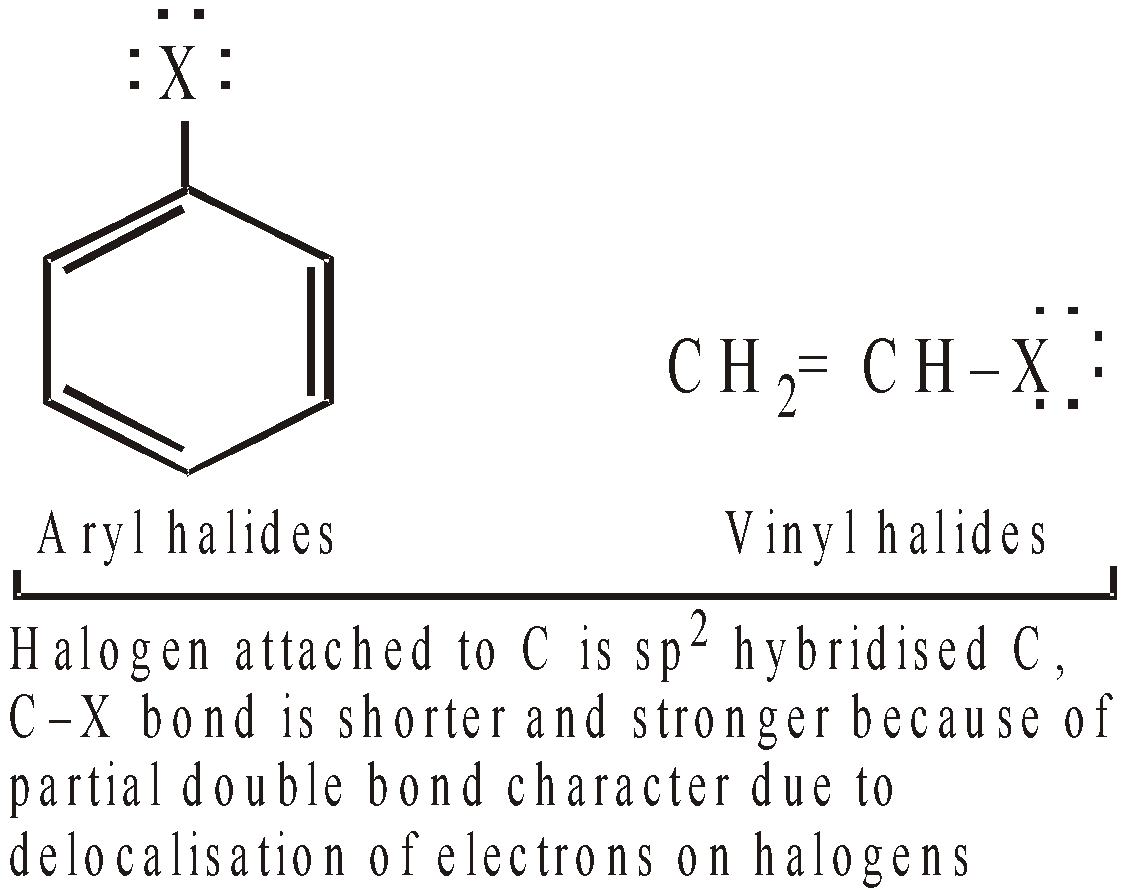
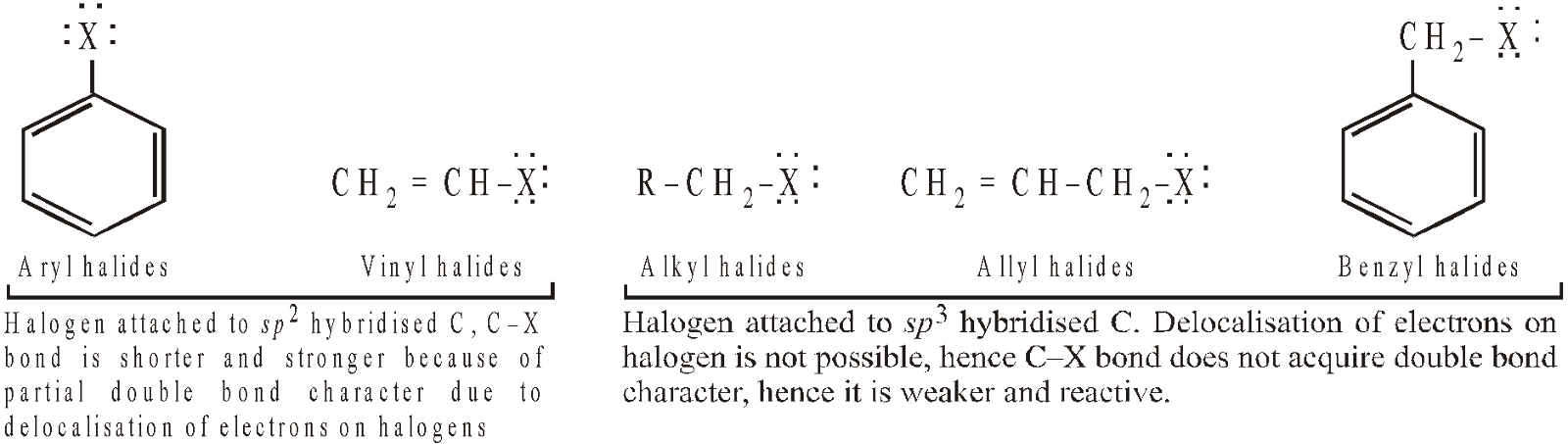
- The strength of the C–X bonds causes aryl halides to react very slowly in reactions in which cleavage of C–X bond is rate determining, i.e. nucleophilic substitution.
PREPARATION
- By direct halogenation of aromatic hydrocarbons
Nuclear halogenation (suitable for aryl chlorides and bromides)
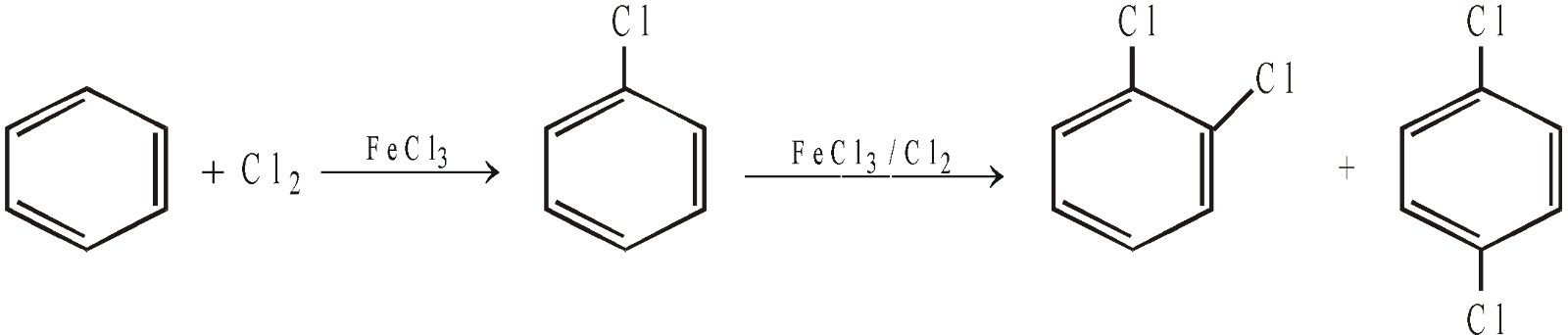
Fluorination is difficult to control, while iodination is too slow to be useful. Moreover, iodination being reversible (because of reducing character of HI), the reaction requires the use of an oxidising agent like HNO3, HIO3, HgO etc.
Introduction of second –Cl is difficult as compared to first, because of electron withdrawing character of chlorine (deactivating nature of halogens).
Nuclear halogenation is an electrophilic substitution reaction.
Side chain halogenation :
Halogenation of higher arenes, i.e. other than benzene, in presence of light or heat and in the absence of a halogen carrier introduces halogen in the side chain.
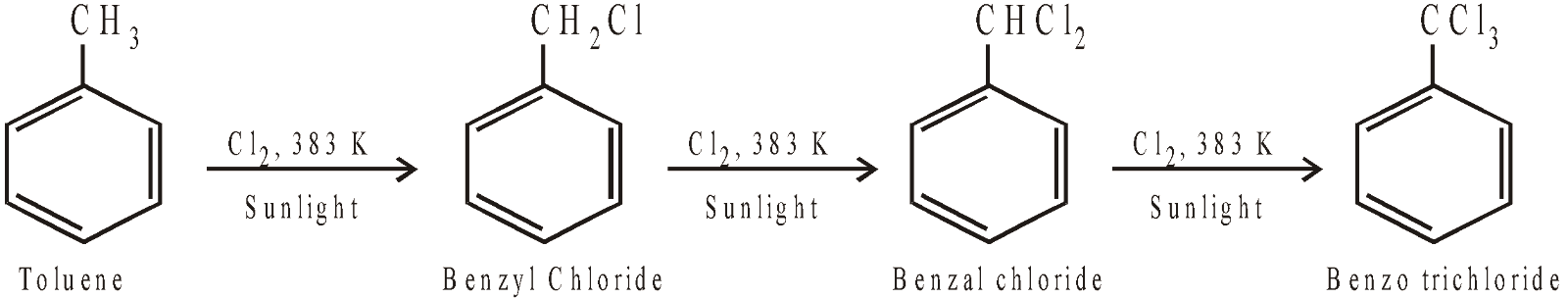
Remember halogenation of toluene at low temperature, in the absence of light and in presence of catalyst, gives nuclear substituted products.
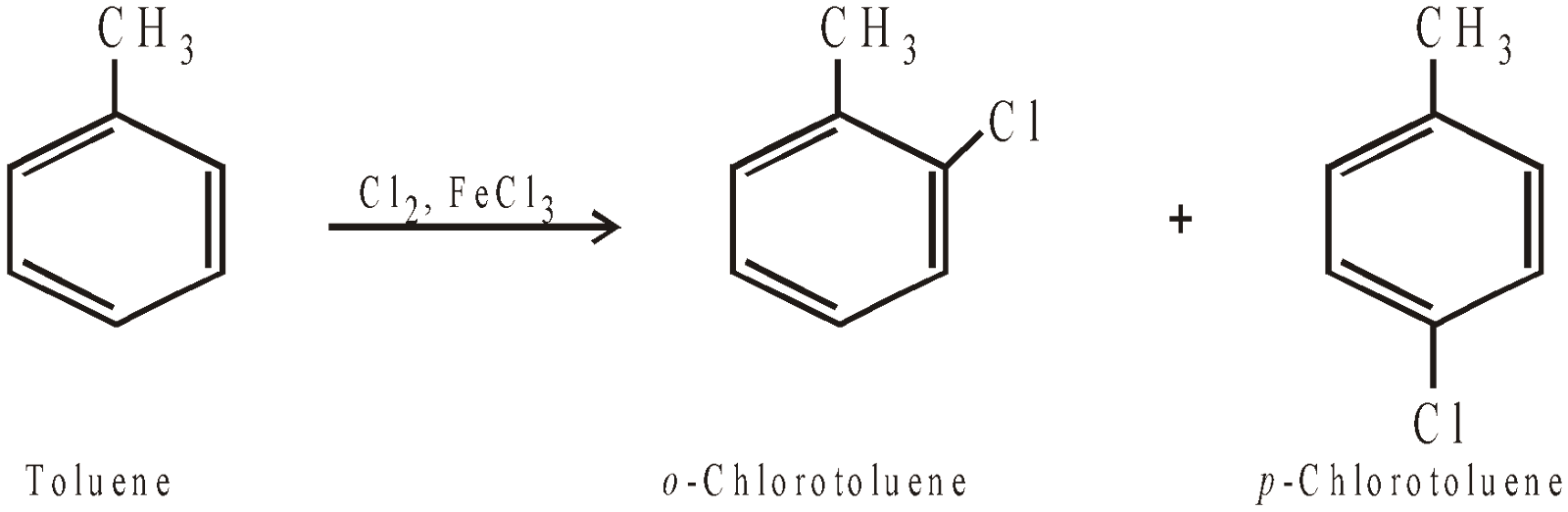
Generally, p-isomer predominates due to steric hindrance in the o-position.
Side chain halogenation occurs by free radical mechanism.
In case, the side chain is larger than methyl group, side chain halogenation mainly occurs at the benzylic carbon (carbon directly attached to benzene nucleus). This is due to stability of benzylic free radical due to resonance.
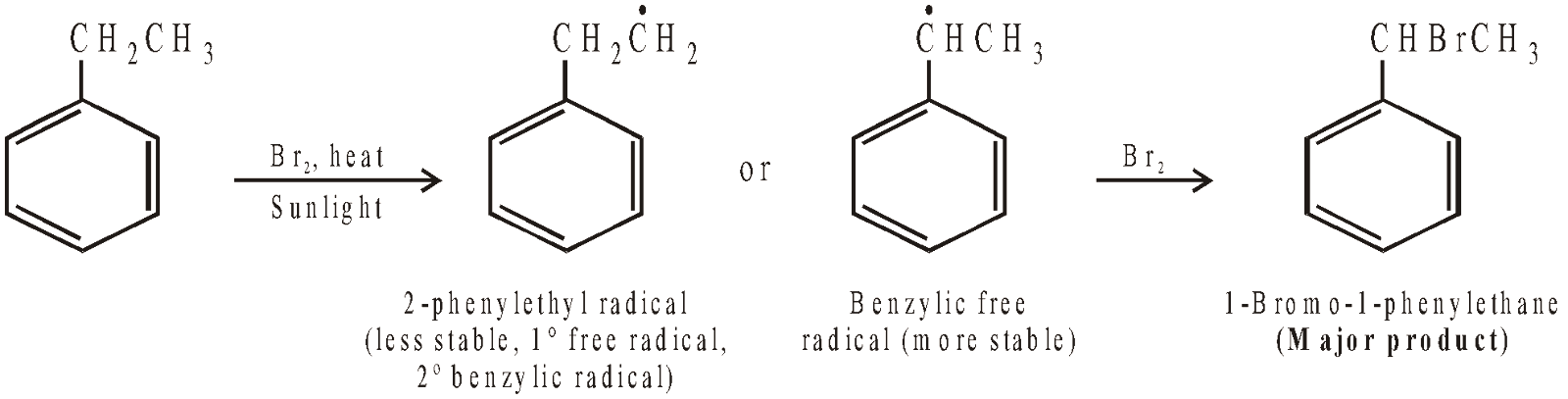
Side chain halogenation can also be carried out with SO2Cl2 in presence of light and a trace of peroxides or with NBS (N-bromosuccinimide) in presence of light and trace of peroxide like benzoyl peroxide.
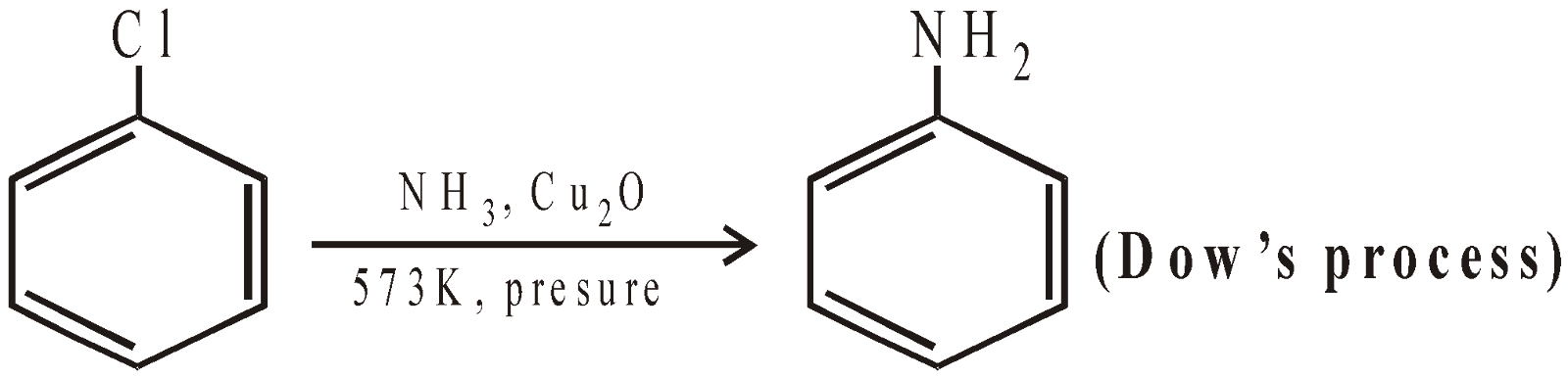
- By the decomposition of diazonium salts
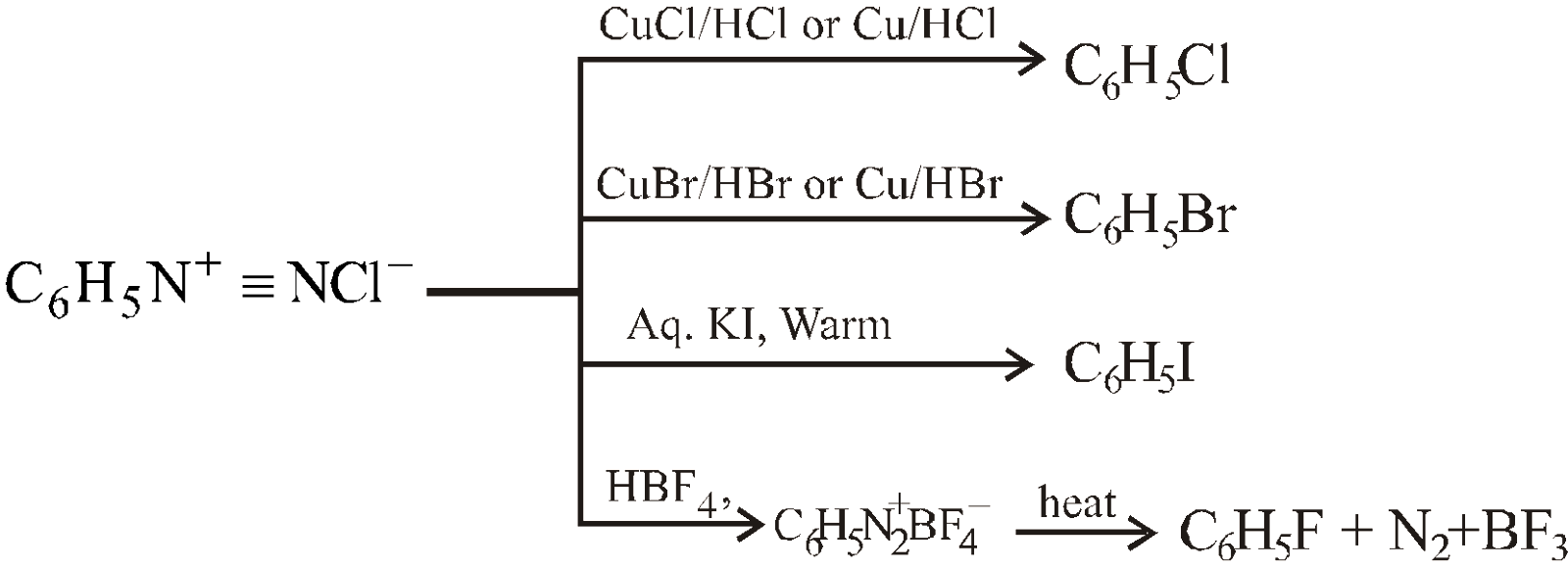
When CuCl/HCl or CuBr/HBr is used, the reaction is called Sandmeyer reaction. It is the halogen attached to cuprous halide which enters the ring.
When Cu/HCl or Cu/HBr is used, the reaction is called Gattermann reaction.
Thermal decomposition of benzenediazonium tetrafluoroborate to give fluorobenzene is called Balz-Schiemann reaction.
This method for preparing aryl halides is more important than direct halogenation of arenes in two respects.
- Fluorides and iodides can be easily prepared.
- Halogenation gives a mixture of o- and p- isomers which are difficult to separate.
- Hunsdiecker method :
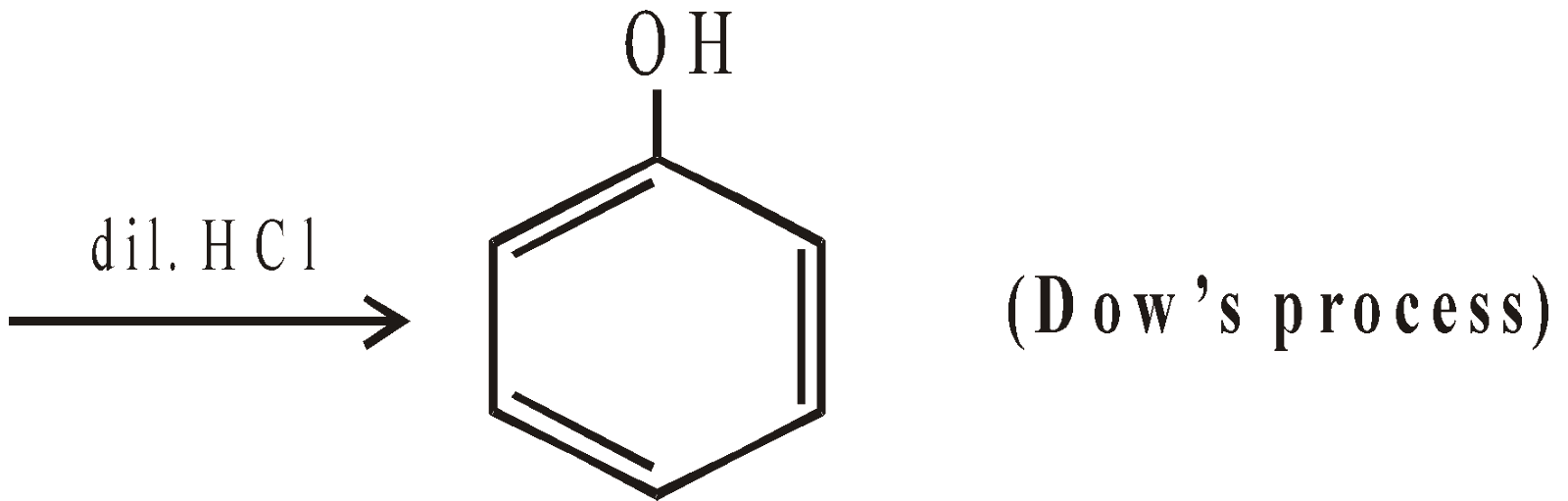
- Industrial method for chlorobenzene (Raschig process)
PHYSICAL PROPERTIES
- Like alkyl halides, aryl halides are insoluble in water due to their incapability of forming H-bonds.
- Aryl halides are less polar than alkyl halides because in aryl halides, halogen is present on sp2 hybridised carbon which is more electronegative than the sp3 hybridised carbon of alkyl halides or halo cyclohexanes. Consequently, the electronegativity difference between C and Cl is low in aryl halides than in alkyl halides.
- Possibility of resonance in chlorobenzene makes the C–Cl bond shorter and hence, its dipole moment is less than that of cyclohexyl chloride.
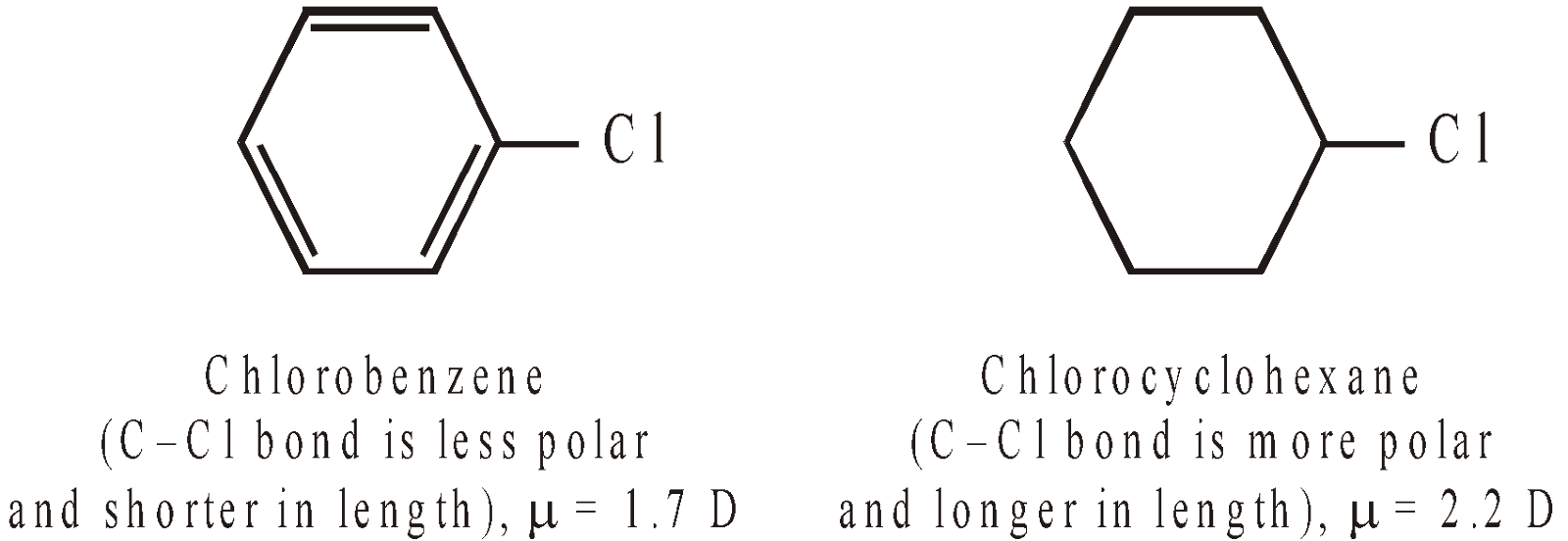
- Although, the three isomeric dihalobenzenes have nearly same boiling points, the p-isomer has higher melting point than the o- and m-isomers. This is due to symmetrical nature of the para isomer due to which it is better packed in the crystal lattice. This explains why only the para isomer crystallises on cooling a solution containing ortho and para isomers.
- Due to strong intercrystalline forces, the higher melting point of para isomer is less soluble in a given solvent than the ortho isomer.
CHEMICAL PROPERTIES
LOW REACTIVITY TOWARDS NUCLEOPHILIC SUBSTITUTION
- Aryl halides (like vinyl halides) are less reactive towards nucleophilic substitutions under ordinary conditions (difference from alkyl halides). This low reactivity is due to
(a) resonance effect
(b) sp2 hybridisation of carbon atom holding the halogen atom
(c) less polarity of the C–X bond.
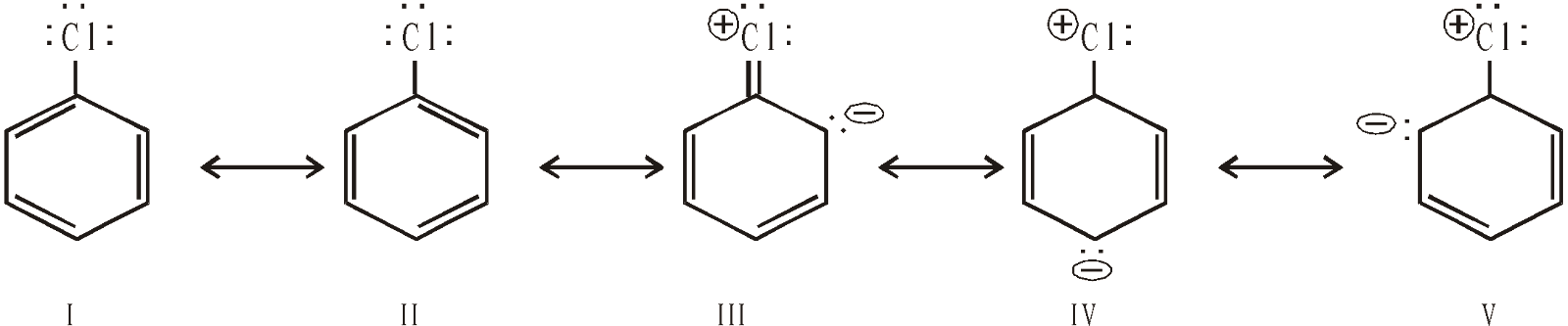
Resonating structures of chlorobenzene
Possibility of resonance in aryl halides produces two results
- Stabilisation of the molecule by delocalisation of electrons.
- The C–Cl bond acquires some double bond character (structures III, IV and V) and thus becomes shorter and stronger than the C–Cl bond in alkyl halides.
On the same ground, low reactivity of vinyl chloride is explained
- Conversely, benzyl halides do not show delocalisation of electrons of the halogen atom, hence its halogen is quite reactive towards nucleophilic substitutions (similarity with alkyl halides). Moreover, benzyl halides are even more reactive than alkyl halides, because the carbocation (benzyl) formed after the removal of halide ion is stabilised by resonance (remember higher is the stability of a species, easier will be its formation).
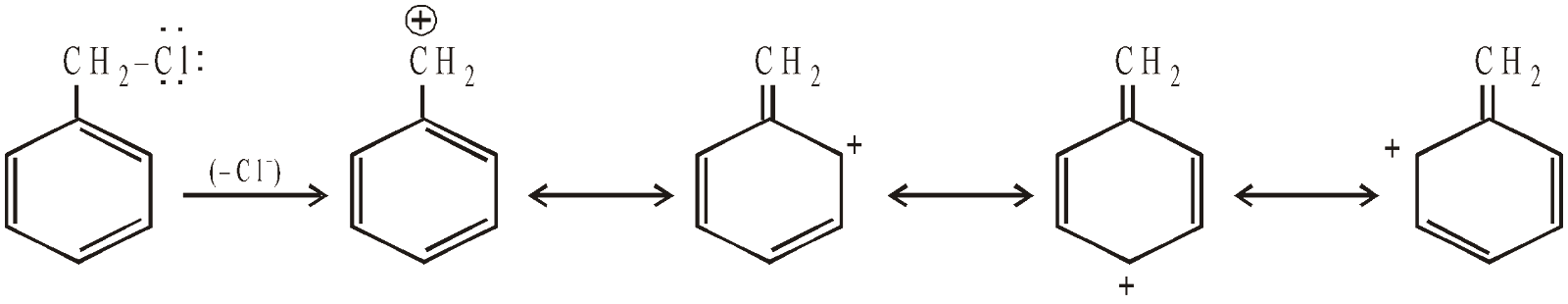
Similar is the case of allyl carbocation formed from allyl halides.
- Thus halogen derivatives can be categorised into two main groups on the basis of reactivity of the halogen atom.
- Those in which halogen is present on sp3-hybridised carbon atom, such halogens and corresponding halides are highly reactive. For example,
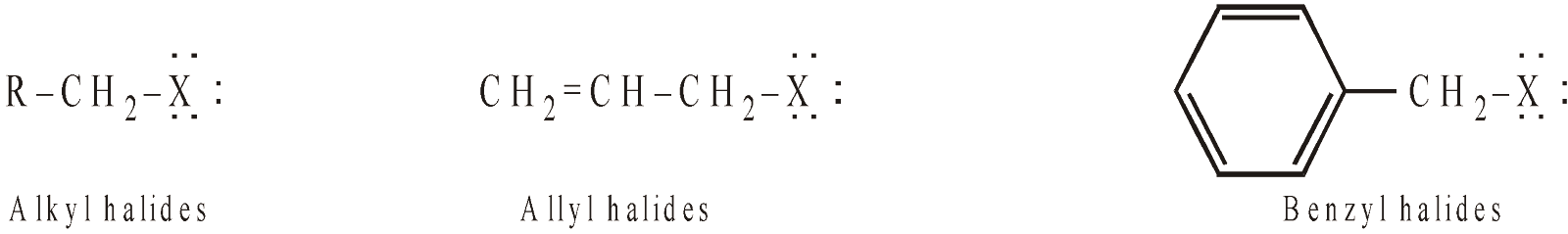
- Those in which halogen is present on sp2-hybridised carbon atom, such halogens are relatively inert. For example,
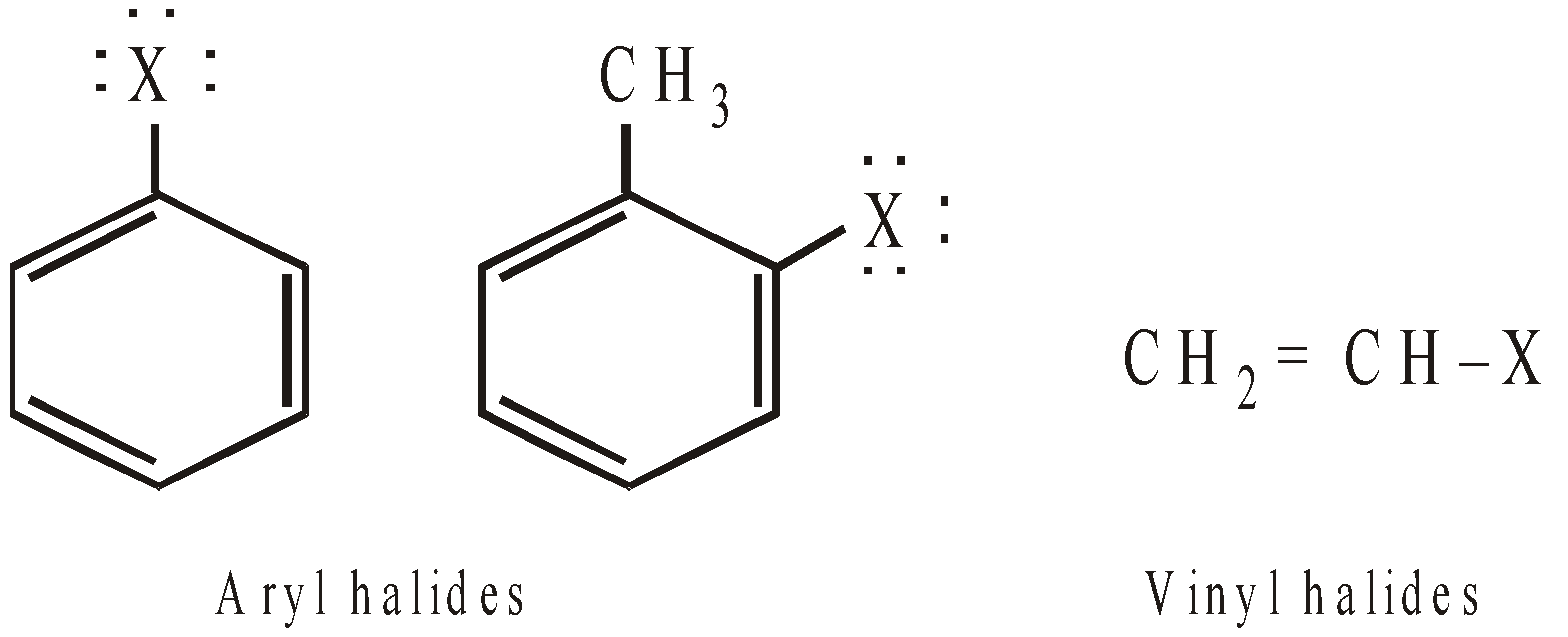
- However, aryl halides having electron-withdrawing groups (like –NO2, –CN, –COOH, –SO3H etc.) in ortho and para positions undergo nucleophilic substitution very easily. Further, greater the number of such groups in o- and p- positions, more rapid is the reaction and hence less vigorous conditions are required. Thus, reactivity of chlorine in the following compounds towards nucleophilic substitution follows the order.
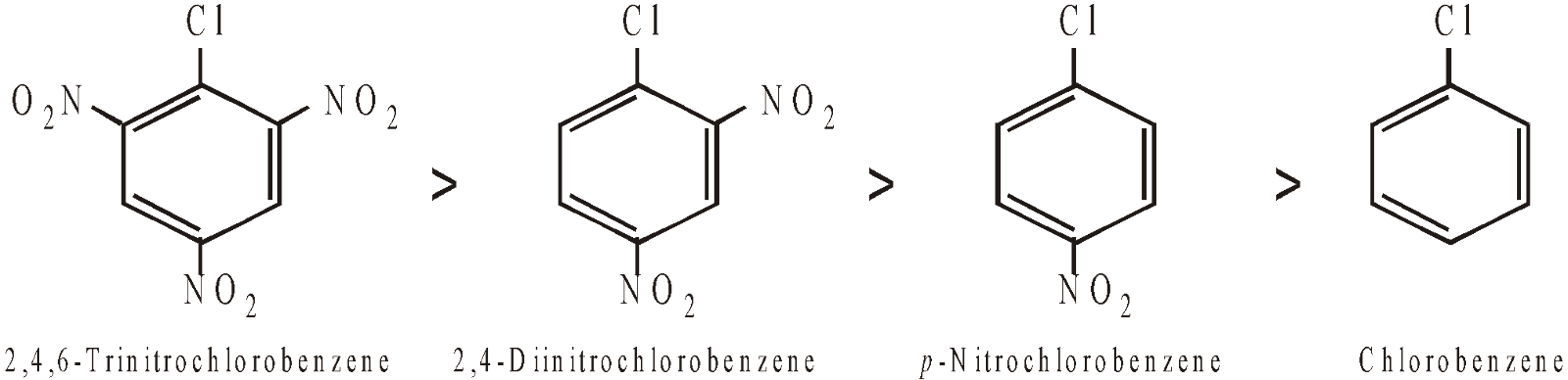
Remember that the electron-withdrawing groups activate chlorine of chlorobenzene towards nucleophilic substitution, they deactivate the benzene nucleus towards electrophilic substitution.
- Since electron-withdrawing groups activate halogen toward nucleophilic substitution, electron-releasing groups (like –OH, –NH2, –OCH3, –R etc.) deactivate toward nucleophilic substitution.
- Aromatic nucleophilic substitution proceeds through mechanisms different from the SN2 and SN1 mechanism of alkyl halides.
- Bimolecular mechanism is applicable to reactions of aryl halides having electron-withdrawing groups with the usual nucleophiles like NaOH, CH3ONa etc. Here carbanion is formed as an intermediate.
- Benzyne mechanism is applicable when aryl halides are treated with a strong base like or with the usual nucleophiles like NaOH, NH3, CH3ONa etc. under drastic conditions.
- Relative reactivity of halogens towards nucleophilic substitution. The order of reactivity of the halogen atom in aryl halides is opposite to that observed in alkyl halides (R – I > R – Br > R – Cl > R – F). In aromatic nucleophilic substitution, fluoride is the most reactive and iodide the least. Ar – F > Ar – Cl > Ar – Br > Ar – I
The unusually high reactivity of aryl fluorides (although having a strong C – F bond) is due to very high electronegativity of fluorine due to which it stabilises the carbanion, formed as intermediate during the reaction, most easily.
IMPORTANT NUCLEOPHILIC SUBSTITUTION REACTIONS
Note that introduction of –NO2 group in p– and o– positions make the substitution of –Cl by –OH easier as it is evident by increased mild conditions.
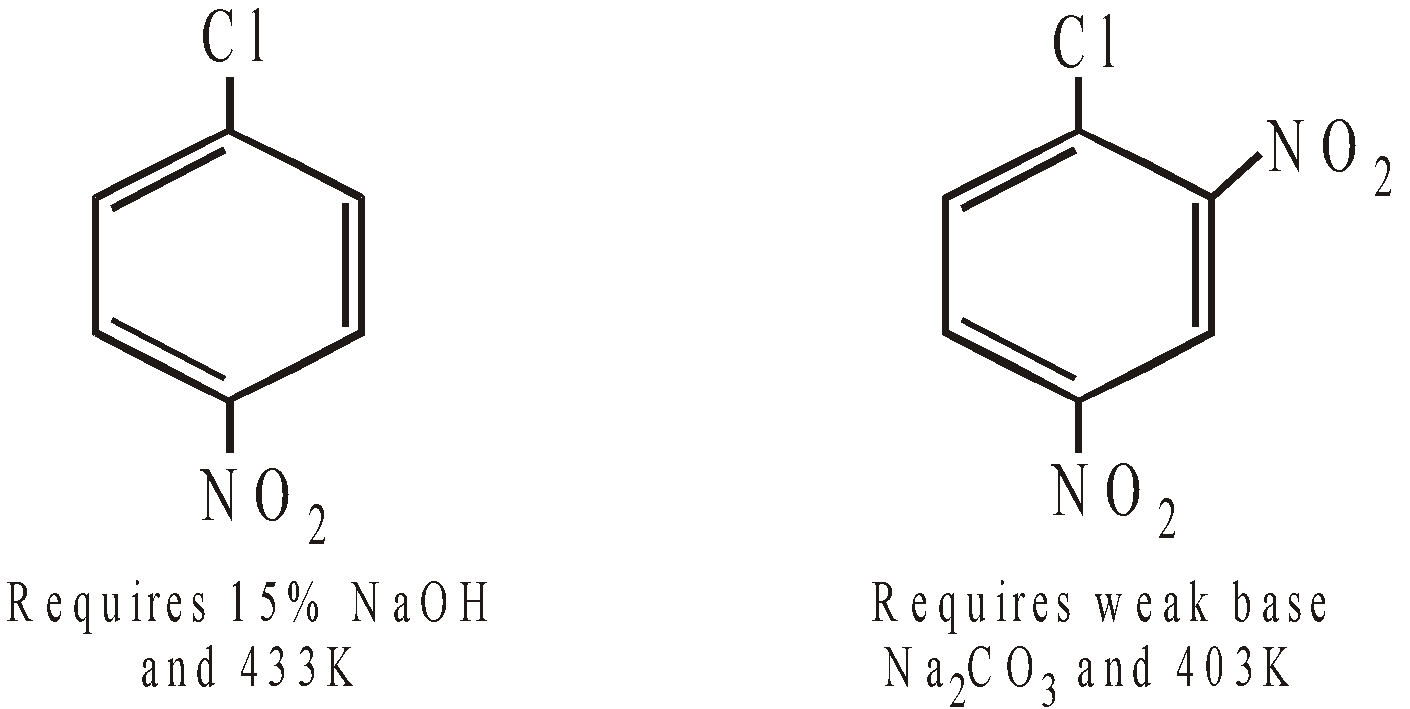

Benzyl chloride undergoes all the above reactions under mild conditions
- However, simple aryl halides undergo nucleophilic substitution very easily by strong basic nucleophiles, like amide ion, via benzyne mechanism.
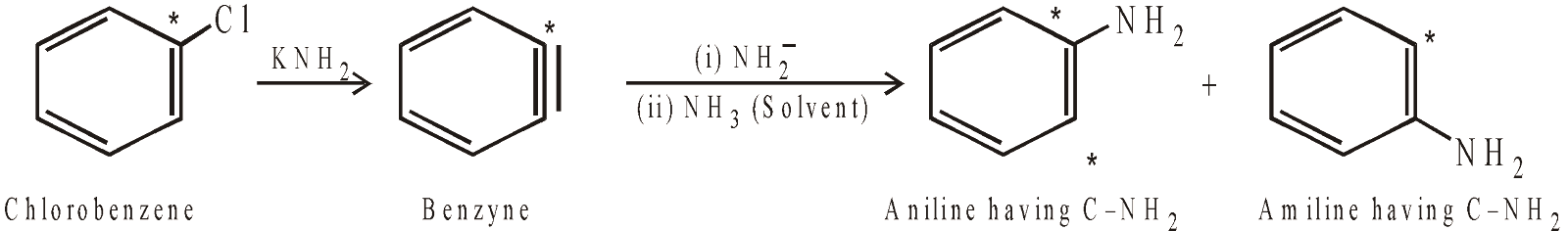
where * indicates isotopically labelled carbon, i.e. C-14.
Benzyl chloride does not form benzyne intermediate.
Remember triple bond of benzyne is different from the triple bond of alkynes, in benzyne one of the p bonds is formed by p–p overlap while the other by sp2–sp2 overlap.
ELECTROPHILIC SUBSTITUTION
Remember that halogens are deactivating but o, p–directing. Thus chlorination, nitration, sulphonation and Friedel-Craft reaction forms a mixture of o- and p-chloro substituted derivatives
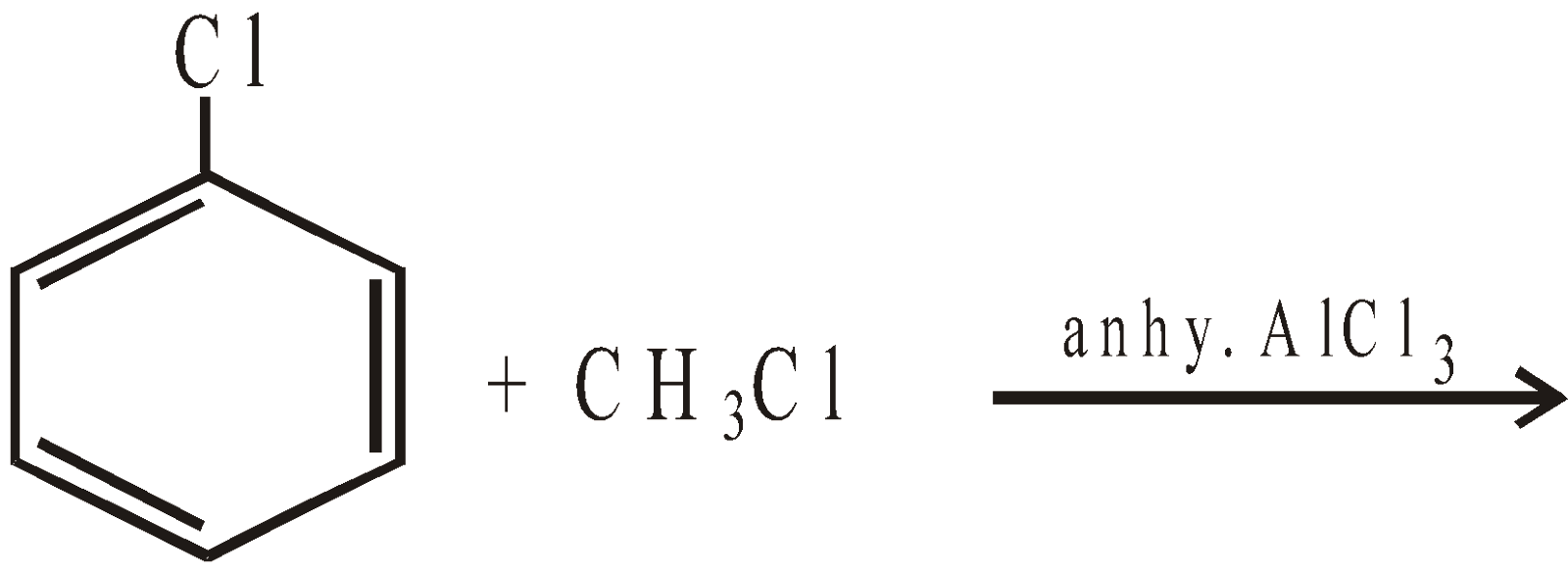
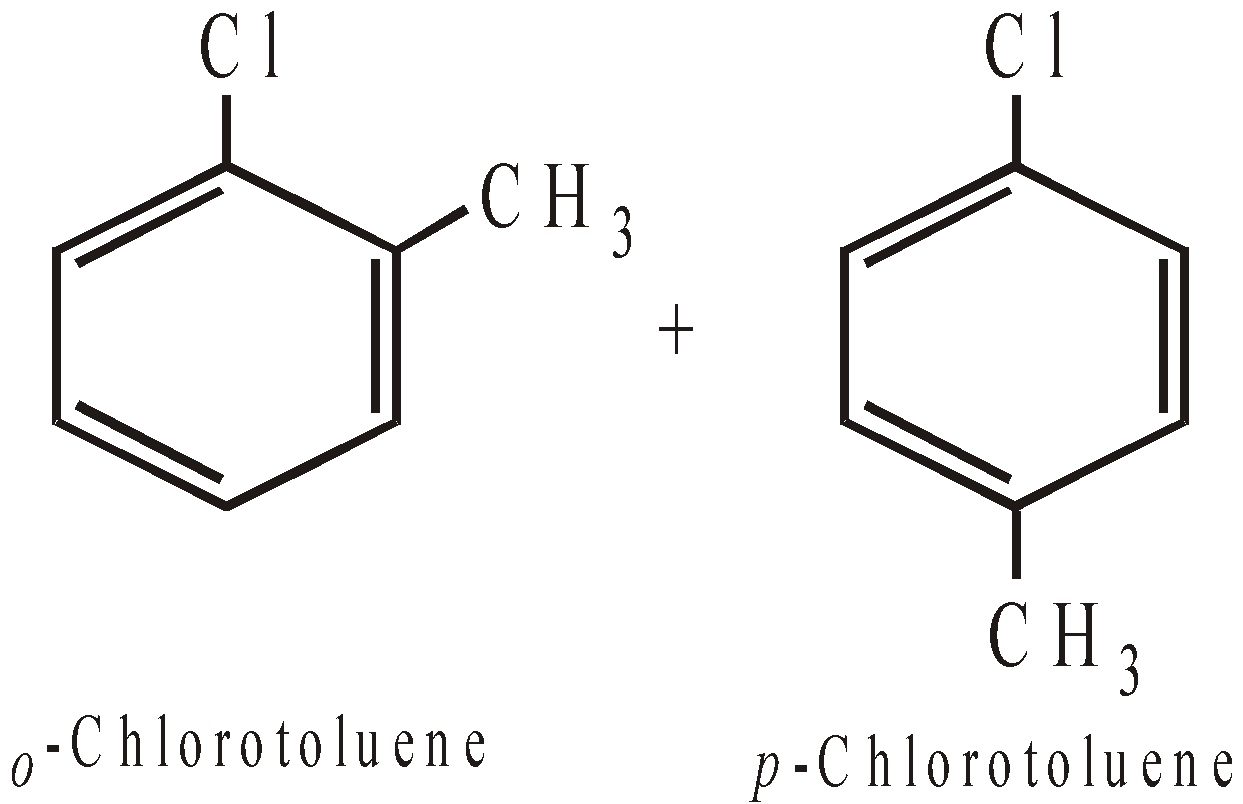
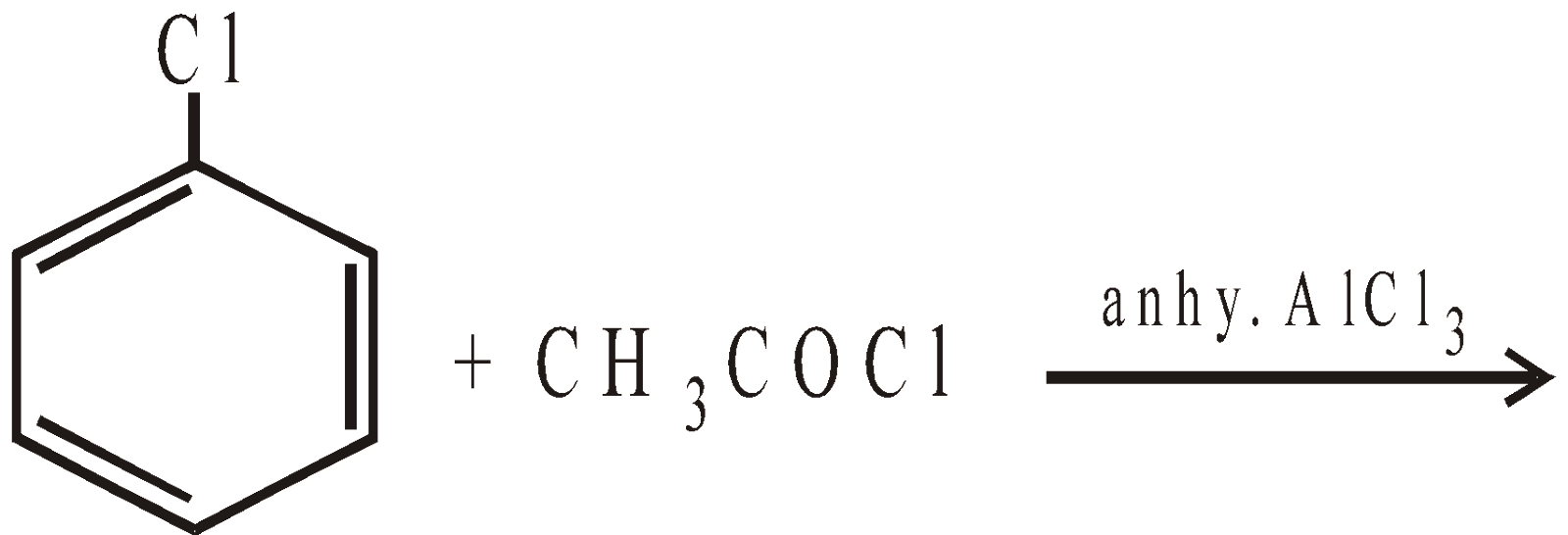
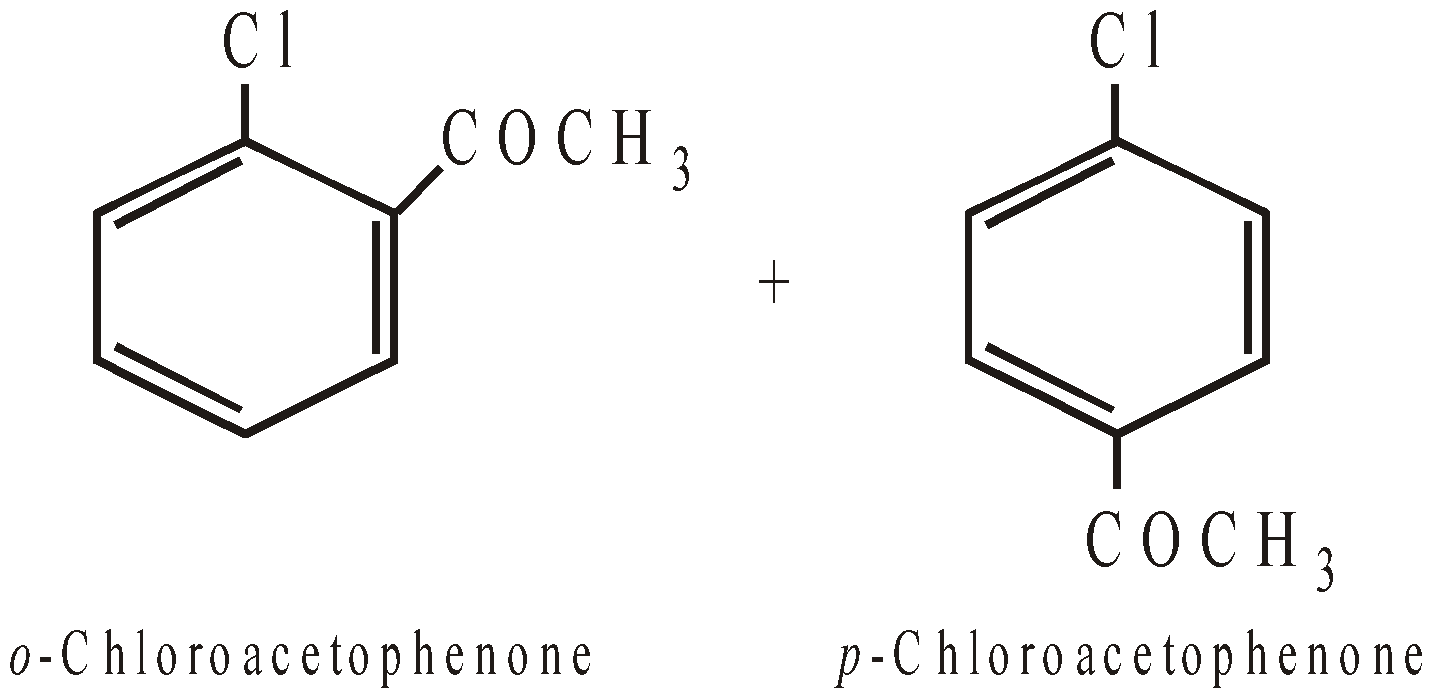
However, unlike CH3Cl, chlorobenzene does not undergo Friedel-Craft reaction with benzene. This is because of instability of C6H5+ cation.
Benzyl chloride also undergoes electrophilic substitution easily and mainly in the o- and p-positions. However, here percentage of m- too is significant (10-15%) as compared to that in toluene. Formation of m-isomer is due to the presence of Cl as Cl which decreases the electron-releasing power of the parent methyl group due to its electron withdrawing nature. Thus we can explain the increased percentage of the m-isomer with the increase in number of Cl atoms in the –CH3 group.
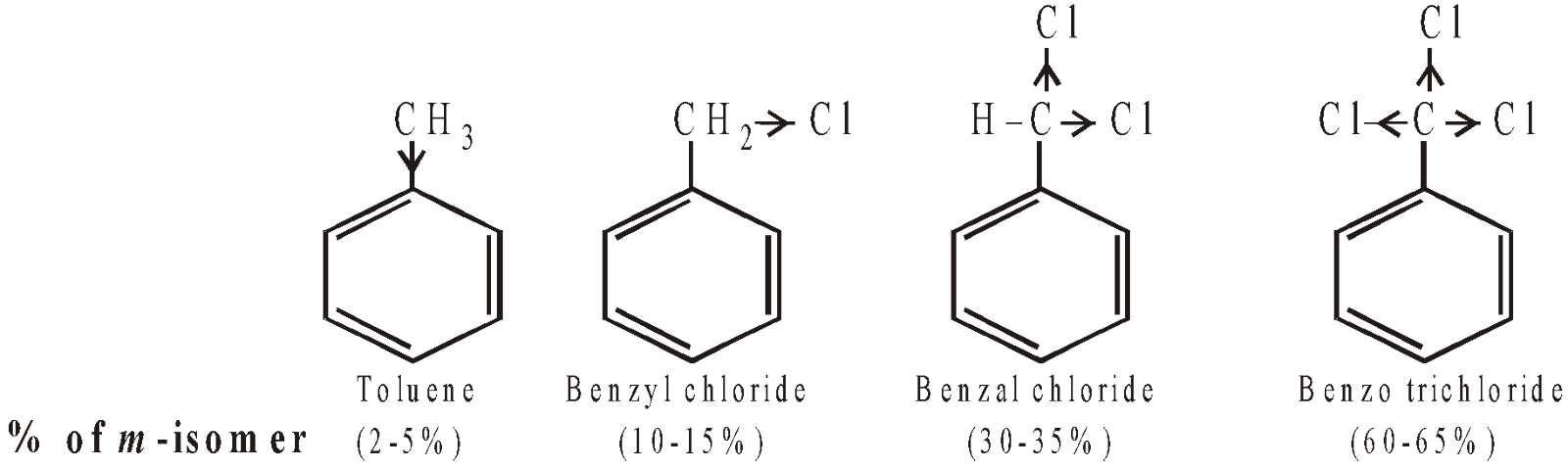
OTHER REACTIONS
- Formation of Grignard reagent : Since chlorobenzene is less reactive than methyl chloride, THF (a higher boiling solvent) is used.
- Fittig reaction :
- Wurtz-Fittig reaction :
- Ullmann reaction :
- Reduction :
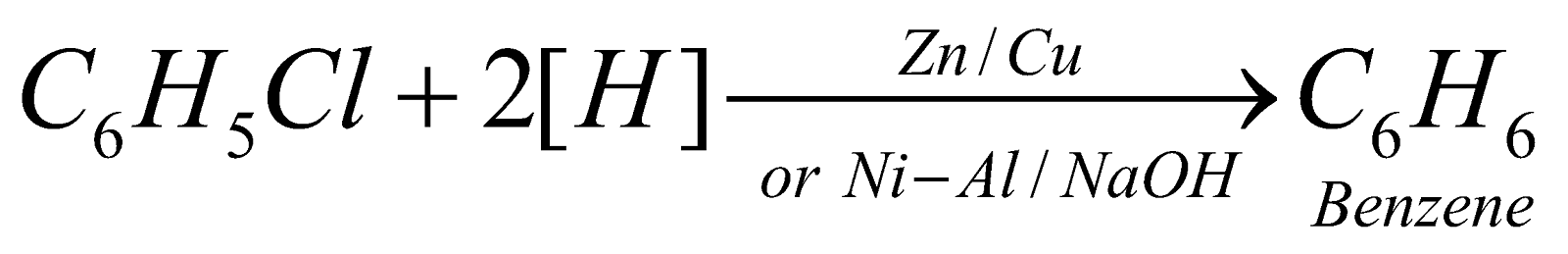
Benzyl chloride undergoes all the above reactions forming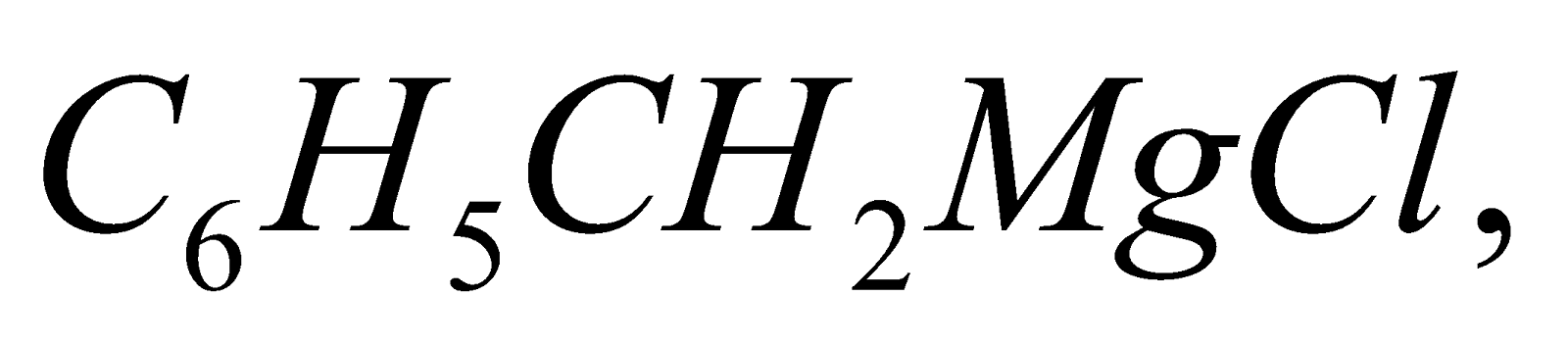
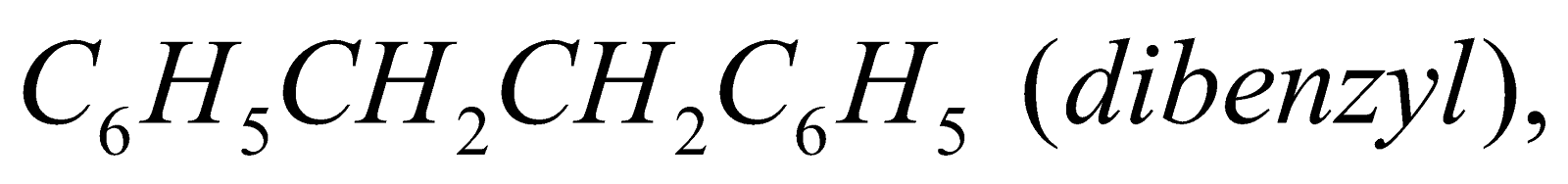
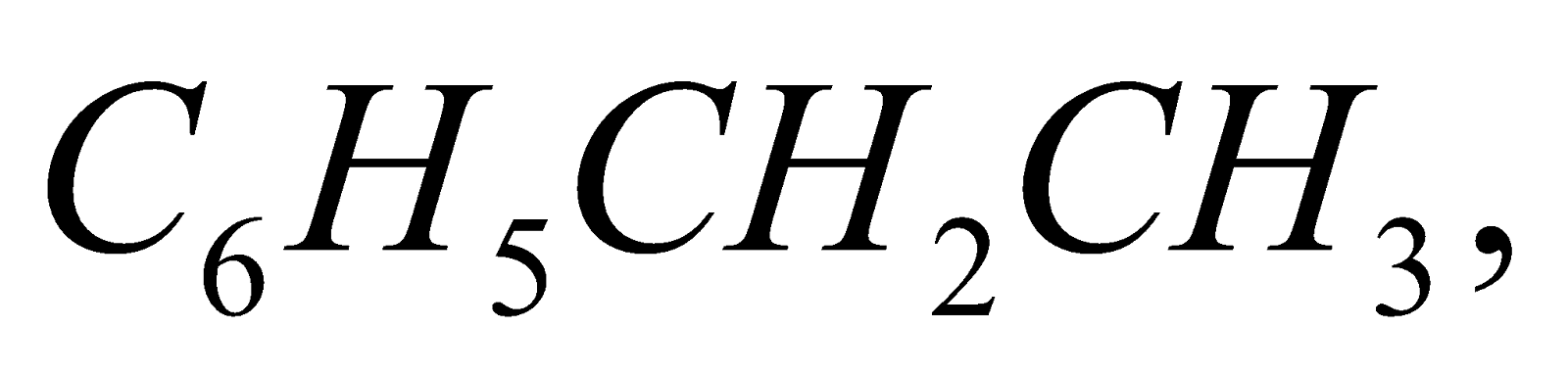
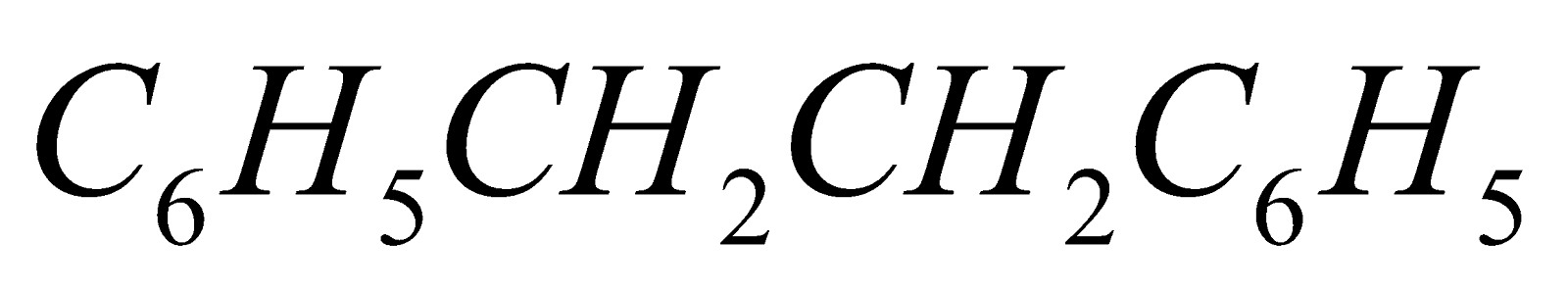 and
and 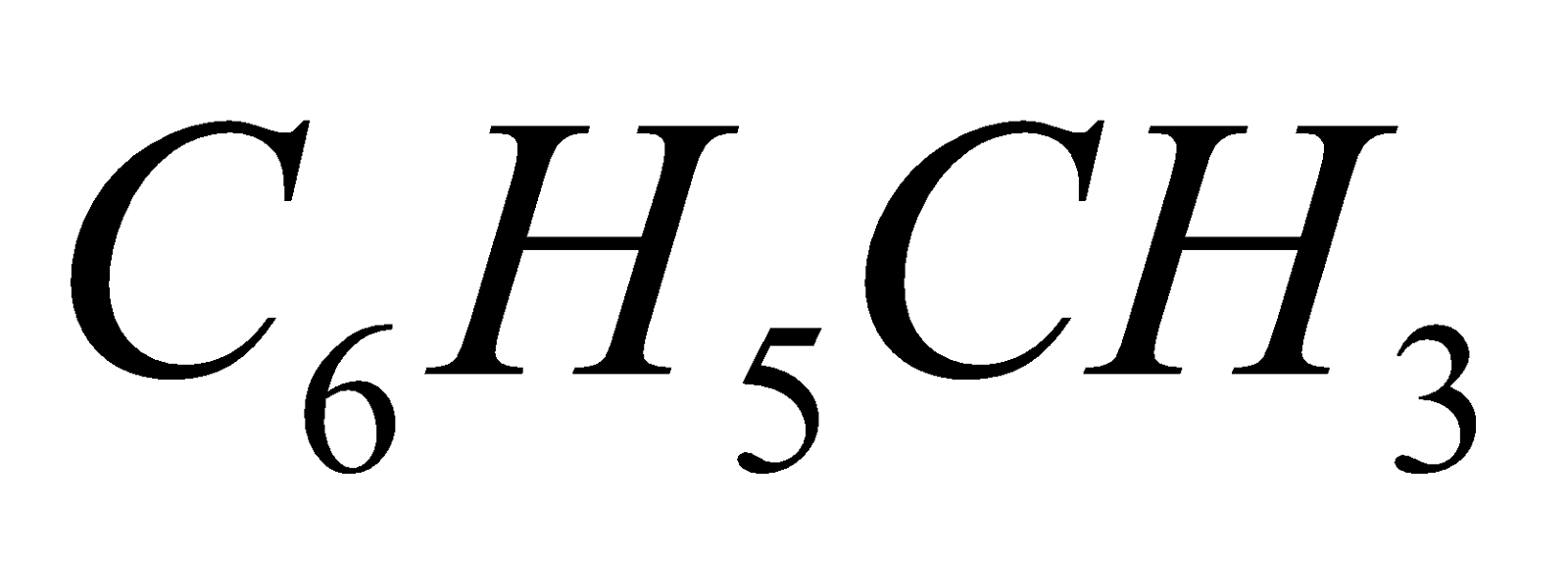 respectively.
respectively.
CONDENSATION WITH CHLORAL TO FORM DDT
DDT or 2,2-bis (4-chlorophenyl) –1, 1, 1-trichloroethane
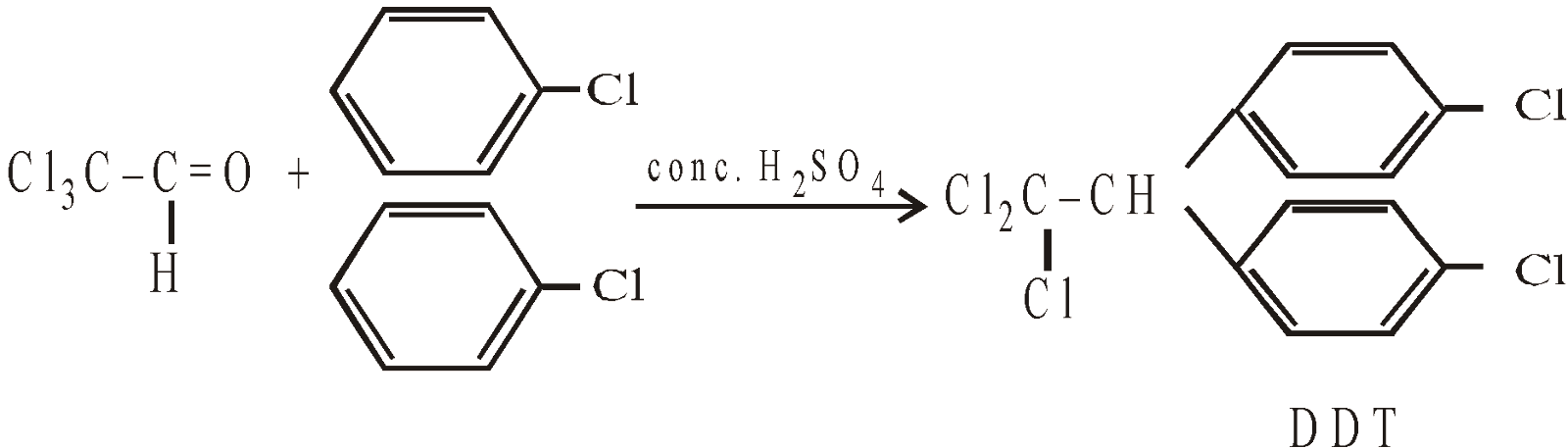
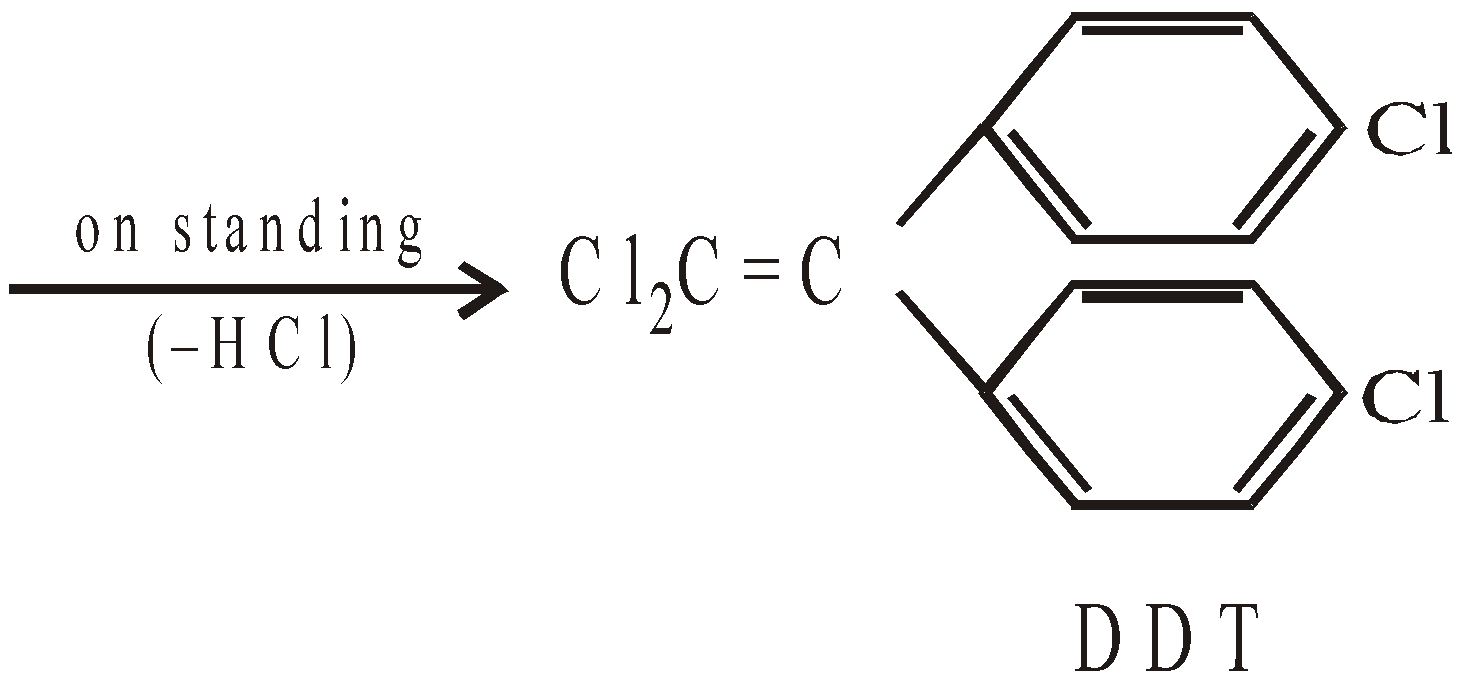
DDT is non-biodegradable and slowly loses a molecule of HCl to form another compound p,p’–dichloro-diphenyldichloroethene (DDE) which hinders with the egg shell formation of birds with the result eggs break off before hatching.
OXIDATION OF THE SIDE CHAIN
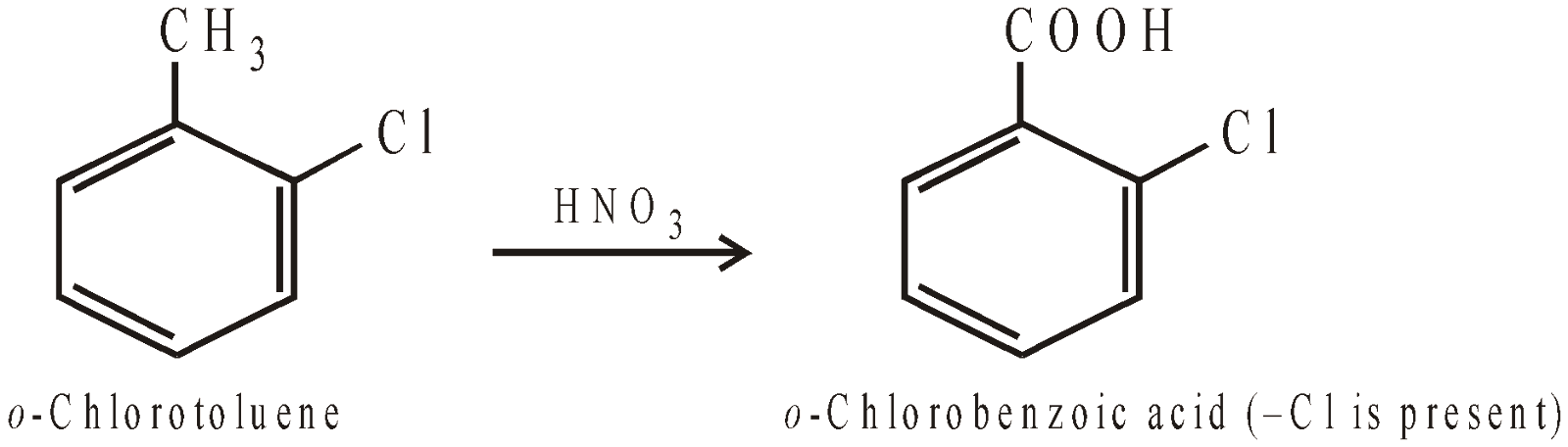
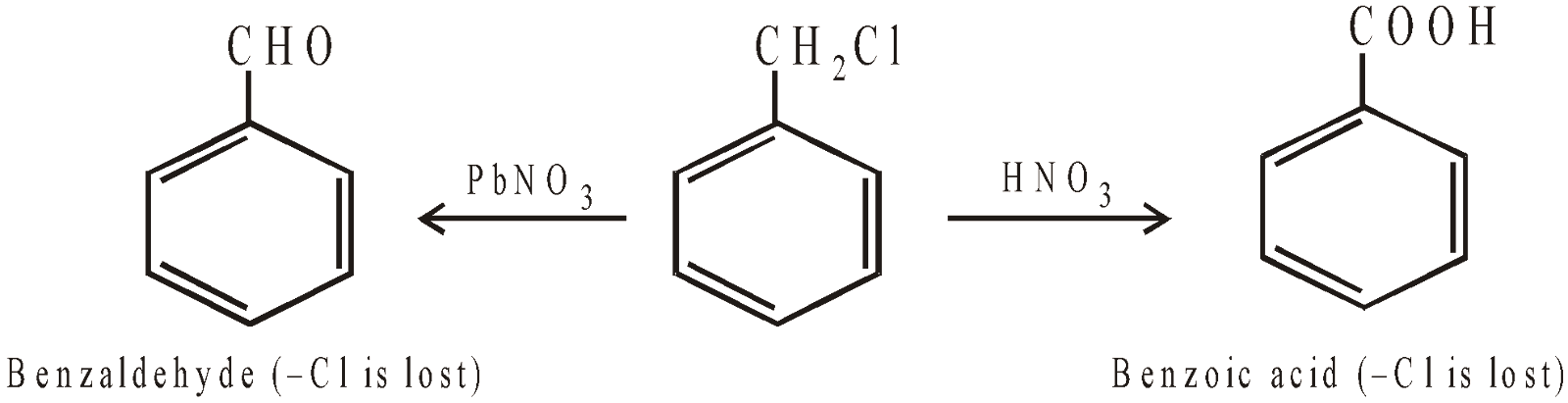
POLYHALOGEN DERIVATIVES
Dihalogen derivatives of alkanes may be vicinal (when two halogen atoms are present on adjacent carbon atoms), gem (when two halogen atoms are present on the same carbon atom) and a, w (when two halogen atoms are present on the two terminal carbon atoms).
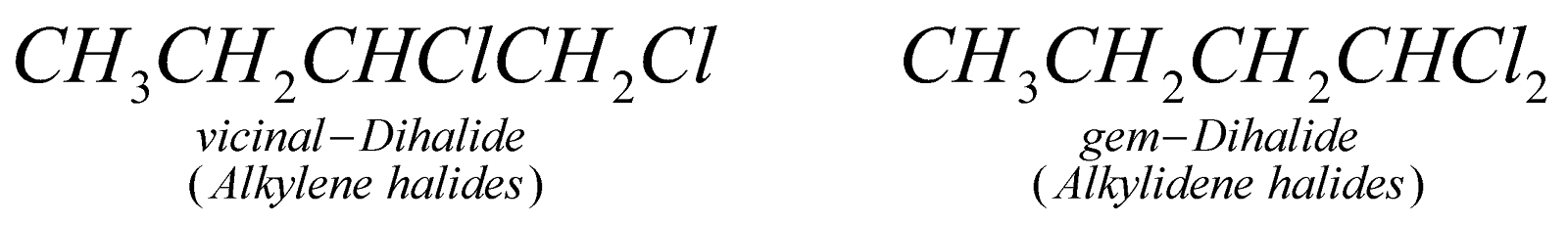
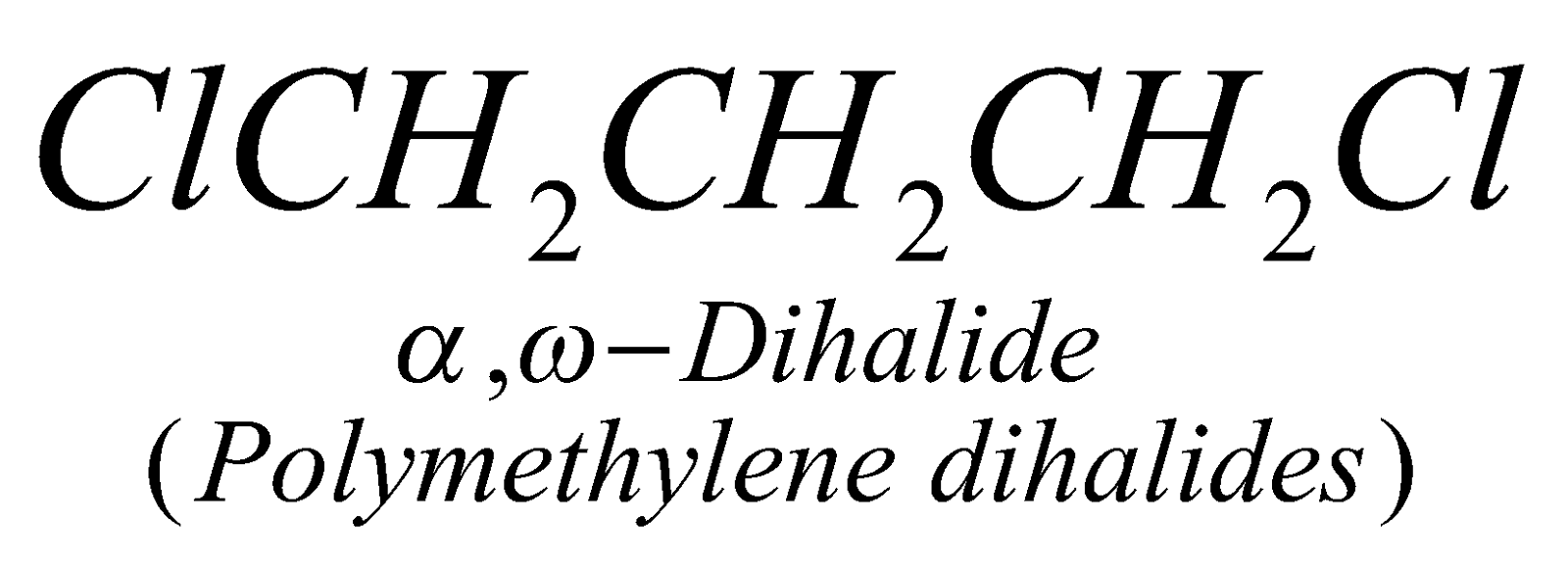
PREPARATION
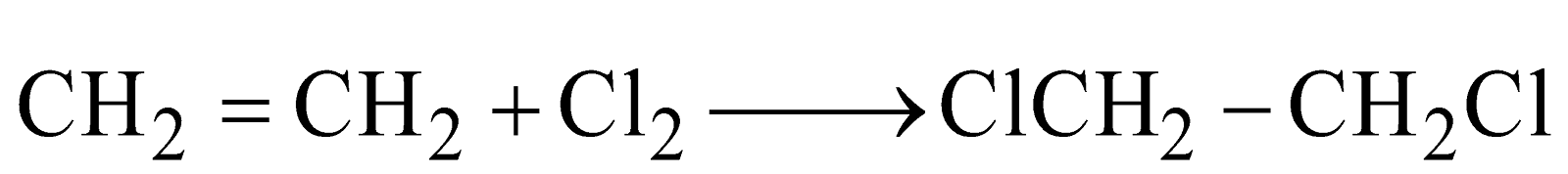 (For vic-dihalides)
(For vic-dihalides)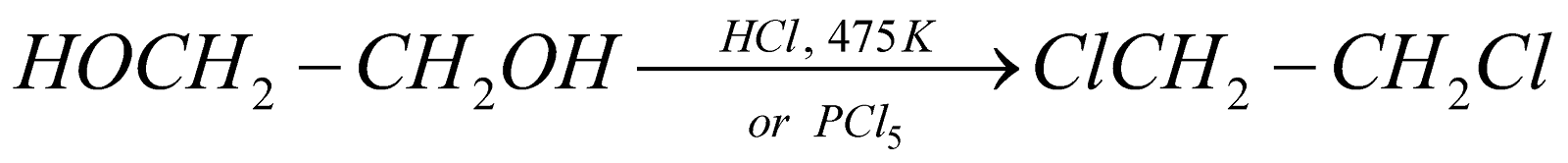 (For vic-dihalides)
(For vic-dihalides)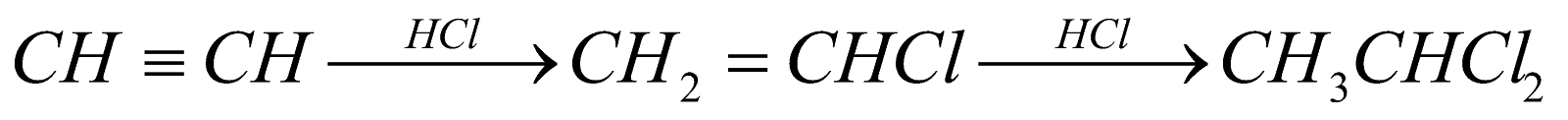 (For gem-dihalides)
(For gem-dihalides)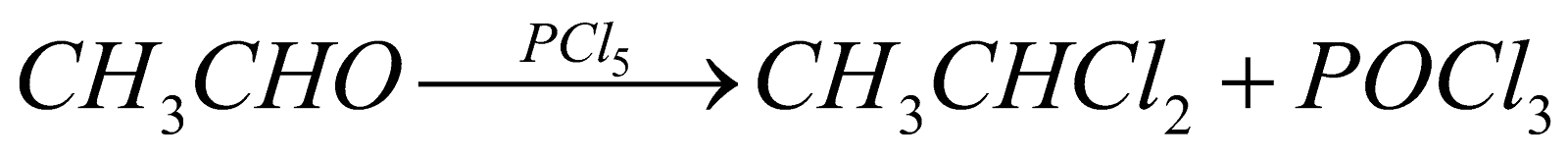 (For gem-dihalides)
(For gem-dihalides)
PROPERTIES
- Dehydrohalogenation : Both give alkynes.
- Dehalogenation : Both give alkenes.
- Hydrolysis : Two give different products.
- Reaction with aq. KCN followed by hydrolysis give different products.
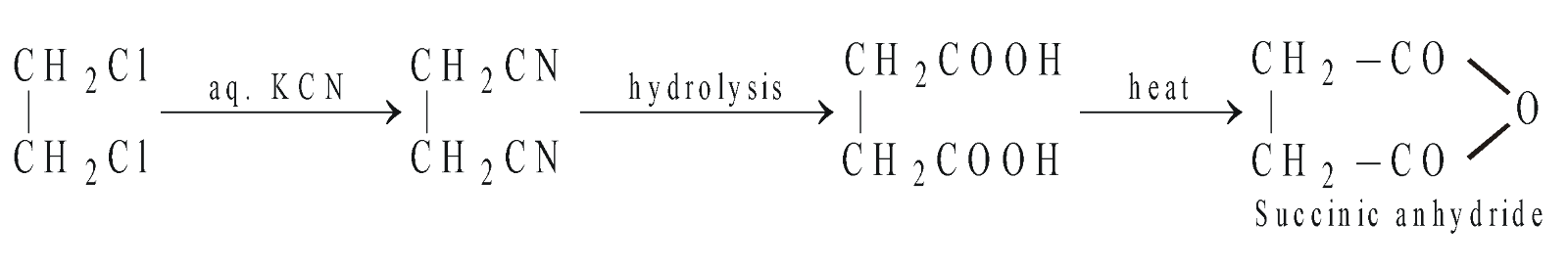
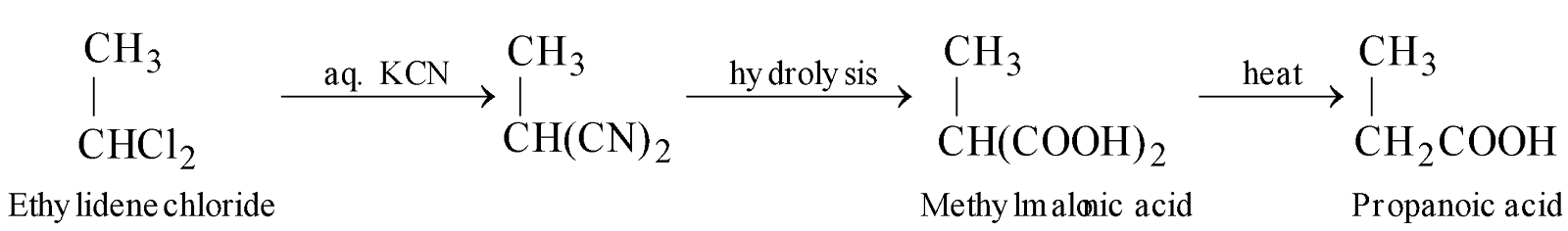
Effect of heating on dicarboxylic acids : Dicarboxylic acids having two –COOH group on the same carbon atom give monocarboxylic acids, those having two –COOH groups on C1 and C2 or on C1 and C3 give anhydrides, those having two –COOH groups on C1 and C4 and C1 and C5 give cyclic ketones (Blanc rule).
CHLOROFORM (TRICHLOROMETHANE)
PREPARATION
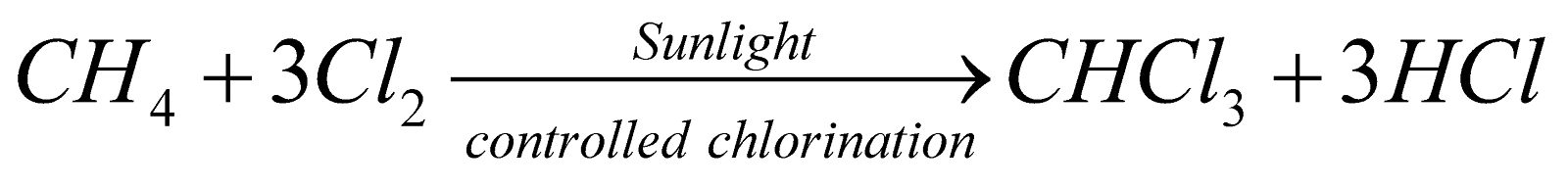
- By distilling ethanol or acetone with a paste of bleaching powder (laboratory and commercial method).
Cl2, so obtained acts as a mild oxidising as well as chlorinating agent
- From ethanol :
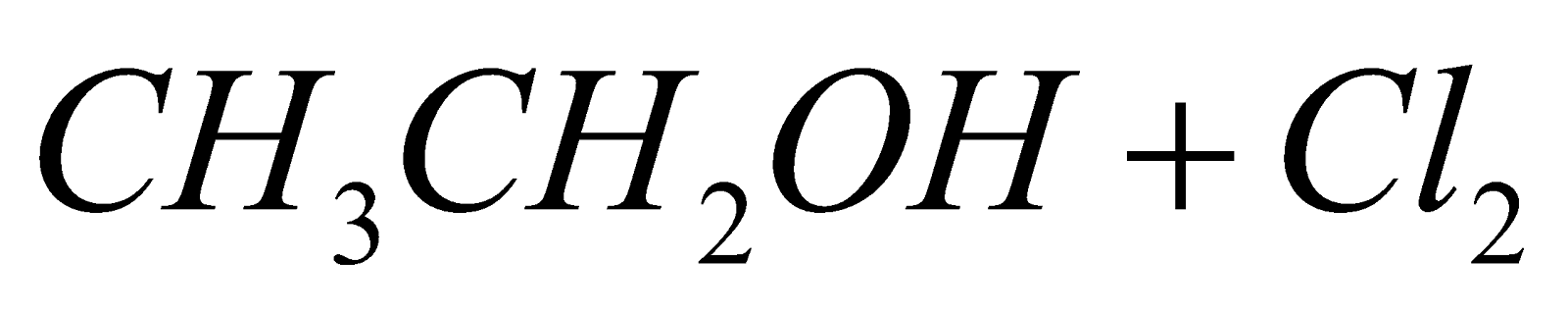
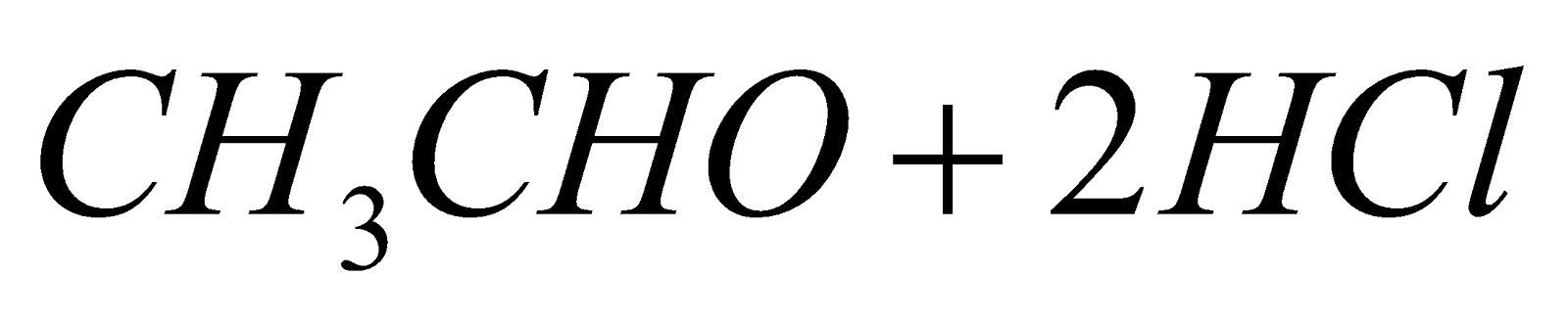 (Oxidation)
(Oxidation)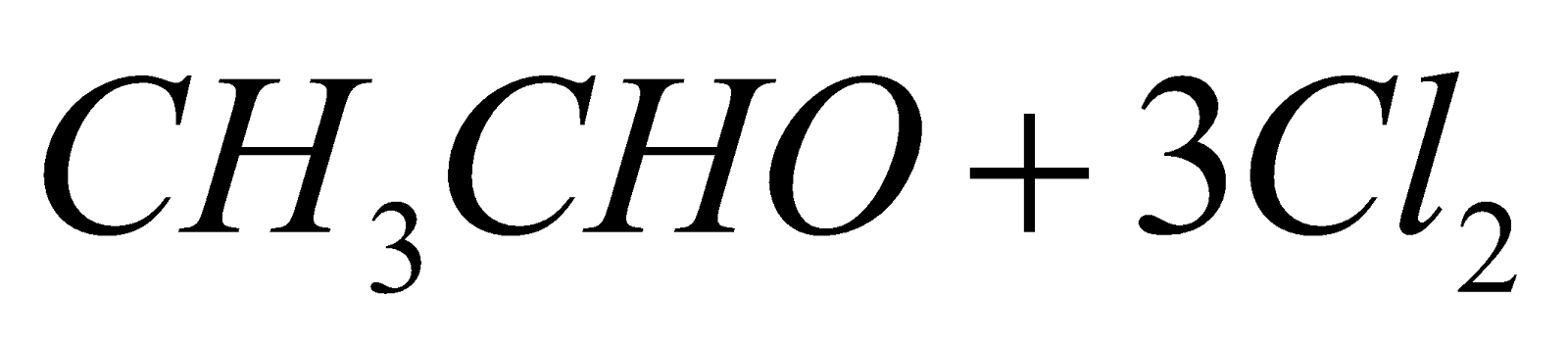

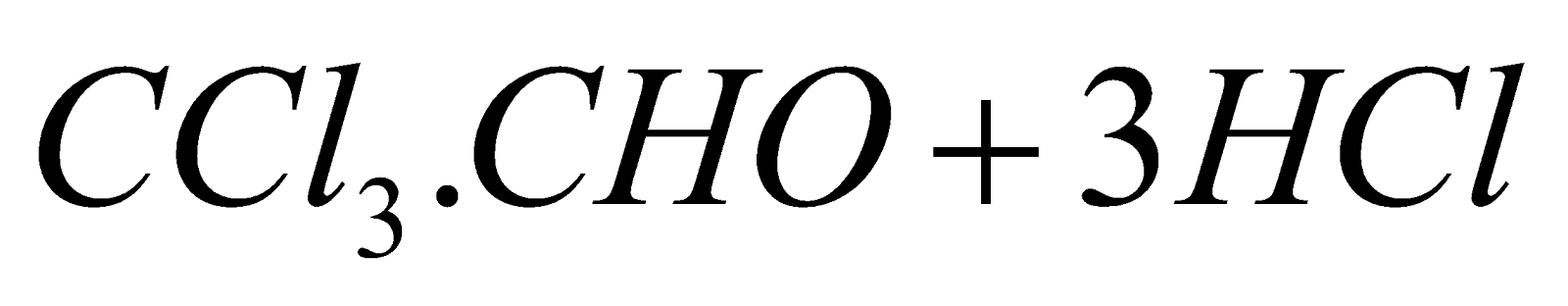 (Chlorination)
(Chlorination)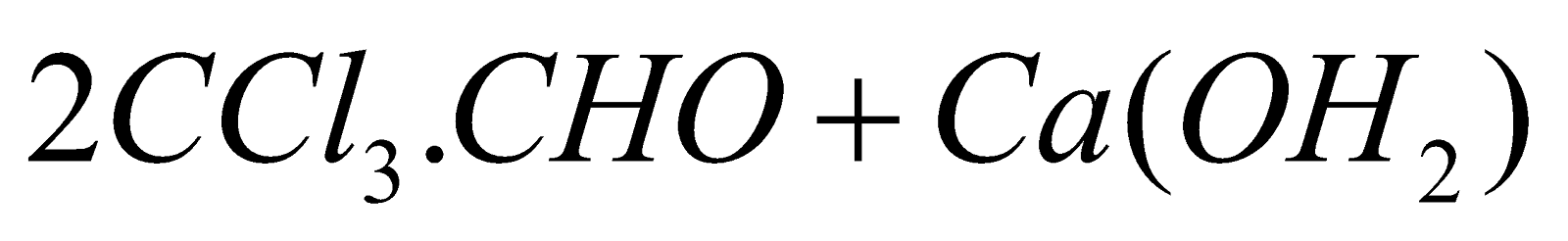

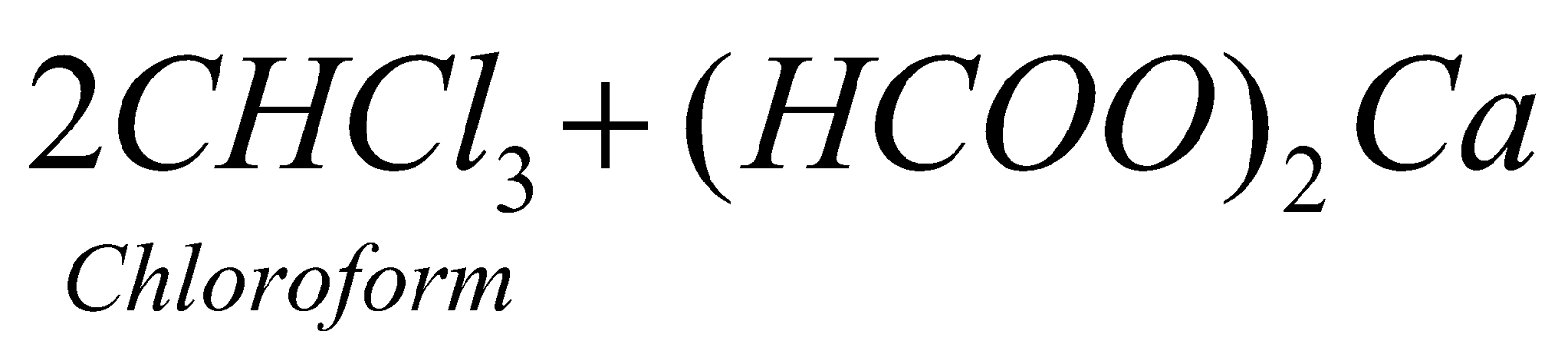 (Hydrolysis)
(Hydrolysis)
- From acetone :


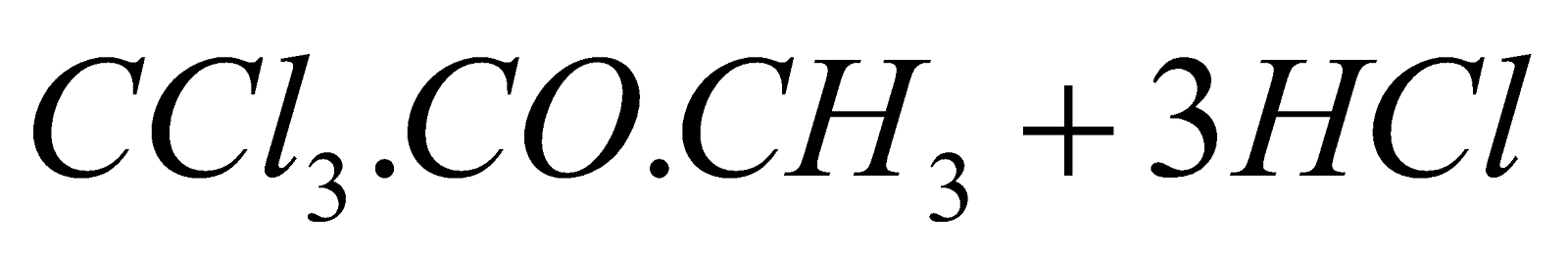 (Chlorination)
(Chlorination)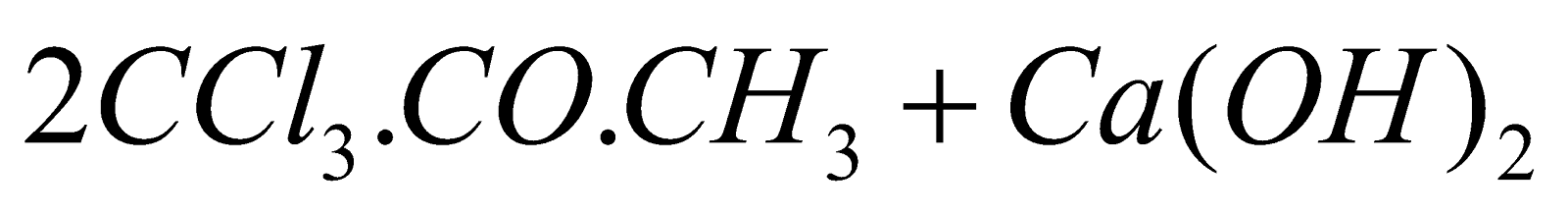

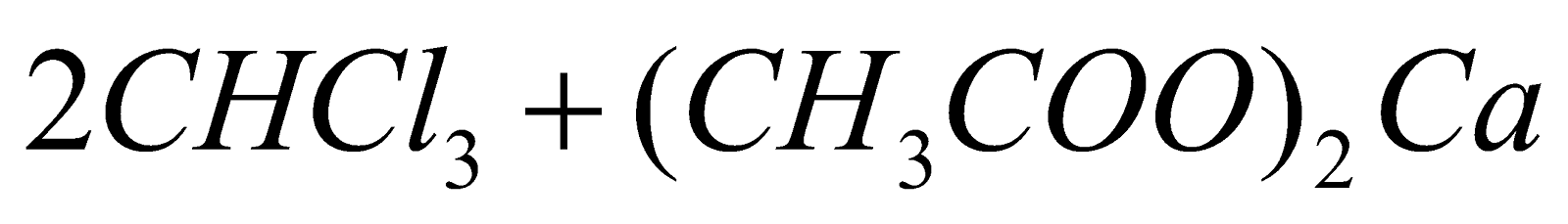 (Hydrolysis)
(Hydrolysis)
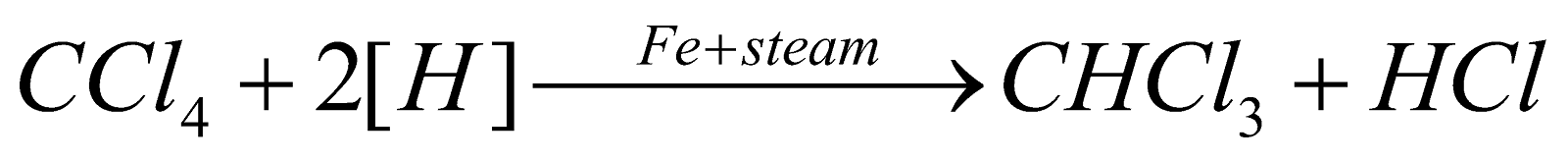 (Commercial method)
(Commercial method)- Pure chloroform, required in anaesthesia, is obtained by chloral hydrate with NaOH.
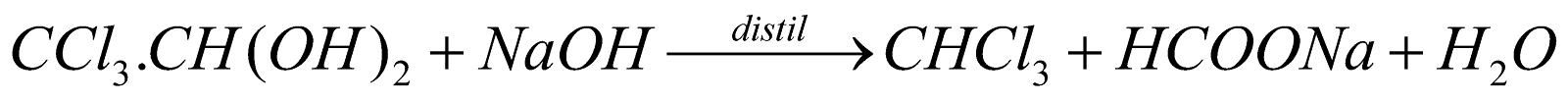
PROPERTIES
- Oxidation
On exposure to air and sunlight, chloroform, a colourless heavy liquid, oxidises to carbonyl chloride (phosgene), a highly poisonous gas used in warfare.
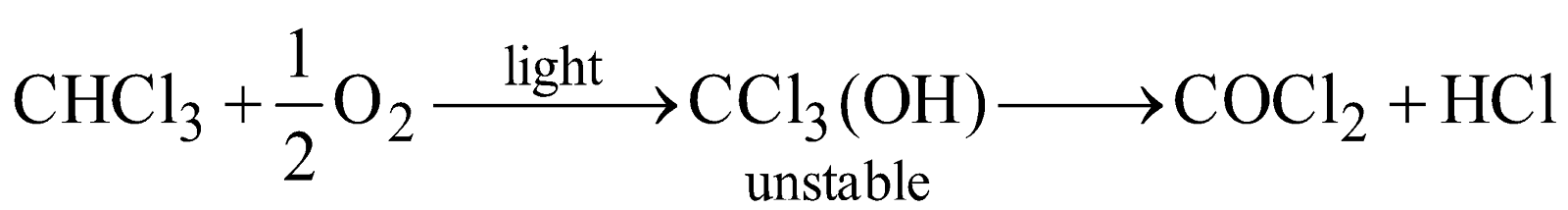
To avoid this oxidation chloroform is always stored in dark coloured bottles filled to the brim to exclude any air. Further nearly 1% alcohol is also added to destroy traces of phosgene, if formed, to harmless diethyl carbonate.
- Reduction
- Chlorination
- Nitration
Chloropicrin (nitrochloroform) is a useful insecticide and lachrymatory (tear producing) substance.
- Hydrolysis
- Carbylamine reaction
Alkyl carbylamines or alkyl isocyanides have extremely bad smell, hence this reaction is used for detecting the presence of a primary amine.
- Reimer-Tiemann reaction
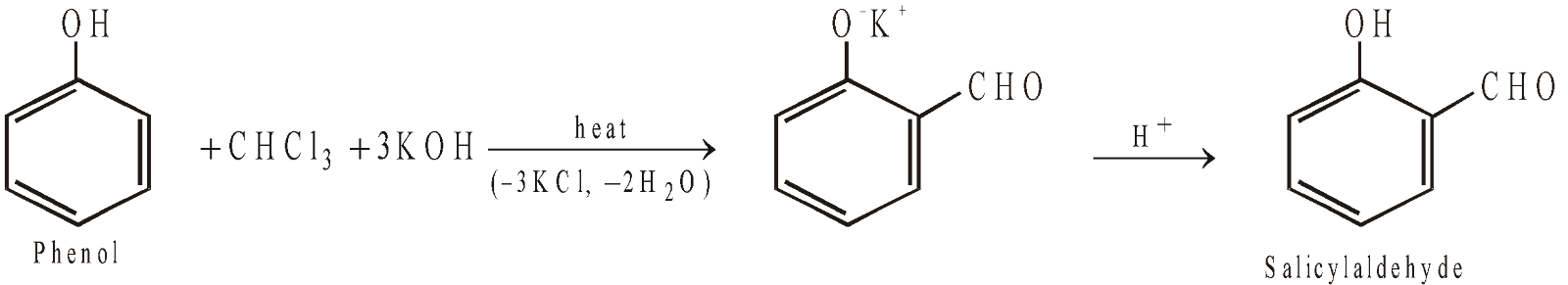
- Condensation with Ketones
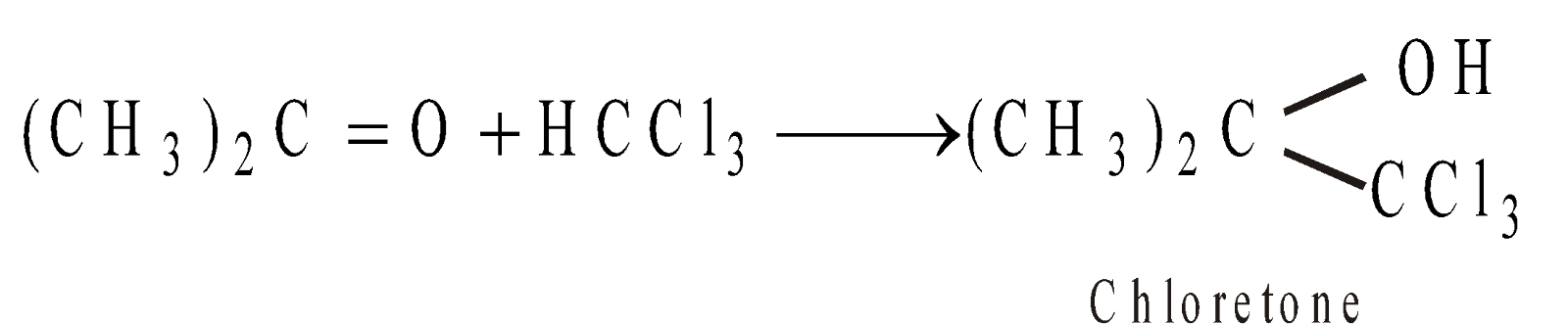
Chloretone is used as a hypnotic (sleep producing substance).
- Dehalogenation
IODOFORM
PREPARATION
Iodoform is prepared by heating ethanol or acetone with NaOH or Na2CO3.
PROPERTIES
Relative reactivity of haloforms : CHI3 > CHBr3 > CHCl3
Being highly reactive,
- iodoform when comes in contact with organic matter, decomposes easily to free iodine, an antiseptic.
- iodoform gives yellow precipitate of AgI, on heating with silver nitrate, while chloroform does not give any precipitate with AgNO3 solution.
IODOFORM TEST
Any compound containing CH3CO– or CH3CH(OH)– group, when heated with iodine and aqueous NaOH or NaOI (sodium hypoiodite) gives yellow precipitate of iodoform, this reaction is called iodoform reaction.
Thus iodoform reaction is used for detecting CH3CO– or CH3CH(OH)– group in an organic compound. Hence iodoform test can be used for distinguishing between
Compound marked with asterisk * responds iodoform test.
CARBON TETRACHLORIDE (TETRACHLORO METHANE OR PERCHLOROMETHANE)
PREPARATION
When all hydrogen atoms of a hydrocarbon are replaced by corresponding number of halogens, halogen derivative is known as perhalohydrocarbon. Perchloroethane, C2Cl6 is a solid and known as artificial camphor, it is used as moth repellents.
PROPERTIES
- It is a colourless, non-inflammable, poisonous liquid, soluble in alcohol and ether.
- On heating with steam at about 773K, it undergoes oxidation forming carbonyl chloride.
- It reacts with HF in presence of SbCl5 forming dichlorodifluoromethane; commonly known as freon-12.
or
Freon is a widely used as refrigerant and propellant in aerosols and foams. However, freons destruct ozone layer in the atmosphere.
- Reimer-Tiemann reaction
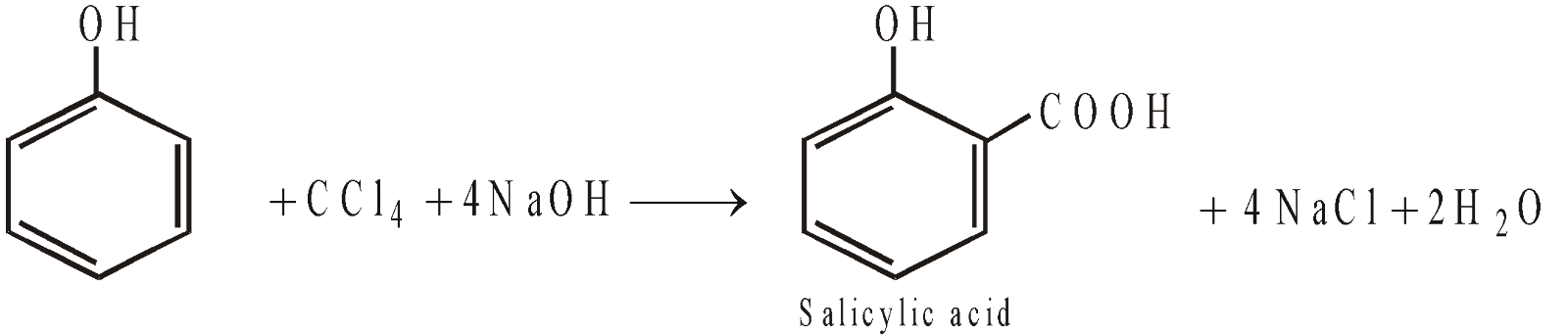
- Reduction
- Hydrolysis
Due to non-availability of d-orbital in C, CCl4 resists hydrolysis with boiling water.
USES
Carbon tetrachloride is used:-
- as a solvent for oils, fats, resins,
- in dry cleaning,
- as a laboratory reagent,
- as anthelmintic (removal of worms) for hookworms
- as a fire extinguisher under the name of pyrene
Carbon tetrachloride gives dense, and incombustible vapours which cover the burning surface and thus prevents oxygen to reach the fire. After the use of pyrene, the room should be well ventilated to remove poisonous phosgene vapours formed by the oxidation of carbon tetrachloride.
UNSATURATED HALOGEN DERIVATIVES
- Those in which halogen atom is attached on unsaturated carbon atoms. Since in such compounds lone pair of electrons on halogen are conjugated with the p-bond, these undergo resonance, the C-Cl bond acquires double bond character, hence it is strong and chlorine is non-reactive.
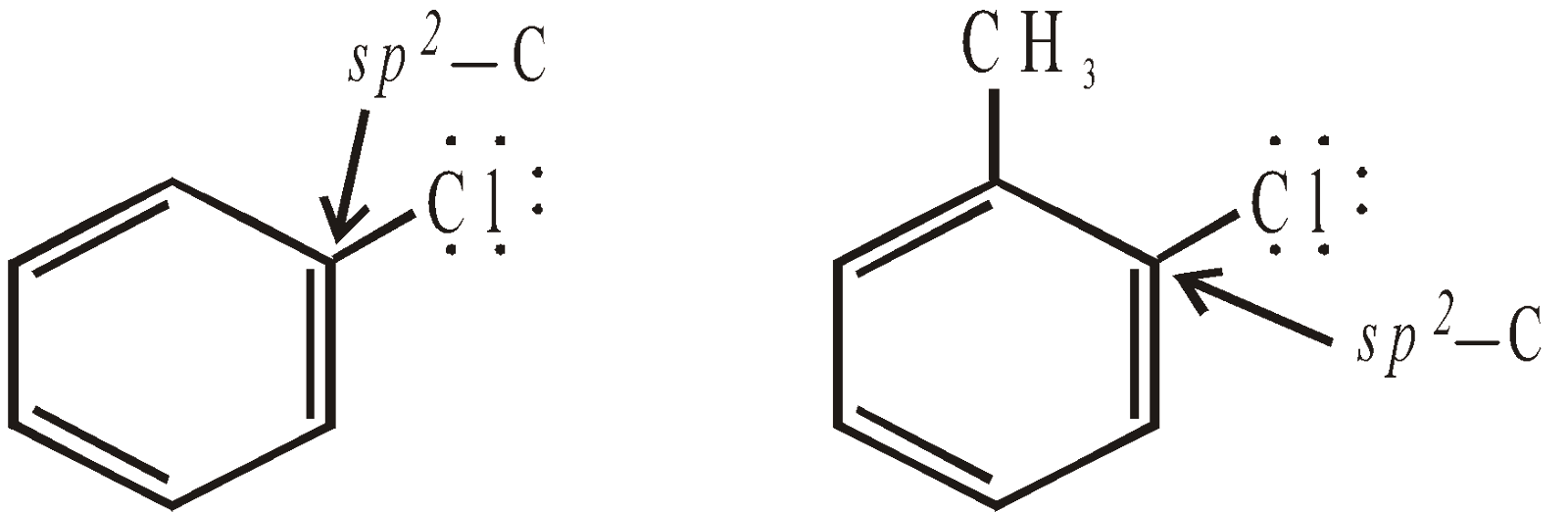
- Those in which halogen is attached on the saturated carbon atom; hence resonance not possible; halogen is reactive.
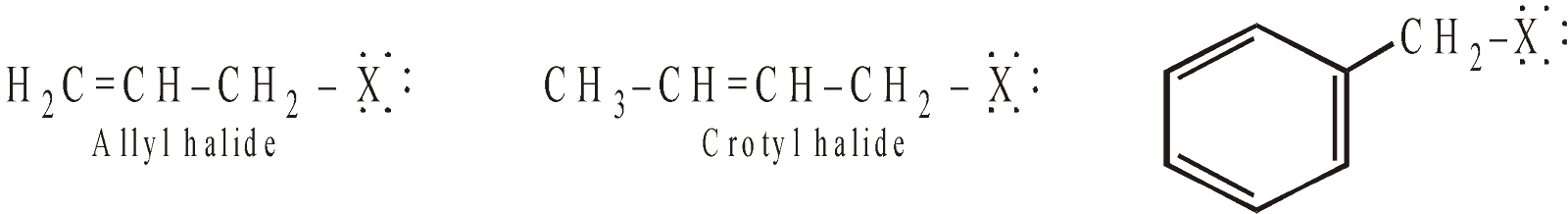
VINYL HALIDES
PREPARATION
PROPERTIES
- Halogen atom of vinyl halides is inert to nucleophilic substitution. However, like alkyl halides they undergo elimination reaction, and form Grignard reagents.
- Like alkenes, it undergoes the usual addition reactions such as addition of H2, X2, HX and polymerisation.
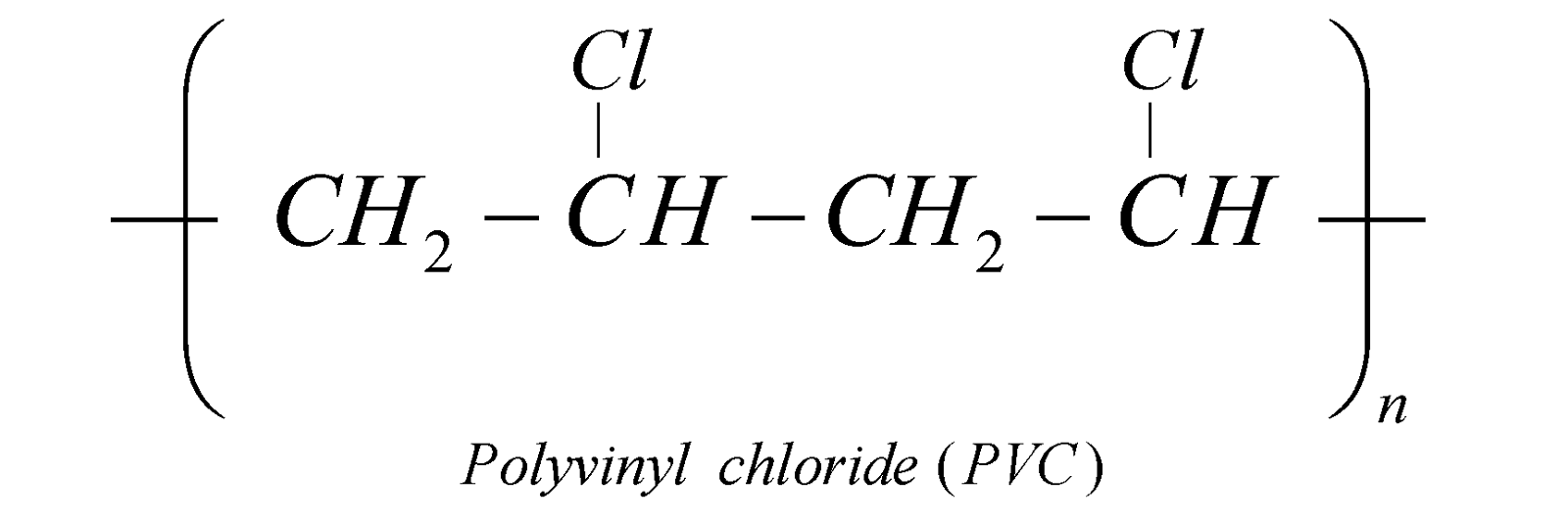
PVC is a plastic of great importance.
ALLYL CHLORIDE
It can best be prepared by allylic chlorination of propene with chlorine at about 673K (a free radical substitution).
ALLYL BROMIDE
It is best prepared by allylic bromination using N-bromosuccinimide (NBS) in presence of sunlight (a free radical substitution).
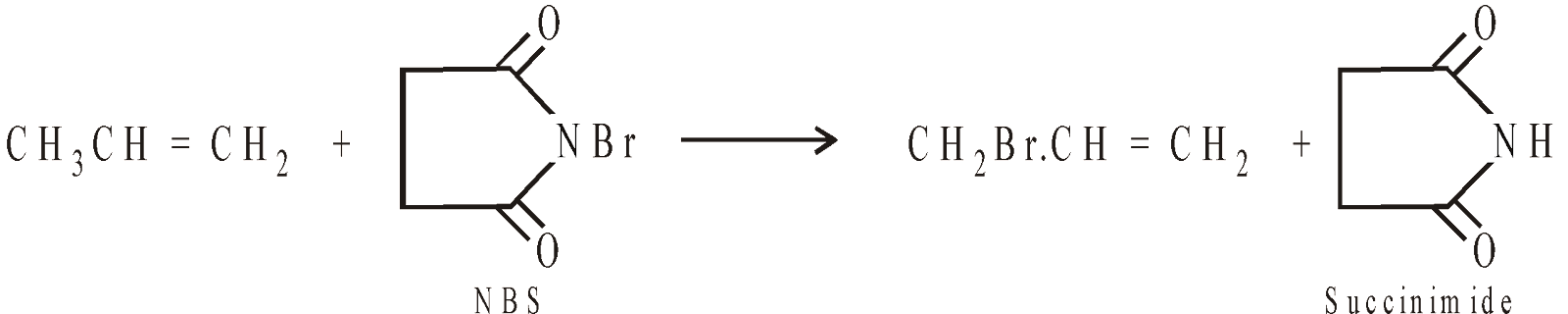
ALLYL IODIDE
It is prepared either from allyl chloride or glycerol.

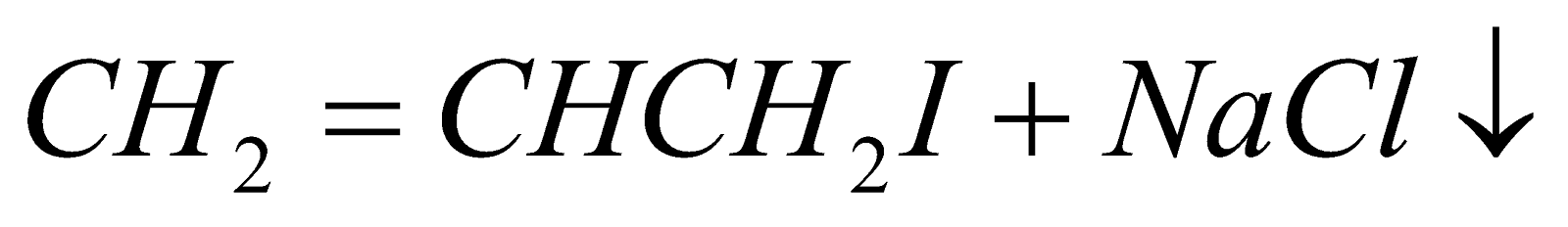 (Insoluble in acetone)
(Insoluble in acetone)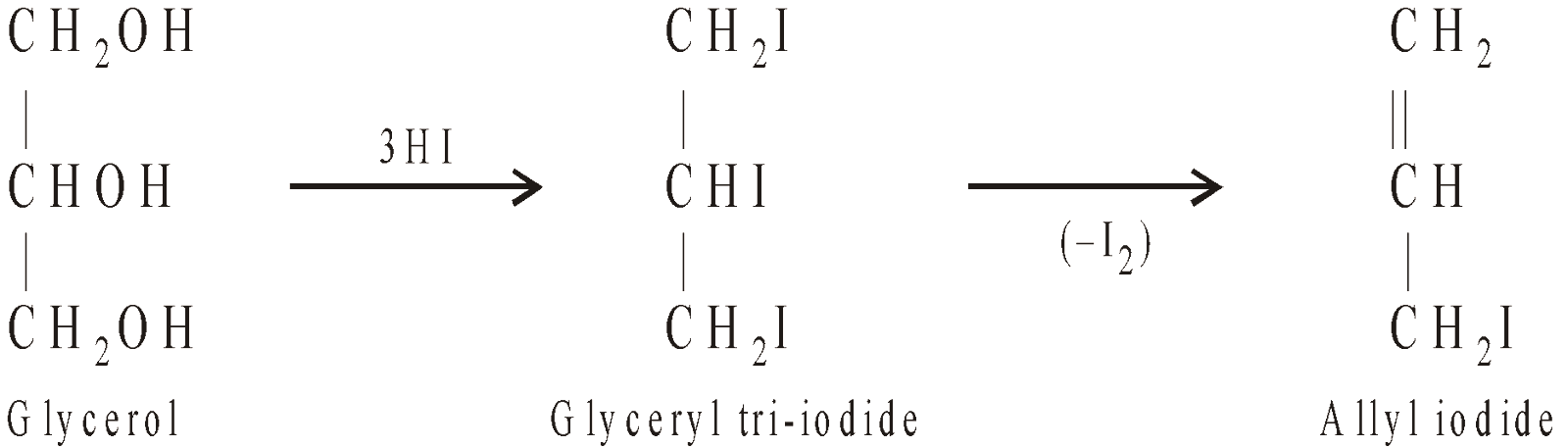
Allyl halides give the usual addition reactions of the double bond and also nucleophilic substitution. Allyl halides undergo nucleophilic substitutions much faster than the alkyl halides because the intermediate allyl carbocation stabilises due to resonance.
ISOMERIC DICHLOROETHENES
1,2-dichloroethene (acetylene dichloride)
1,2-Dichloroethene exists in two geometrical isomeric forms, i.e. cis- and trans-.
It is used as a solvent.
1,1-dichloroethene (vinylidene dichloride)
1,1-Dichloroethene does not show cis, trans-isomerism.
It is used in the manufacture of saran wrap used for food packaging.
It is used in the manufacture of saran wrap used for food packaging.
TYPES OF HALOGENS
Halogen present in organic compounds can be of three types-
- Ionic halogens as in benzene diazonium halides and quaternary ammonium halides . Aqueous solution of such halides when treated with AgNO3 solution in presence of dil. HNO3 give white or yellow precipitate in cold.
- Labile halogens as in alkyl halides (R – X), allyl halides and benzyl halides (C6H5CH2X). These halogens are also precipitated as silver halides but under strong conditions. Organic halide is boiled with aqueous KOH solution, cooled, acidified with dil. HNO3 and then treated with AgNO3 solution; appearance of a white or yellow precipitate indicates labile halogen.
- Inert halogen as in aryl halides (ArCl) and vinyl halides (CH2=CHX). These compounds give precipitate with AgNO3 solution neither in cold nor on heating.