About this unit
Tetravalency of carbon; Shapes of simple molecules – hybridization (s and p). Classification of organic compounds based on functional groups: – C = C – , – C ? C – and those containing halogens, oxygen, nitrogen and sulphur; Homologous series. Isomerism: structural and stereoisomerism. Nomenclature (Trivial and IUPAC): Covalent bond fission – Homolytic and heterolytic: free radicals, carbocations and carbanions; stability of carbocations and free radicals,electrophiles and nucleophiles. Electronic displacement in a covalent bond: Inductive effect, electromeric effect, resonance and hyperconjugation Common types of organic reactions: Substitution, addition, elimination and rearrangement.
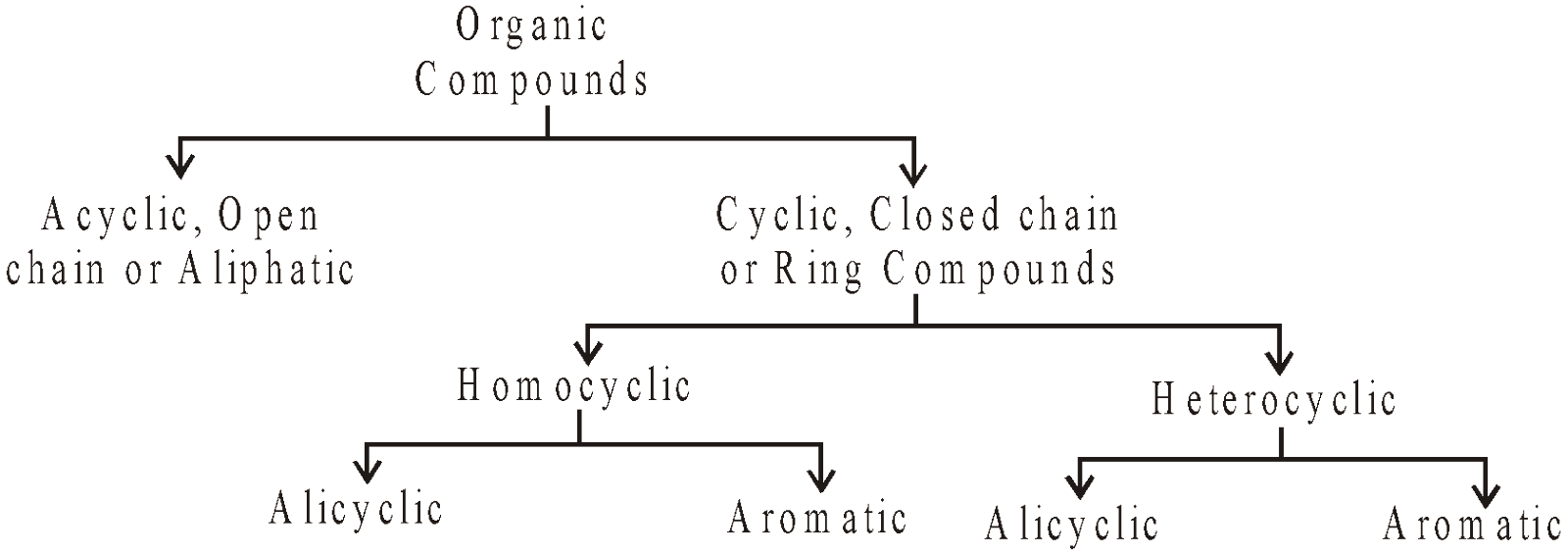
CLASSIFICATION AND NOMENCLATURE OF ORGANIC COMPOUNDS
CLASSIFICATION OF ORGANIC COMPOUNDS
The ability of carbon to combine with large number of elements especially O, N, S, X etc, to undergo catenation to form chains of varying lengths and shapes and existence of isomers has led to the formation of more than five million organic compounds. These have been classified into the following main groups
ACYCLIC OR OPEN CHAIN COMPOUNDS
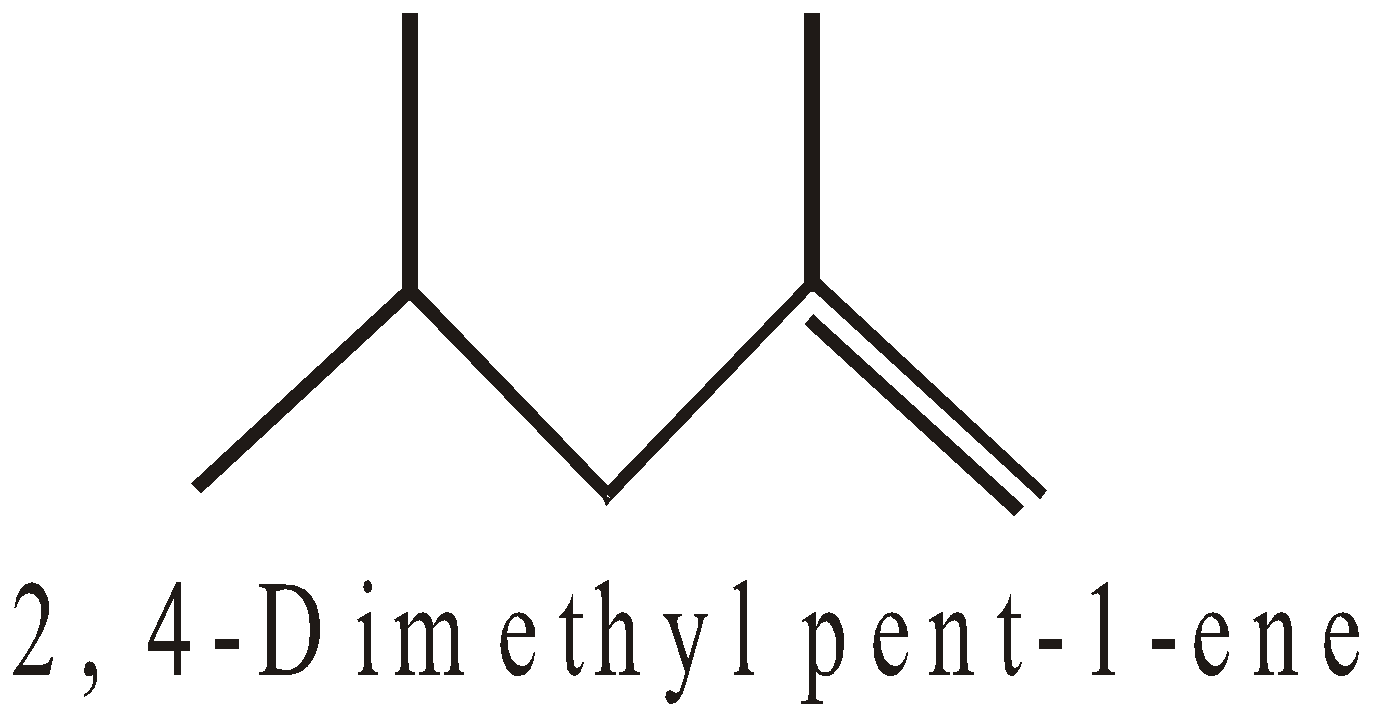
CYCLIC OR CLOSED CHAIN COMPOUNDS


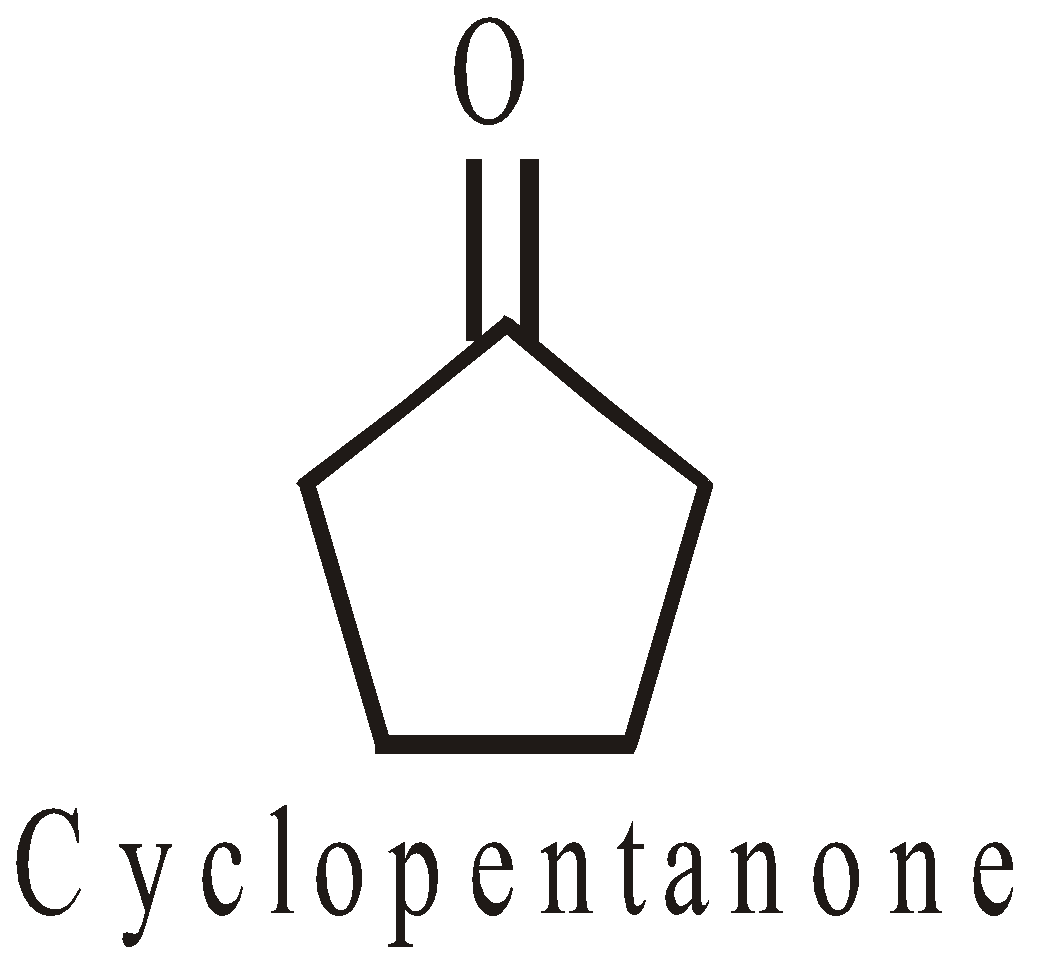
HOMOCYCLIC COMPOUNDS
The ring system is made up of one type of atoms generally carbon
- Alicyclic : The cyclic compounds resembling open chain aliphatic compounds. For example: Cycloalkanes
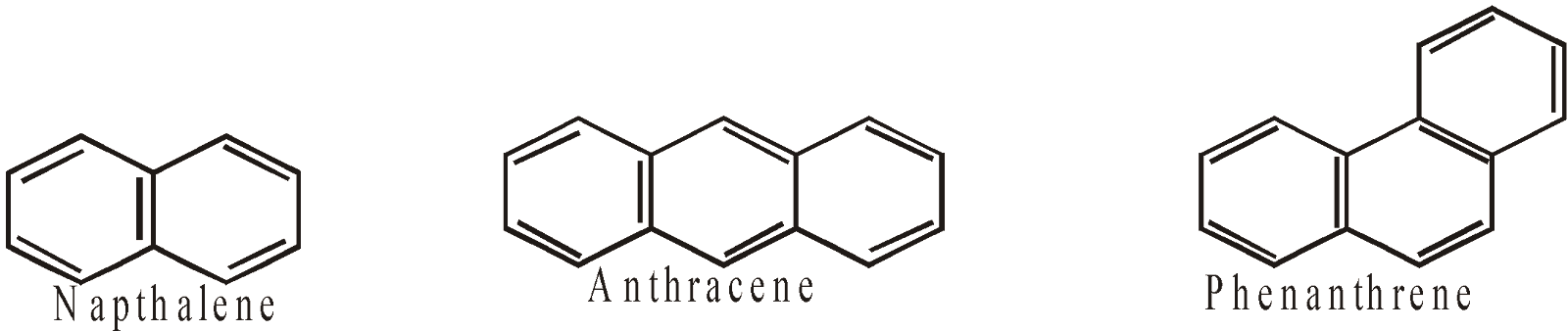
- Aromatic : The benzene, naphthalene and their derivatives etc are homocyclic aromatic compounds
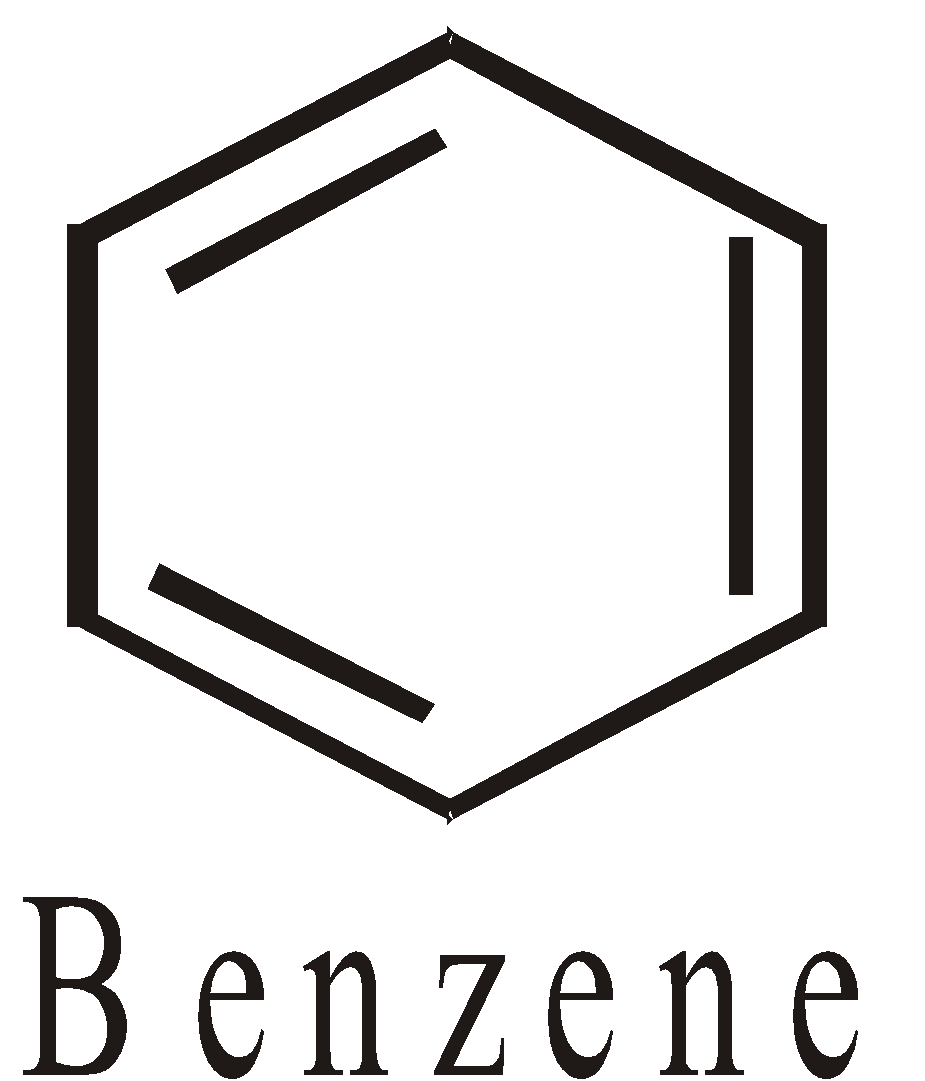
HETEROCYCLIC COMPOUNDS
The ring system is made up of two or more than two types of atoms. They may be
- Alicyclic

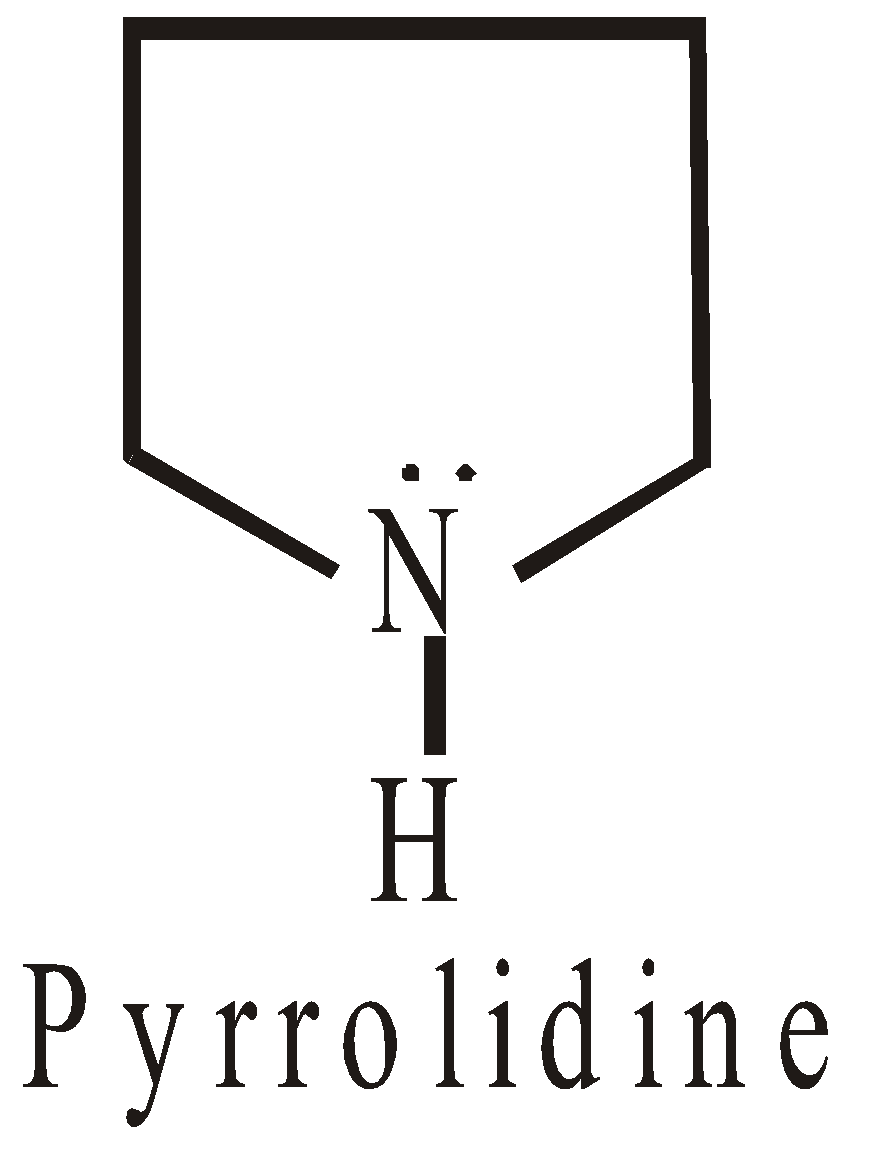
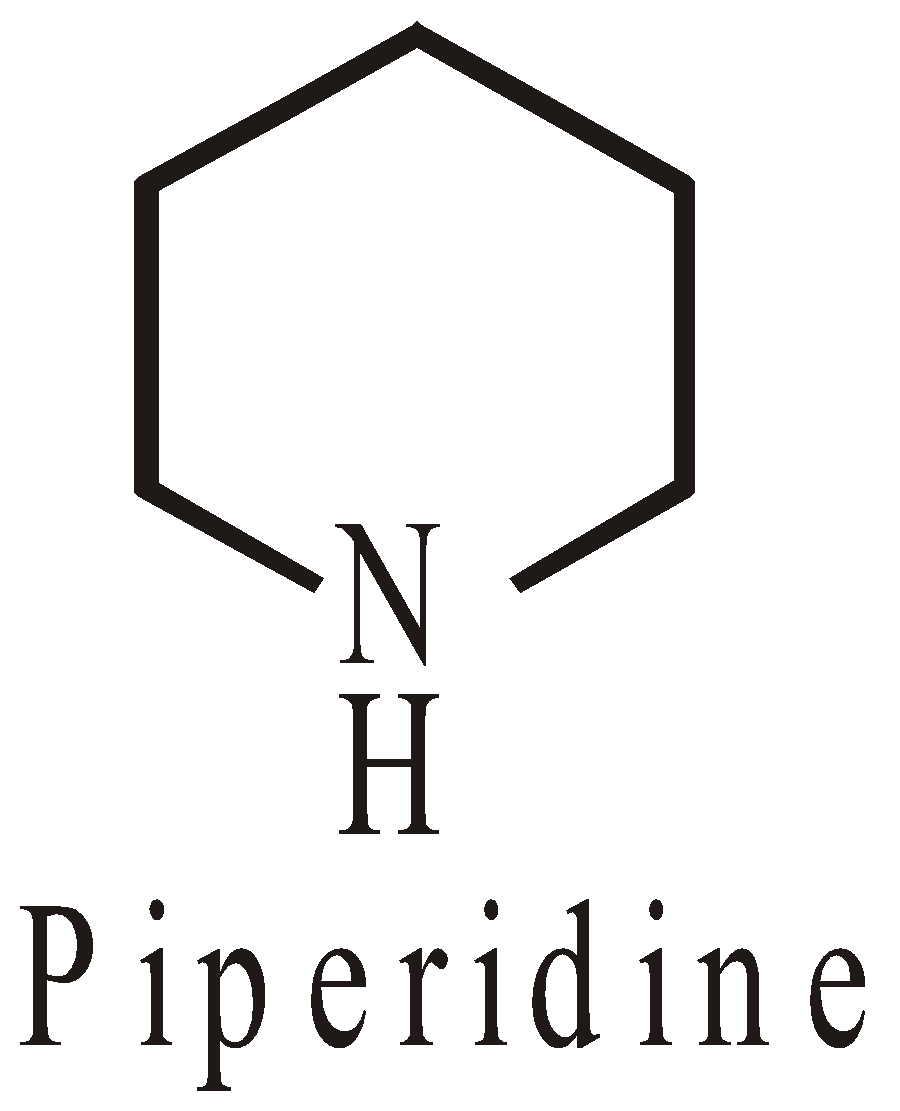

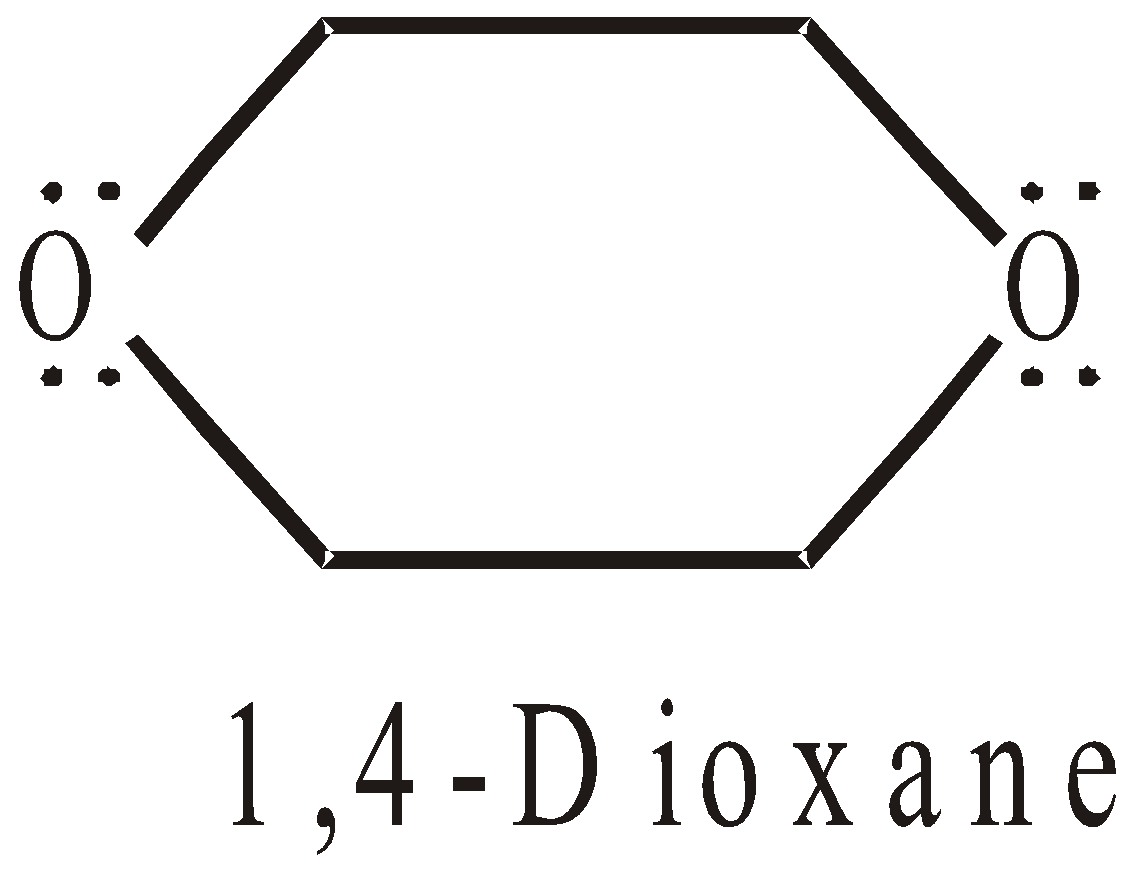
- Aromatic
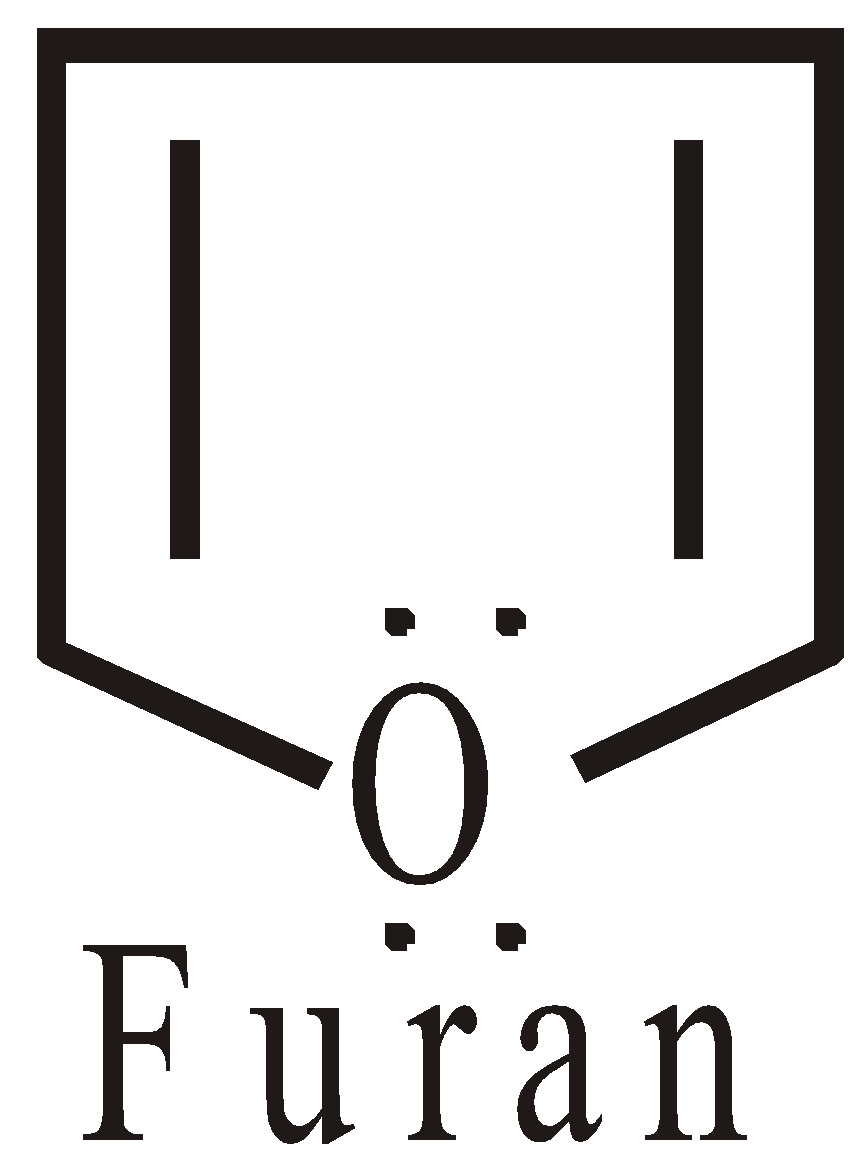
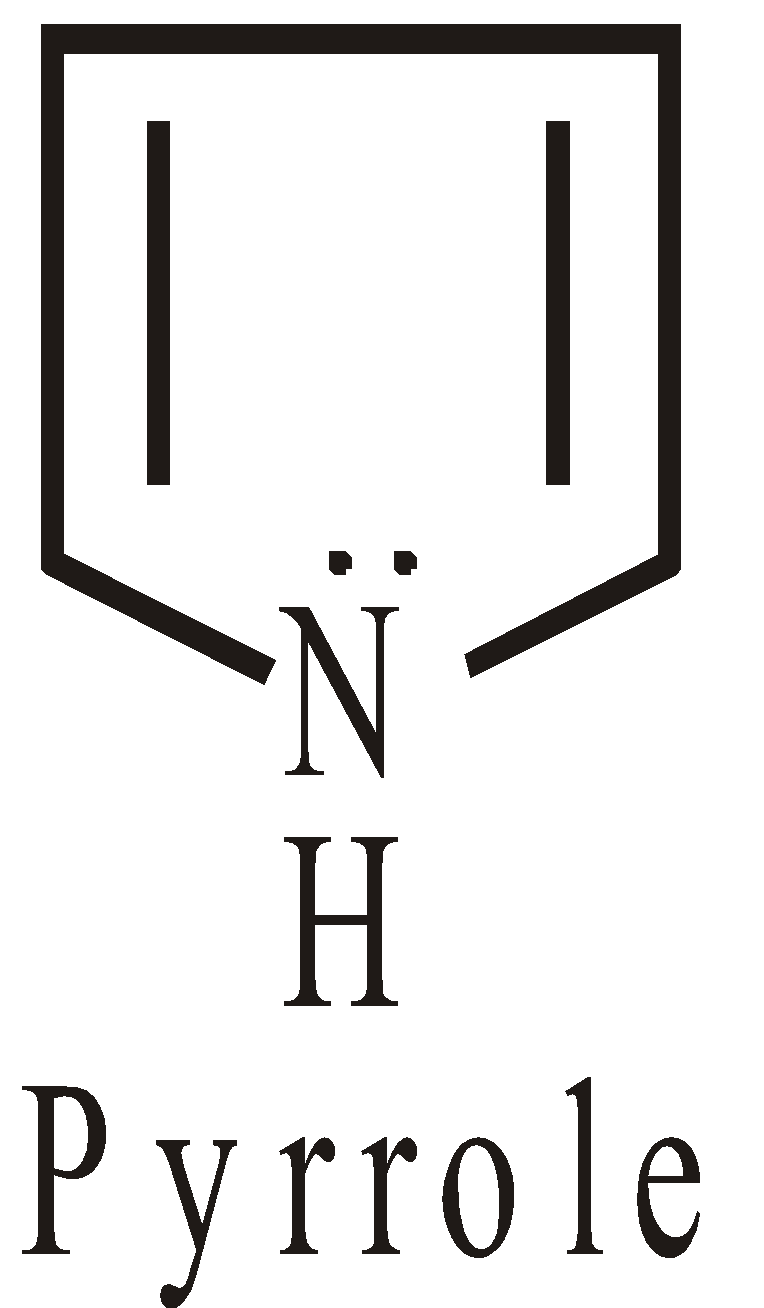


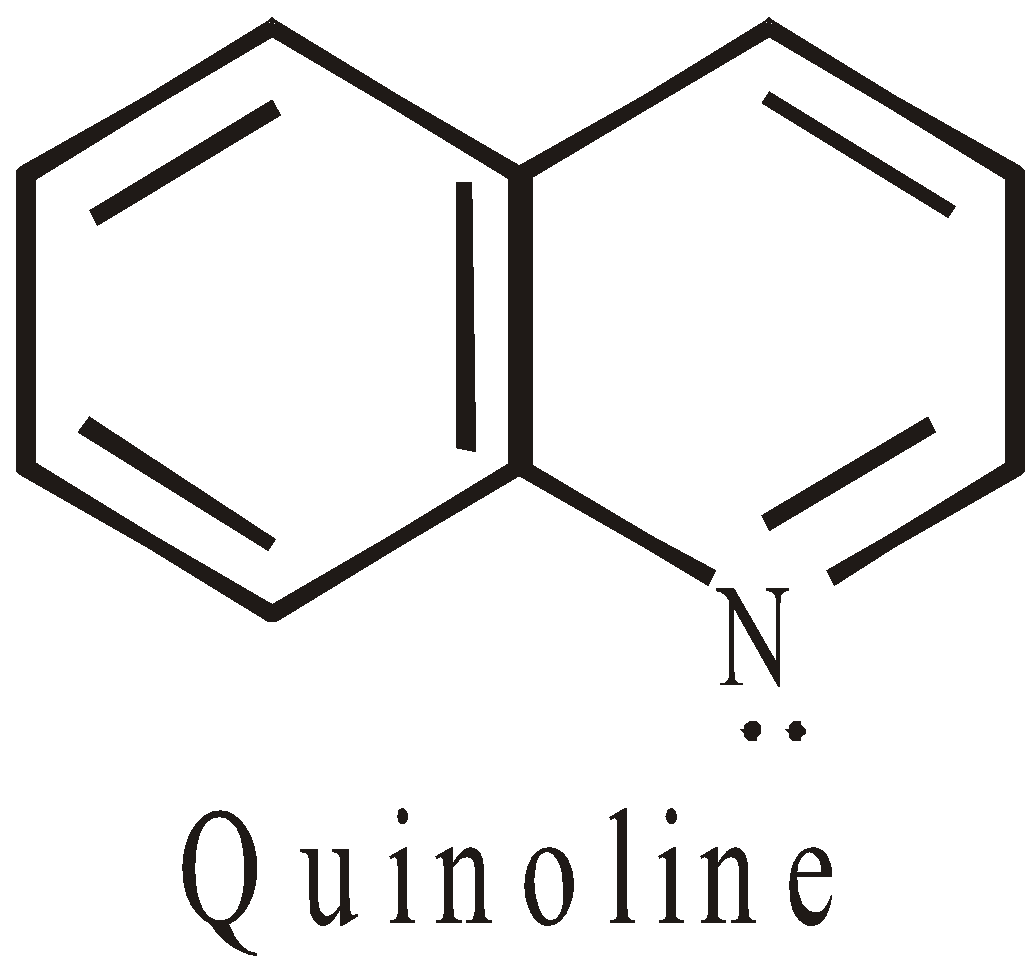
CLASSIFICATION BASED ON FUNCTIONAL GROUPS
On the basis of functional groups which confer characteristic properties on them, the organic compounds have been classified as follows
Class | Functional group |
Halides | X (Cl, Br, I) Halo |
Esters | |
Olefins Alkenes | >C = C< |
Acid halides | |
Acetylenes/ Alkynes | |
Anhydrides | |
Alcohols | —OH (Hydroxy) |
Amines | —NH2 |
Aldehydes | |
Ketones | |
Sulphonic acid | —SO3H |
Acids | |
Amides |
HOMOLOGOUS SERIES
A group of a particular class of compounds where a preceding or succeeding member differ by one–CH2. The members of the series are known as homologues. The homologues
- have the same general formula CnH2n+2 or CnH2n+1 X
- molecular weight differing by 14 of two successive members
- can be prepared by general methods of preparation
- have almost similar chemical properties
- show regular gradation in physical properties such as mpt, bpt, density etc
NOMENCLATURE
The most widely accepted and the latest system of naming organic compounds is IUPAC (International Union of Pure and Applied Chemists) system, according to which the name essentially consists of three parts.
WORD ROOT
It indicates the nature of the basic carbon skeleton. From C1 to C4 common names have been retained and from C5 upwards Greek number roots have been used
Chain length | Word root | Chain length | Word root |
C1 | Meth- | C7 | Hept- |
C2 | Eth- | C8 | Oct- |
C3 | Prop- | C9 | Non- |
C4 | But- | C10 | Dec- |
C5 | Pent- | C11 | Undec- |
C6 | Hex- | C12 | Dodec- |
The generic word root for any carbon chain is “alk”.
SUFFIX
These are of two types
PRIMARY SUFFIX
It is added to the word root to designate saturation or unsaturation in a carbon chain
Type of Carbon chain | Primary Suffix | Generic name |
Saturated | – ane | Alkane |
Unsaturated with one C=C | – ene | Alkene |
Unsaturated with one C≡C | – yne | Alkyne |
SECONDARY SUFFIX
It is added to indicate the functional group present in the compound. The terminal ‘e’ is dropped, if secondary suffix begins with a vowel (a, e, i, o, u, y) but it is retained if the secondary suffix begins with a consonant.
Functional Group | Secondary Suffix | Generic name |
– OH | – ol | Alkanol |
– CHO | – al | Alkanal |
>C = O | – one | Alkanone |
– COOH | – oic acid | Alkanoic acid |
– COX | – oyl halide | Alkanoyl halide |
– CONH2 | – amide | Alkanamide |
– COOR | – alkyl — – oate | Alkyl alkanoate |
– (CO)2O | – oic anhydride | Alkanoic anhydride |
– CN | – nitrile | Alkane nitrile |
– SH | – thiol | Alkanethiol |
– NH2 | – amine | Alkanamine |
PREFIX
They are of two types
PRIMARY PREFIX
It is for cyclic nature of the compound and primary prefix cyclo is used immediately before the word root. eg.:
Primary prefix | Word root | Prim. suffix | Sec. suffix | IUPAC name |
Cyclo | hex | ane | – | Cyclohexane |
SECONDARY PREFIX
The certain atoms and groups which are not considered as functional groups but are treated as substituents are called secondary prefixes. They are added before the word root in case of acyclic compounds and before the primary prefix in case of cyclic compounds in alphabetical order.
The important secondary prefixes are
Substituent | Sec. prefix |
– X (F, Cl, Br, I) | Halo |
– NO2 | Nitro |
– NO Nitroso | |
Diazo | |
| Alkoxy |
–R (CH3, C2H5, C3H7, etc) | Alkyl |
Thus the complete IUPAC name of an organic compound consists of the following parts
- Secondary prefix
- Primary prefix
- Word root
- Primary suffix
- Secondary suffix
For example:
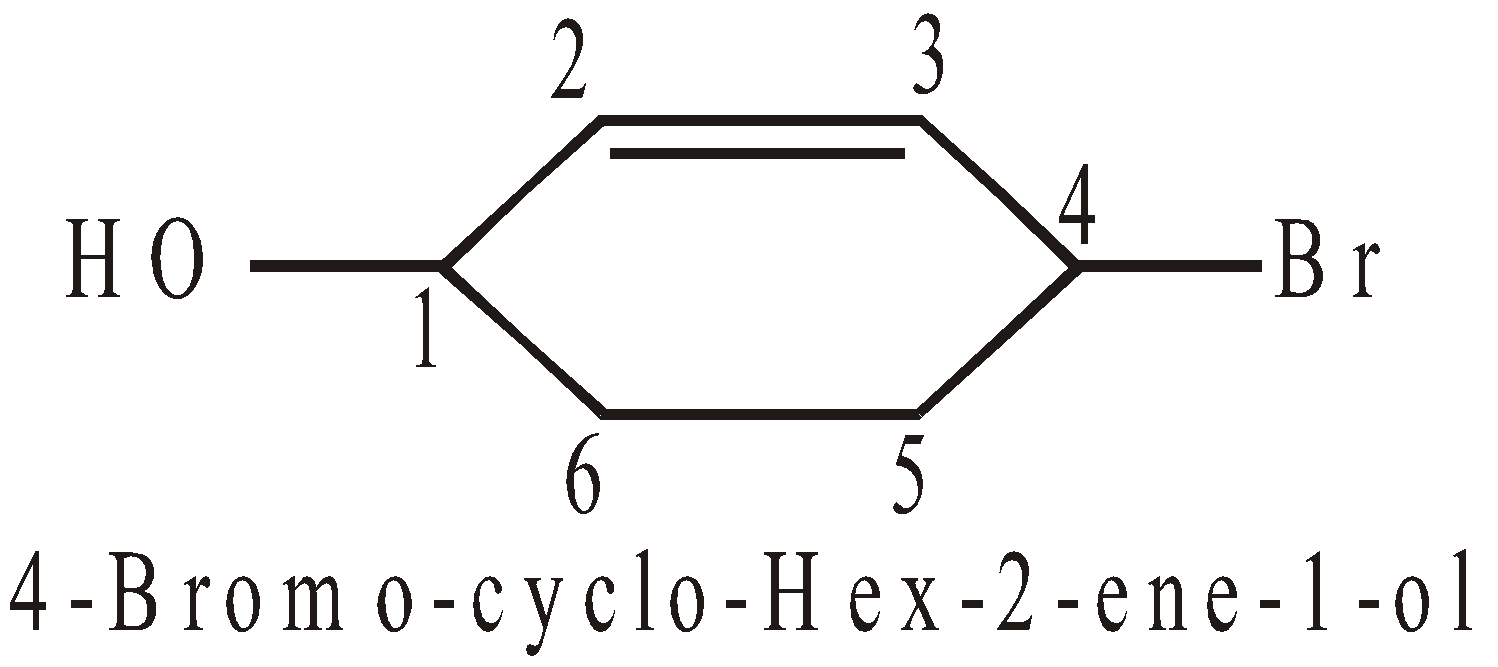
IUPAC name is 4-Bromocyclohex-2-ene-1-ol or 4-Bromo-2-Cyclohexenol
Primary prefix = Cyclo
Secondary prefix = 4-bromo
Word root = hex
Primary suffix = ene
Secondary suffix = ol
ALKYL GROUPS
Univalent groups formed by the removal of one hydrogen atom from an alkane are known as alkyl groups or alphyl groups. Their names are obtained by changing the suffix –ane of parent hydrocarbon by –yl.
Alkane | Group | Shorthand notation | IUPAC Name |
Methane | Methyl CH3– | Me | Methyl |
Ethane | ethyl C2H5– | Et | Ethyl |
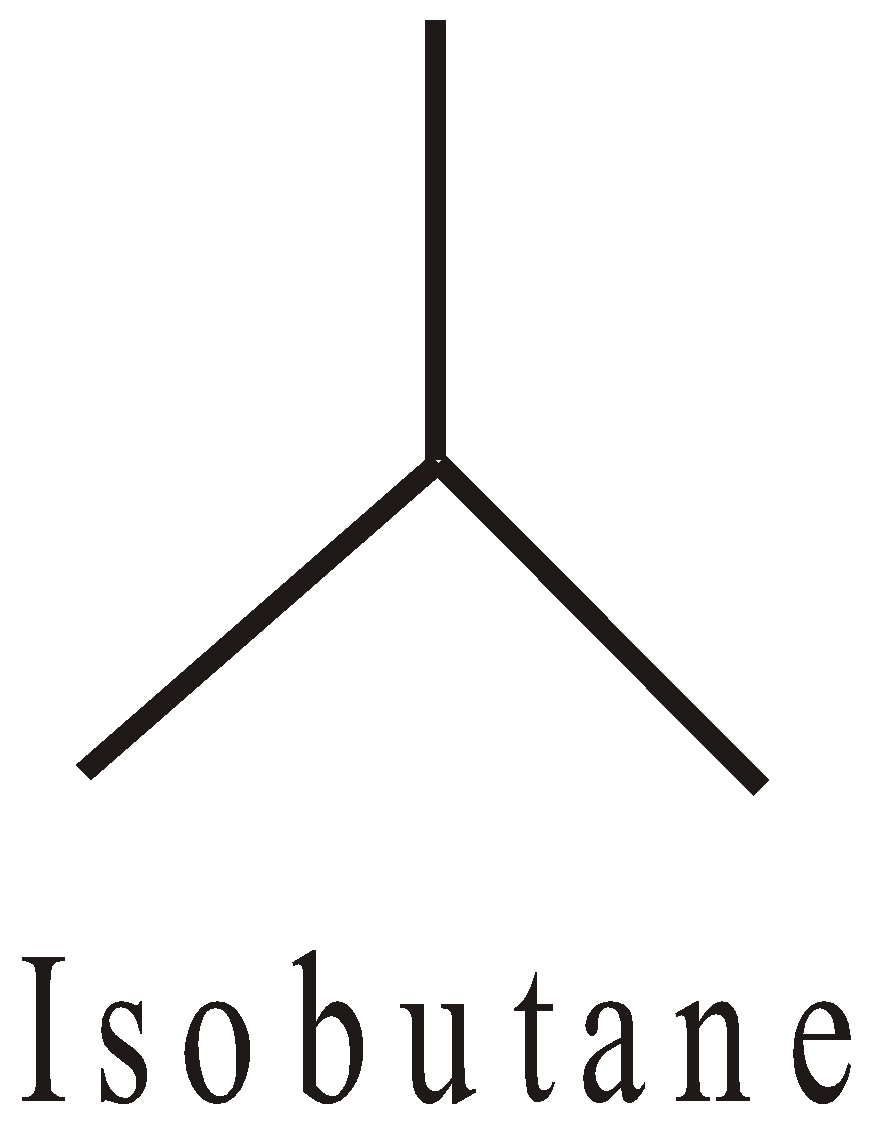
Propane | n-propyl CH3CH2CH2– Isopropyl | n-Pr, Iso- | 1-propyl 2-propyl |
Butane | n-butyl CH3CH2–CH2–CH2– s-butyl Iso-butyl (CH3)2CH–CH2– t-butyl (CH3)3C– | –n-Bu, s-Bu, or Iso-Bu, or Bui t-Bu, But | 1-butyl 1-methyl propyl 2-methyl propyl 1,1-dimethyl ethyl |
LINE ANGLE FORMULA
Bonds are represented by lines, carbon atoms are assumed to be present at the start and finish of a line. Nitrogen, oxygen and halogens are labelled, but hydrogens are only shown when bonded to a drawn atom. Each atom is assumed to have sufficient hydrogen atoms around it to make it neutral. For example:
n-Hexane CH3(CH2)4CH3 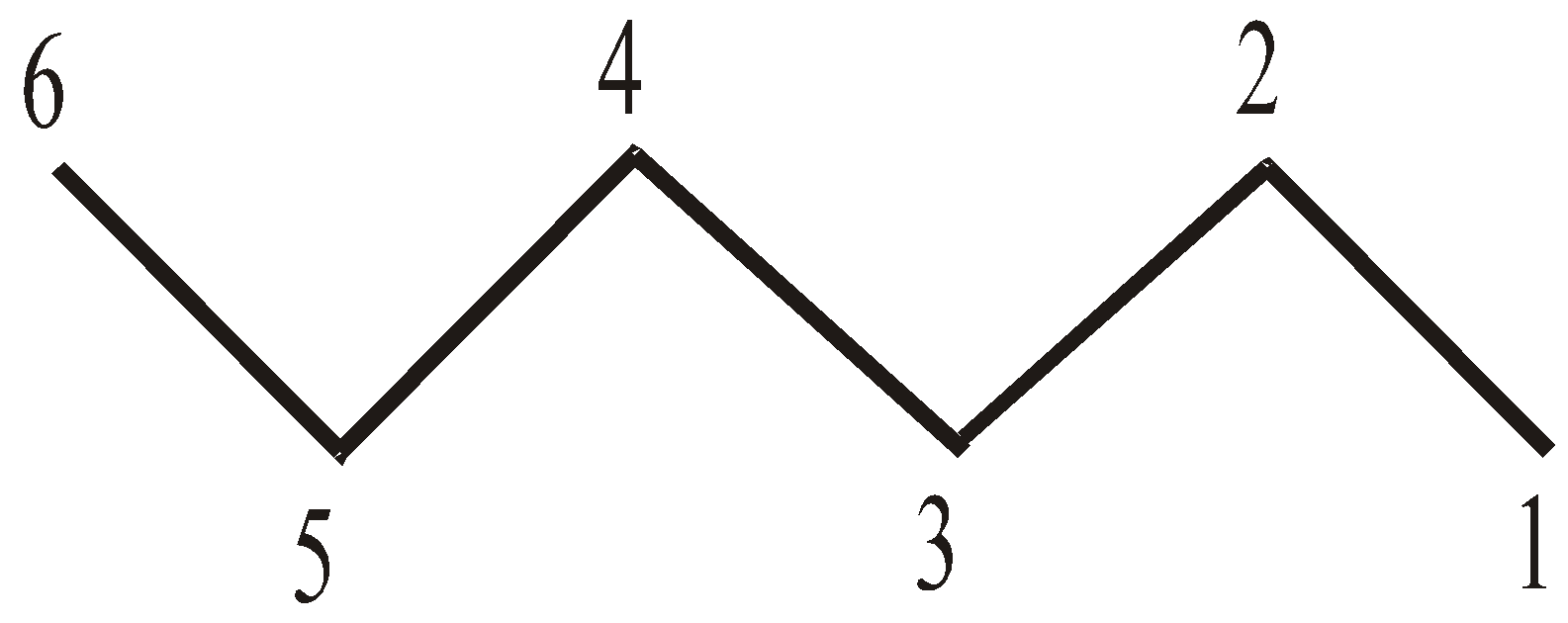
2-Cyclohexenone 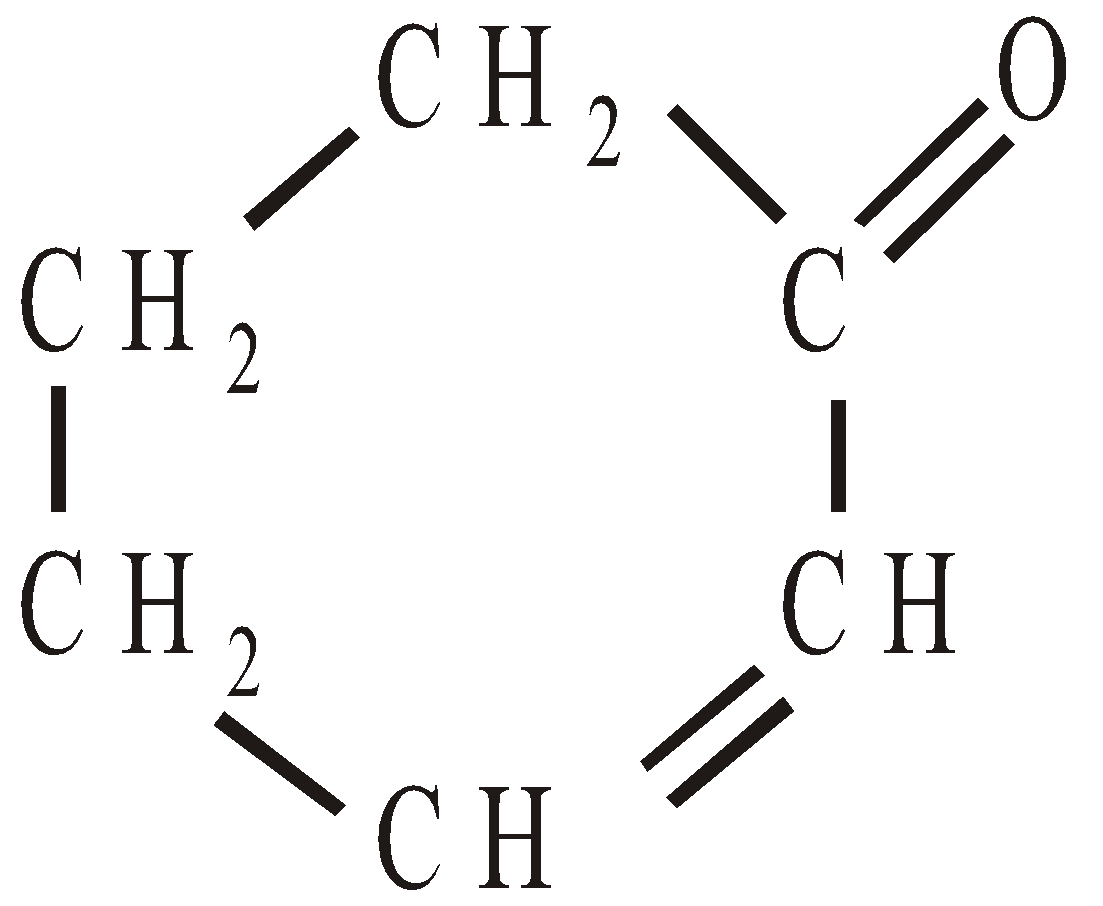
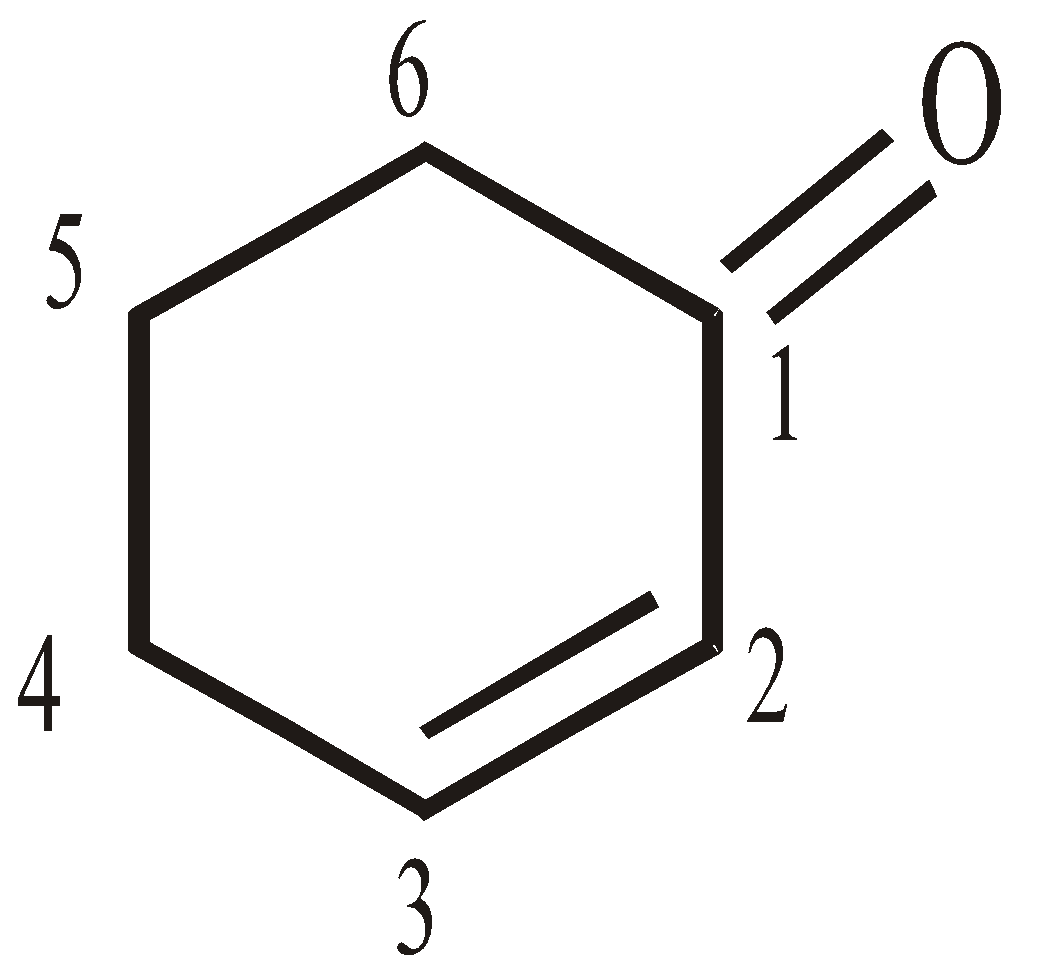


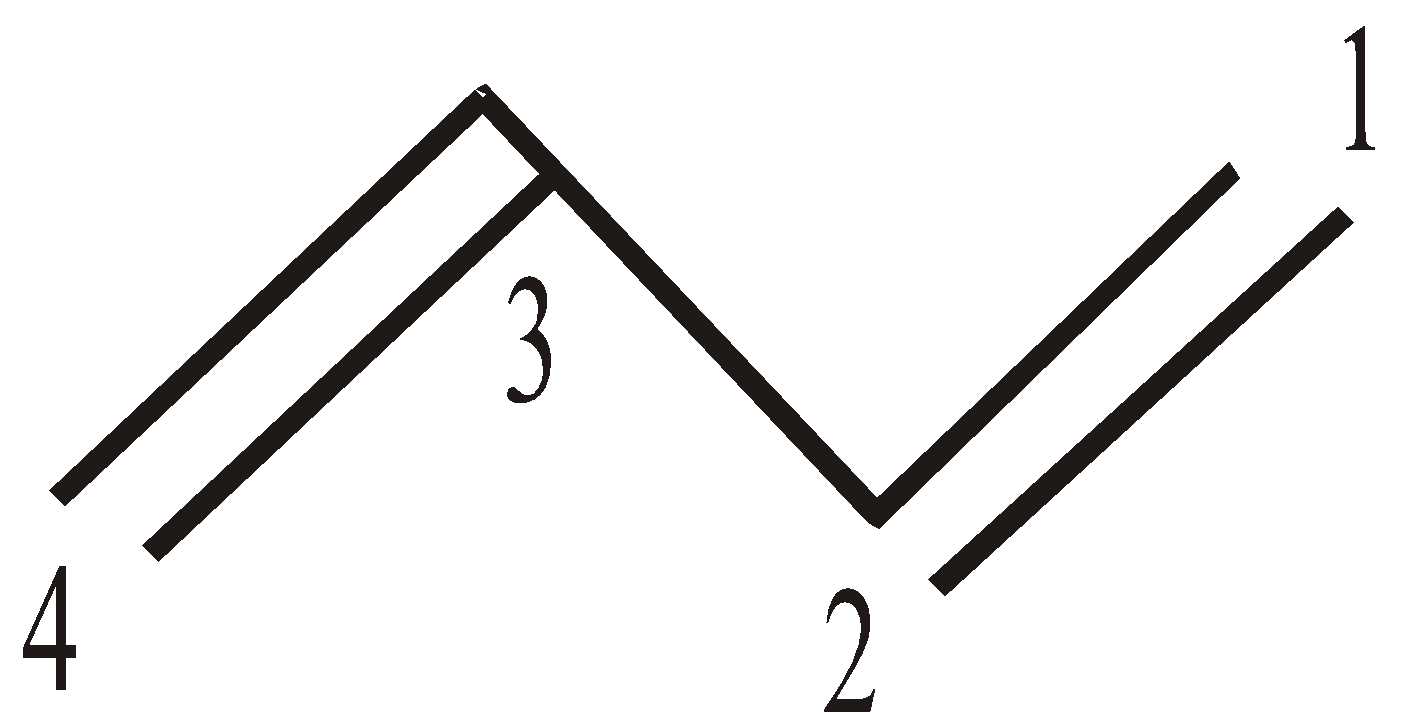
But-1, 3-diene 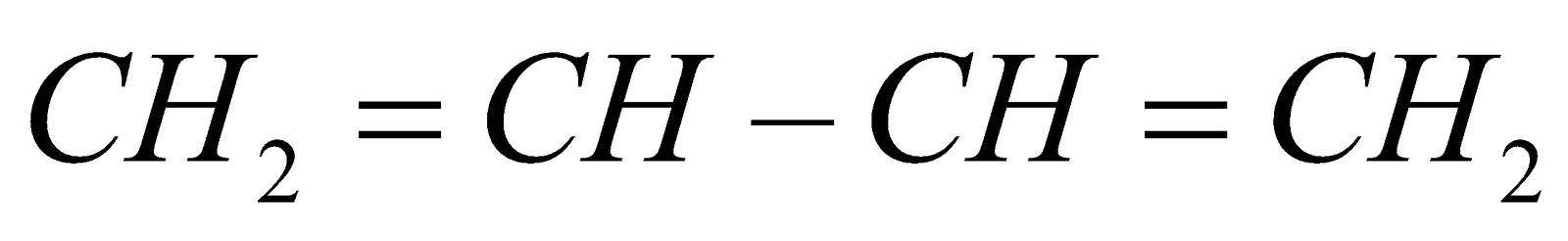
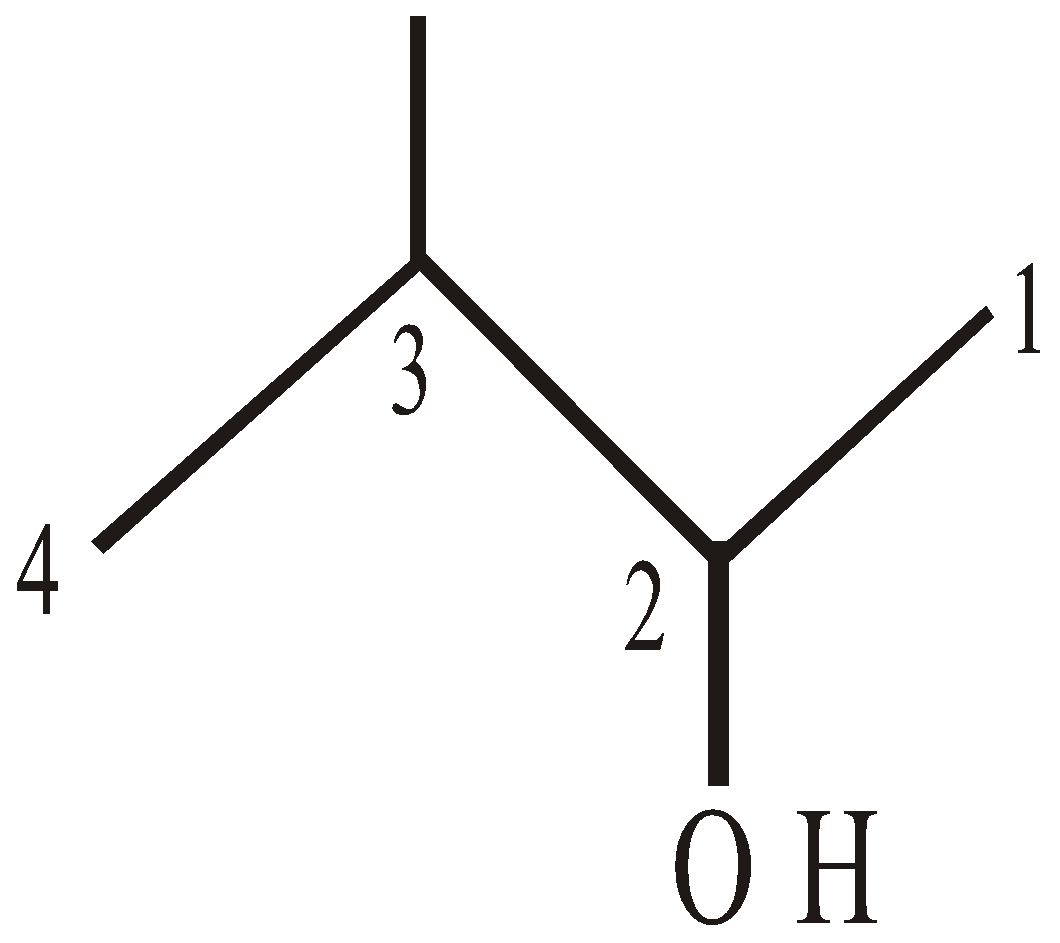
3-methyl but-2-ol 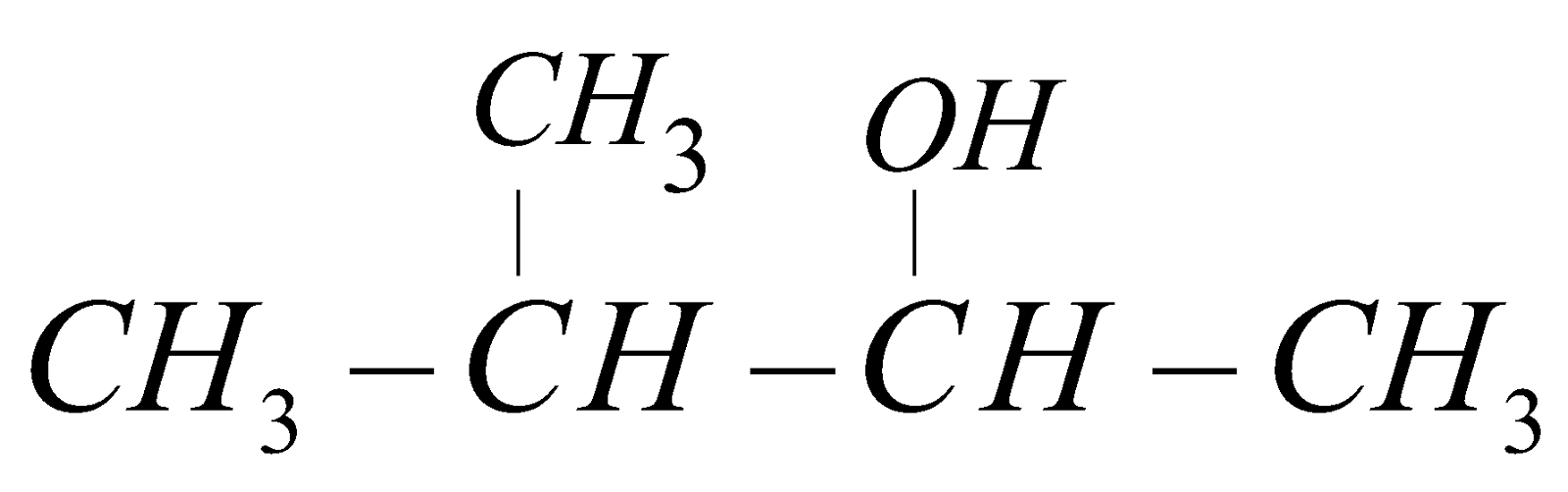

NOMENCLATURE OF COMPLEX HYDROCARBONS
The following rules are followed
- Longest chain rule : The longest continuous chain of carbon atoms is picked up which forms the base name of the compound.
- Numbering : The longest chain is numbered by arabic numerals beginning with the end nearest a substituent.
- If two or more side chains are in equivalent positions, then the one cited first in the name is assigned the lower number.
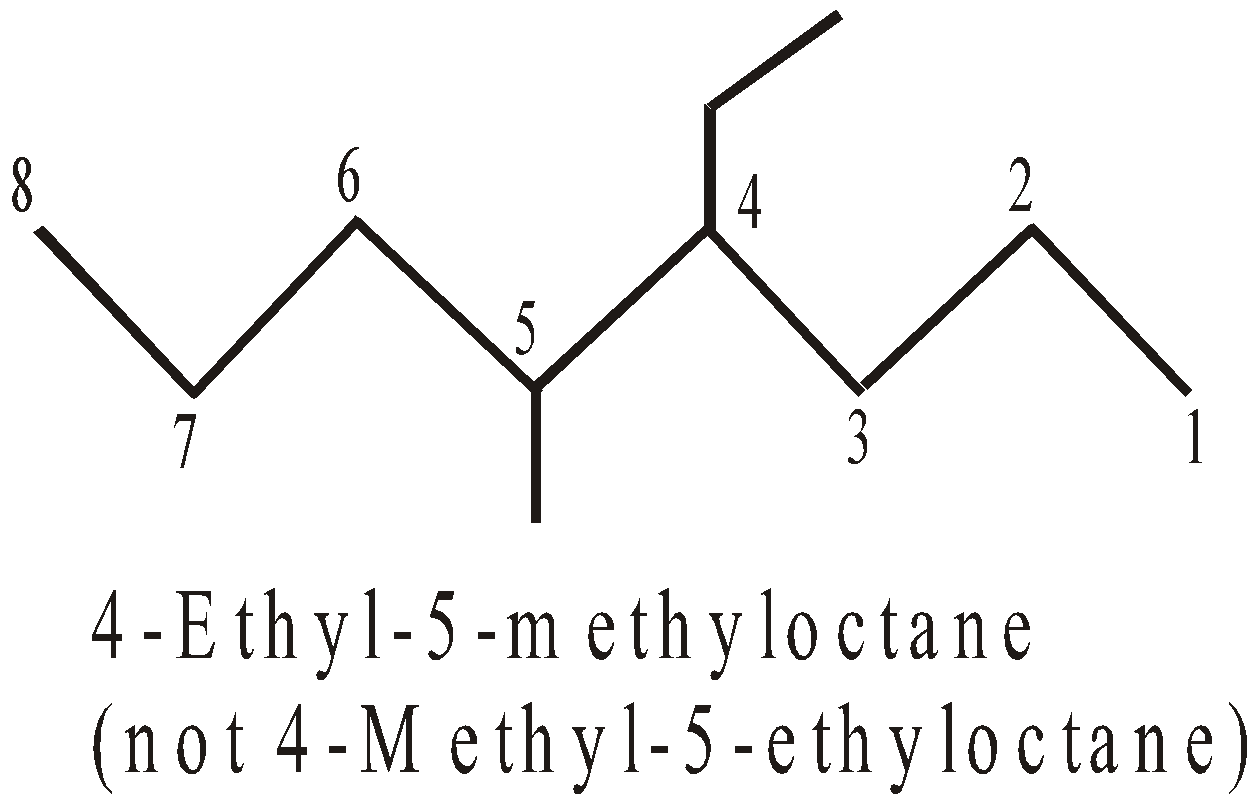
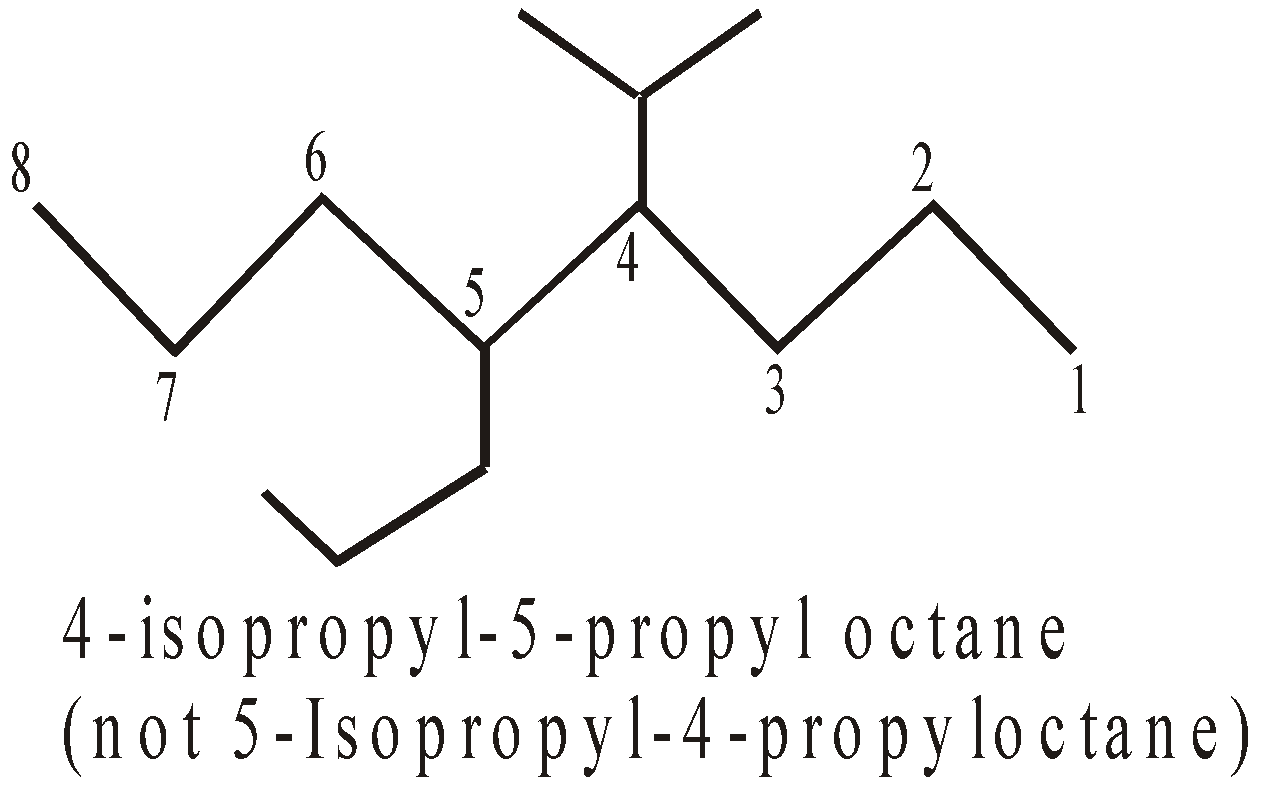
- If two or more of the same alkyl groups are present, use the prefixes di, tri etc to avoid repetition
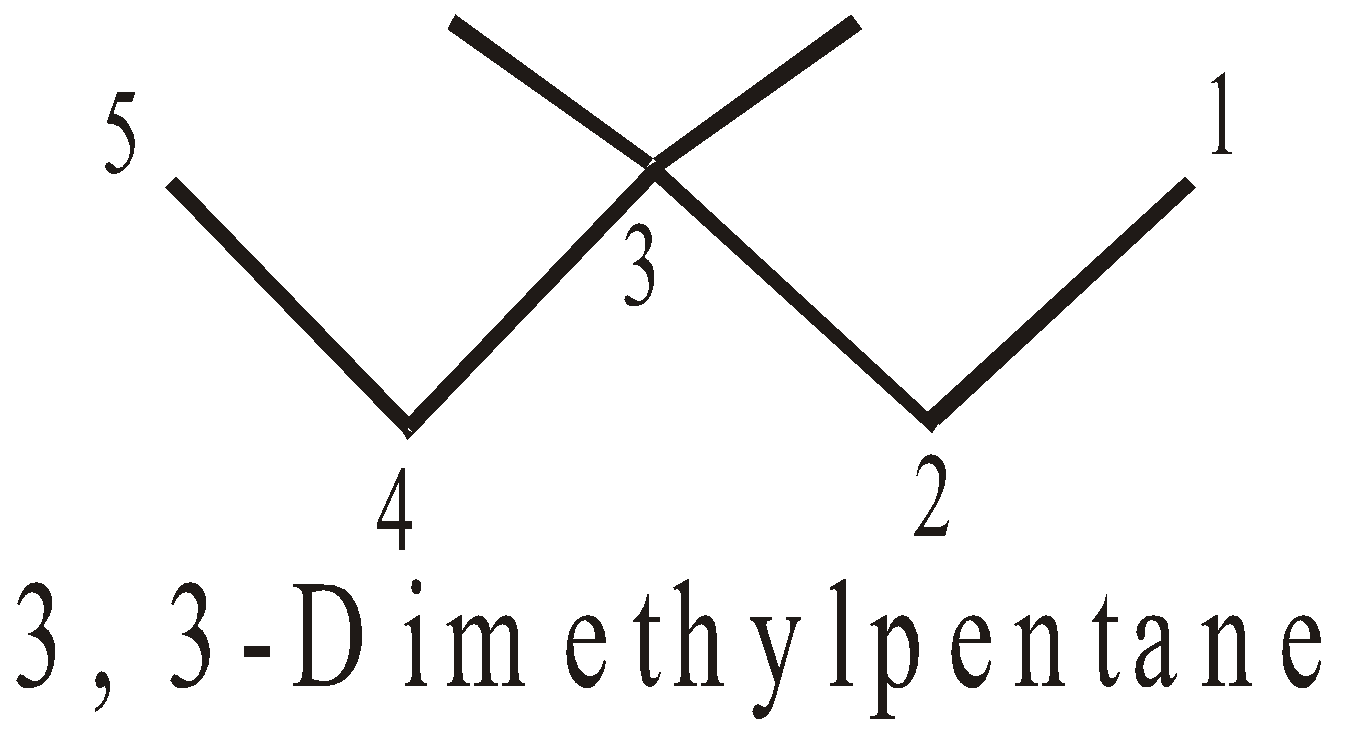
- Alphabetical order : The side chains are cited in alphabetical order
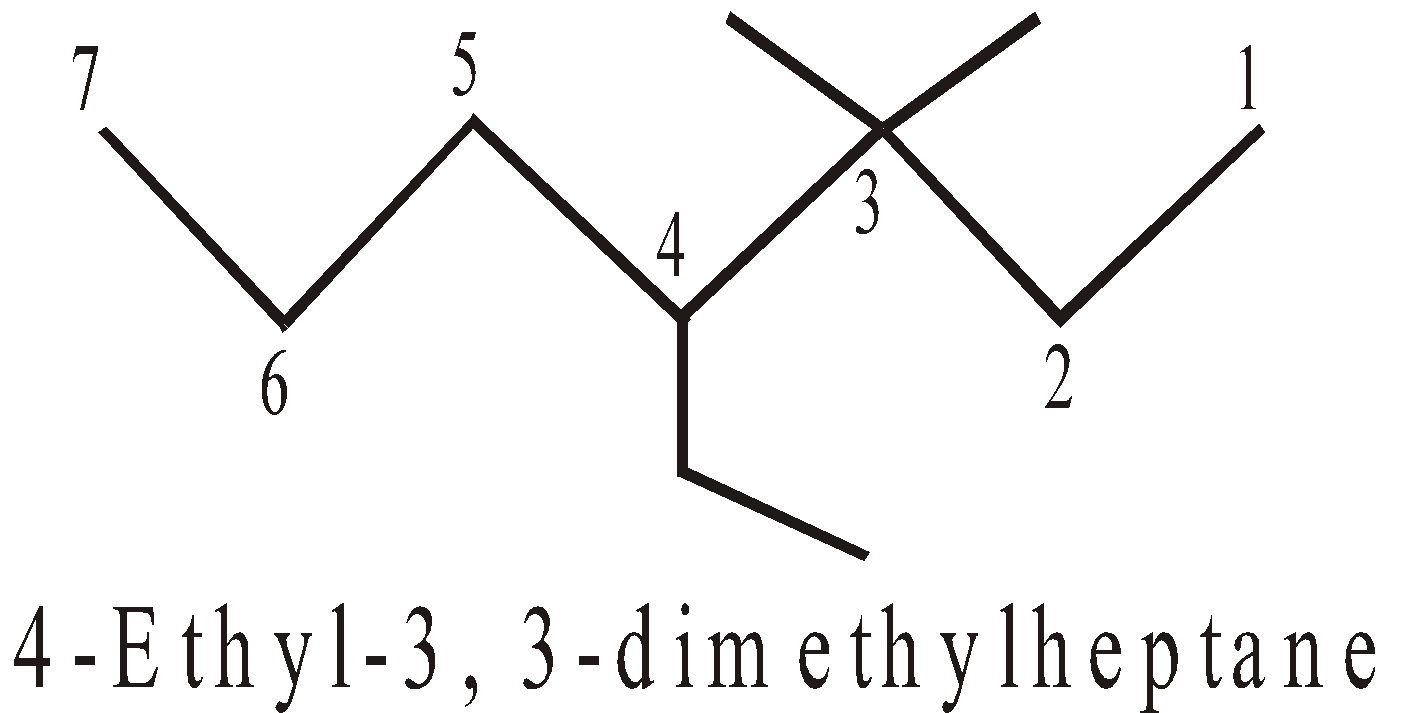
- Longest chain with maximum number of side chains : If two or more chains of the same length are possible, choose the one with maximum number of side chains.
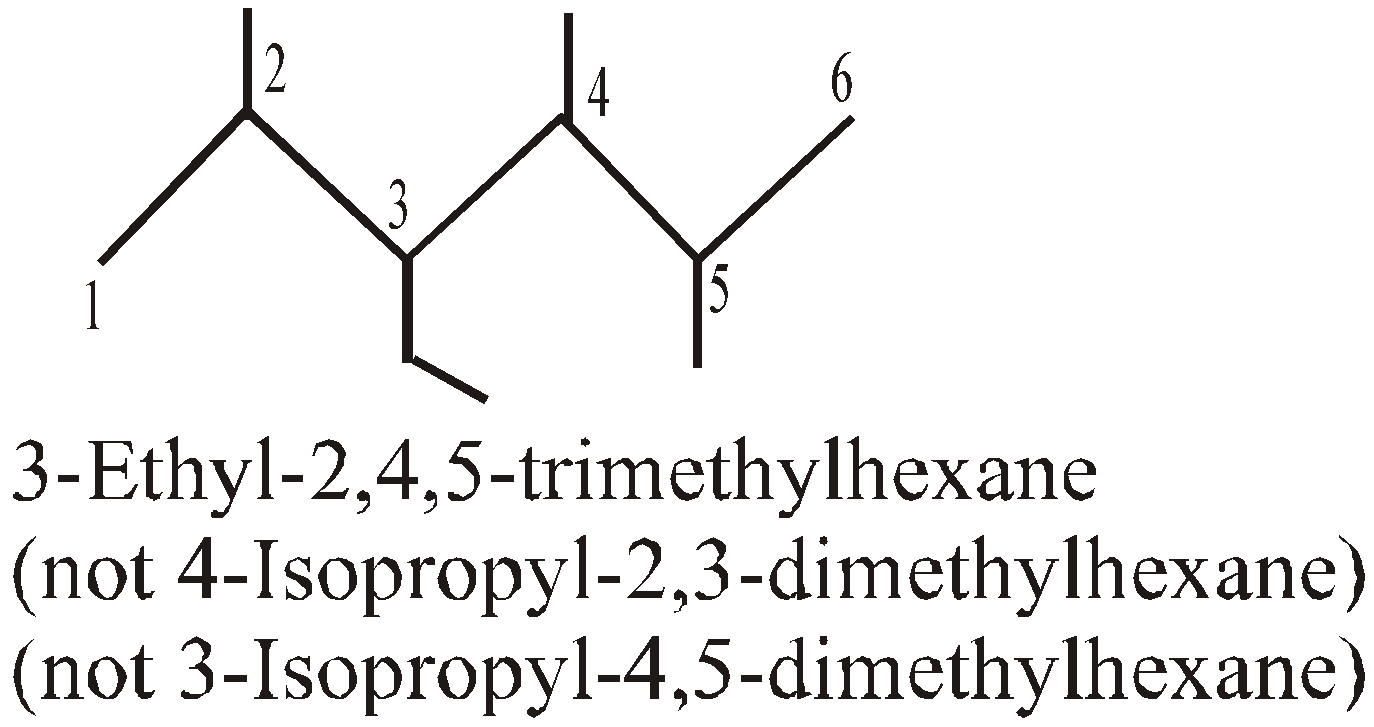
- Locant sum : Sum of the locants must be minimum. But out of two sets of the sum of the locants, the set having the lowest number when compared by term is preferred. For example out of (2+6+7=15) and (3+4+7=14), the first set is correct.
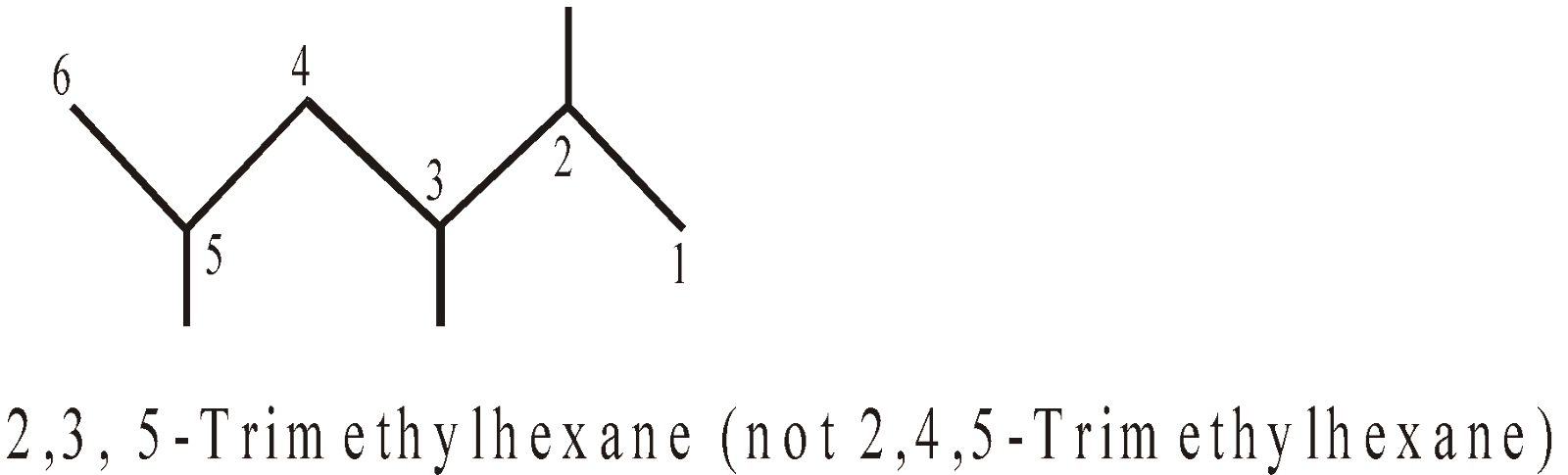
- The name of a complex radical is considered to begin with the first letter of its complete name i.e. including the numerical affix (di, tri, tetra etc are numerical affix) for alphabetical order.
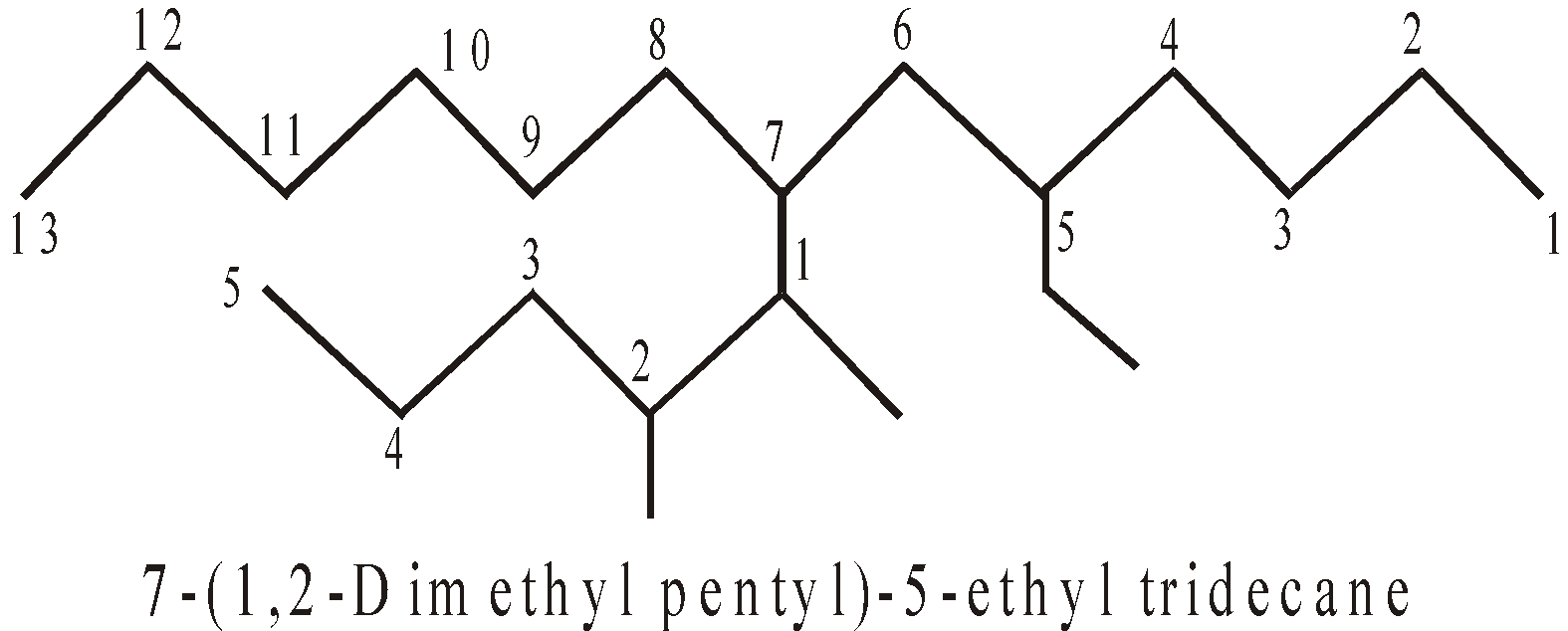
- When the side chains have the identical name the priority is given to side chain having lowest locant
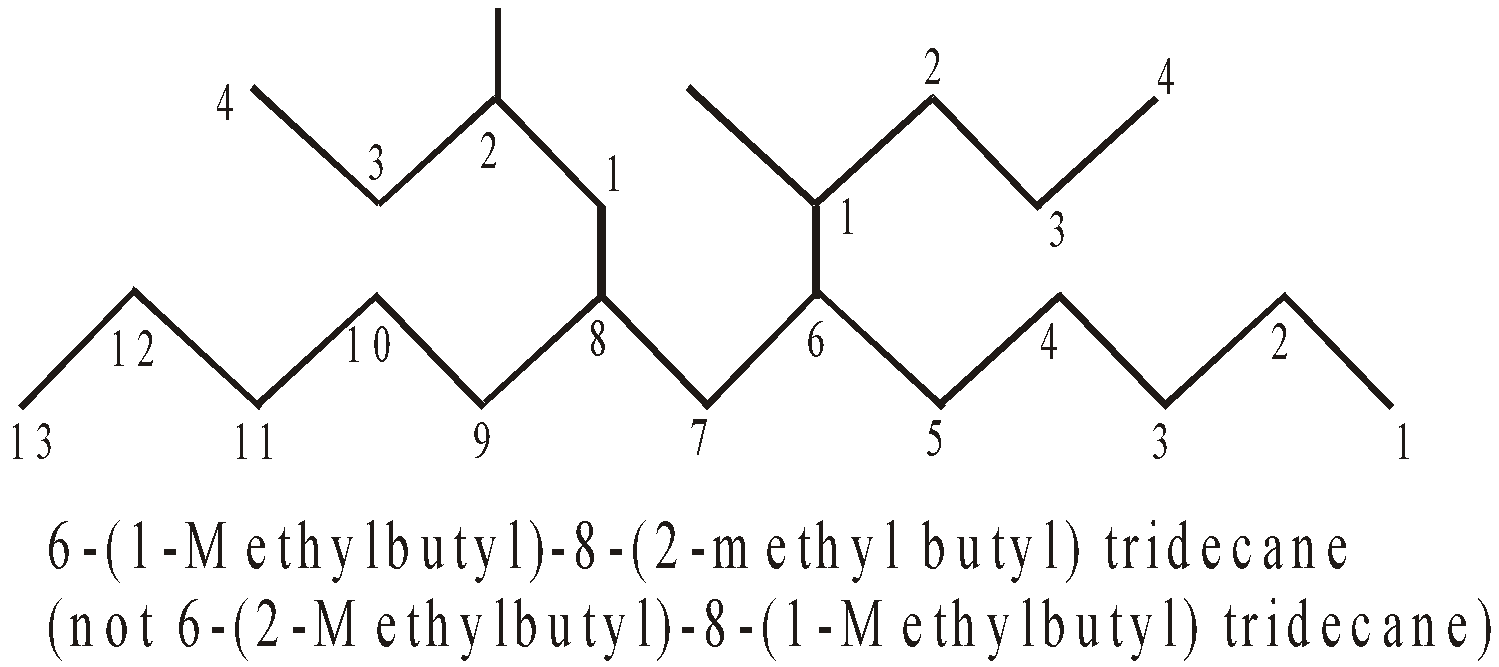
- The numerical prefixes bis, tris tetrakis are used to indicate the multiplicity of substituted substituent
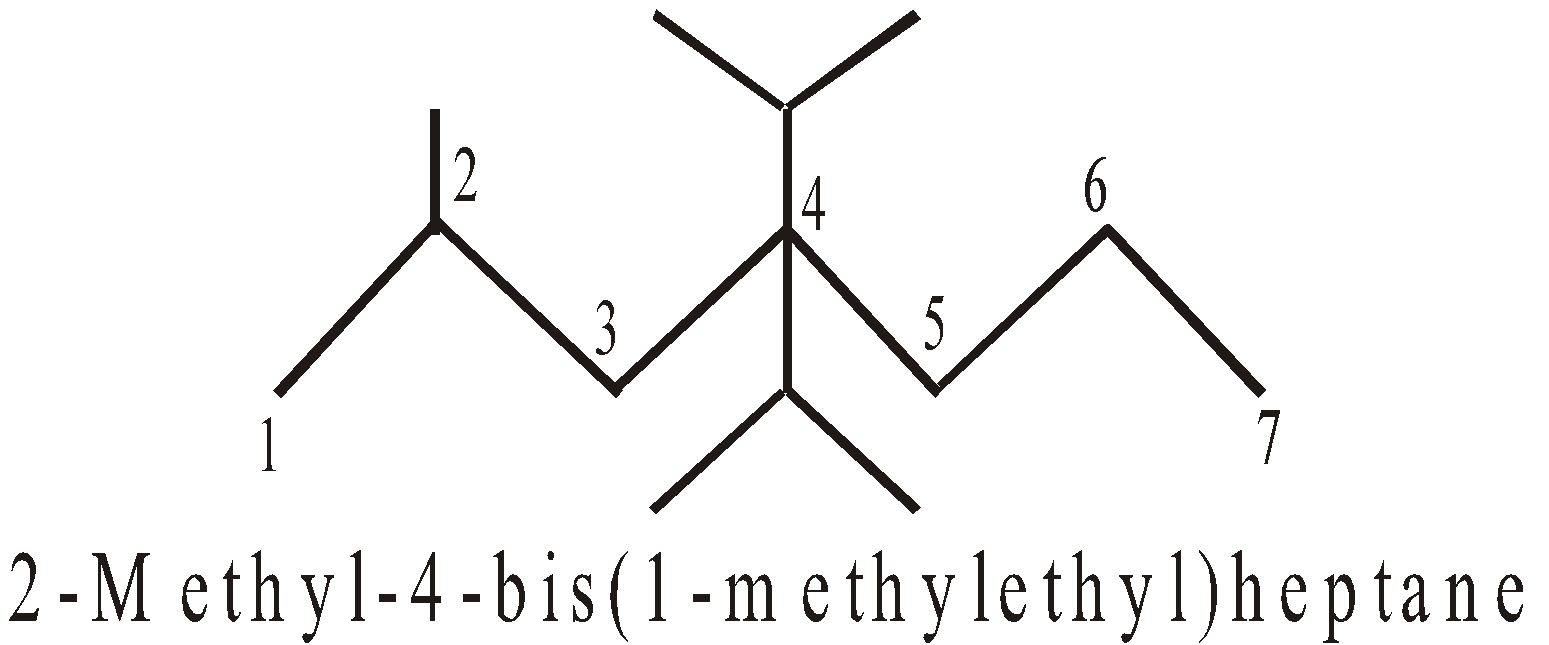
NOMENCLATURE OF COMPLEX ALKENES AND ALKYNES
- Selection of longest chain containing maximum number of double or triple bonds (sometimes longest chain rule is violated)
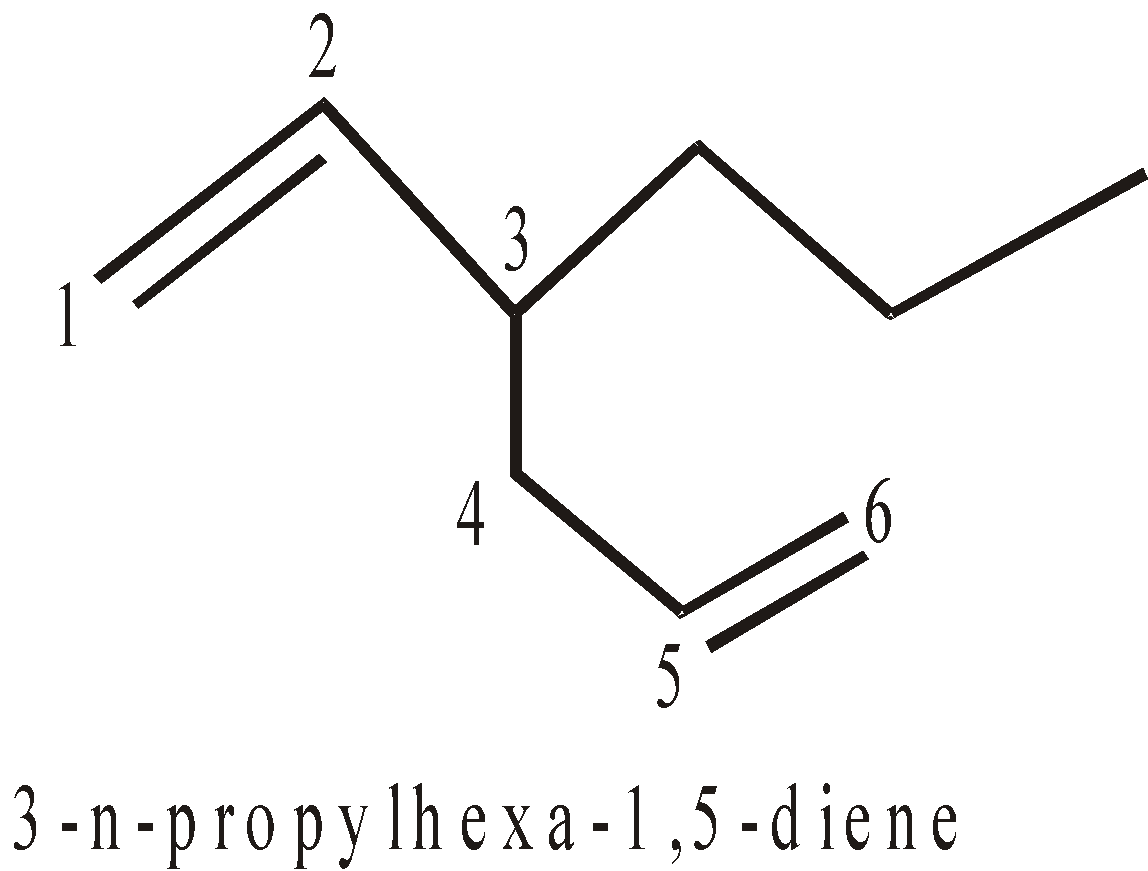
It contains longest chain of 7C atoms, but both the double bonds are not included. Hence longest chain of 6C catoms is picked up)
- If both, the double and triple bonds, are present the compound is regarded as derivative of alkyne. In such cases the terminal ‘e’ of -ene is dropped if it is followed by suffix starting with a,i,o,u,y. For example :
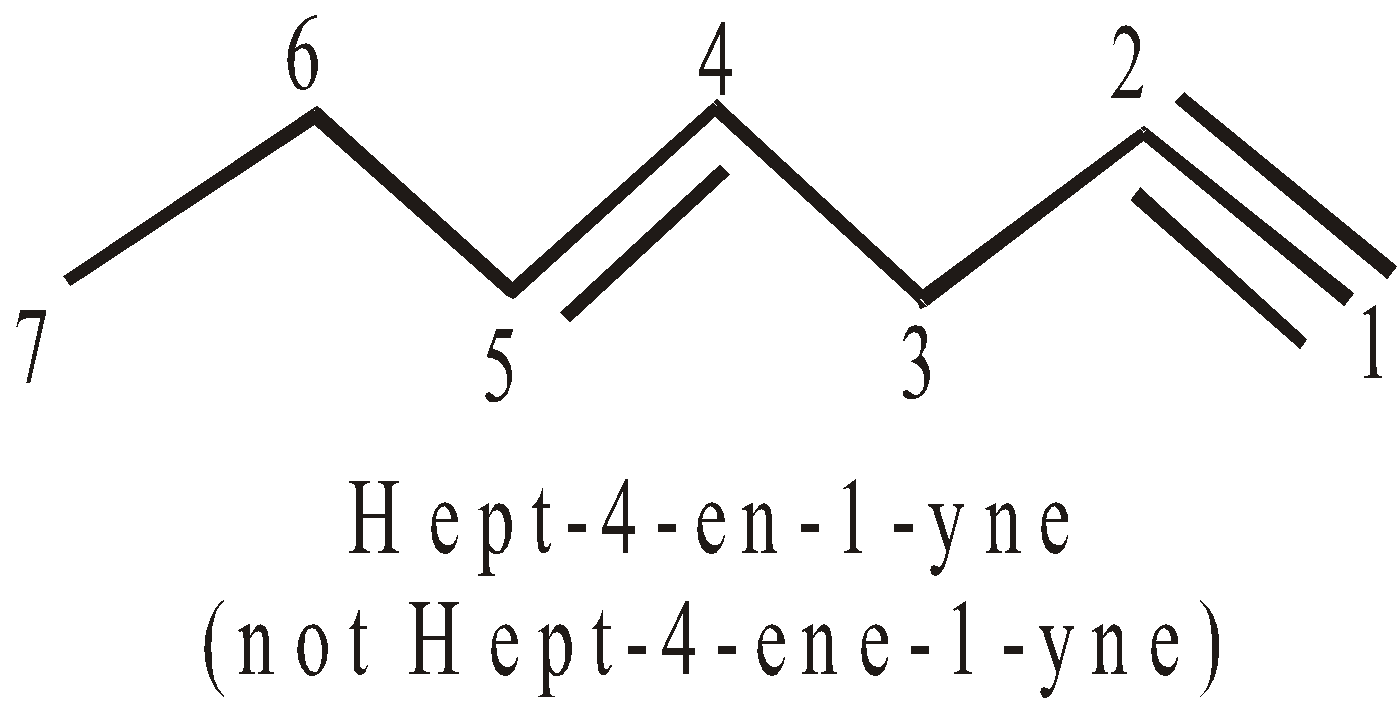
- If double and triple bonds are at equidistant from either side, the preference is given to double bond.
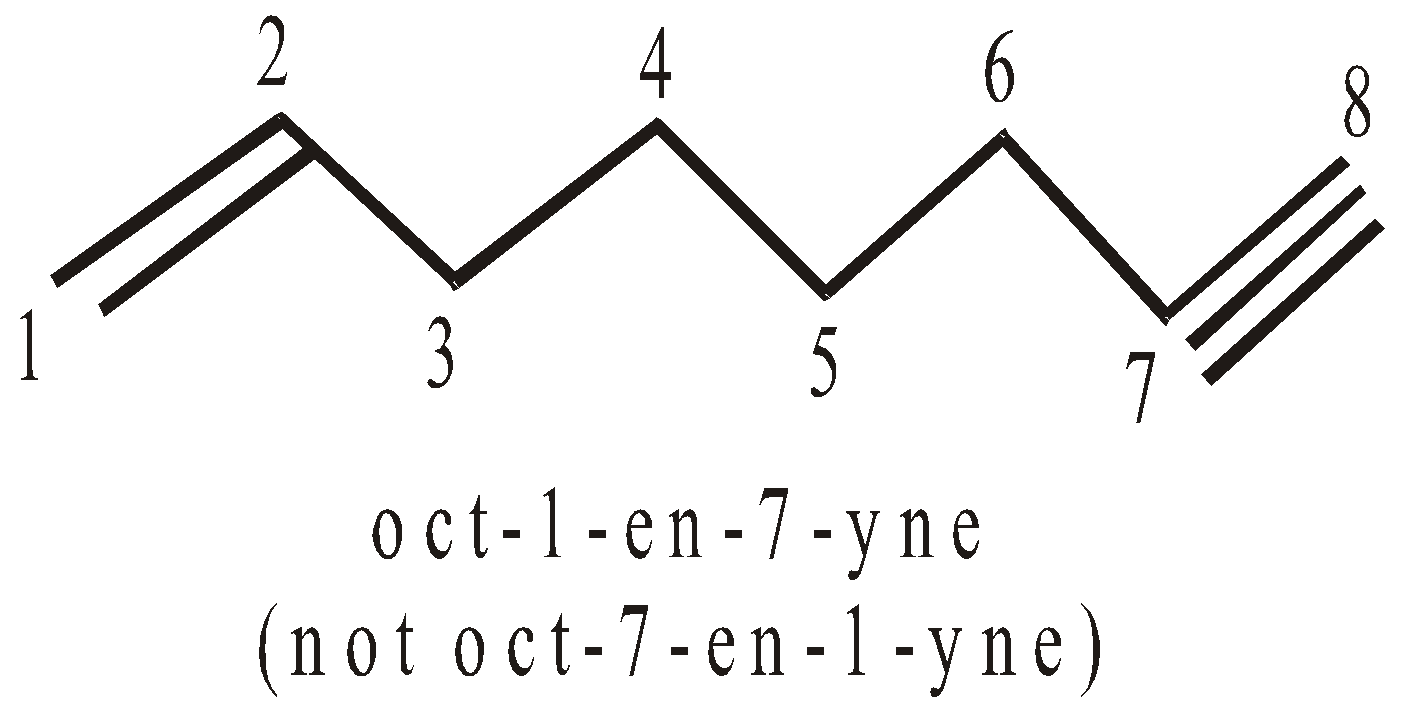
- If the compound contains two or more double or triple bonds a terminal “a” is added to the word root.
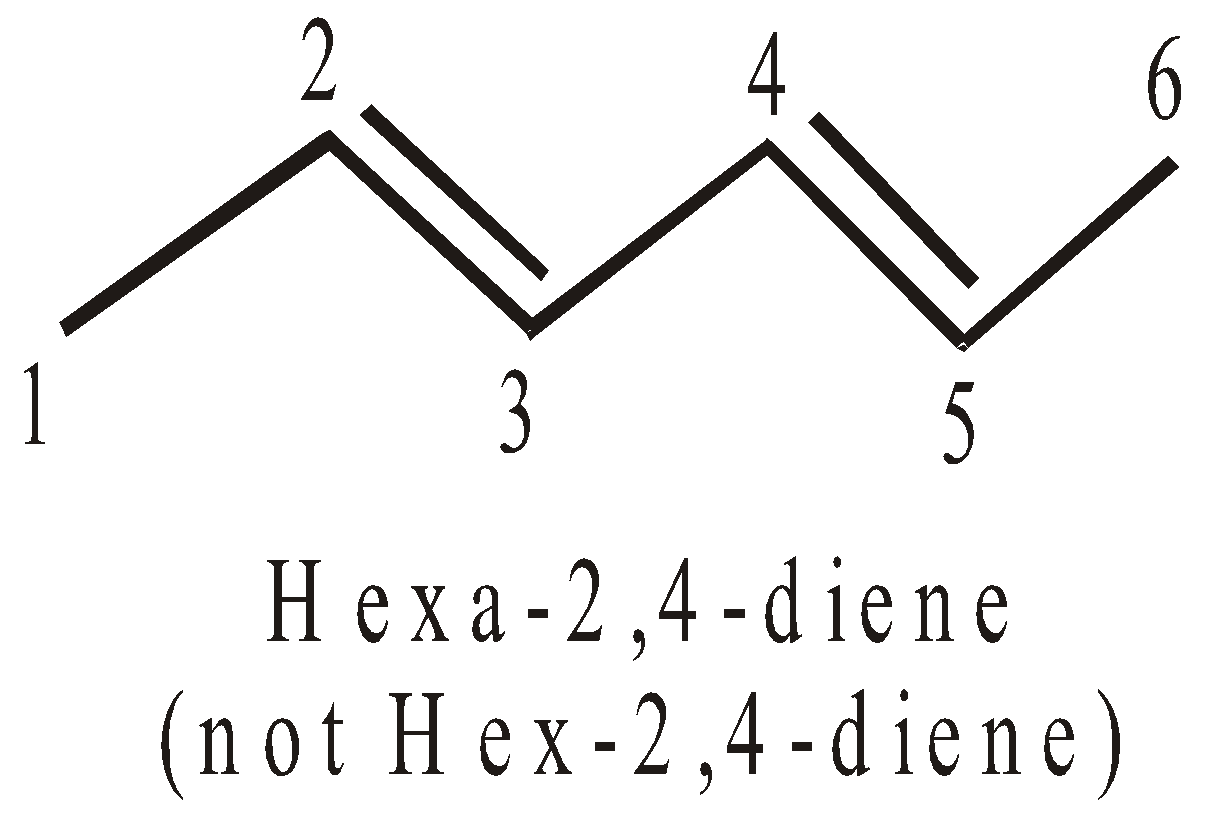
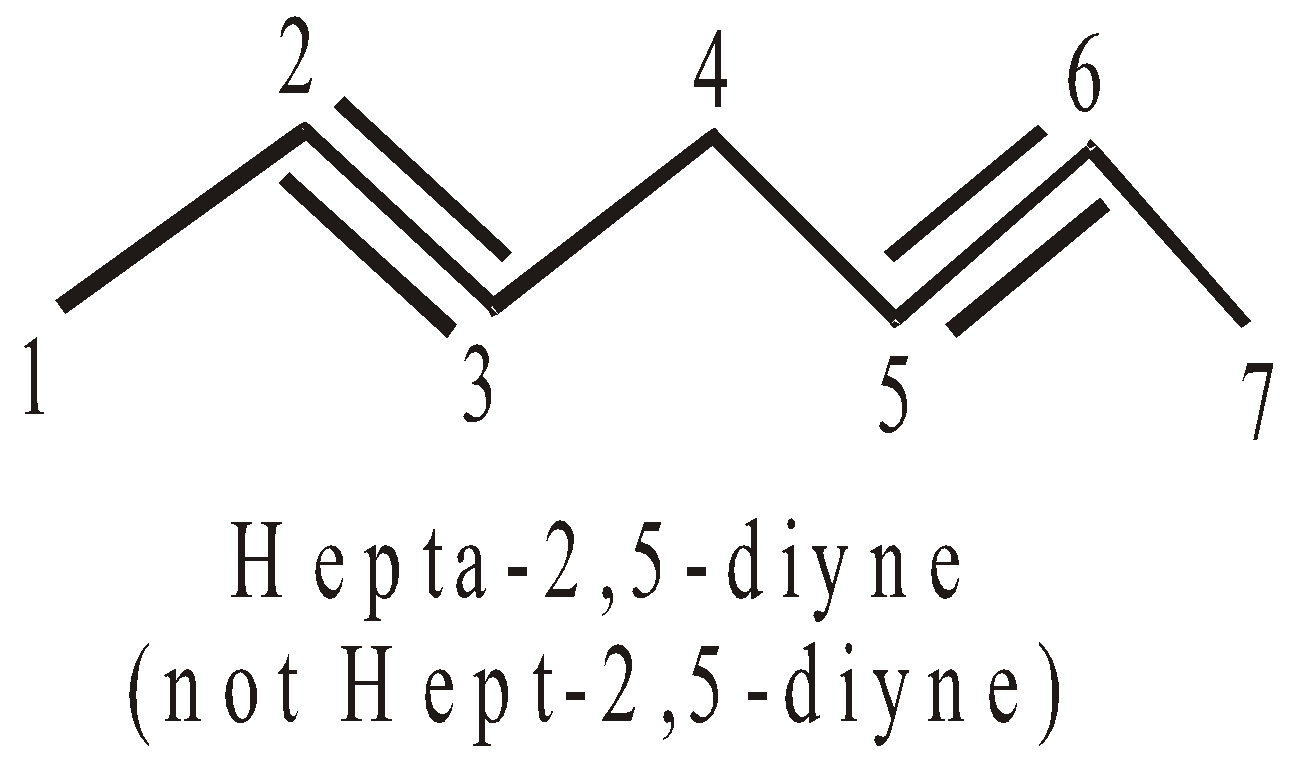
- The terminal ‘a’ is not added to the word root when the complete primary suffix do not start with a numerical affix
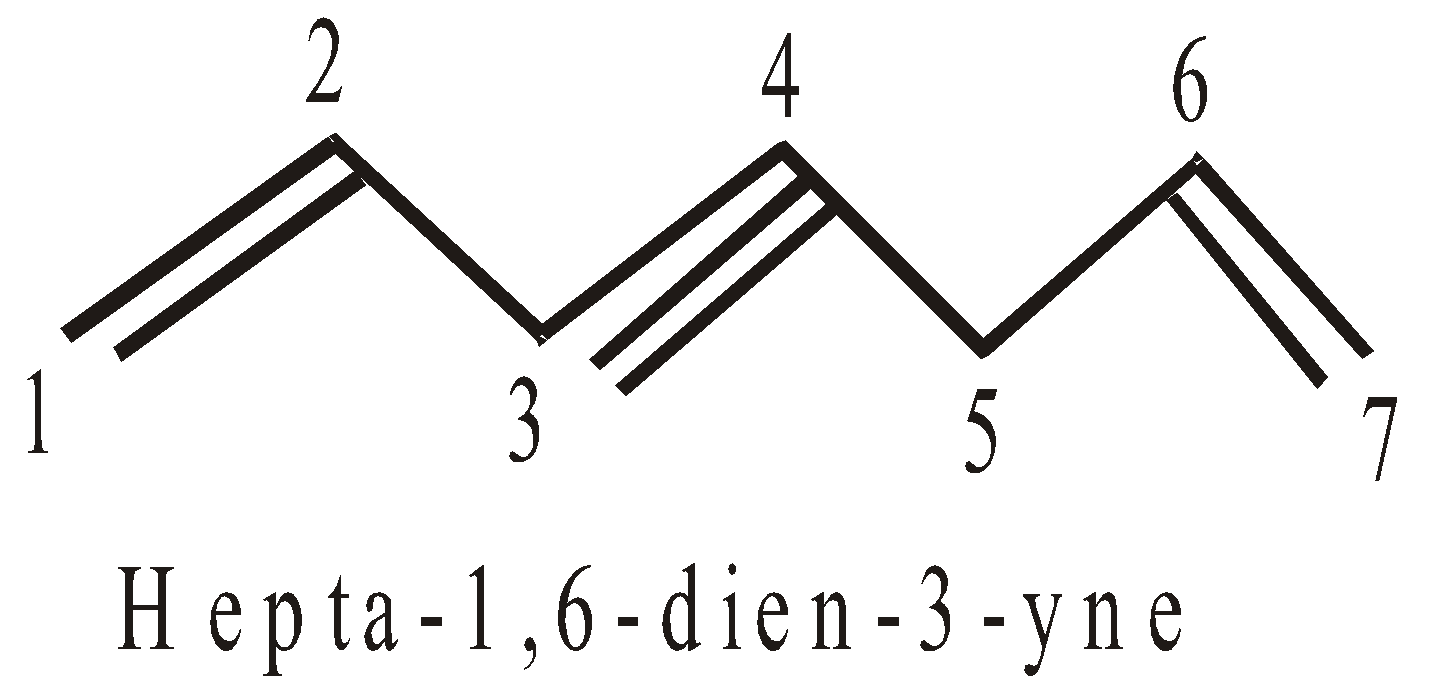
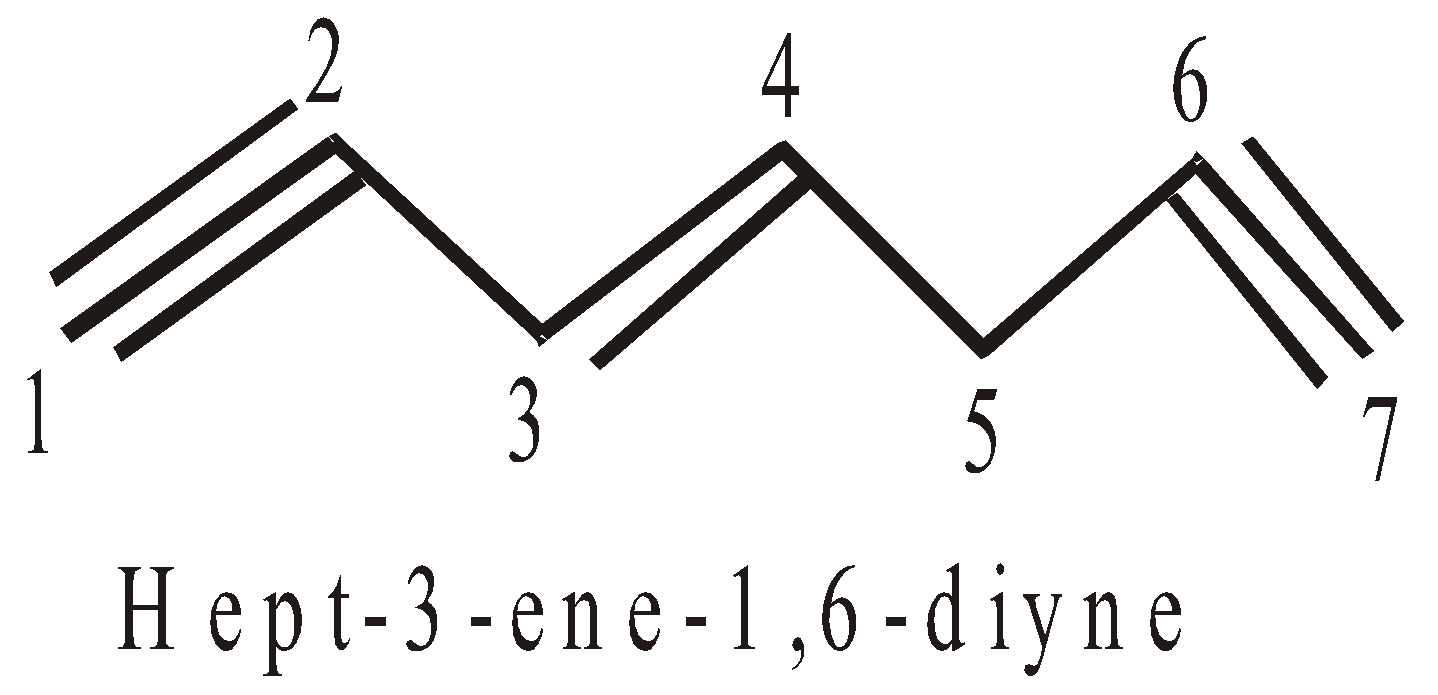
(Note that di, tri, tetra.. are numerical affix)
- Side chains containing multiple bonds are named as follows
Allyl CH2= CH2=CH–CH2–
Ethylidene CH3–CH=
Vinyl CH2=CH–
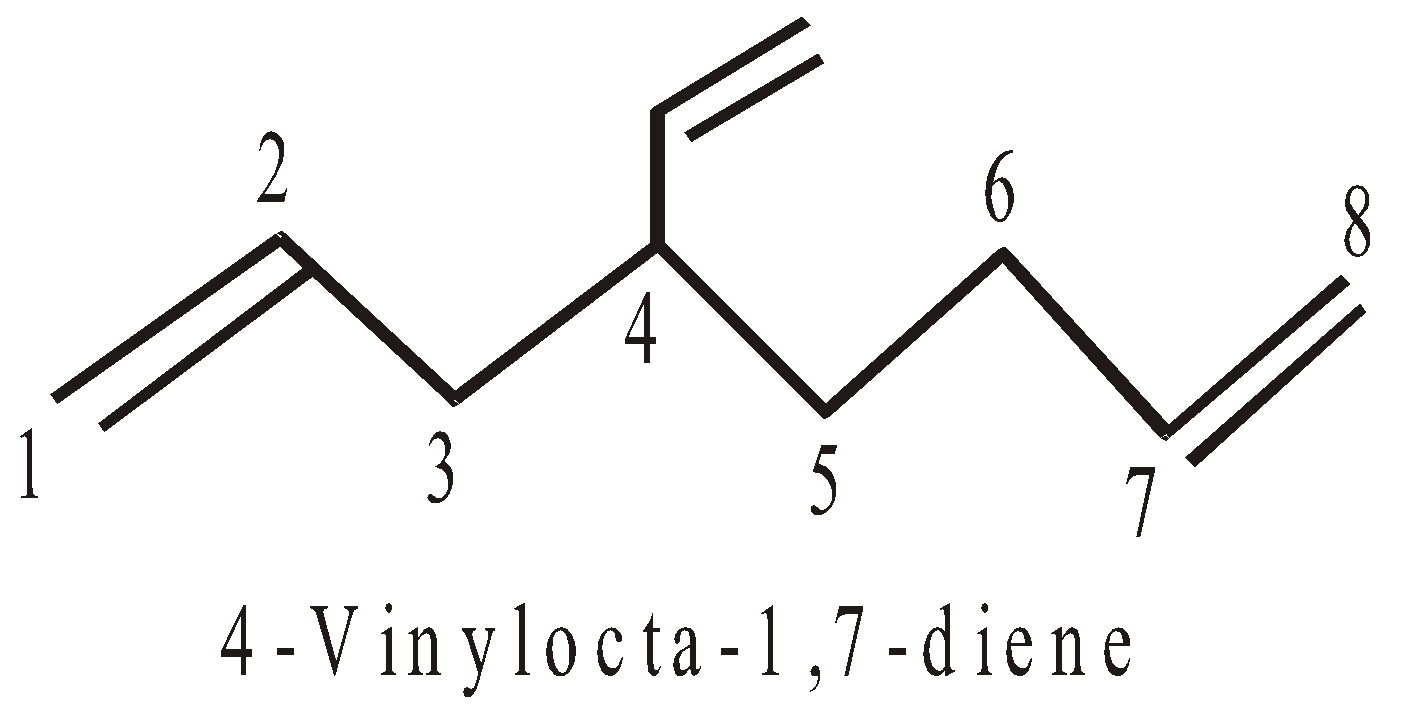
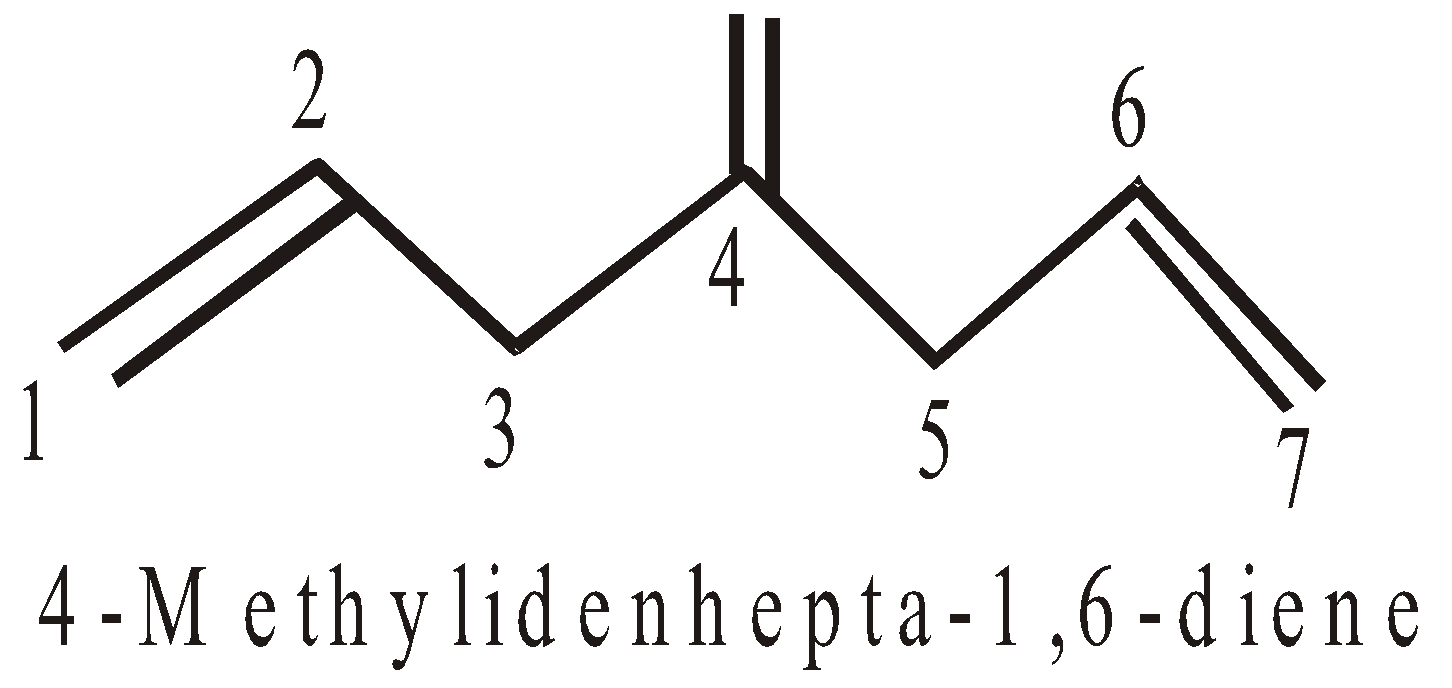
NOMENCLATURE OF CYCLOALKANES (ALICYCLIC COMPOUNDS)
- The base name is decided by the number of carbon atoms which the cyclic or acyclic portion contains. If the ring contains more or equal number of carbon atoms as alkyl then it is regarded as derivative of cycloalkane
- Carbons are numbered to give lowest numbers to substituted carbons. For example
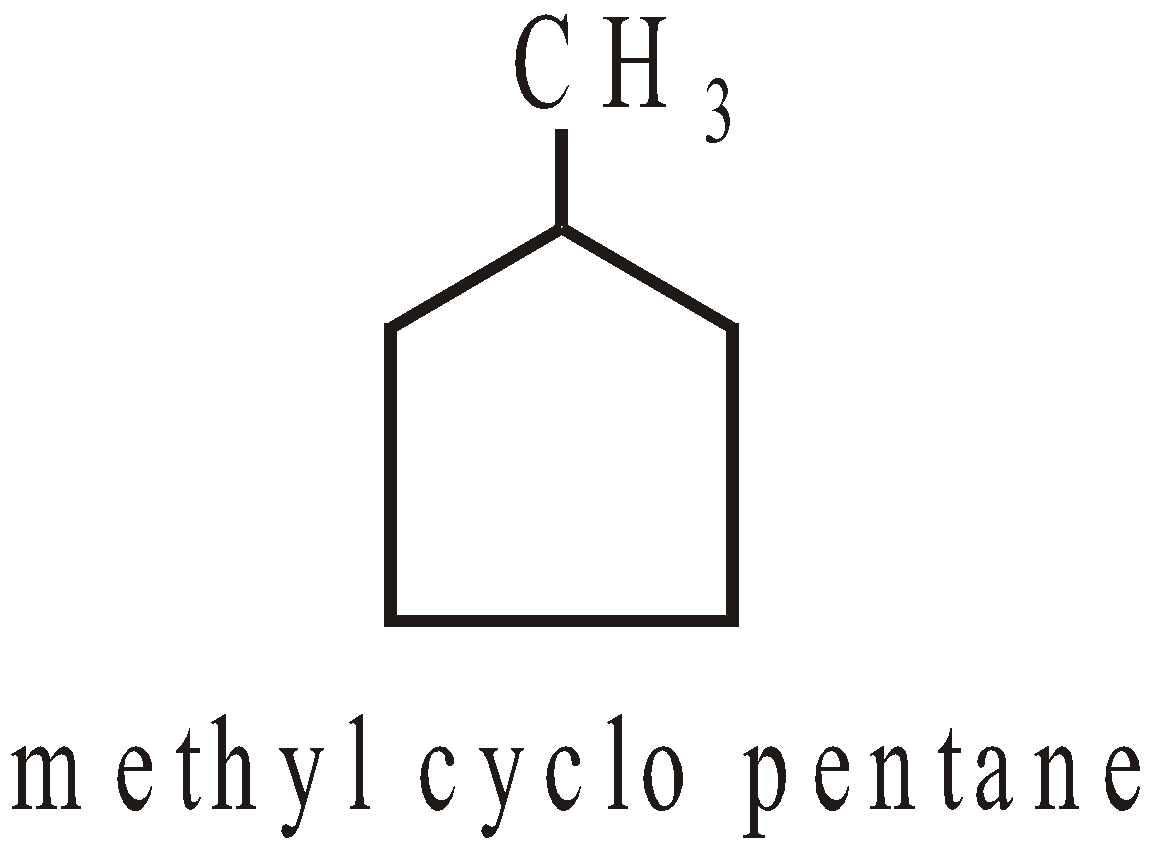
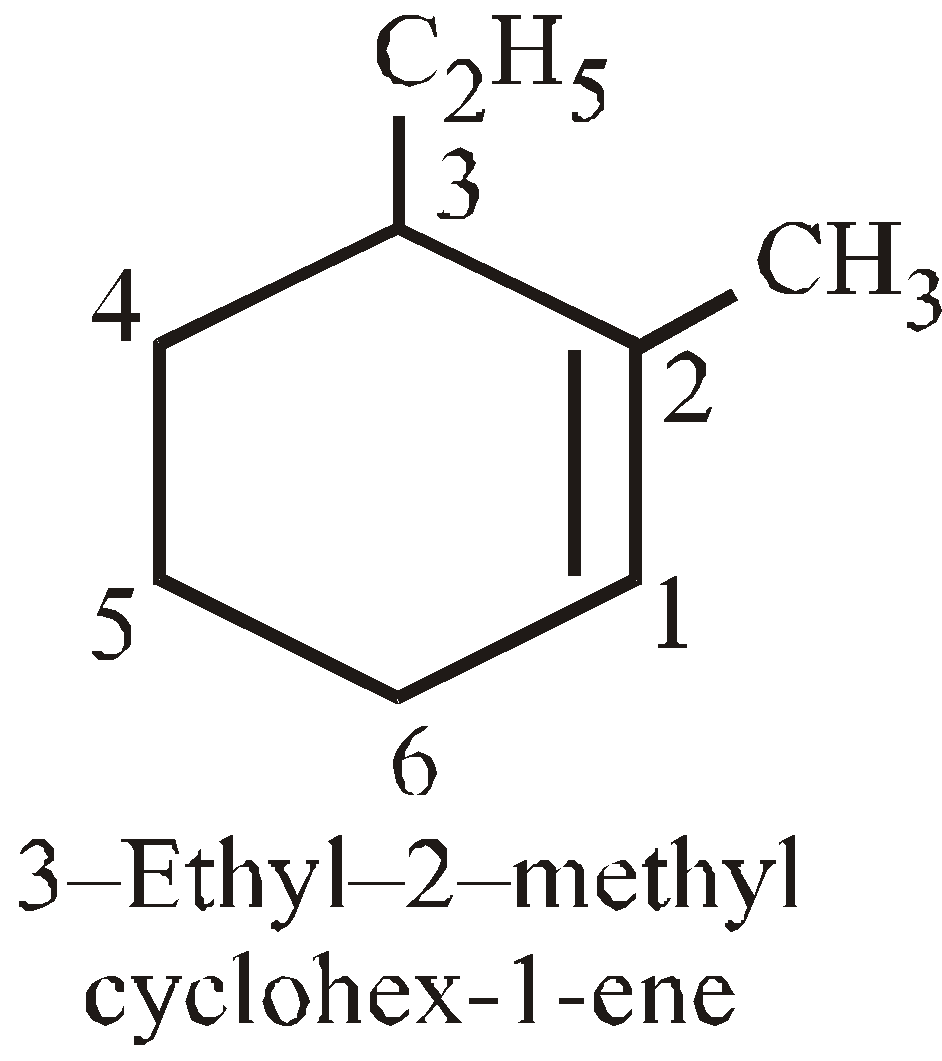
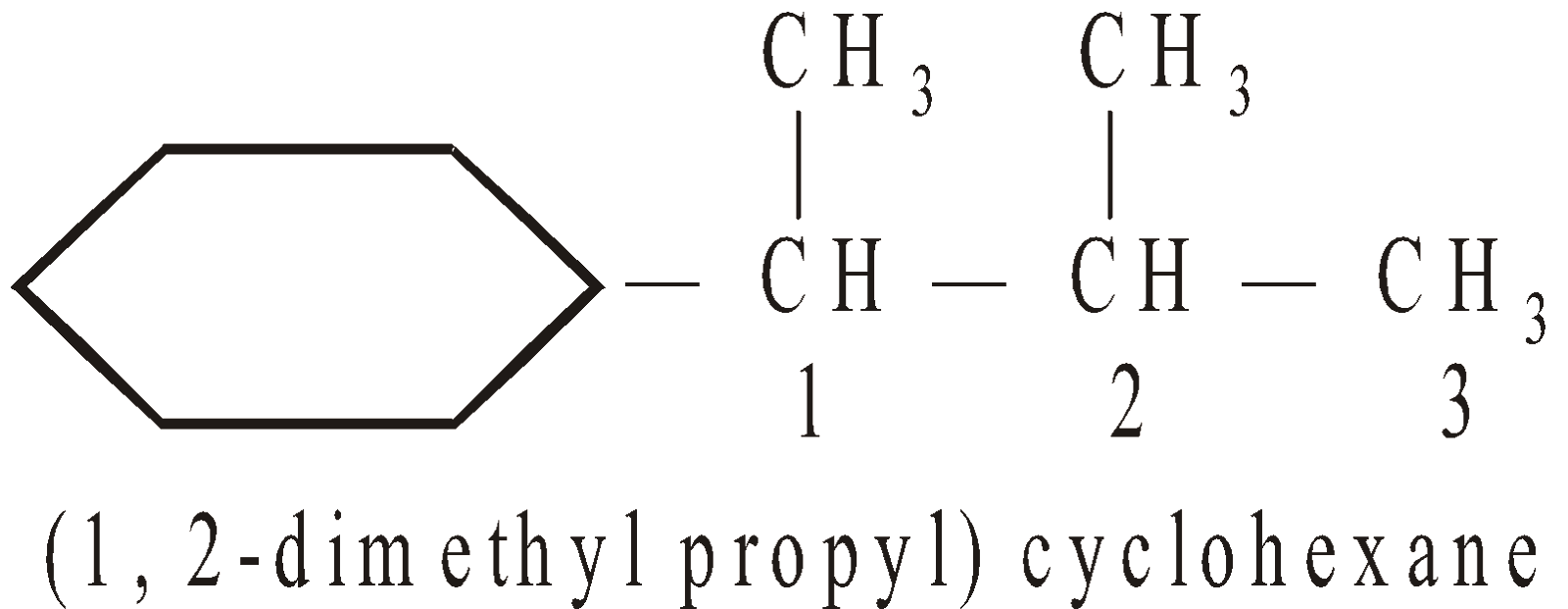
- When there are more acyclic than cyclic carbons the cyclic part becomes cycloalkyl substituent
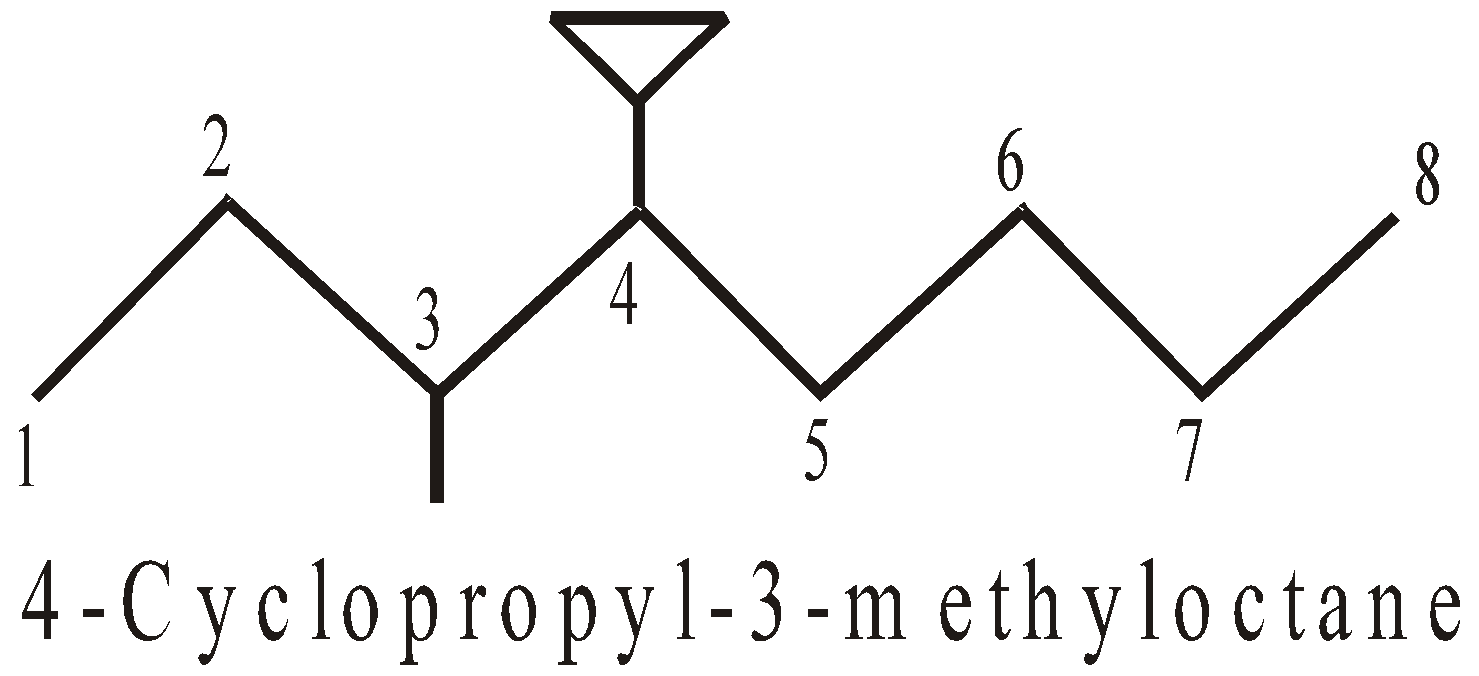
- When acyclic portion contains a multiple bond or a functional group, the cyclic portion is treated as substituent.
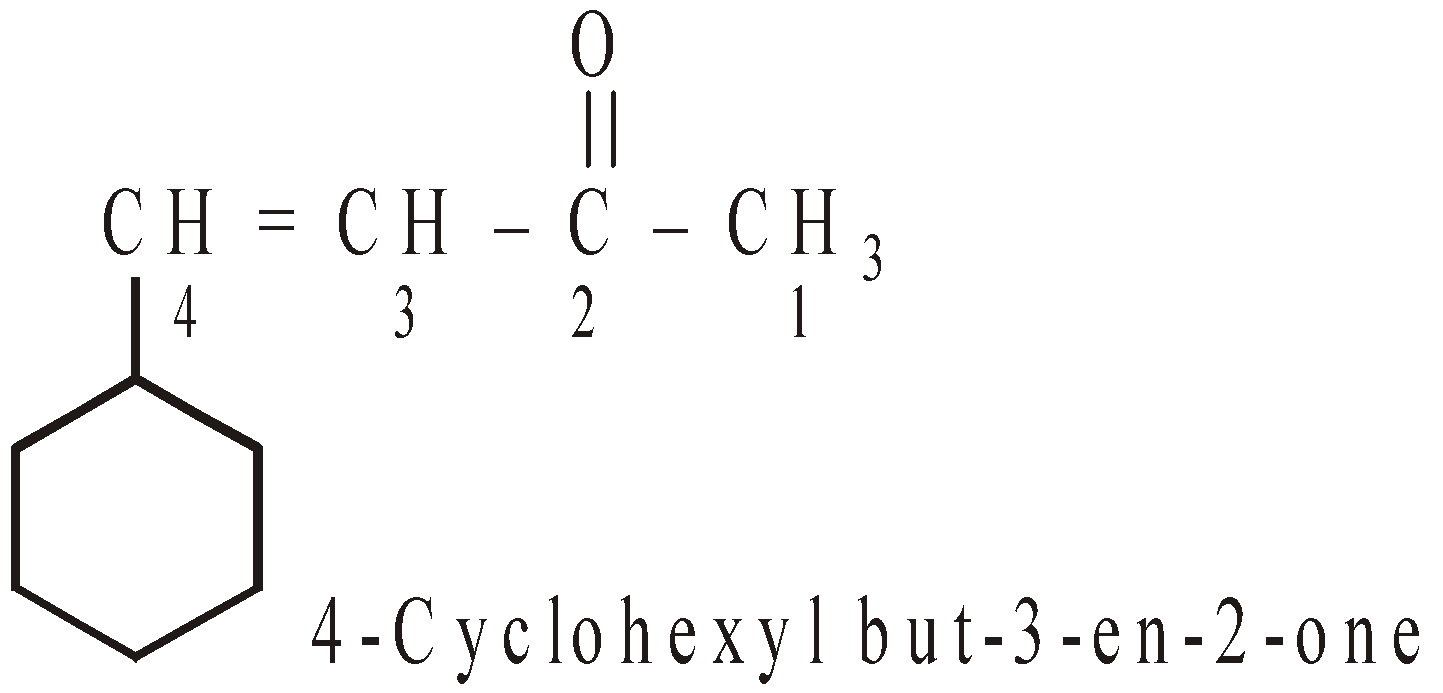
- In case when both contain the same functional group, the base name is decided by the number of c-atoms.
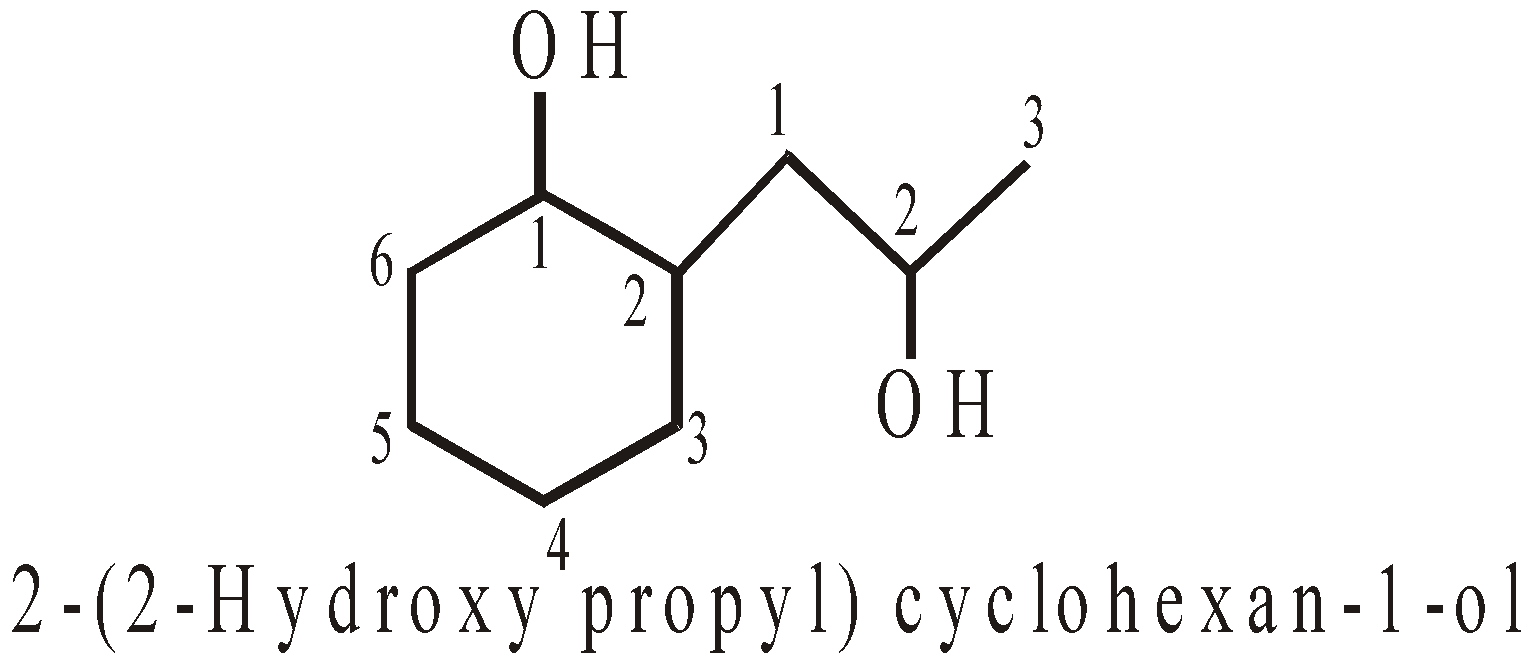
- When both contain the different functional groups, the base name is decided by principal characteristic group
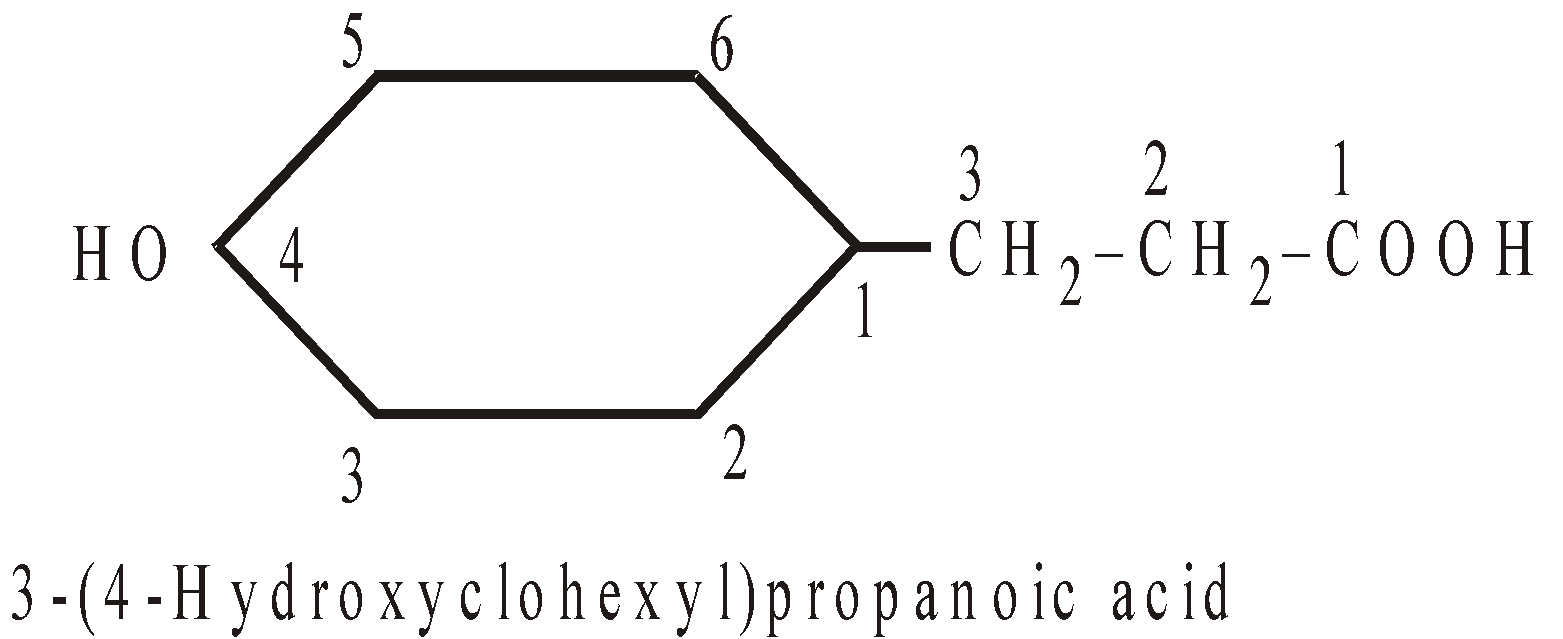
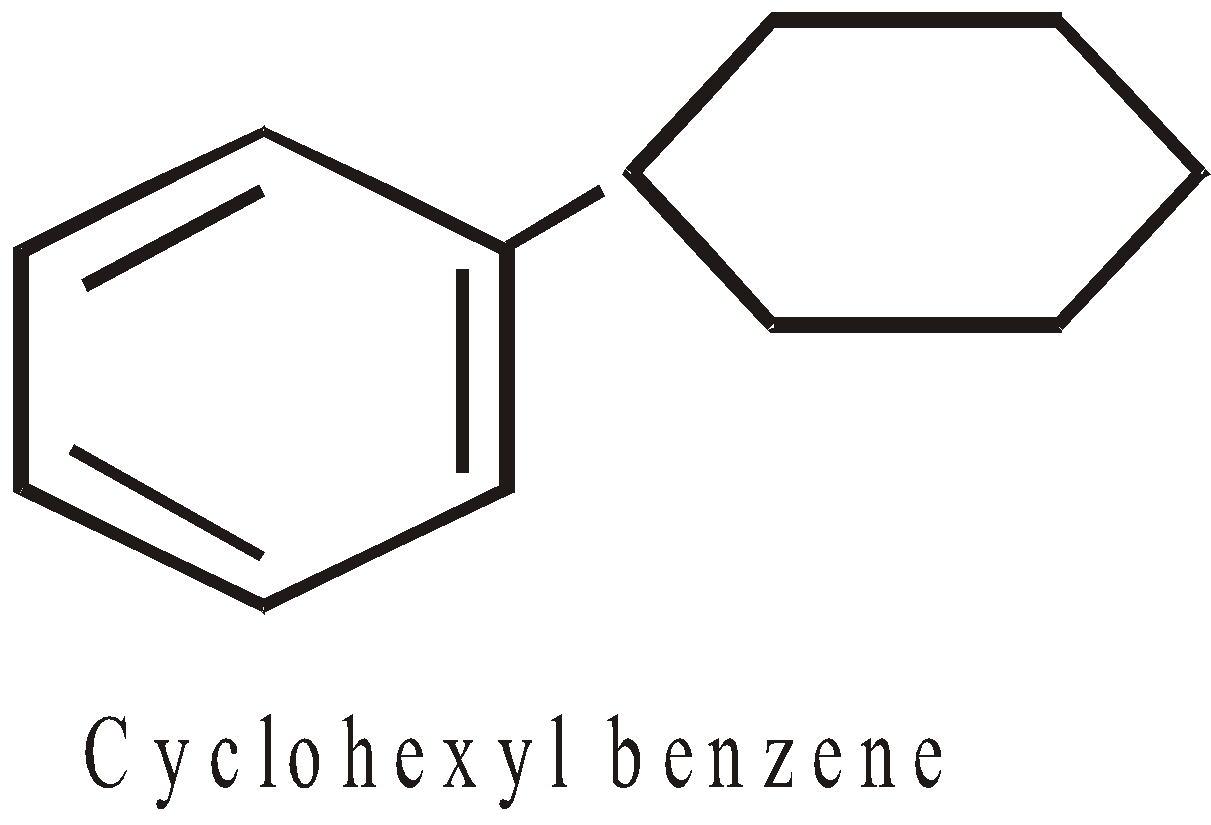
- When the acylic ring is directly attached to benzene ring, it is named as derivative of benzene
- Presence of certain groups
Functional gp. | Suffix |
–COOH | Carboxylic acid |
–COOR | Alkyl carboxylate |
–COX | Carbonyl halide |
–CONH2 | Carboxamide |
Carbonitrile | |
–CHO | Carbaldehyde |
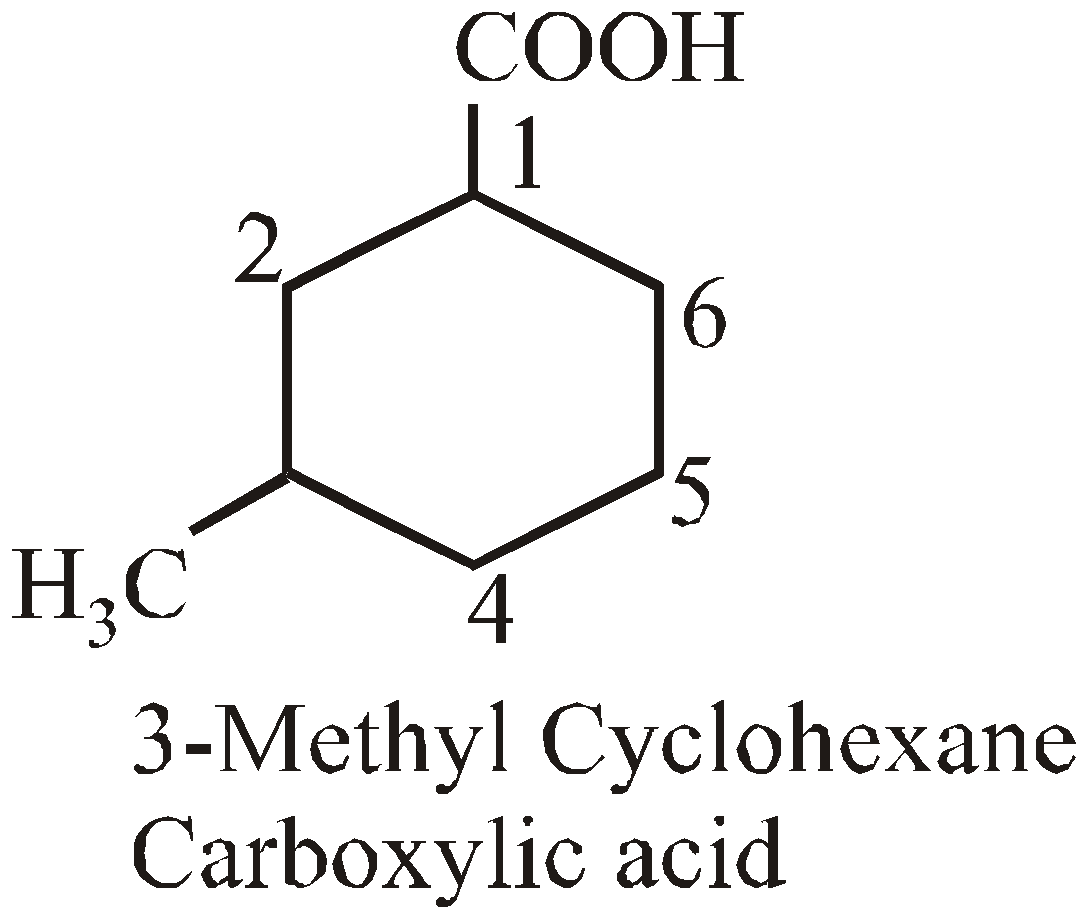
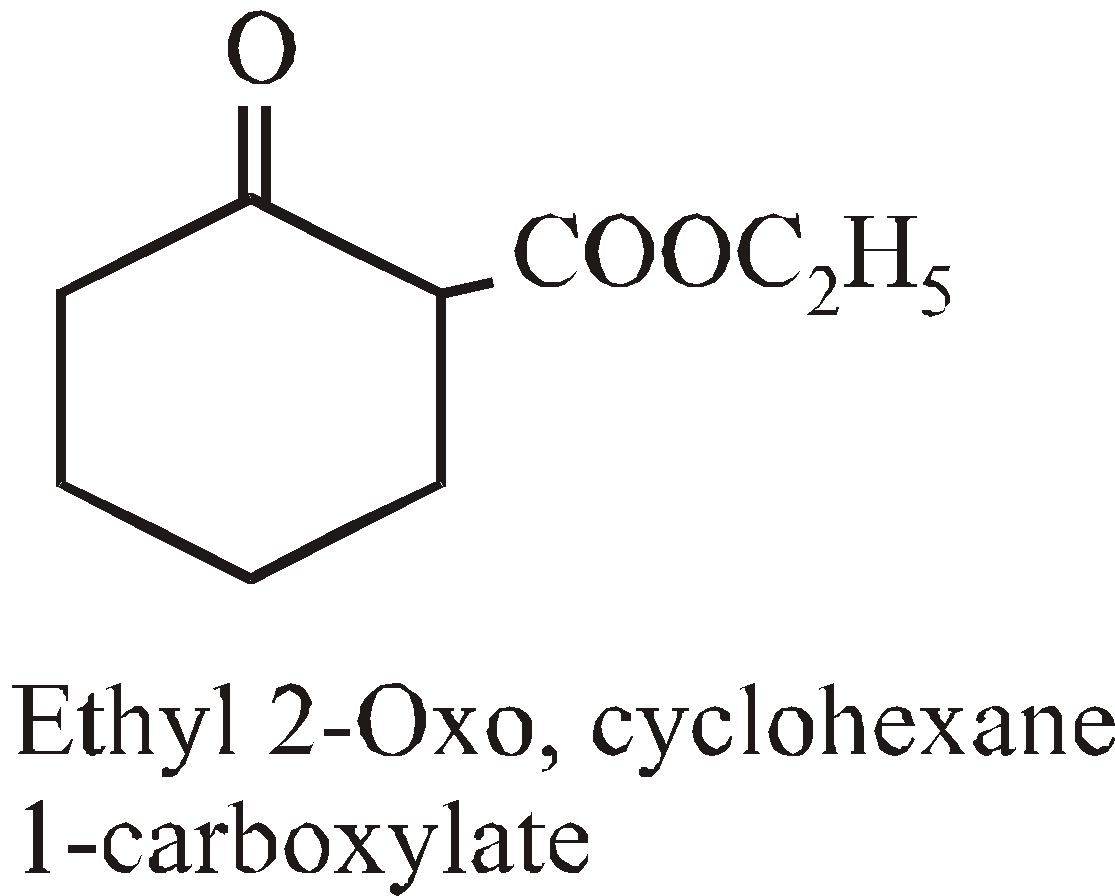
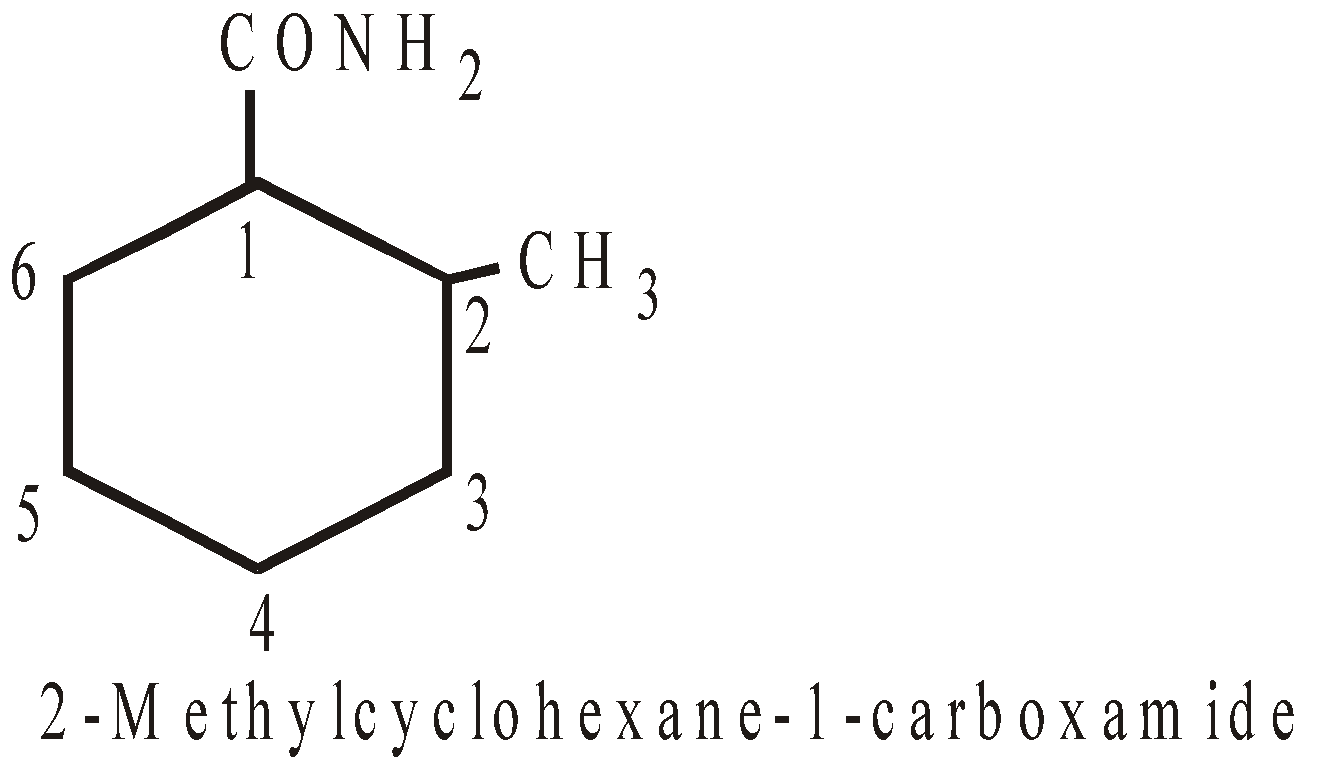
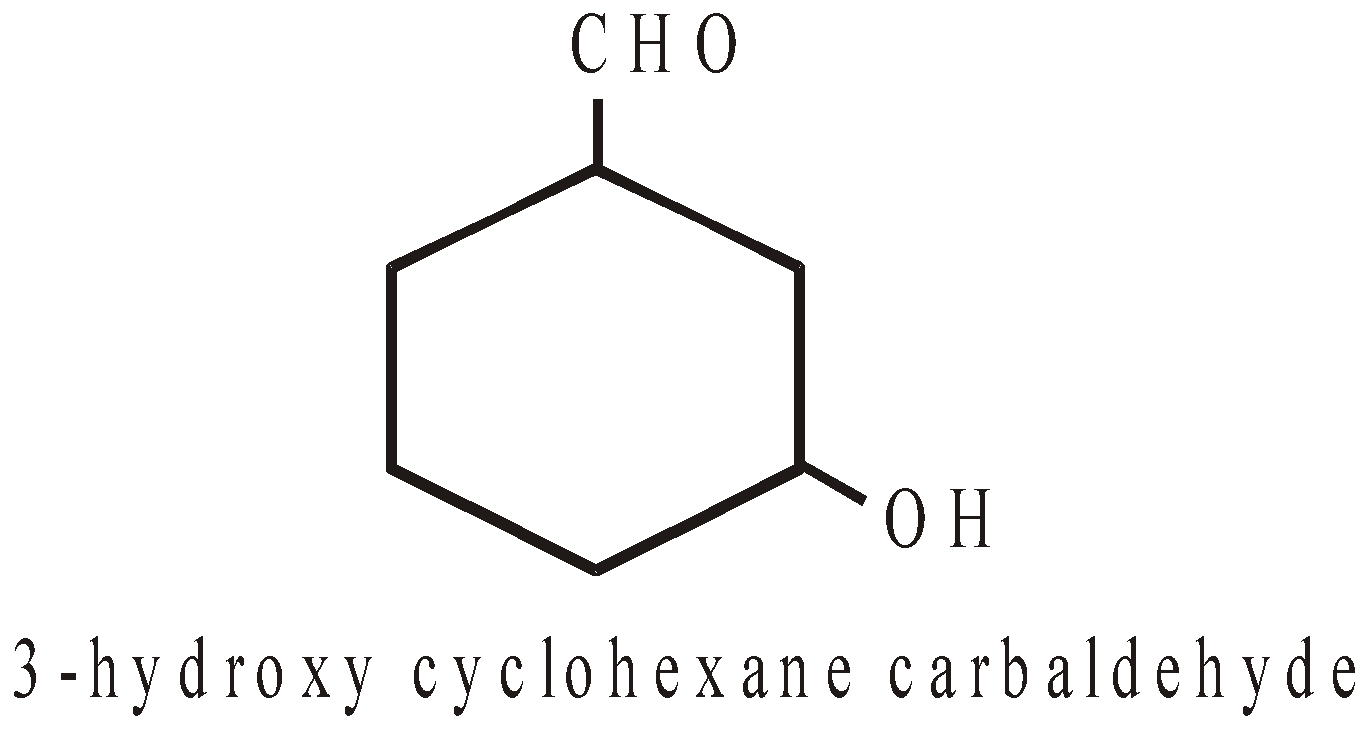
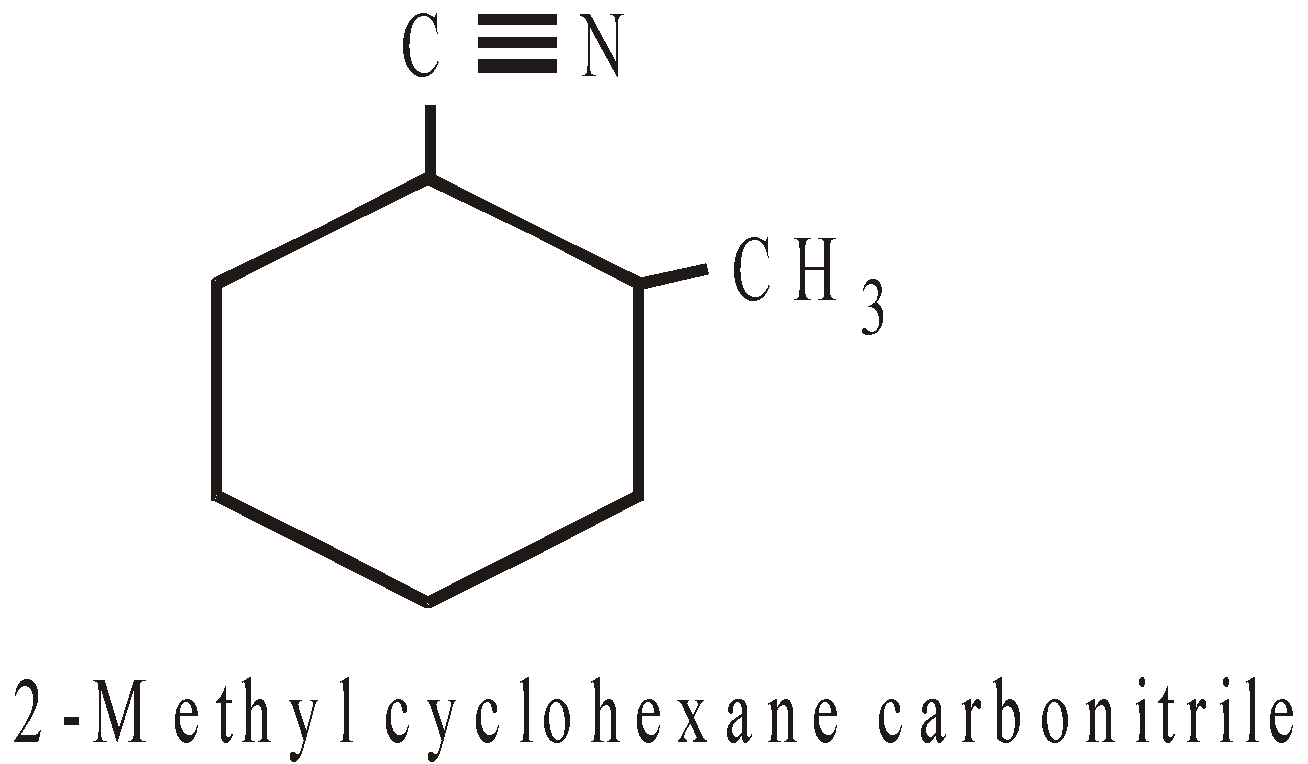
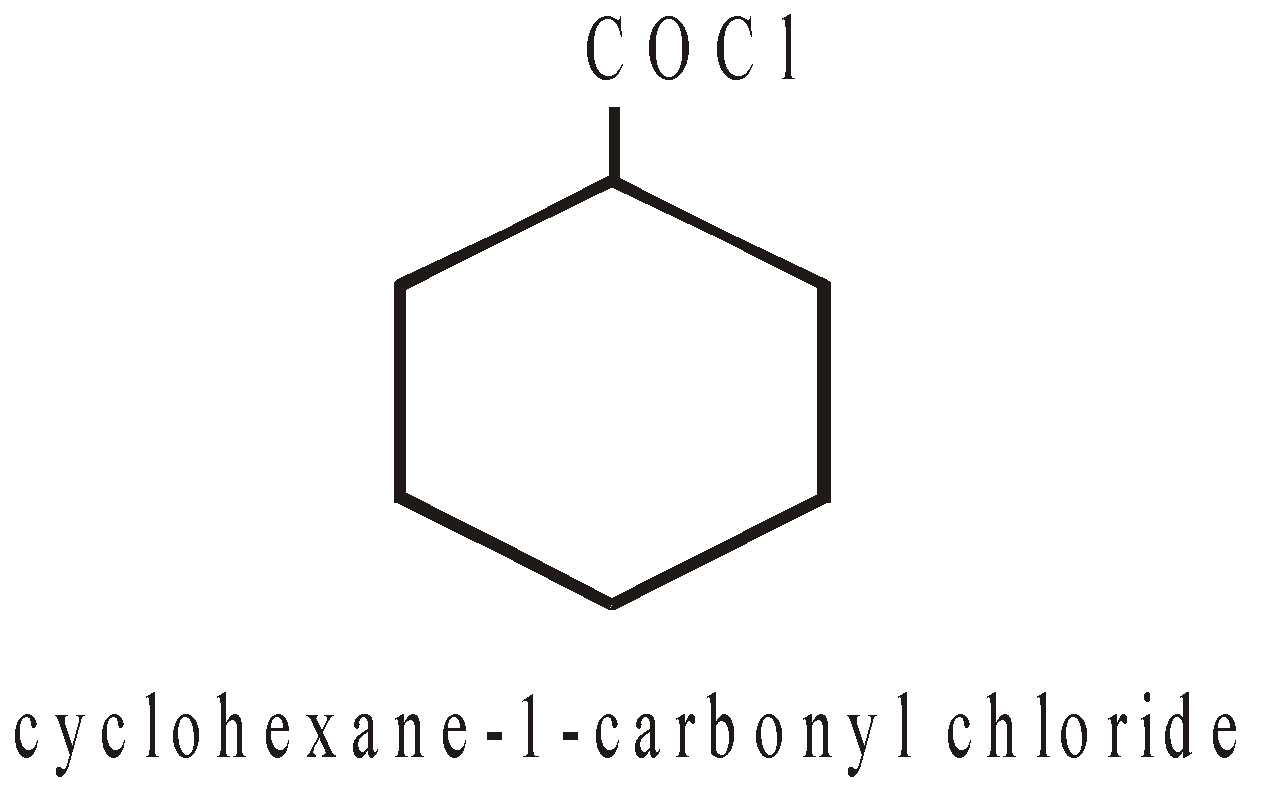
NOMENCLATURE OF POLYCYCLIC ALKANES
There are three ways that rings can be joined.
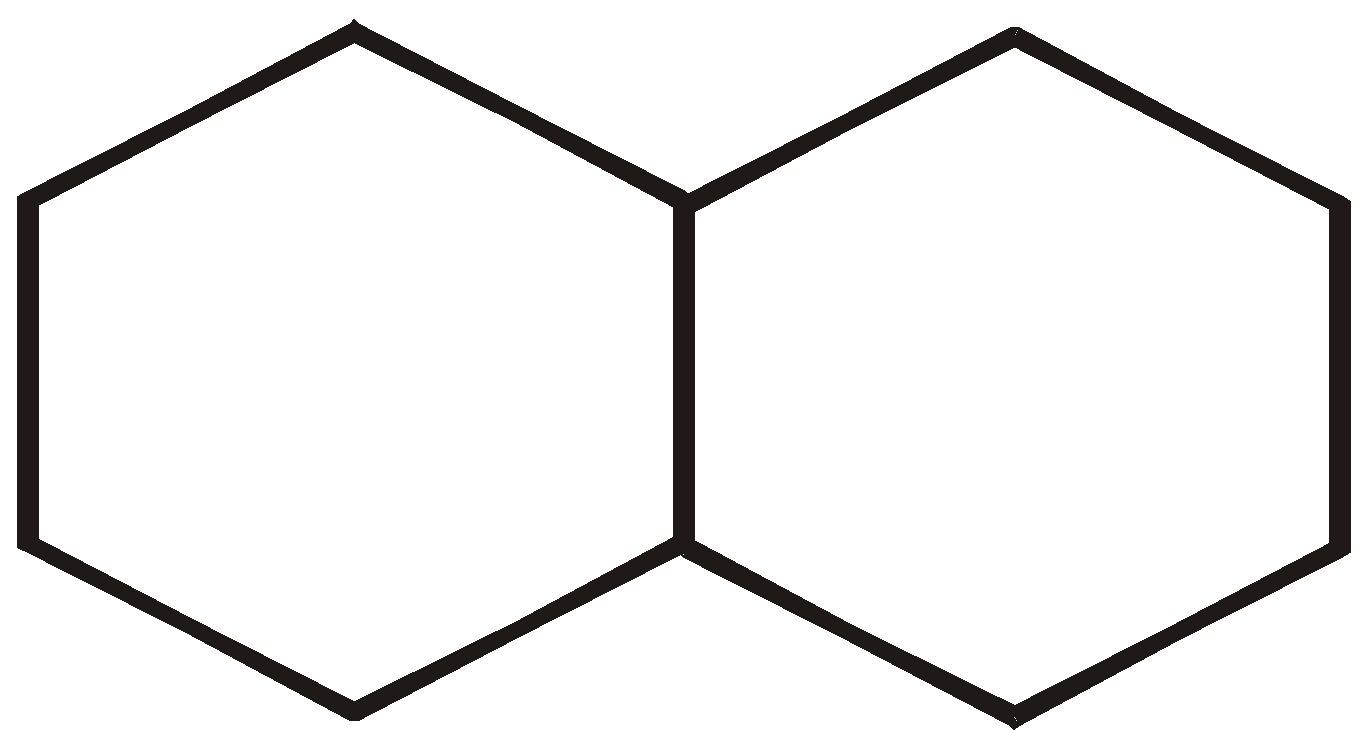
- Fused rings
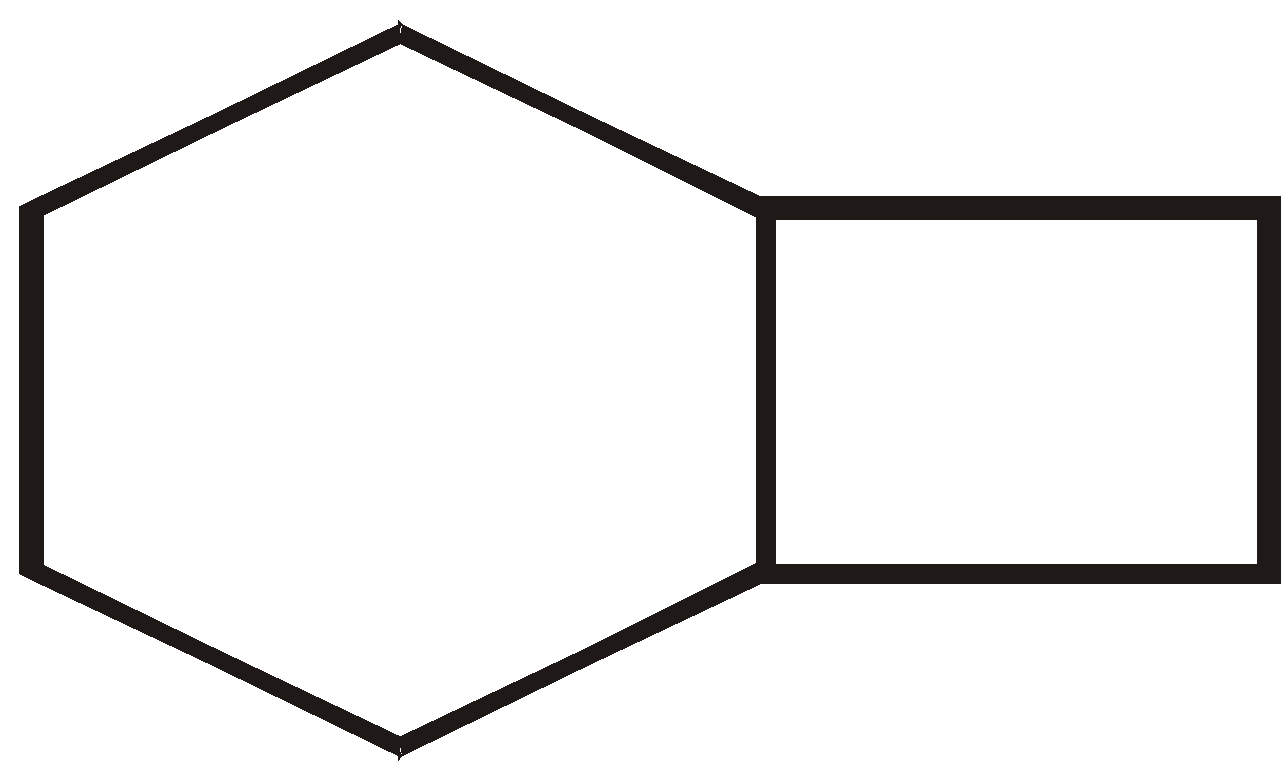
- Bridged rings
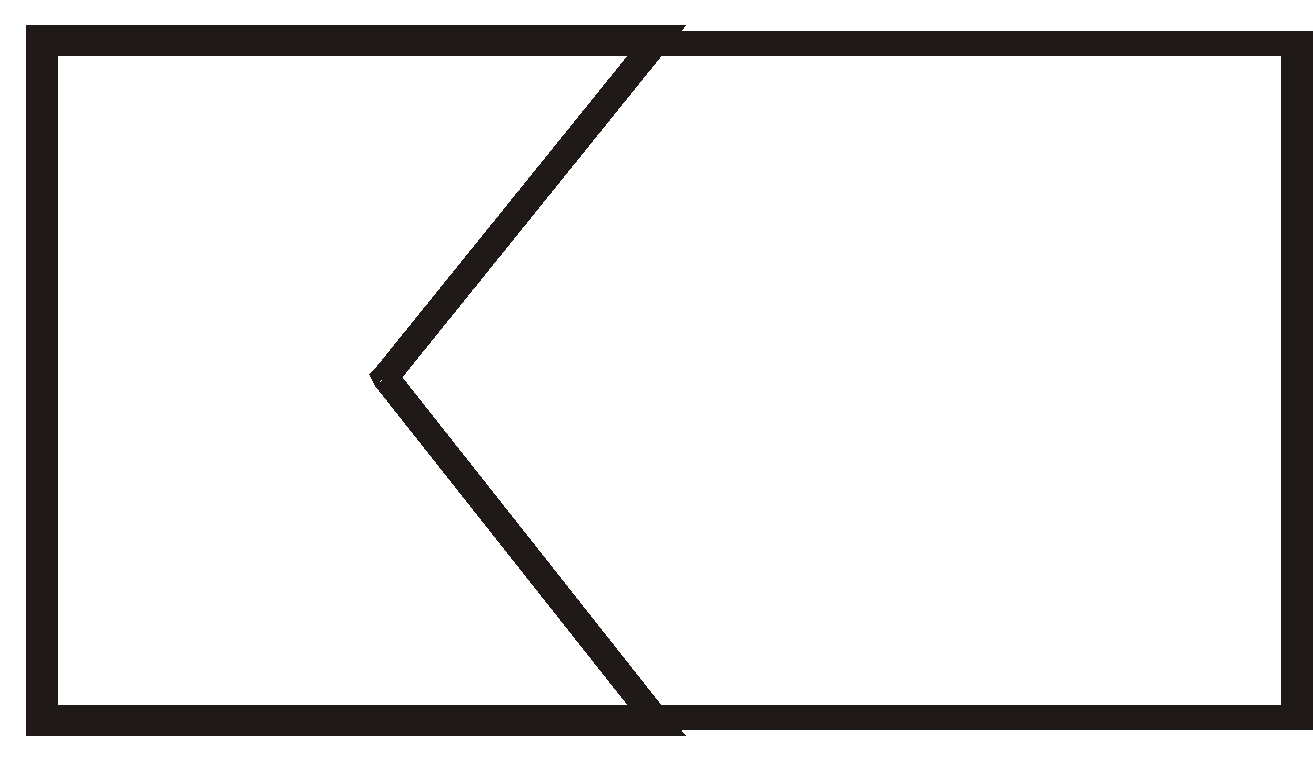
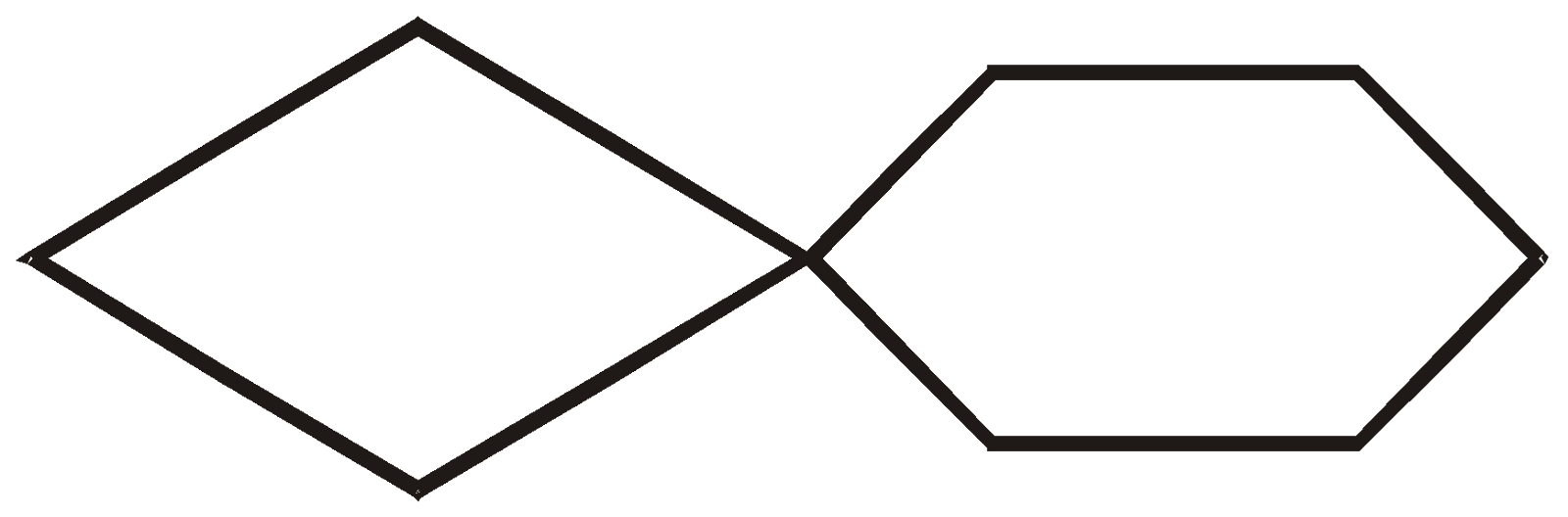
- Spirocyclic compounds
The carbon atoms common to both the rings are called bridge head atoms. The chain of carbon atoms connecting the bridge head atoms is called a bridge.
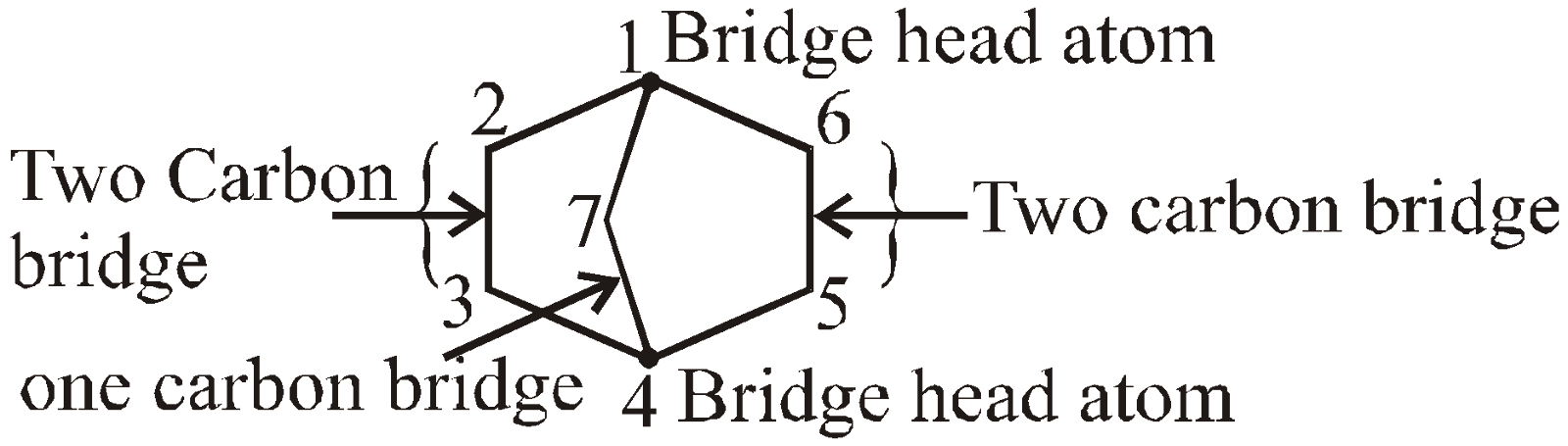
Numbering of C-atoms in fused rings and bridge rings : The numbering starts from bridge head carbon, proceeds along the longest bridge passing through the second bridge head atom, proceeds to the next longest bridge and completed along the shortest path.
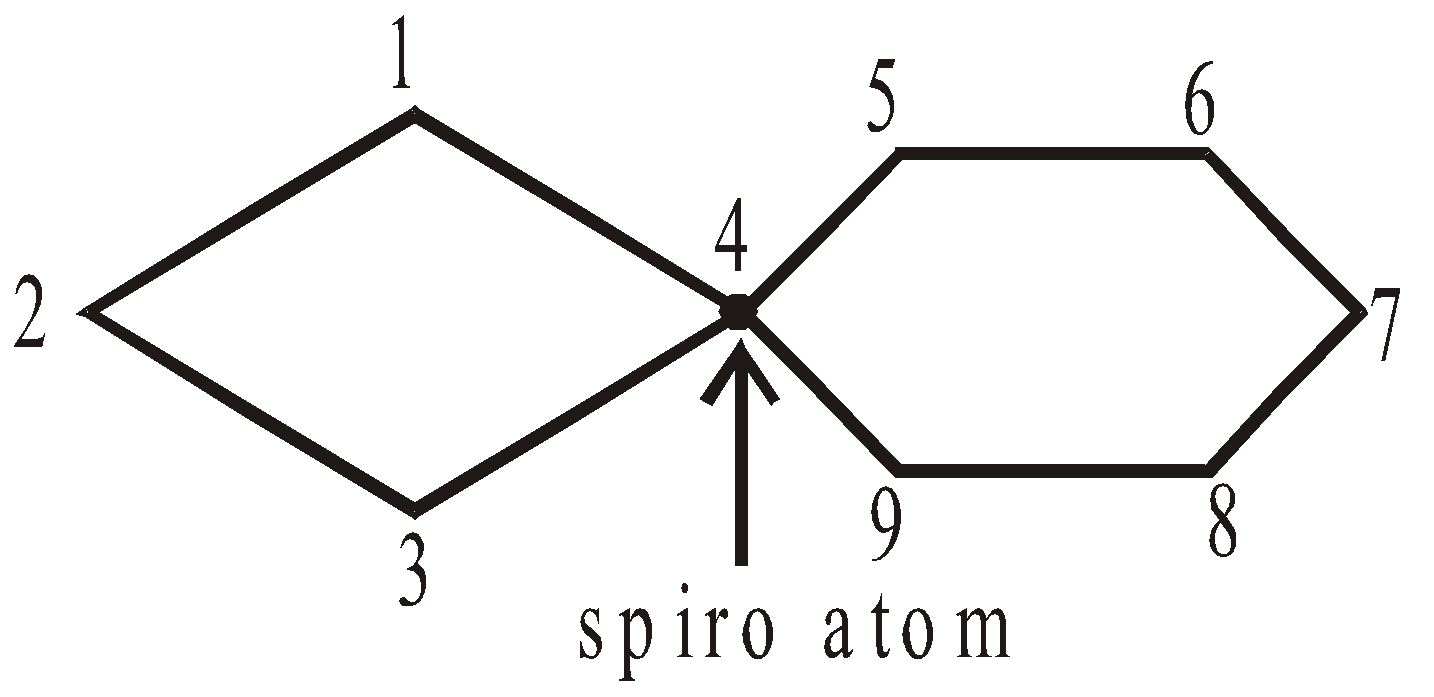
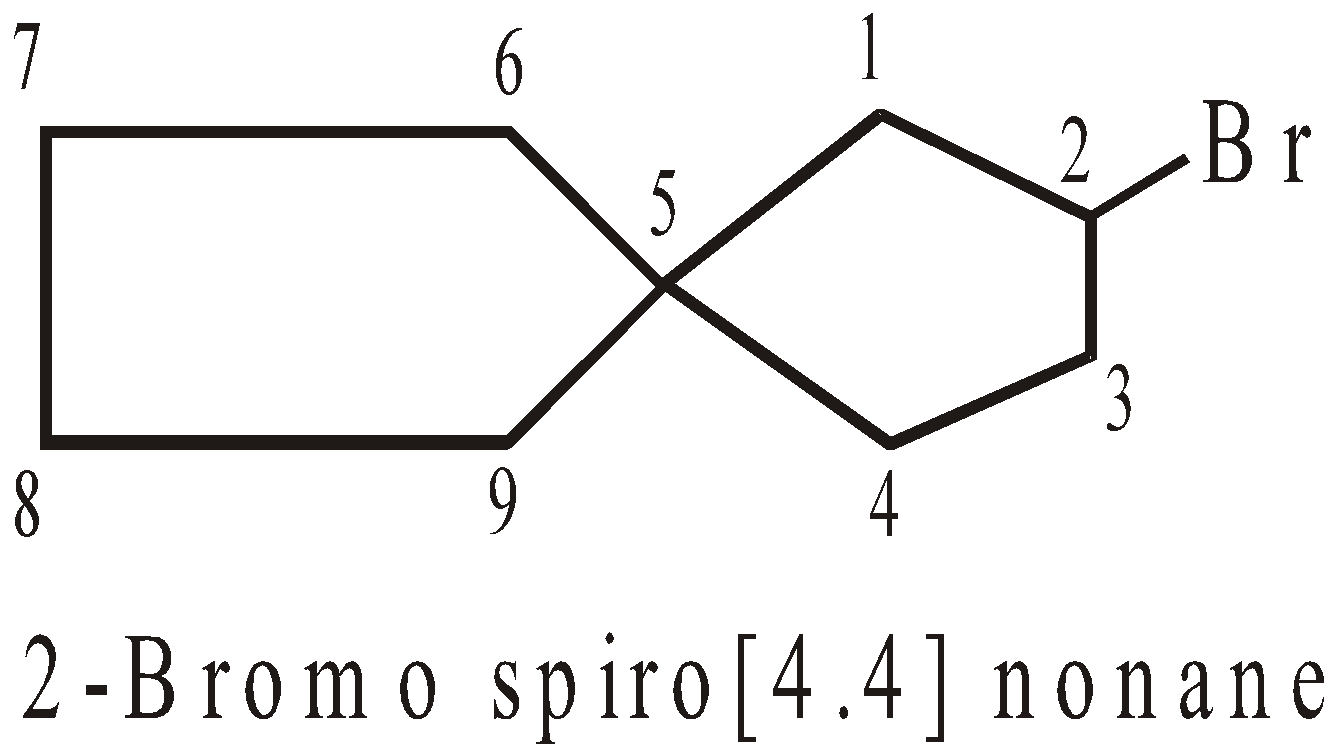
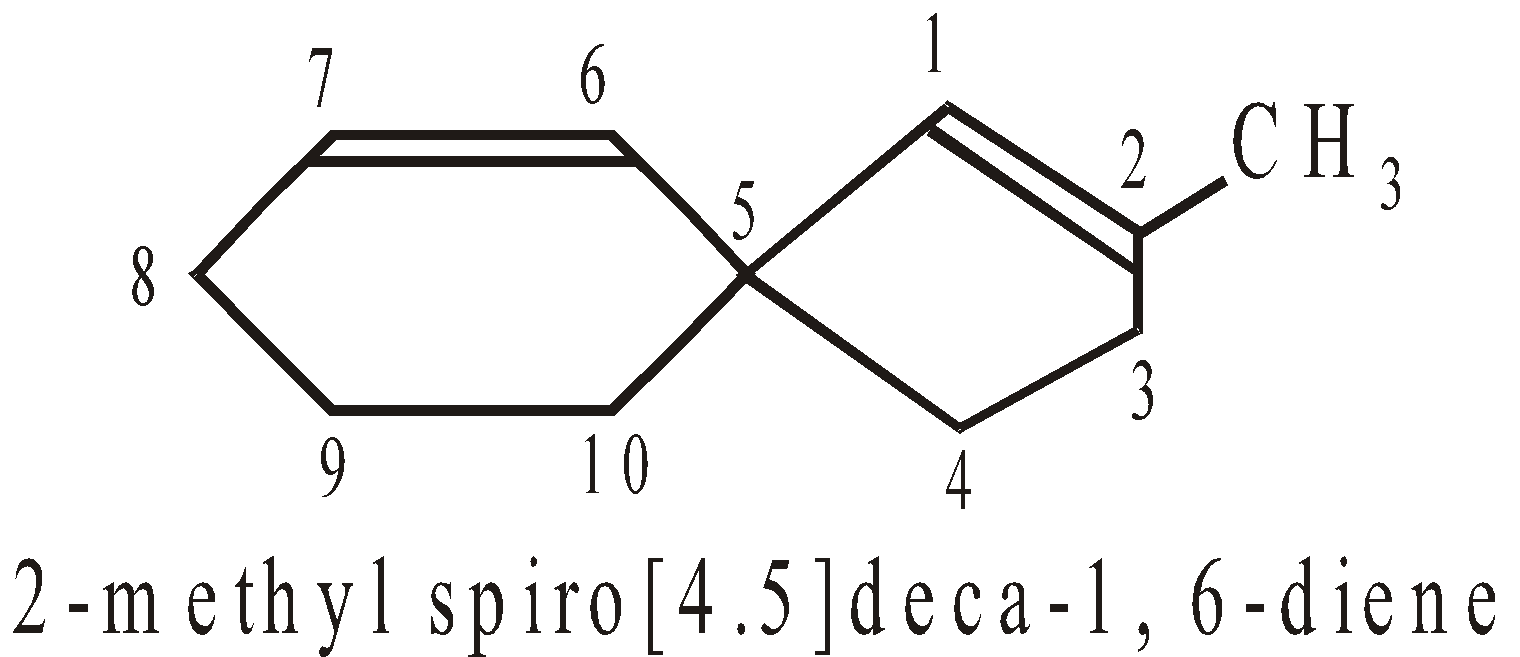
Numbering of C-atoms in spiro compounds : The numbering starts from the carbon atom, next to spiro atom, present in the smaller ring giving minimum number to atoms containing functional groups.
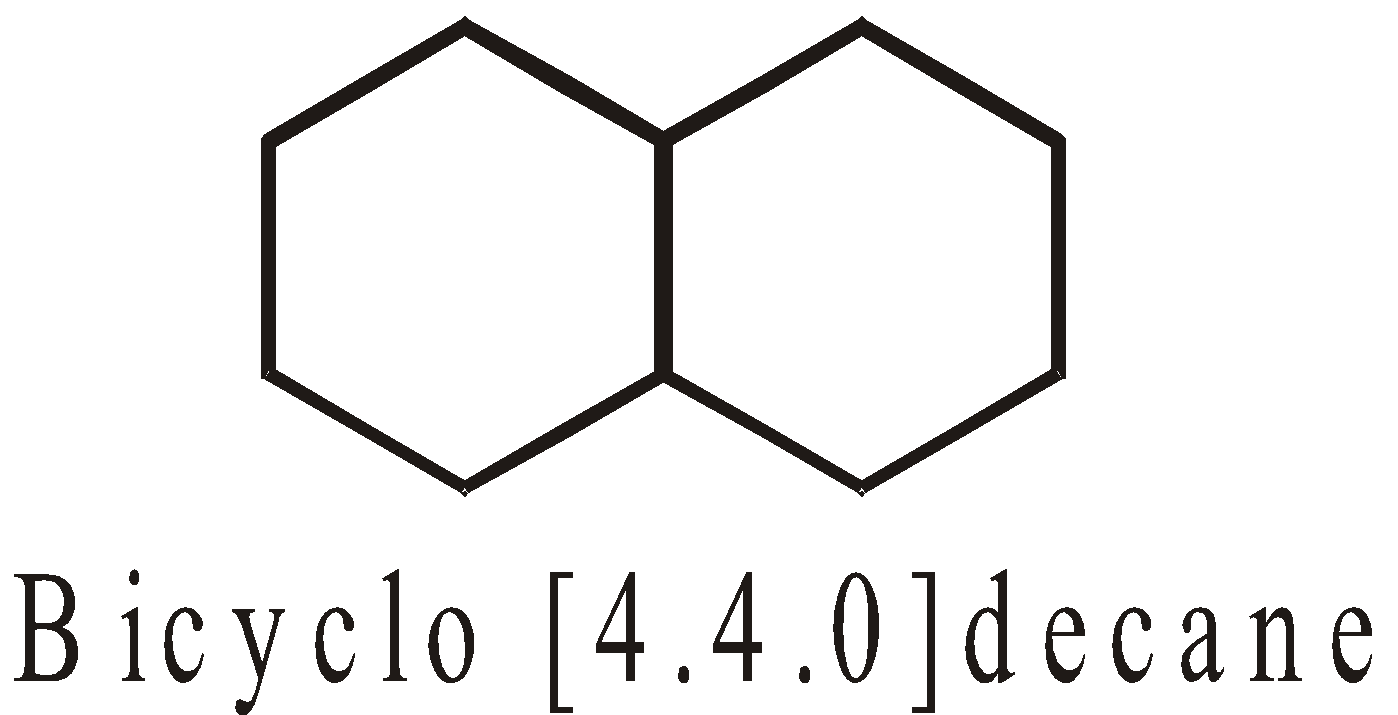
FUSED RINGS
Fused rings share two adjacent carbon atoms and the bond between them eg. :
BRIDGED RINGS
These share two non adjacent carbon atoms (the bridge head carbons) and one or more carbon atoms between them
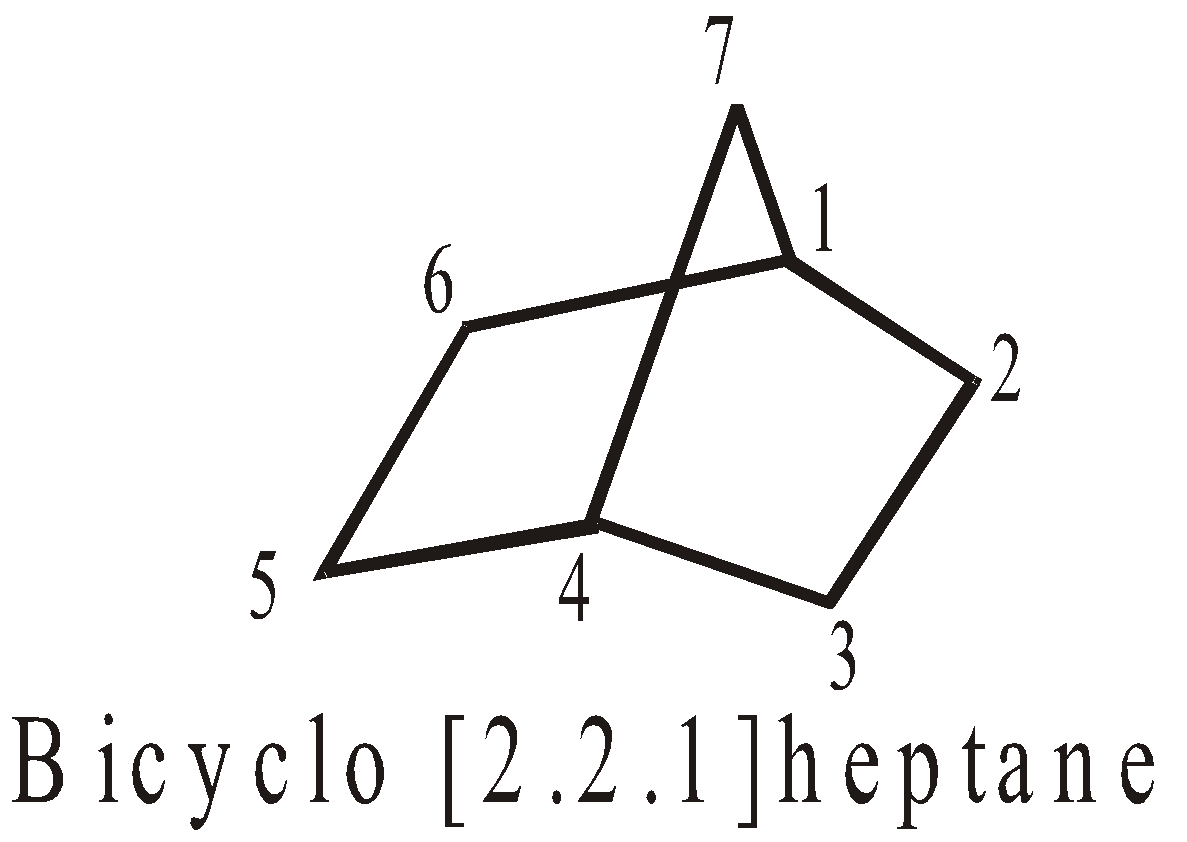
More examples of bridge Compounds
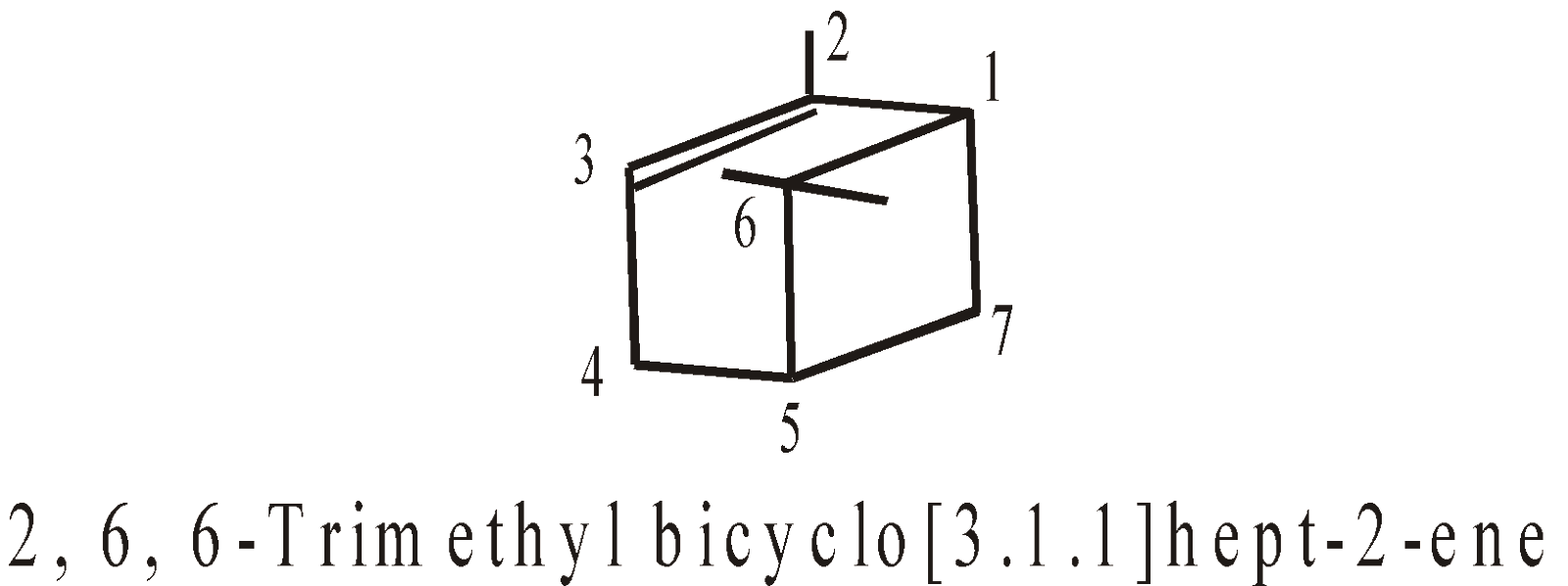
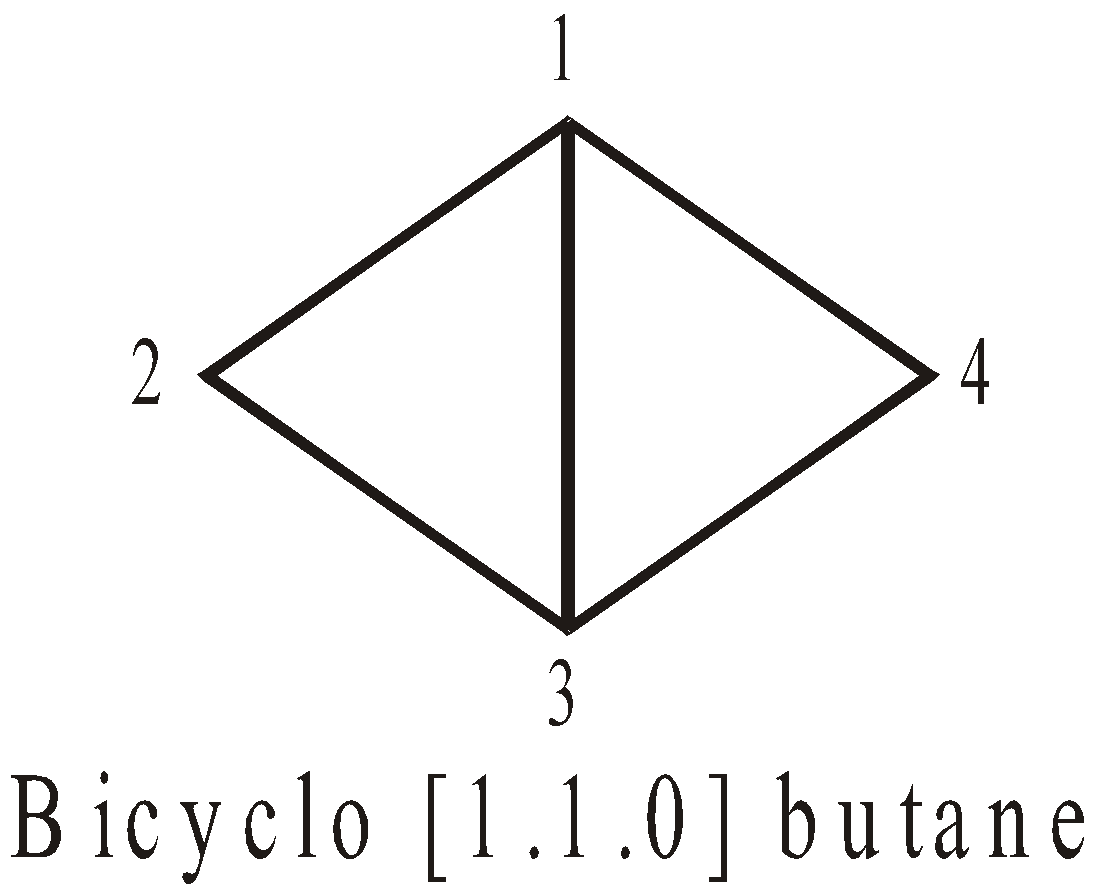
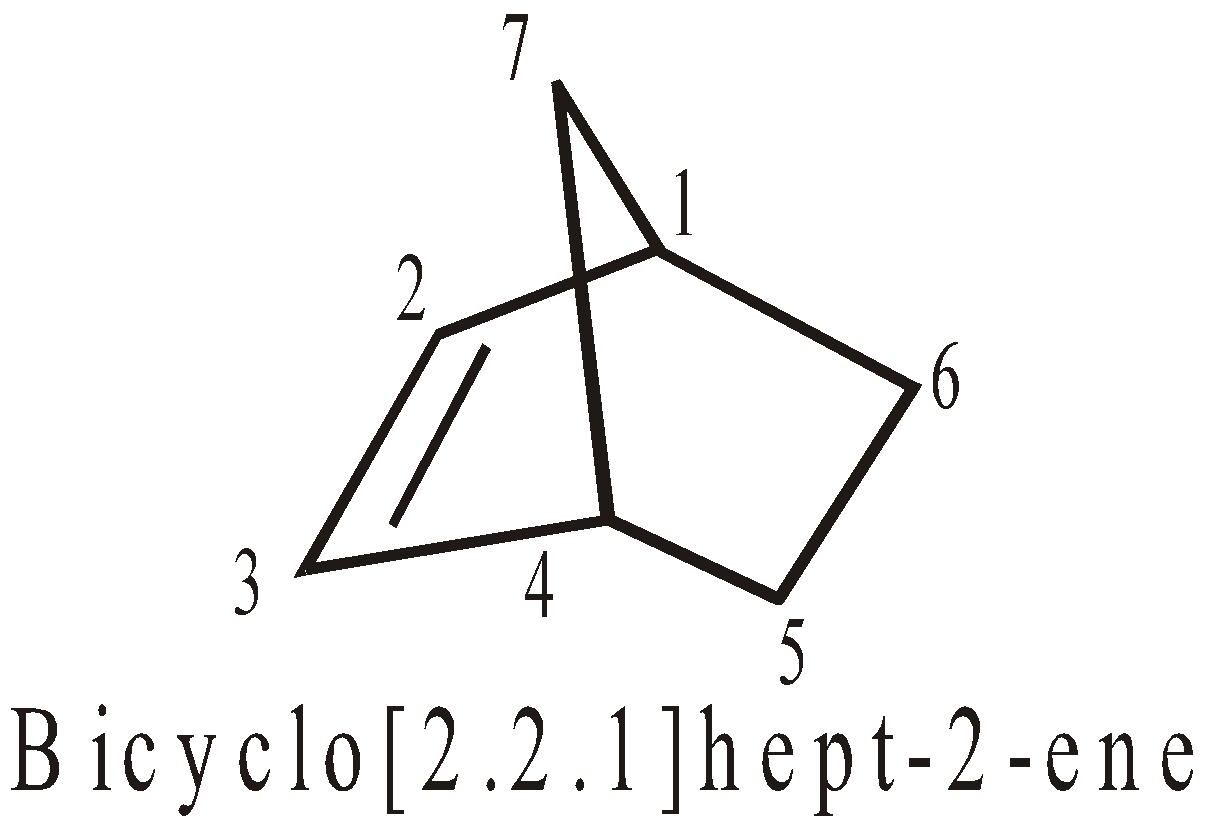
SPIROCYCLIC COMPOUNDS
The two rings share one carbon atom
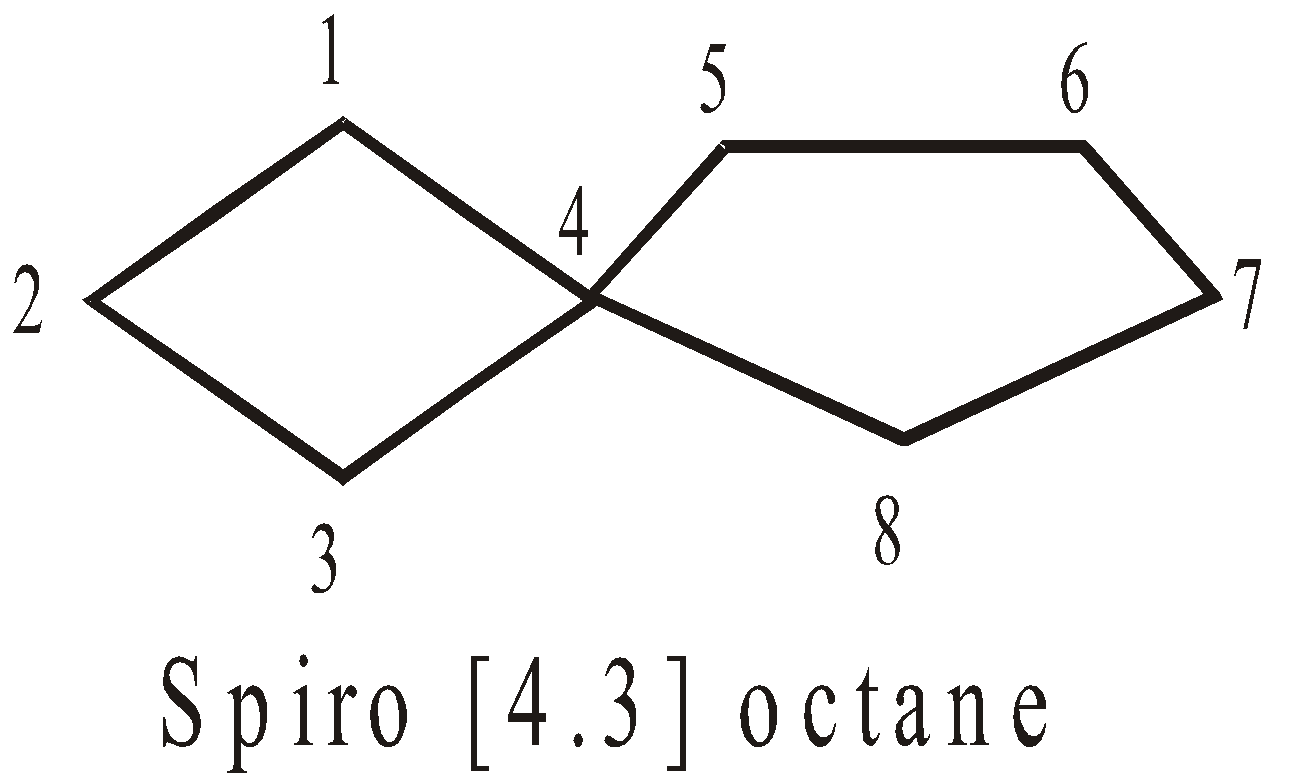
More examples of spiro Compounds
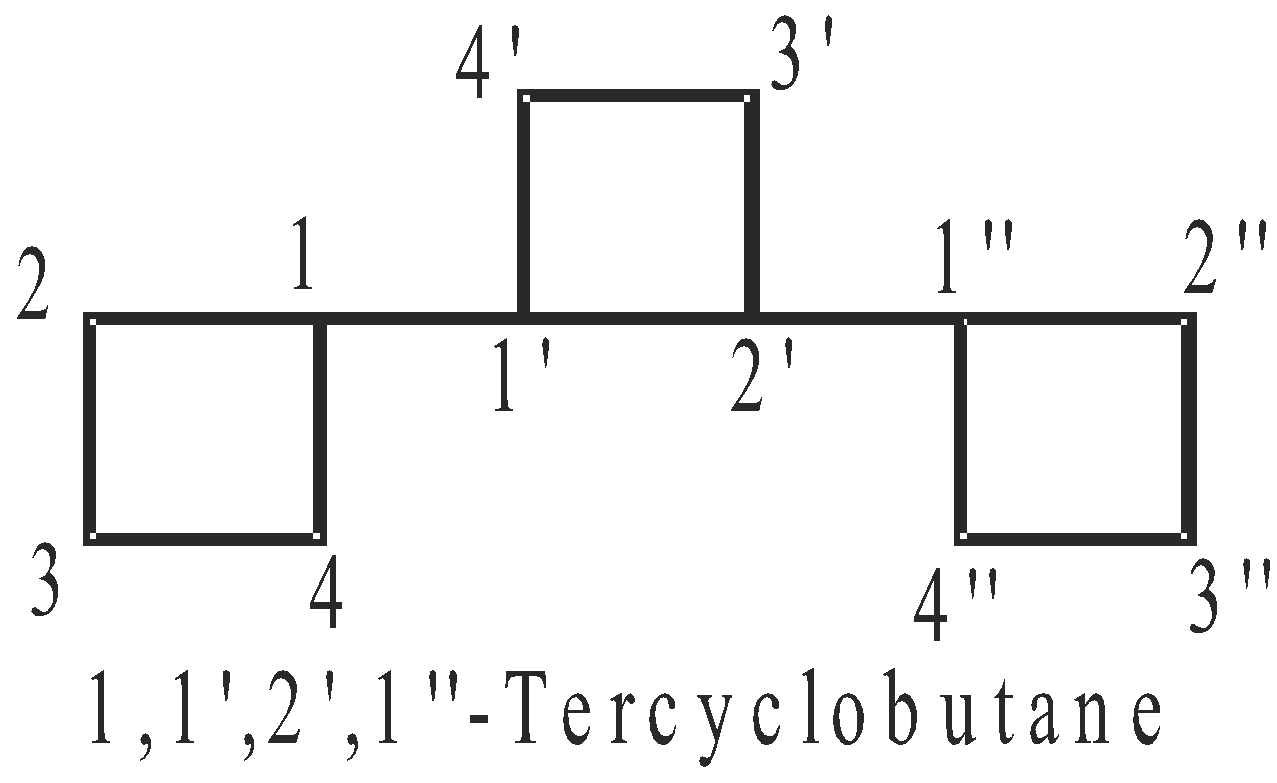
NOMENCLATURE OF COMPOUNDS CONTAINING IDENTICAL CYCLIC UNITS JOINED BY A SINGLE BOND
No. of cyclic hydrocarbon units | Two | Three | Four |
Prefix | bu | ter | quarter |
Numbering of C-atoms : The numbering starts from the C-atom joining the rings.
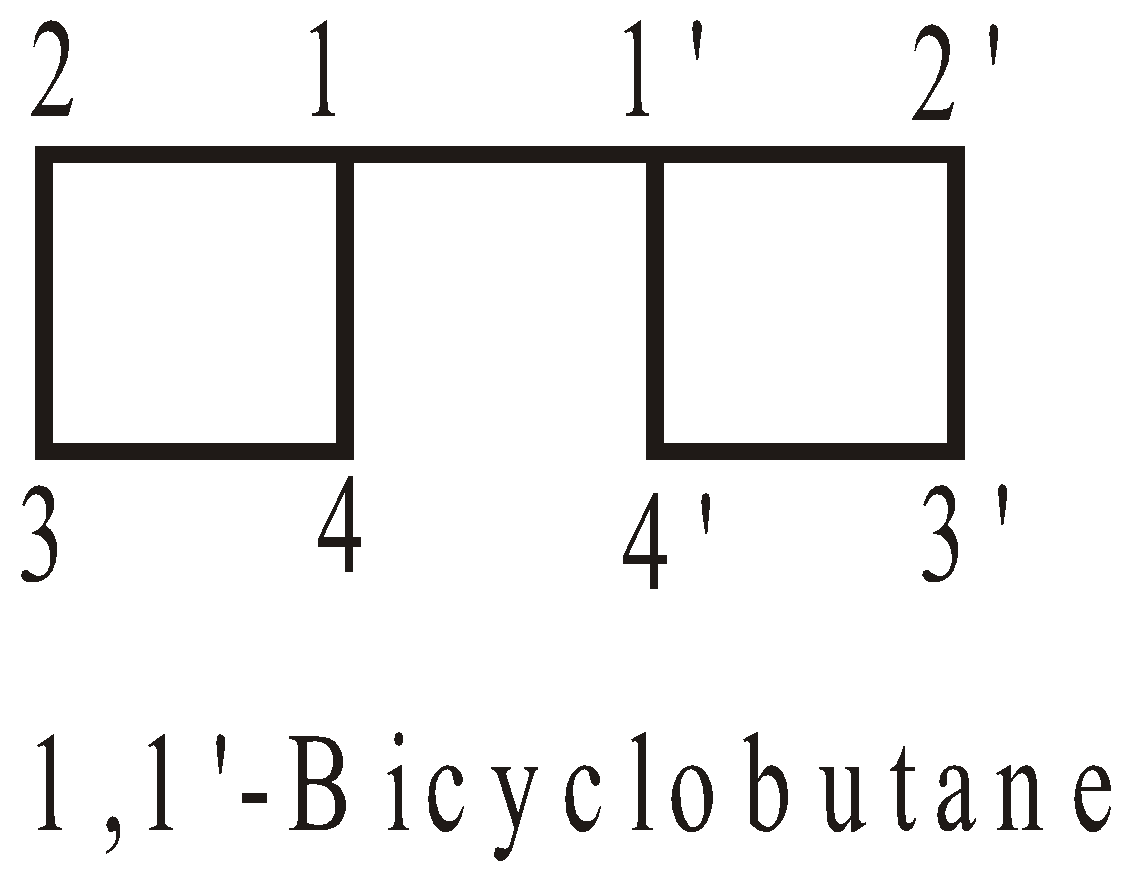

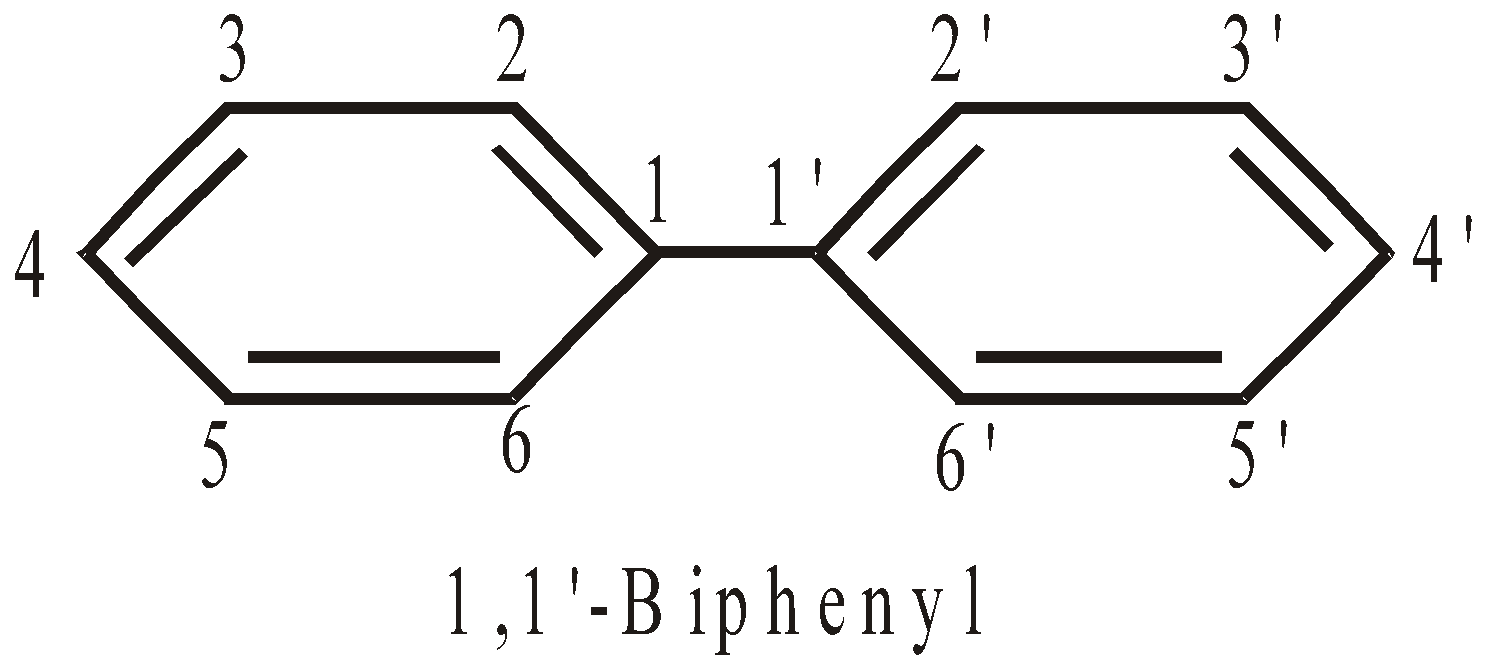
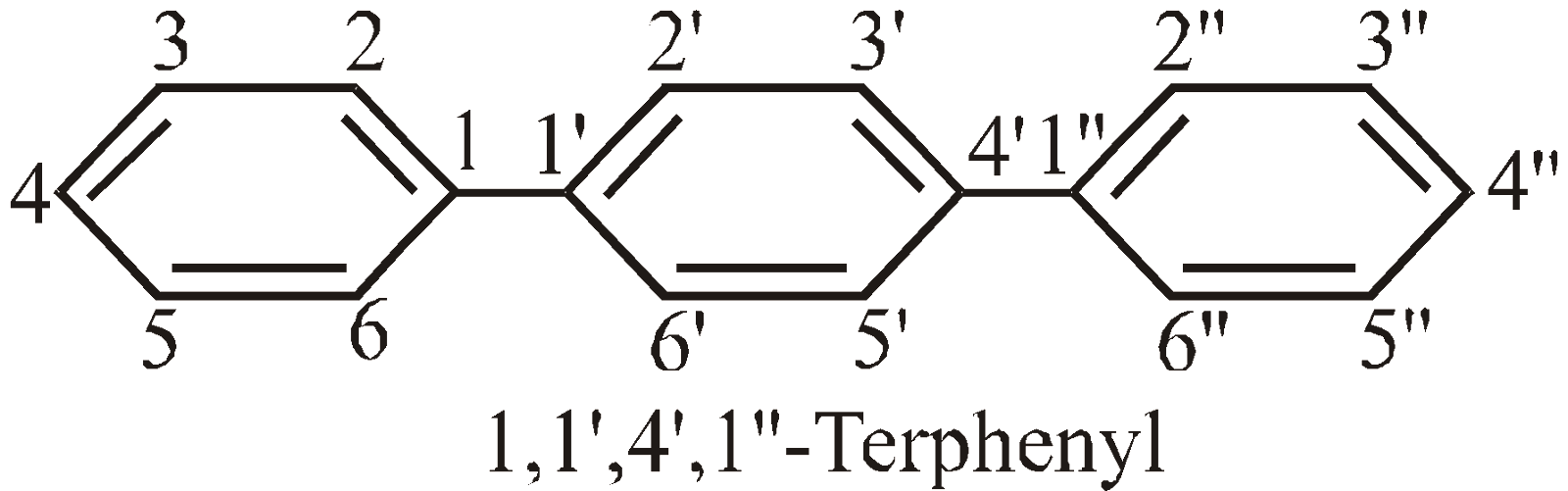
NOMENCLATURE OF COMPOUNDS CONTAINING TERMINATING FUNCTIONAL GROUPS
If only one group such as –COOH, –CHO, –COOR, –CONH2, –COCl or – is present in the molecule it is always given number 1 and 1 is never written when there is no ambiguity.
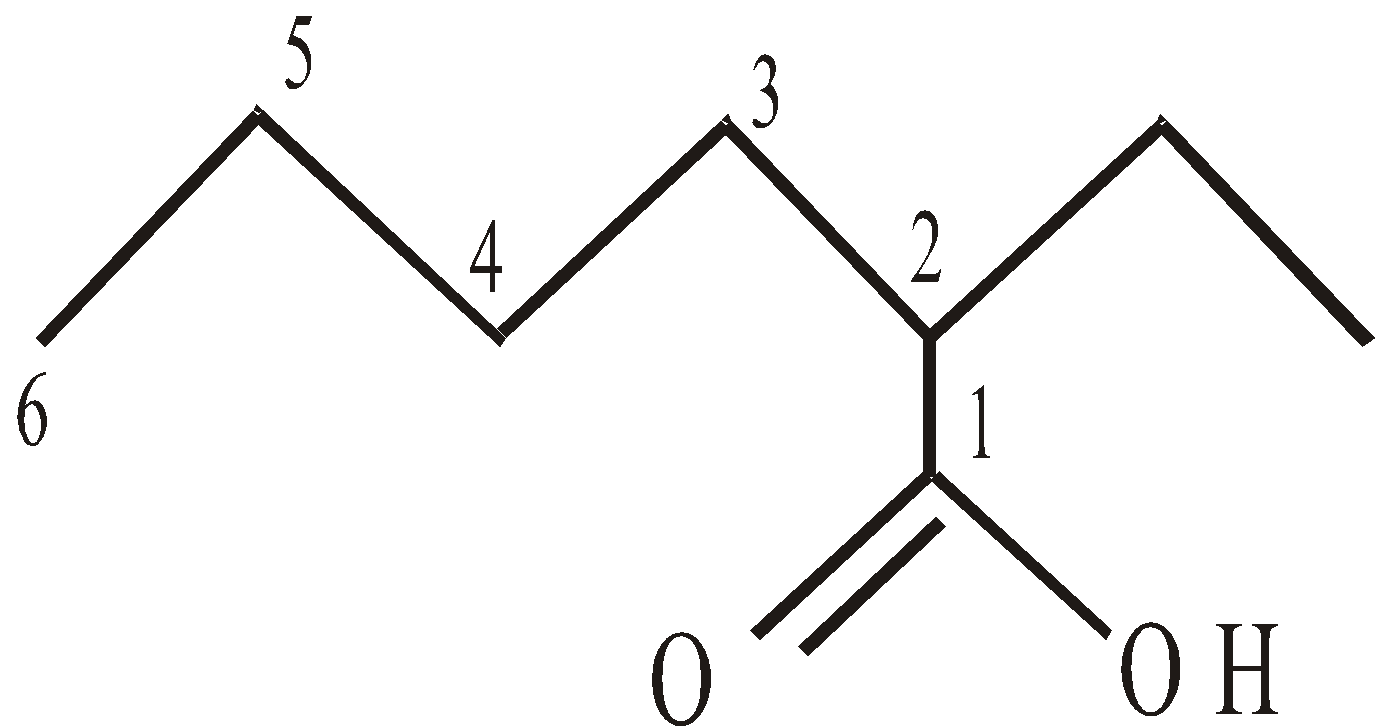
2-Ethylhexanoic acid
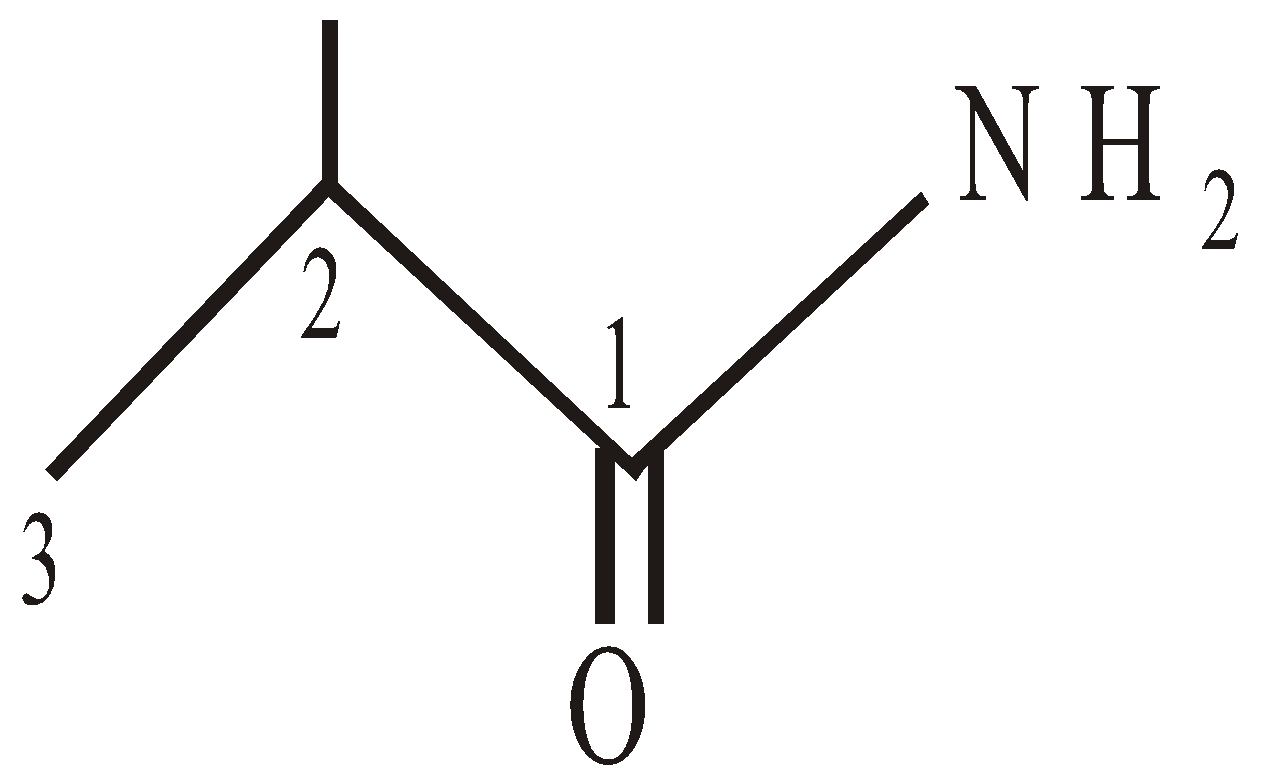
2-Methylpropanamide
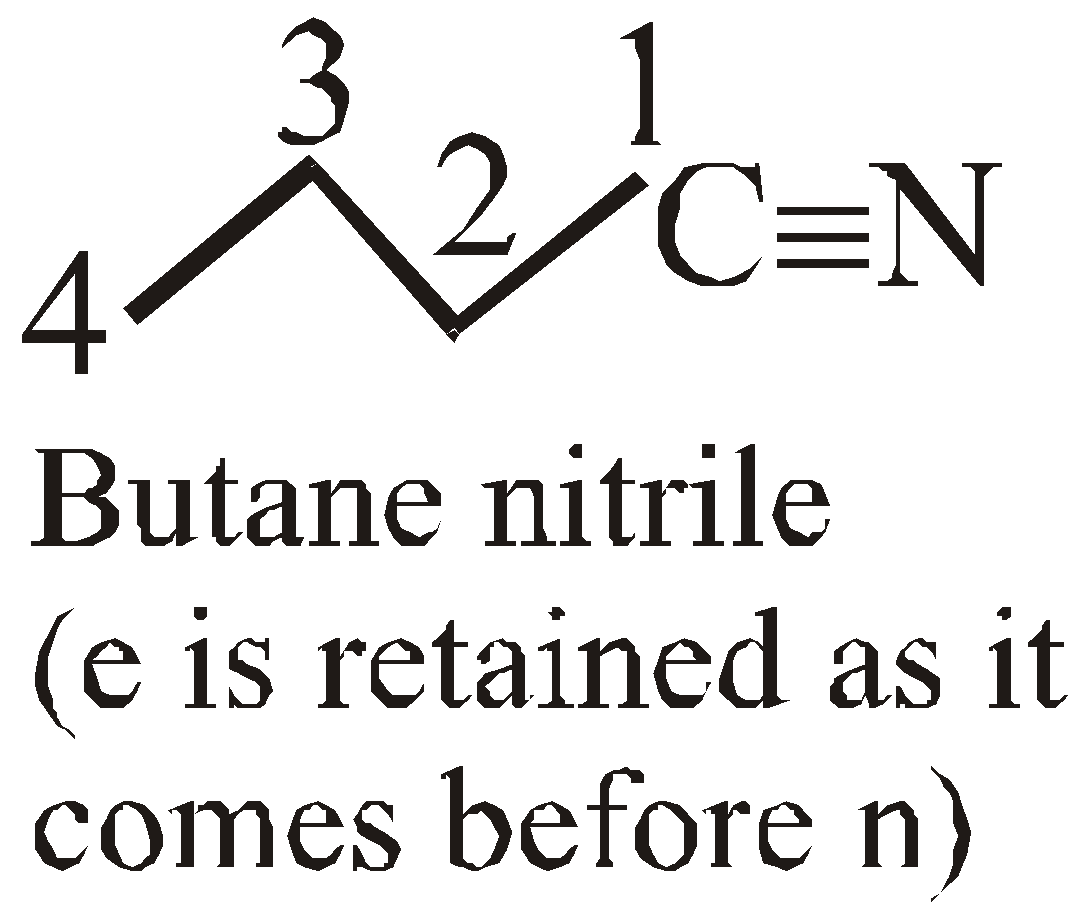
Methylpentanoate
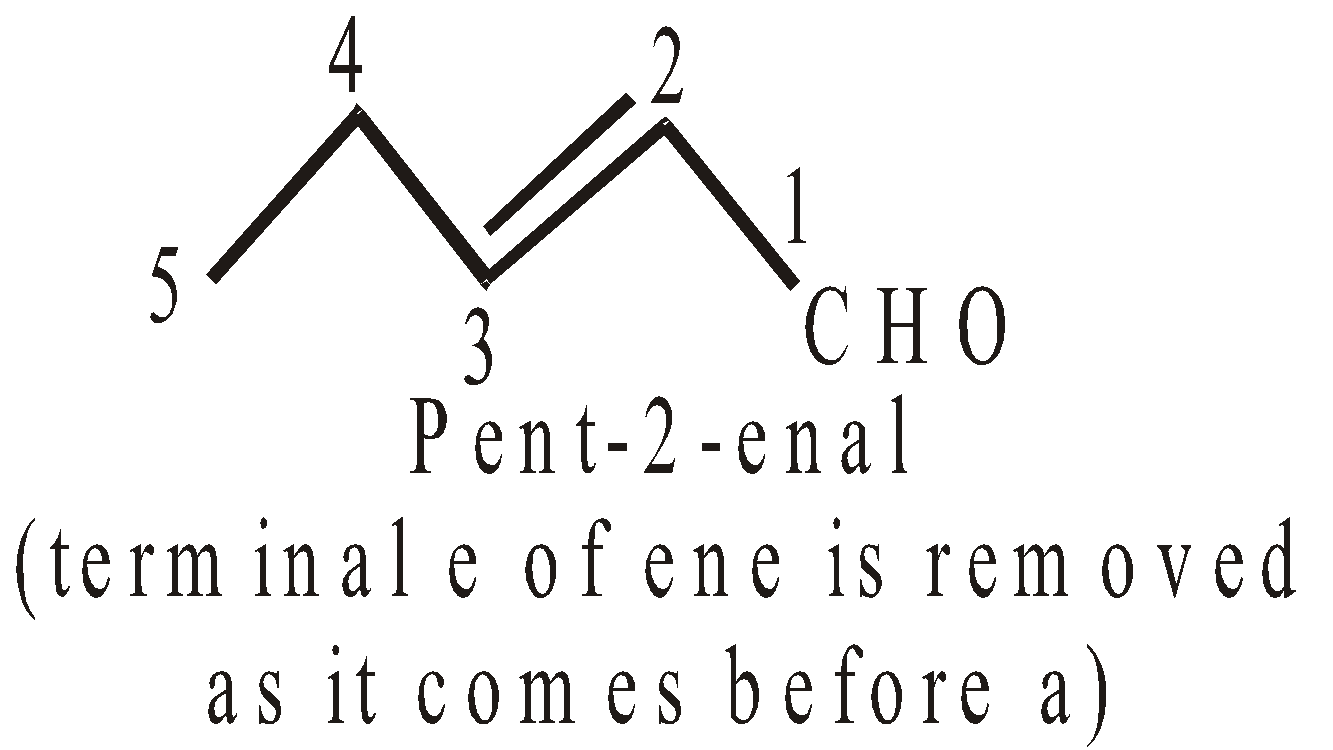
NOMENCLATURE OF COMPOUNDS CONTAINING TWO OR MORE THAN TWO SIMILAR TERMINAL GROUPS
PRESENCE OF ONLY TWO SIMILAR TERMINAL GROUPS
The carbon atoms of such groups are included in the principal chain. For example
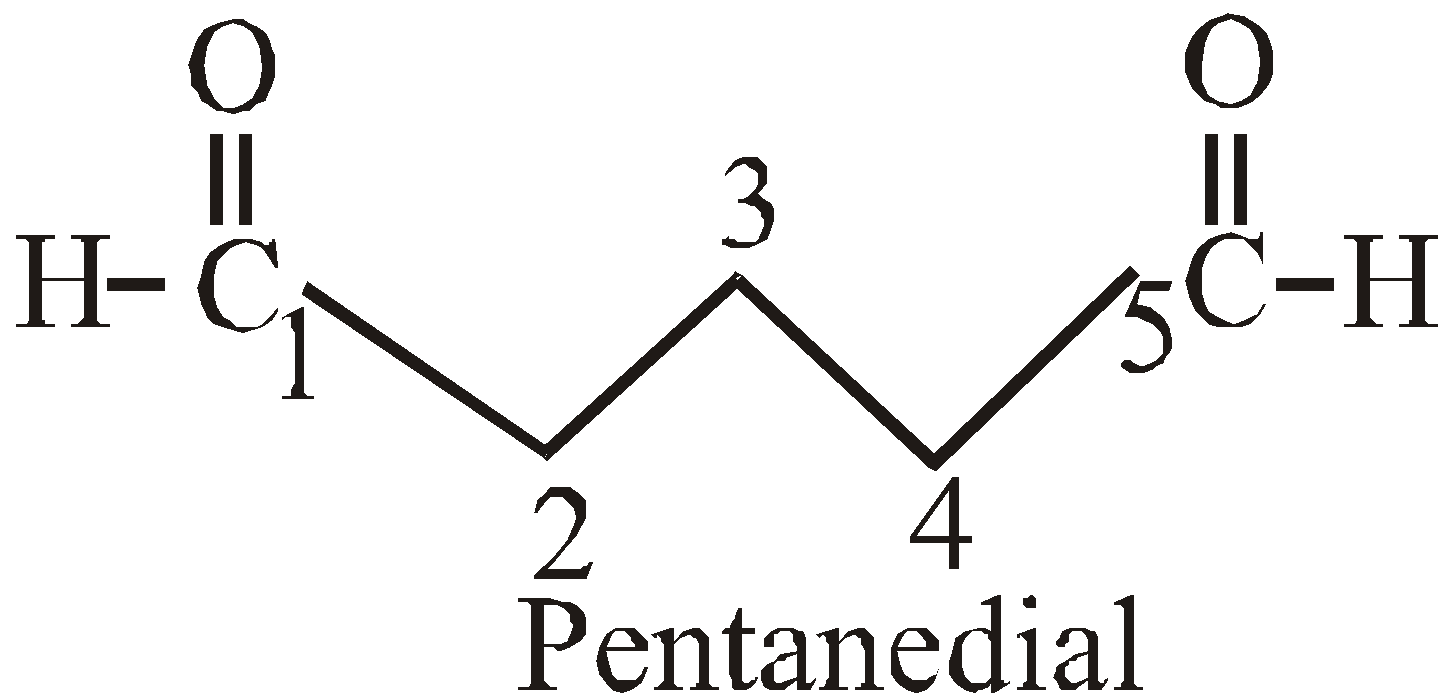
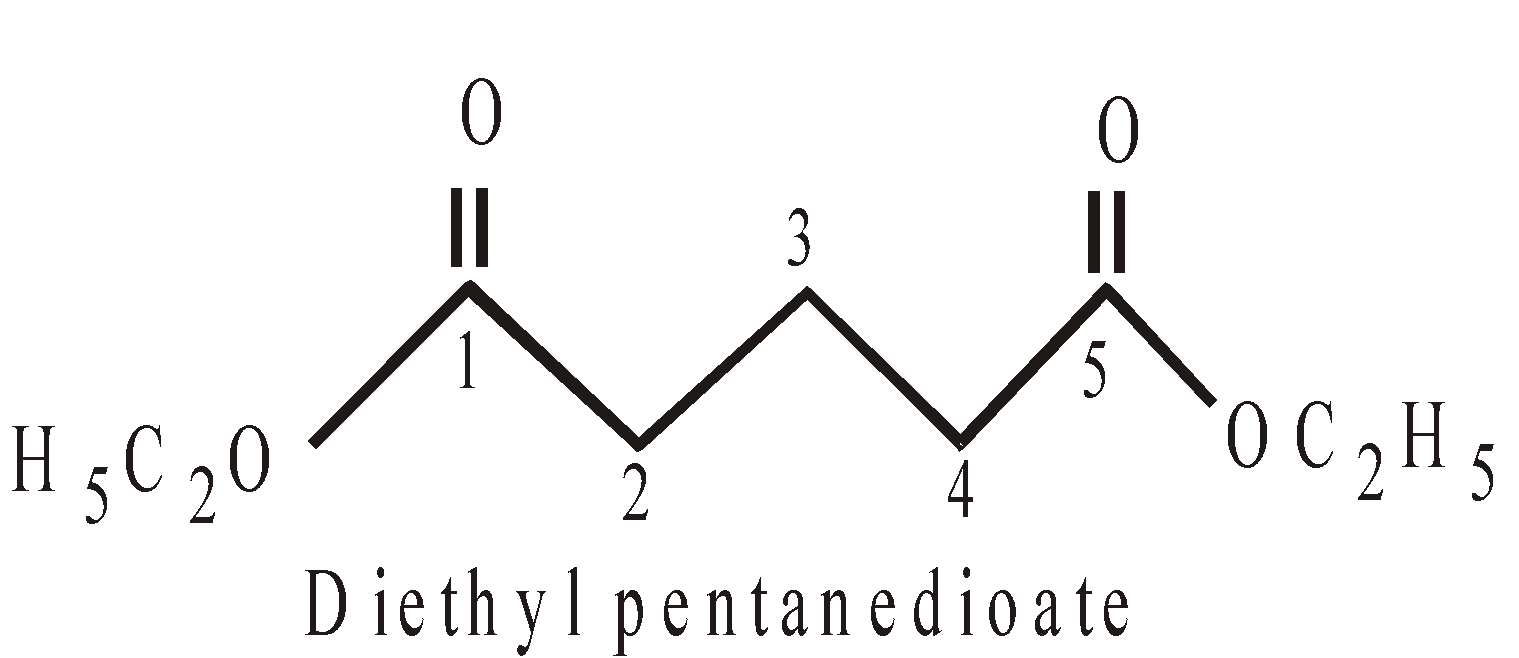
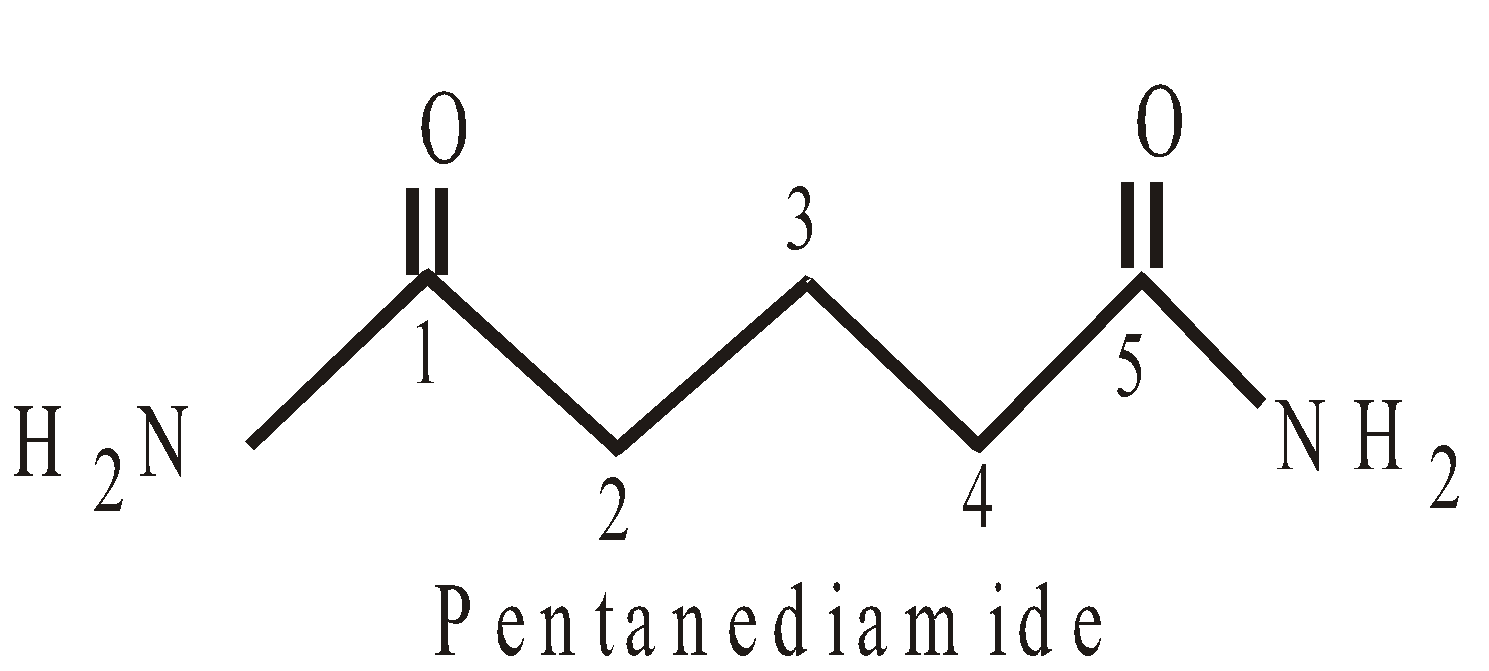
PRESENCE OF MORE THAN TWO SIMILAR TERMINAL GROUPS ATTACHED TO THE MAIN PRINCIPAL CHAIN
In this case special suffixes are used and carbons of terminal groups are not counted in the principal chain
Functional groups | Suffix |
–COOH | – Carboxylic acid |
–CHO | – Carbaldehyde |
–COX | – Carbonylhalide |
–CONH2 | – Carboxamide |
–COOR | – Alkyl carboxylate |
–CN | – Carbonitrile |
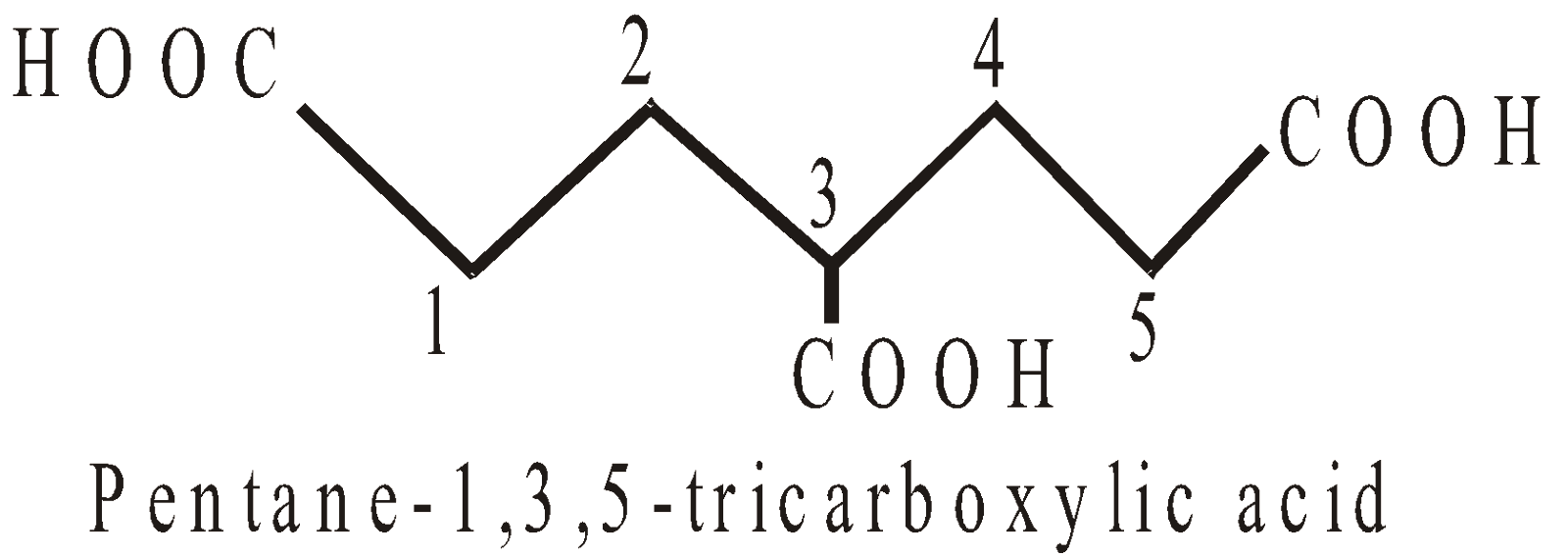
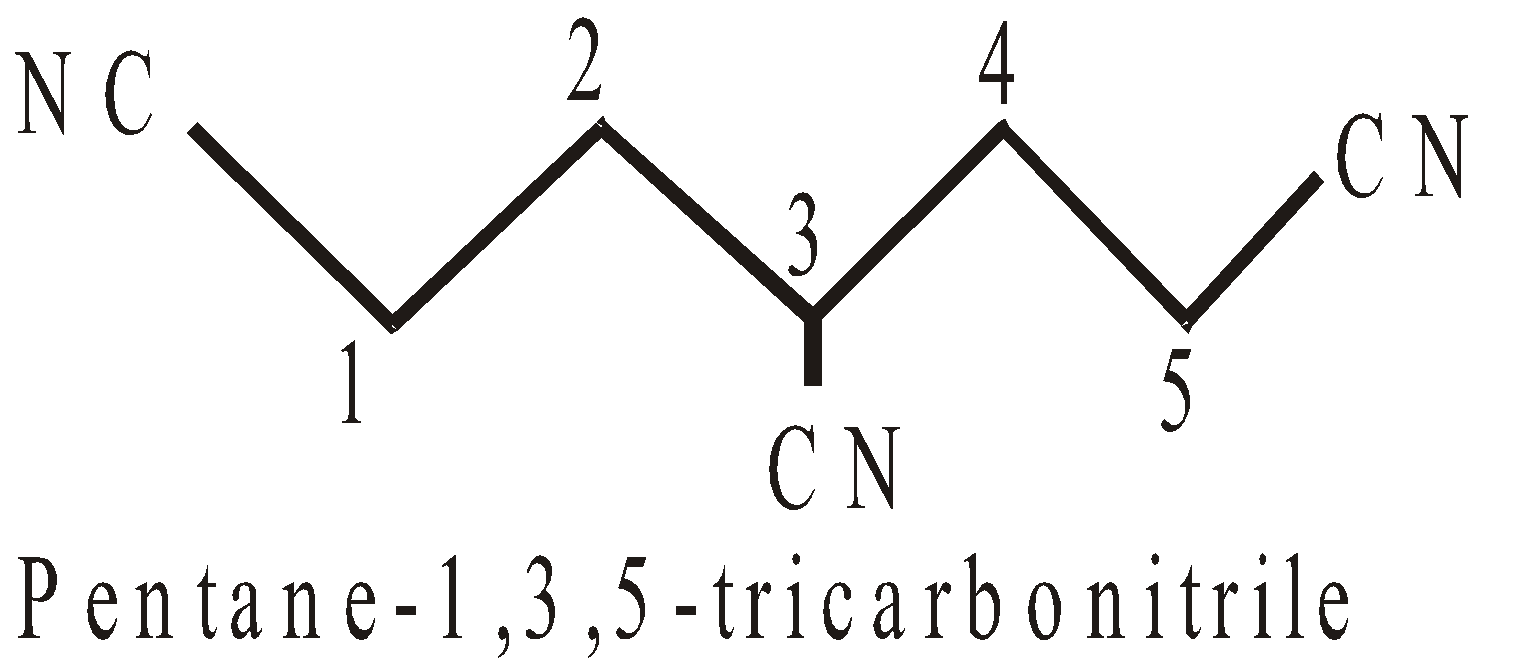
PRESENCE OF MORE THAN TWO SIMILAR TERMINAL GROUPS NOT DIRECTLY ATTACHED TO THE PRINCIPAL CHAIN
In such case the longest chain with two similar terminal groups is selected and carbons of groups are counted in the principal chain.
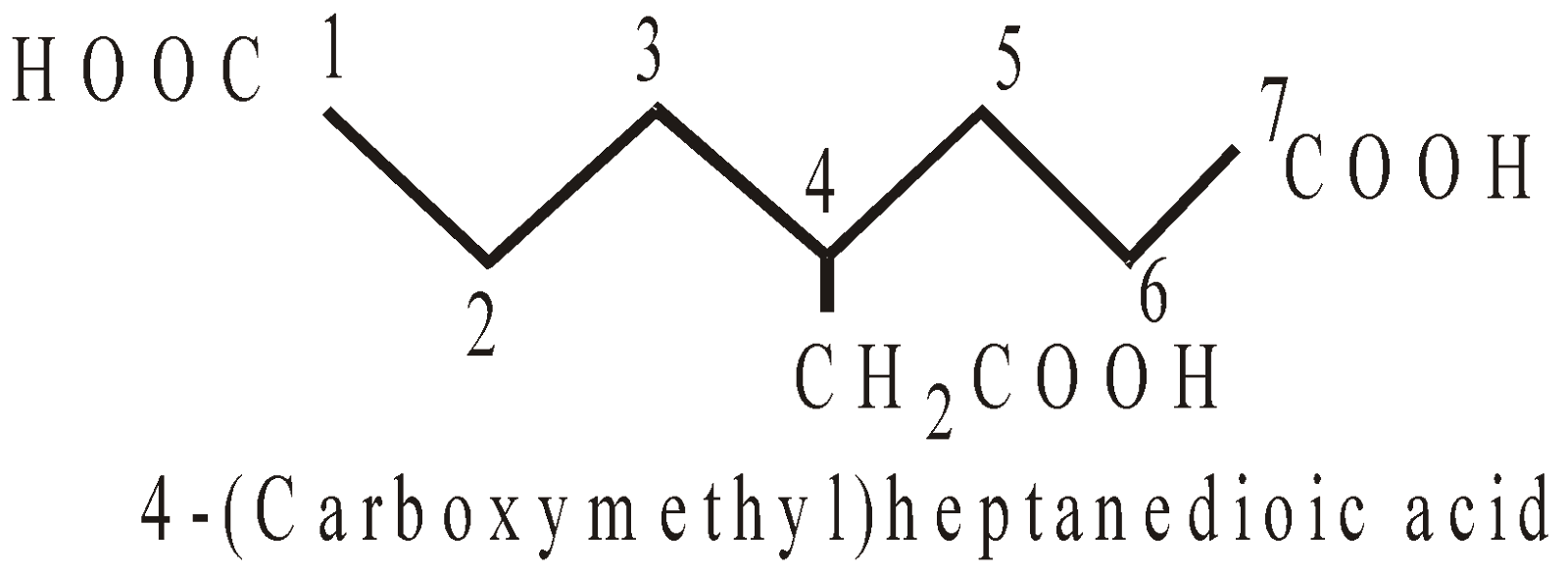
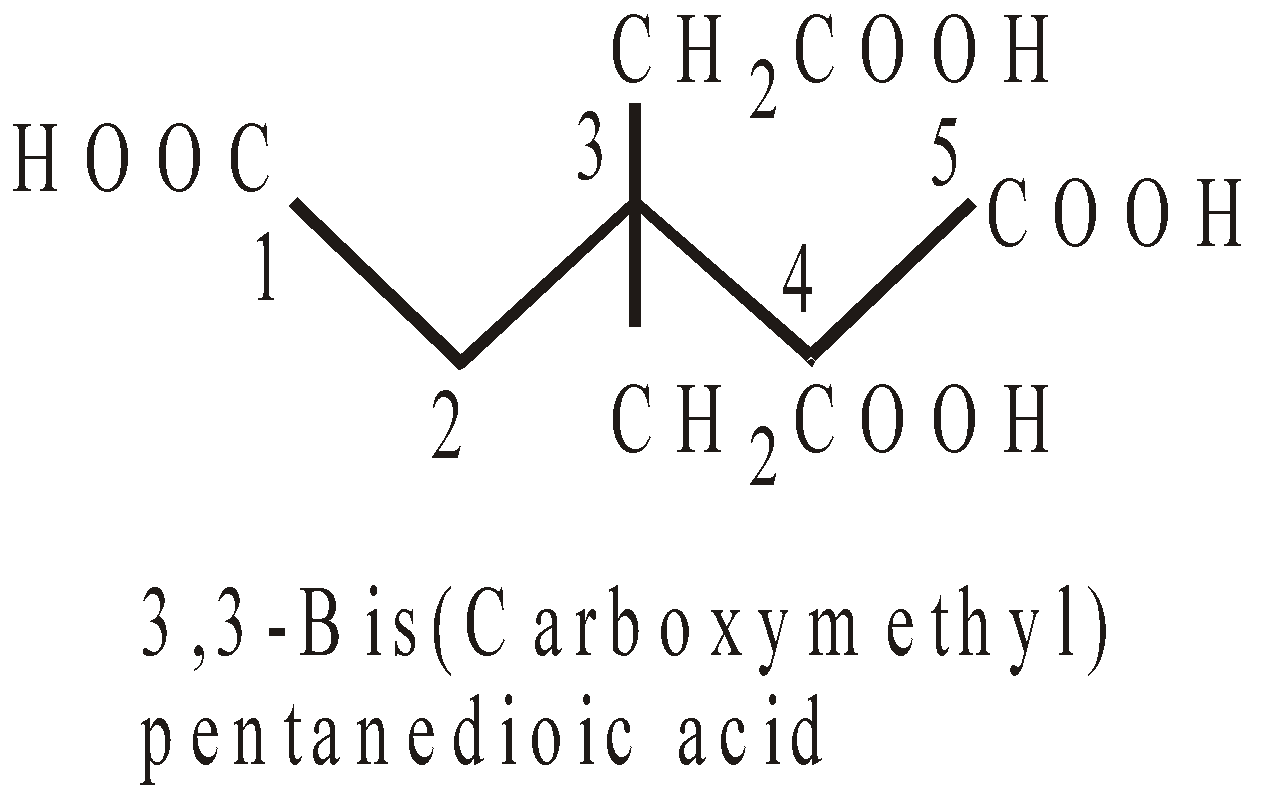
NOMENCLATURE OF COMPOUNDS CONTAINING SUBSTITUENTS (NOT REGARDED PRINCIPAL FUNCTIONAL GROUPS)
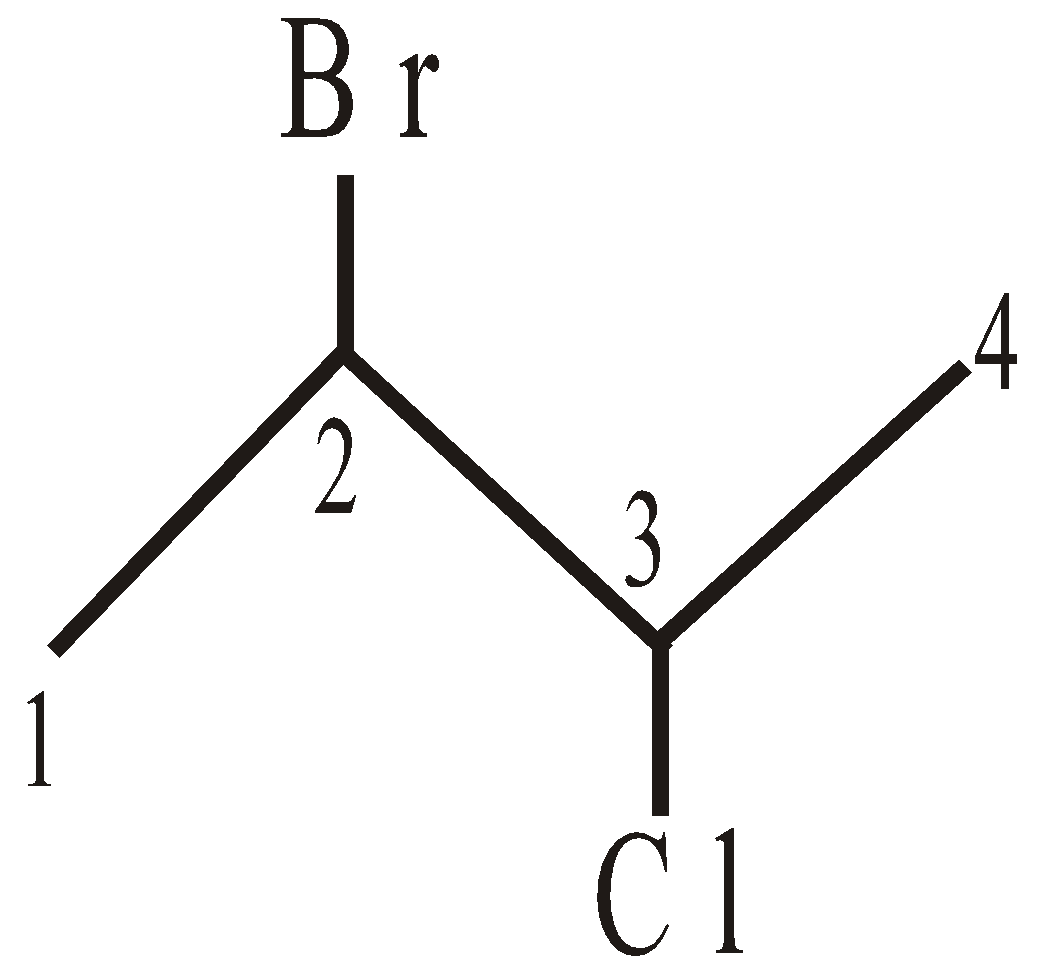
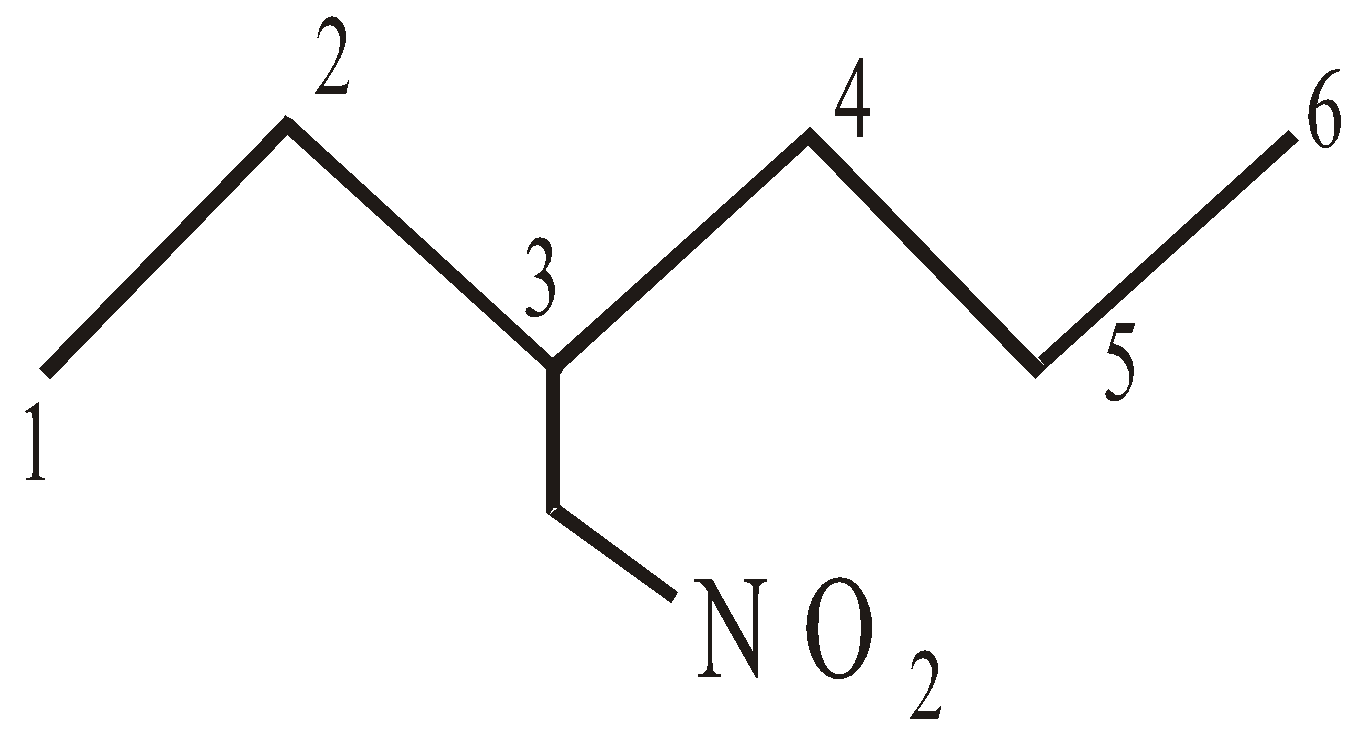
2-Bromo-3-Chlorobutane 3- Nitromethylhexane
(follow alphabetical order)
NOMENCLATURE OF COMPOUNDS CONTAINING MORE THAN ONE TYPE OF FUNCTIONAL GROUPS
In such case the compound is regarded as derivative of senior functional group and the other functional groups are regarded as substituents. The numbering of the parent chain is done in such a way so that the functional group of highest priority gets the lower number and the chain contains the maximum number of functional groups.
The seniority of functional groups (highest priority) follow the following order:-
Group | Prefix name | 2º Suffix name |
– SO3H | Sulpho | Sulphonic acid |
– COOH | Carboxy | Oic acid |
– COOR | alkoxy carbonyl | Oate |
– COX | Halo carbonyl/Halo formyl | –oyl halide |
– CONH2 | Carbamoyl | amide |
– CHO | Aldo or formyl | al |
– CN | Cyano | nitrile |
– NC | Isocyano | Isonitrile |
>C = O | Keto or oxo | one |
– OH | Hydroxy | ol |
– SH | Mercapto | thiol |
– NH2 | Amino | Amine |
– OR | Alkoxy | – |
Epoxy | – | |
> C = C < | – | ene |
– | yne | |
– N = N | Azo | – |
– NO2 | Nitro | – |
– NO | Nitroso | – |
– X (Cl, Br, I) | Halo (Cl, Br, I) |
The terminal e of the primary suffix is replaced by the suffix name of functional group.
Alphabetical order for substituents : These should be placed in alphabetical order.
Naming of substituted substituents : In this case the subsidiary substituents are named as prefixes. For example
NOMENCLATURE OF AROMATIC COMPOUNDS
Generally Benzene and its derivatives are known as aromatic compounds. They are of two types
- Nuclear substituted : The functional group is directly attached to the benzene nucleus e.g. phenol, toluene, chlorobenzene etc.
- Side chain substituted : The functional group is present in the side chain e.g. Benzyl alcohol, Benzylamine etc.
In the first case the compounds are named as derivatives of benzene and in the second case as derivatives of aliphatic compounds (except arenes).
The IUPAC name of benzene is cyclohex-1,3,5-triene, but now aromatic compounds have their popular common name adopted by IUPAC. In IUPAC system the position of functional groups are indicated by arabic numerals i.e. 1, 2, 3 instead of o, m and p.
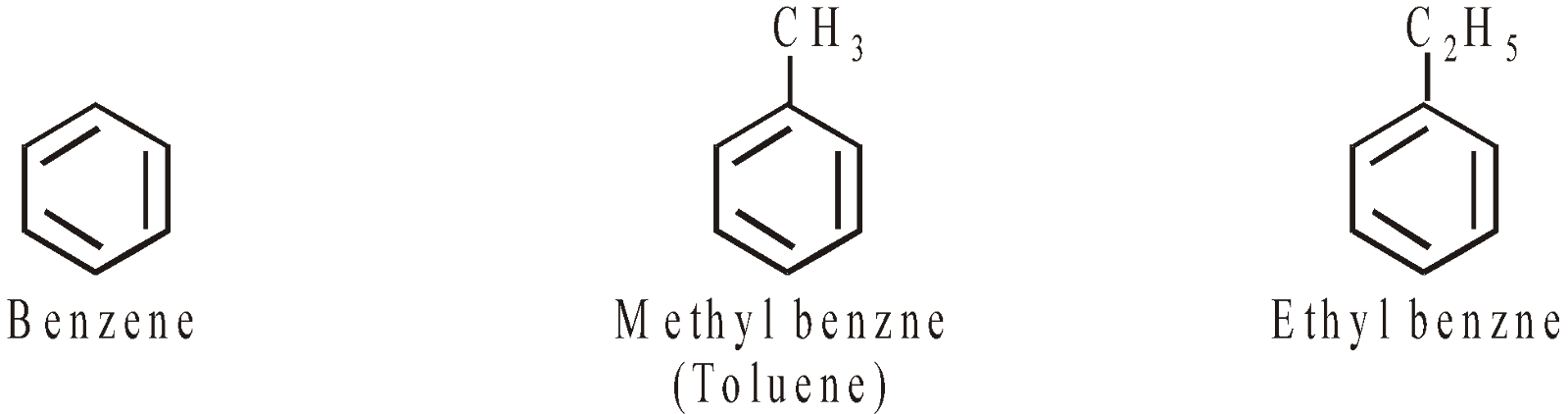
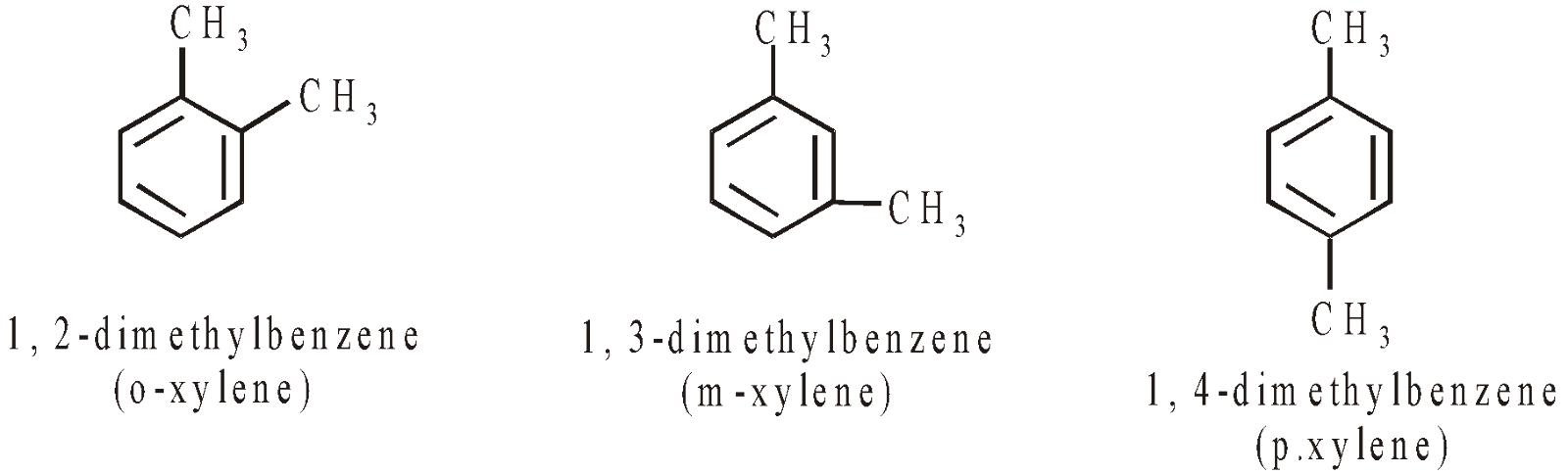
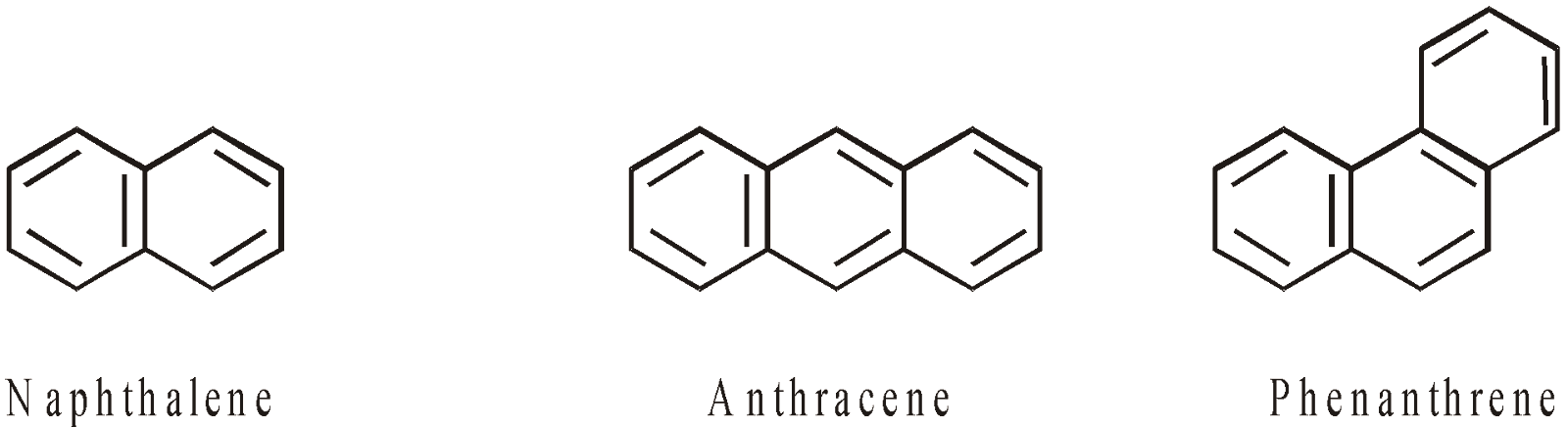
AROMATIC HYDROCARBONS (ARENES)
CONTAINING ONE RING
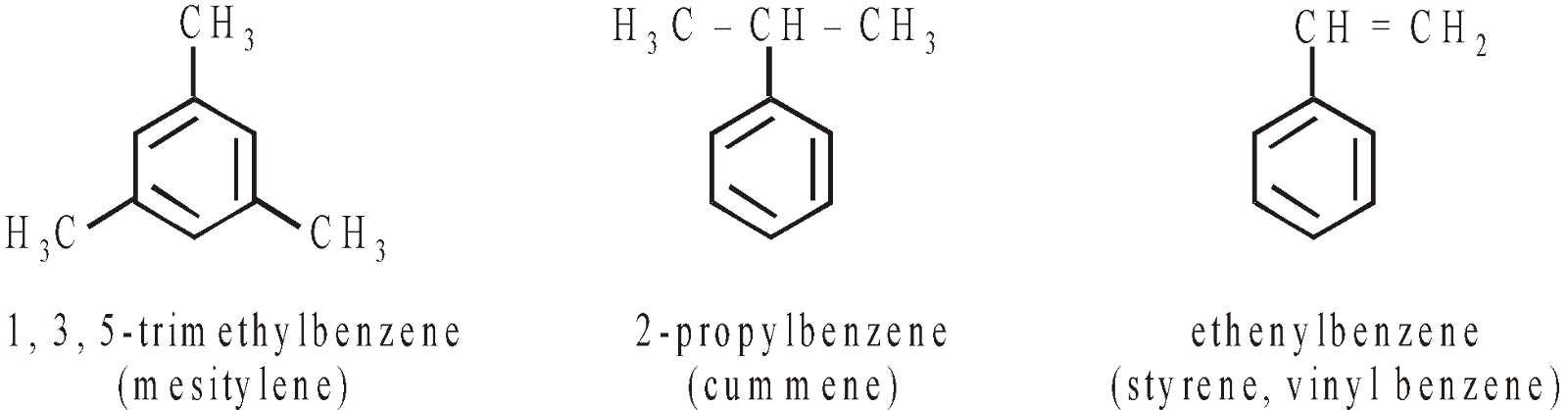
CONTAINING MORE THAN ONE RING
ARYL GROUPS
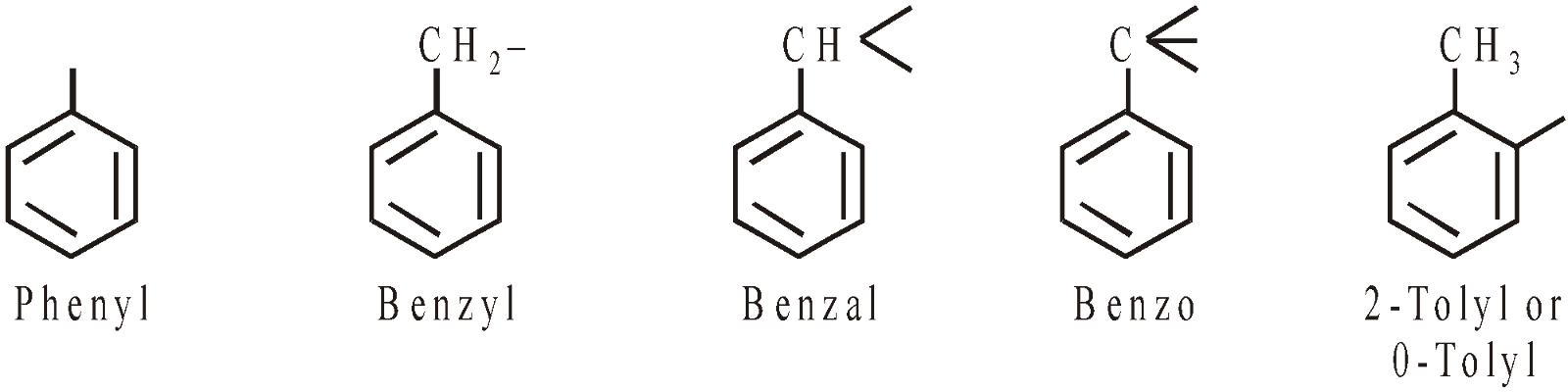
HALOGEN DERIVATIVES
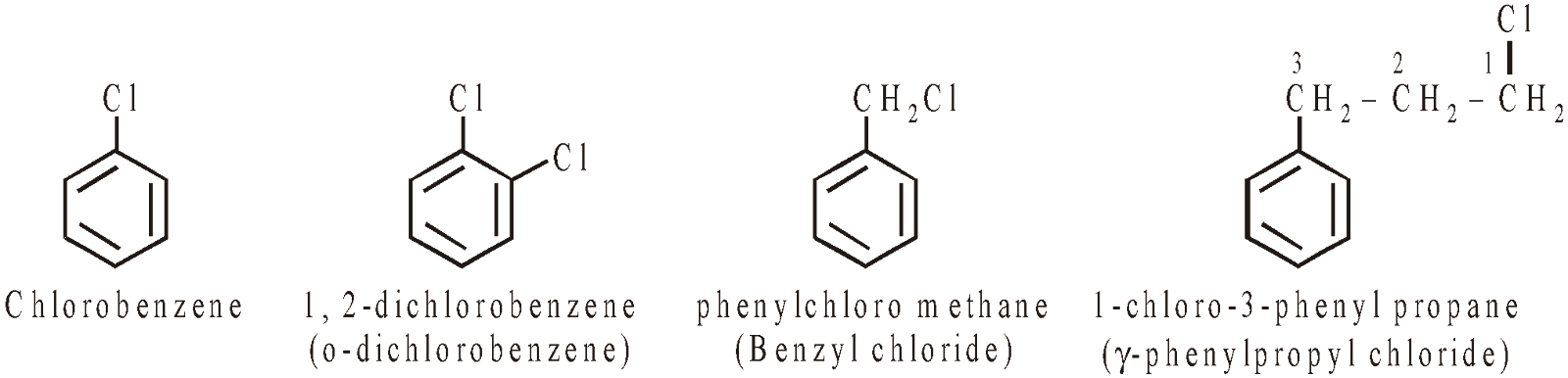
PHENOLS
Nuclear substituted hydroxy derivatives are known as phenols
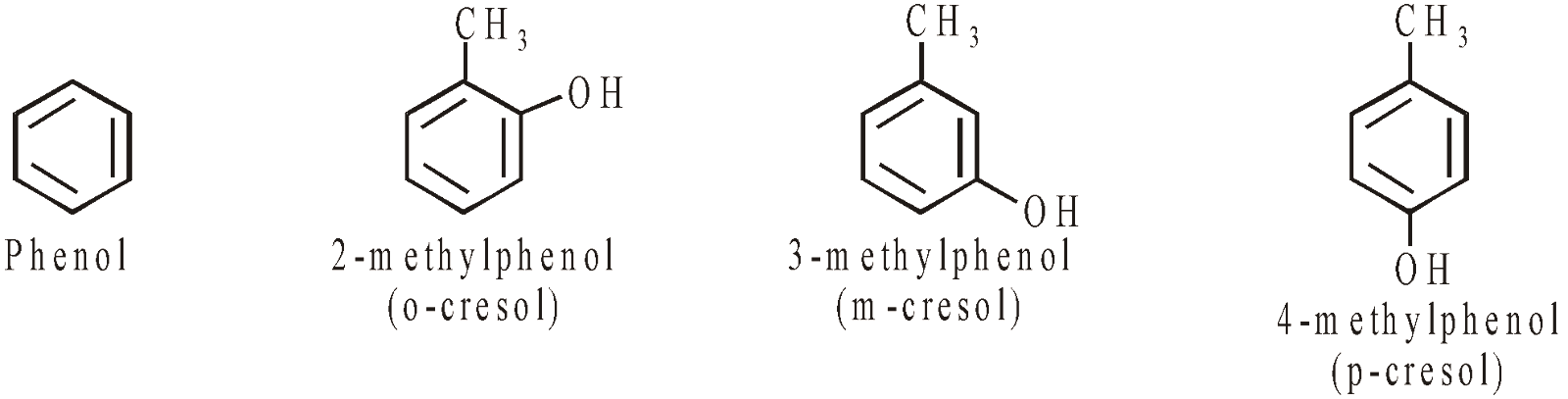
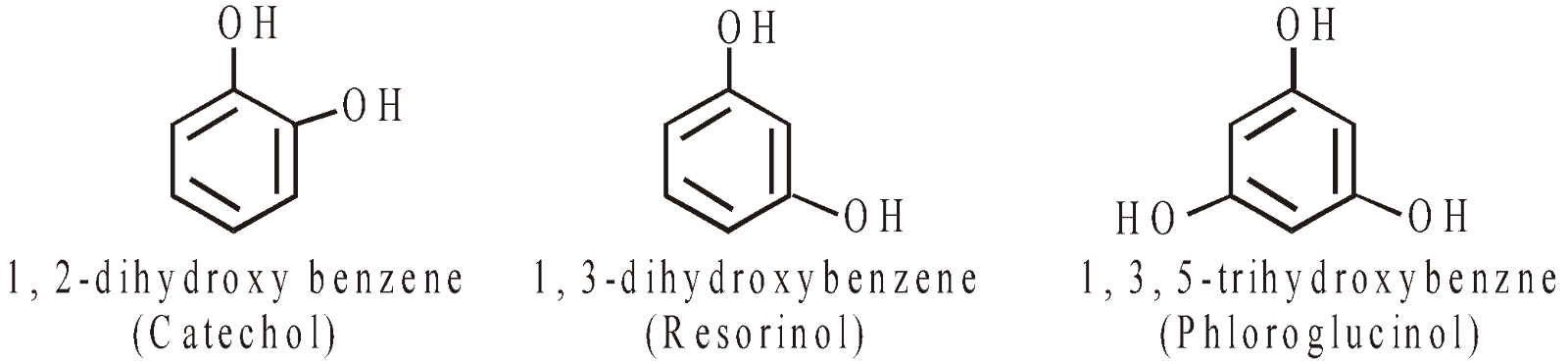
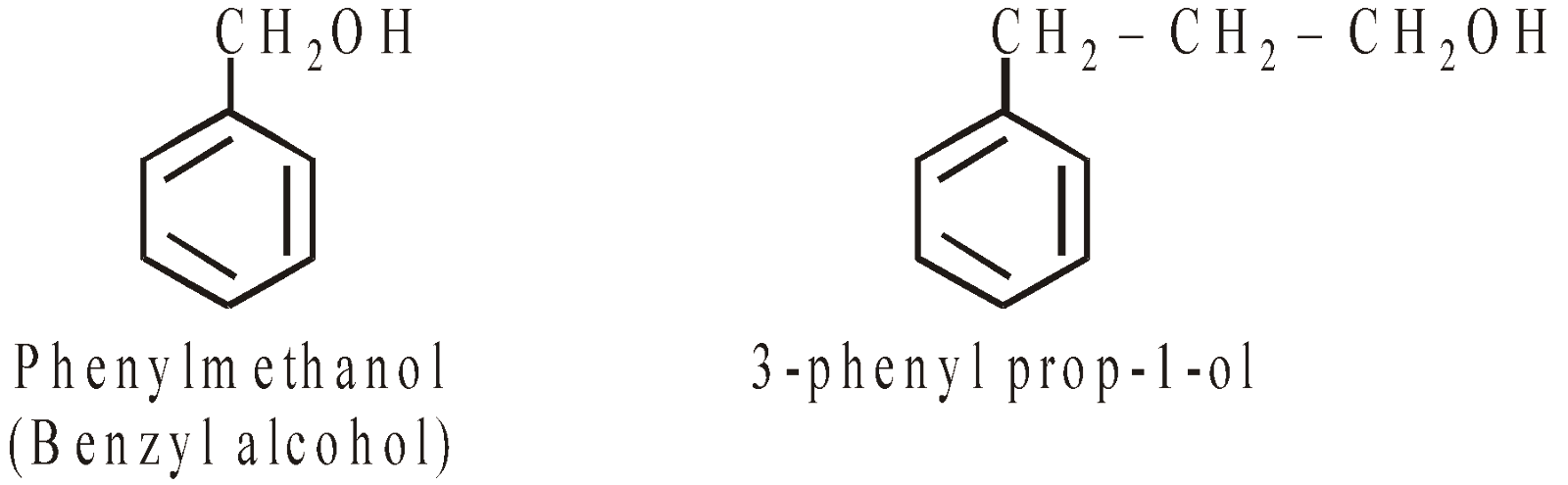
Side chain substituted hydroxy derivatives are known as alcohols
AROMATIC ETHERS
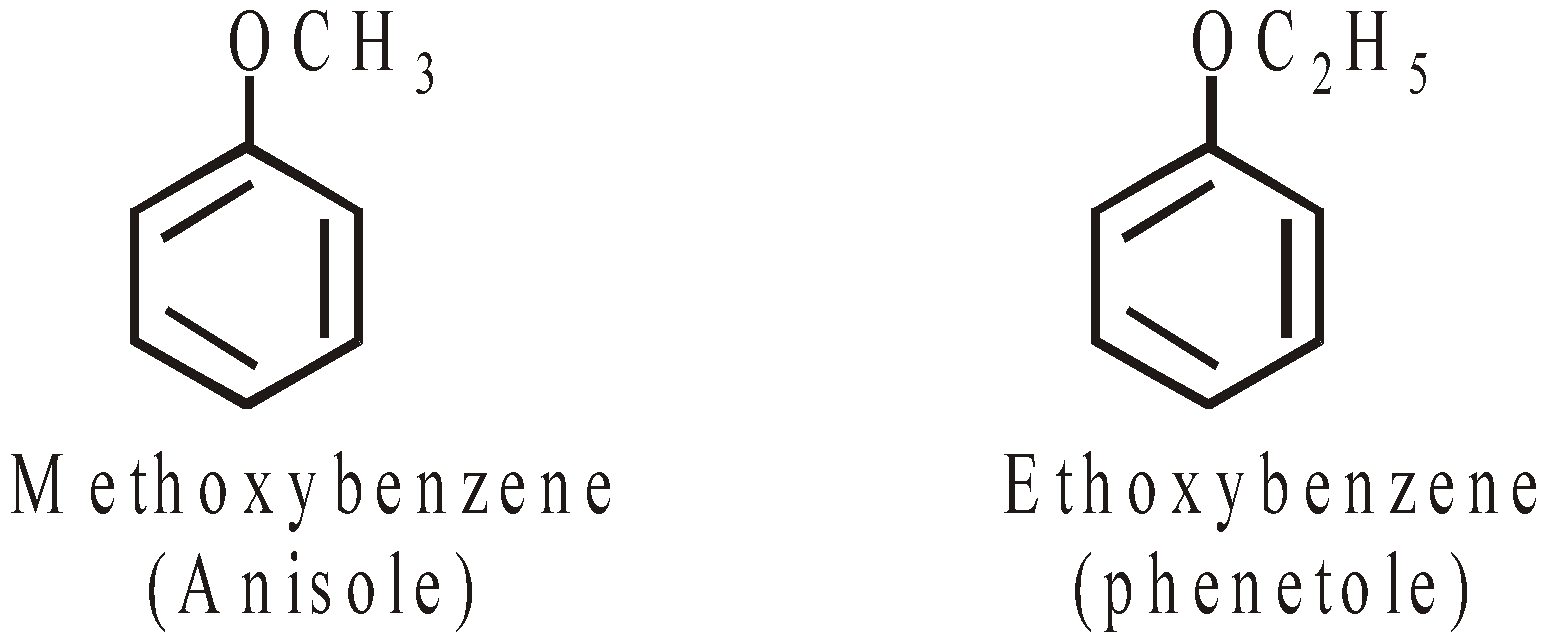
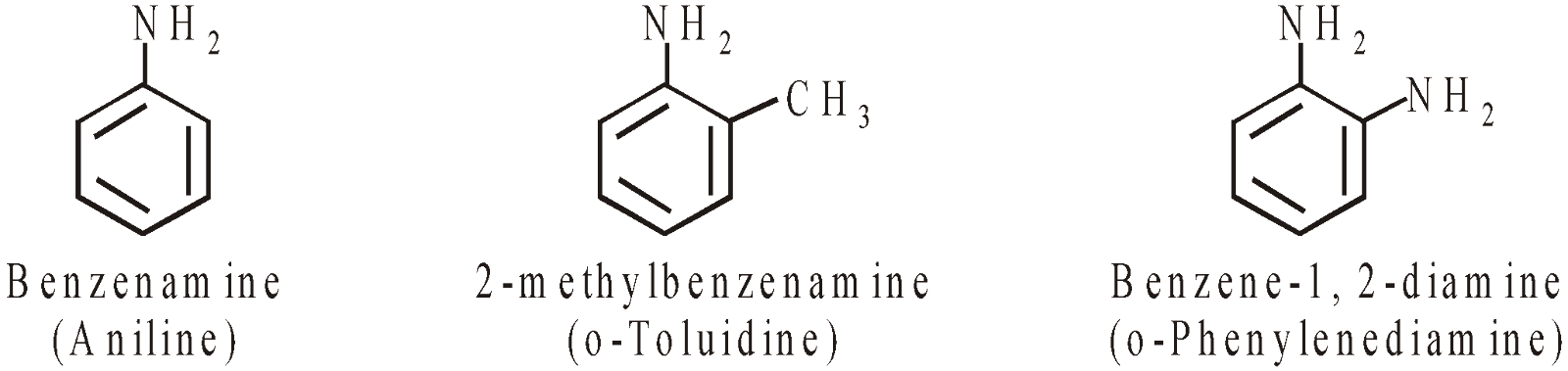
AMINES
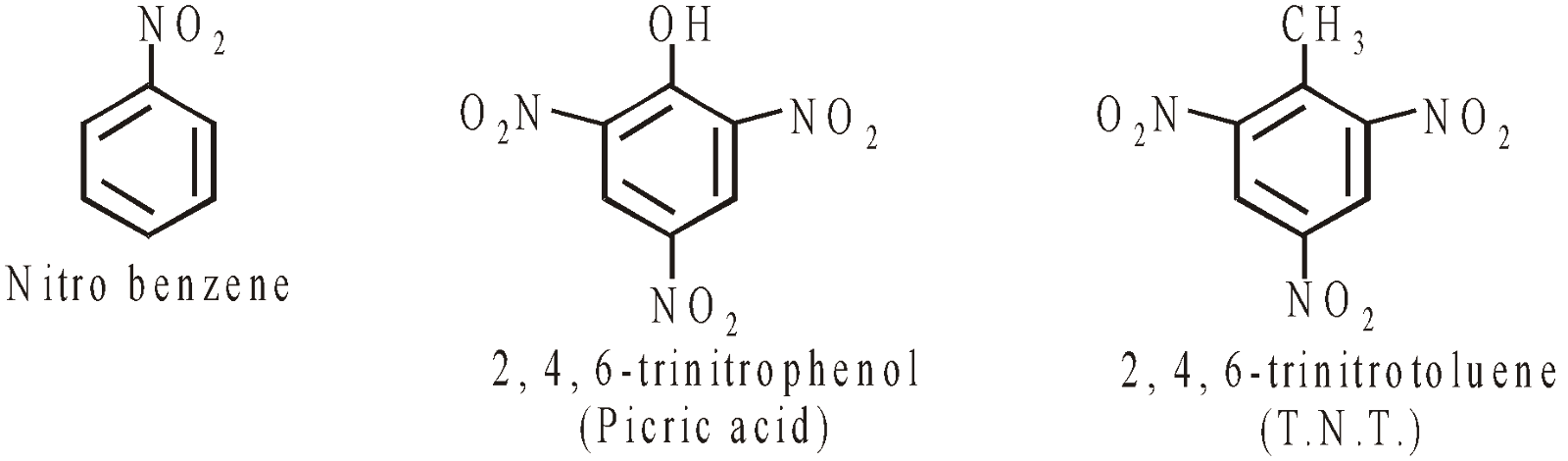
NITRO COMPOUNDS
ALDEHYDES
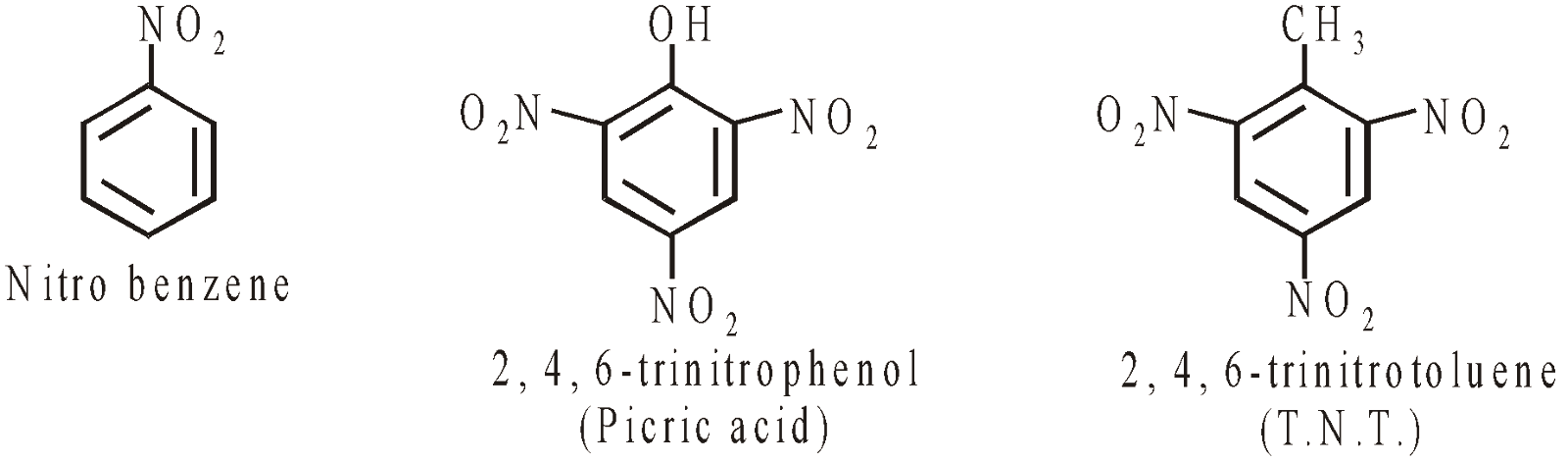
KETONES
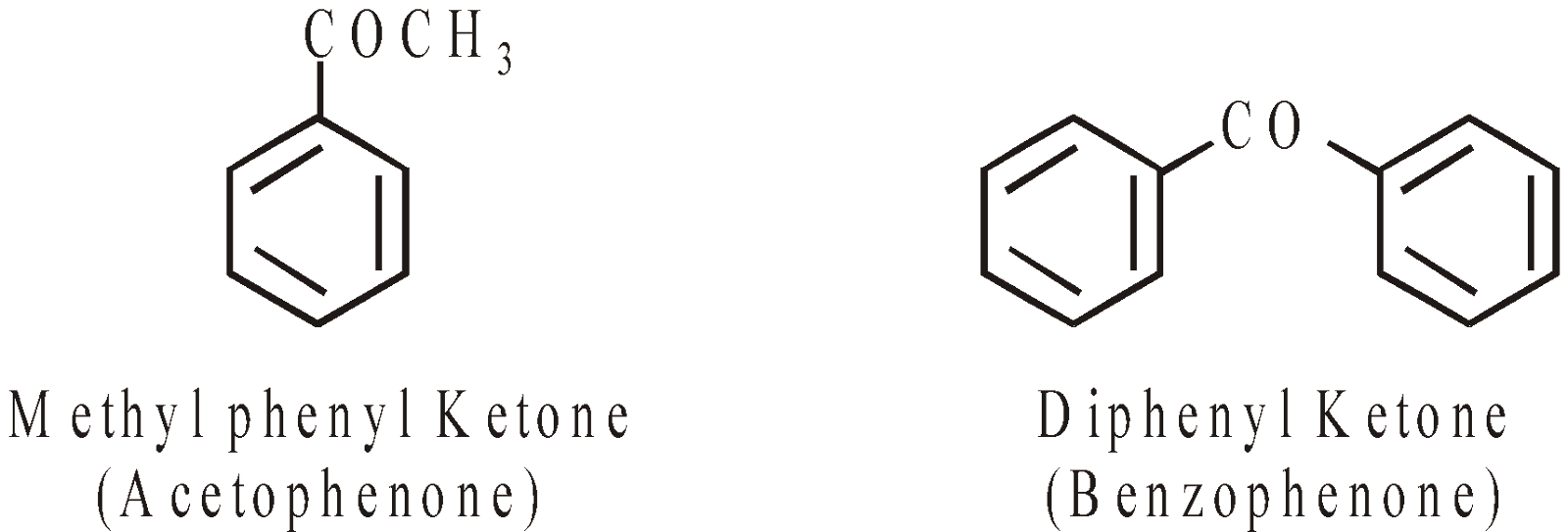
ACIDS
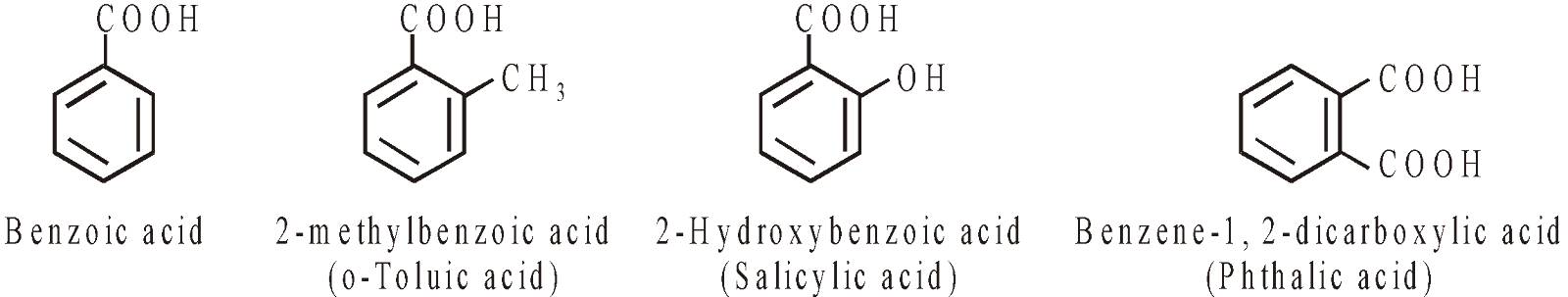
ACID DERIVATIVES
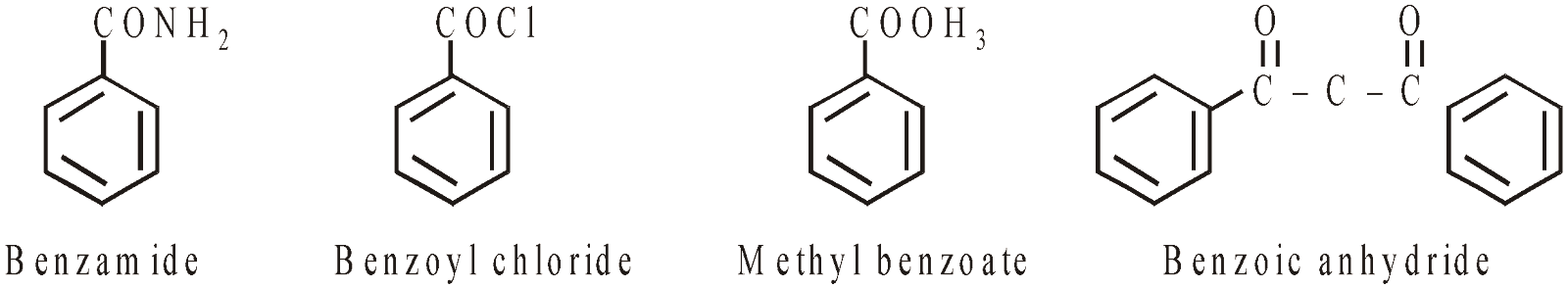
SULPHONIC ACIDS
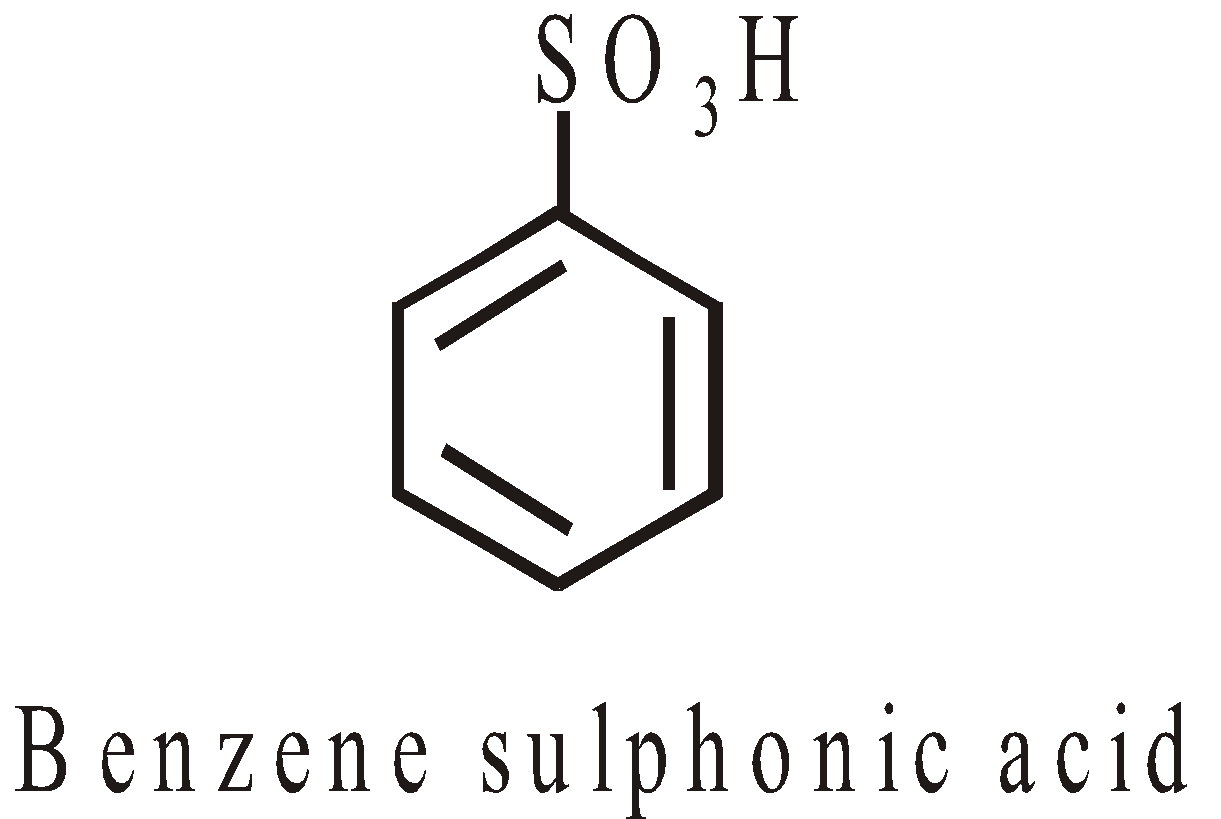
HYBRIDISATION AND SHAPES OF ORGANIC MOLECULES
HYBRIDISATION
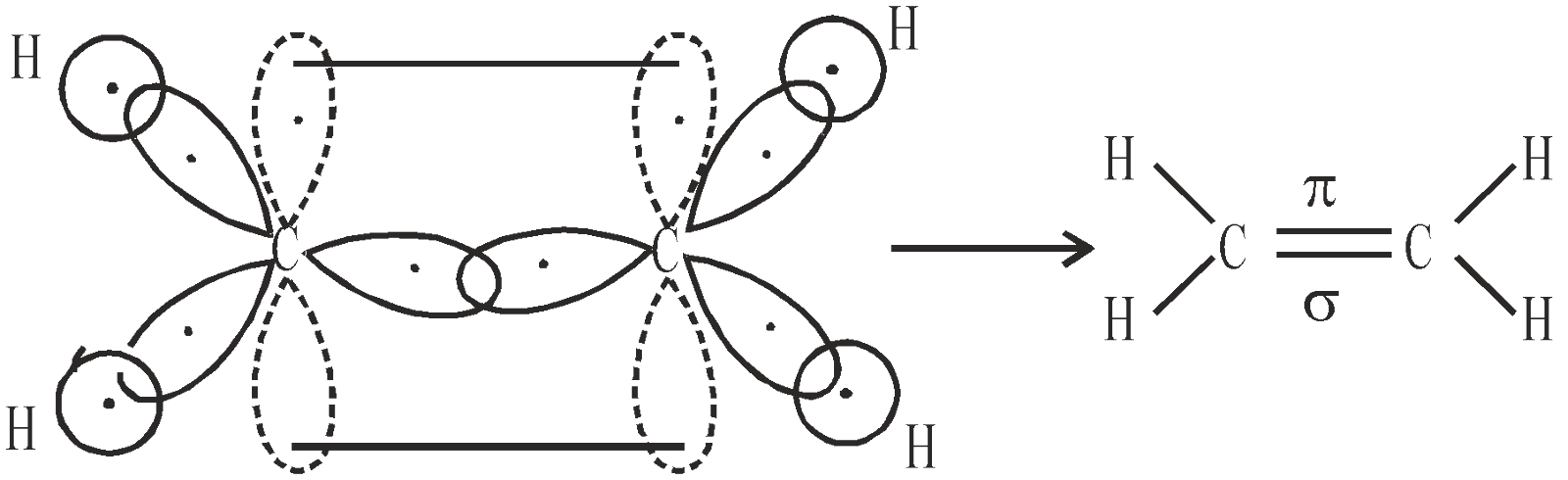
Sigma bonds are the most common bonds in organic chemistry. All single bonds are sigma (σ) bonds and formed by the overlapping between s-s, s-p and p-p (head on) atomic orbitals present on different atoms. A pi (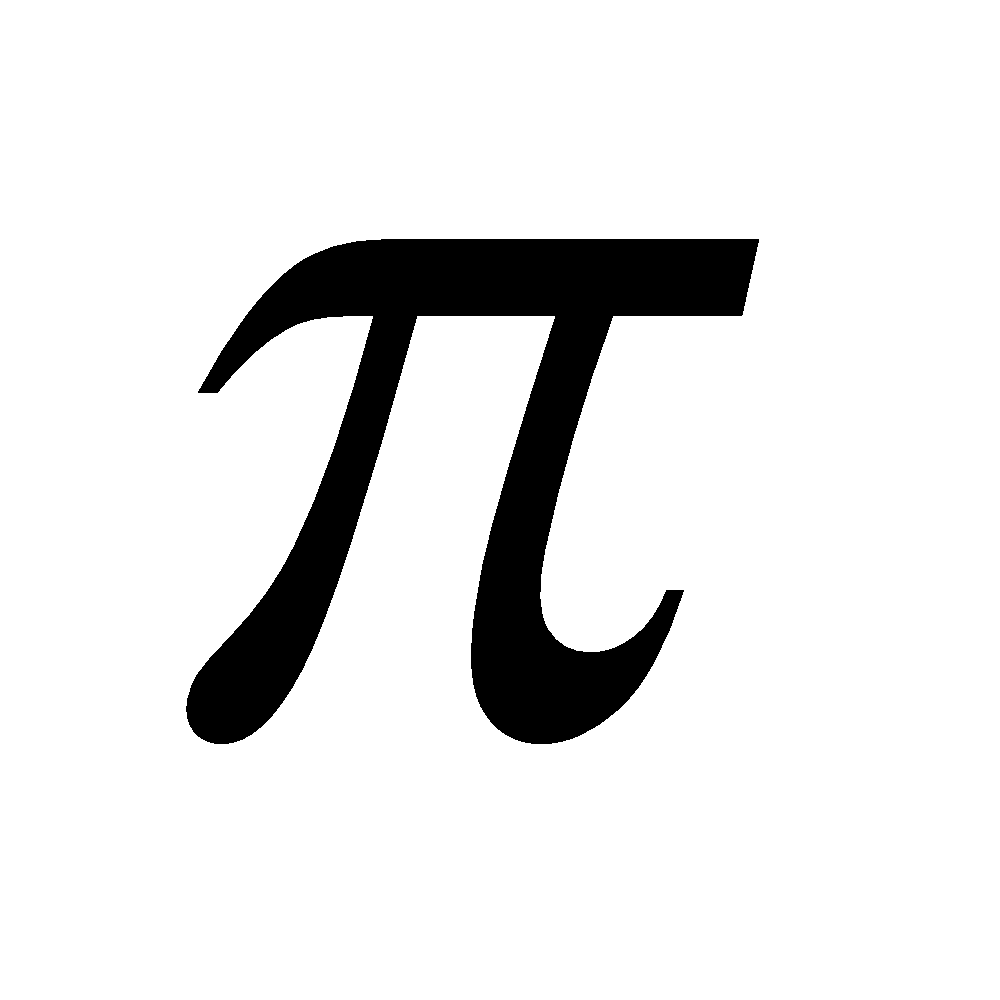 ) bond results from the overlap of two p-orbitals that are oriented perpendicular to the axis of the nuclei. A p bond is not cylindrically symmetrical. A σ bond is stronger than p bond due to better overlap. All multiple bonds contain one σ bond and others
) bond results from the overlap of two p-orbitals that are oriented perpendicular to the axis of the nuclei. A p bond is not cylindrically symmetrical. A σ bond is stronger than p bond due to better overlap. All multiple bonds contain one σ bond and others 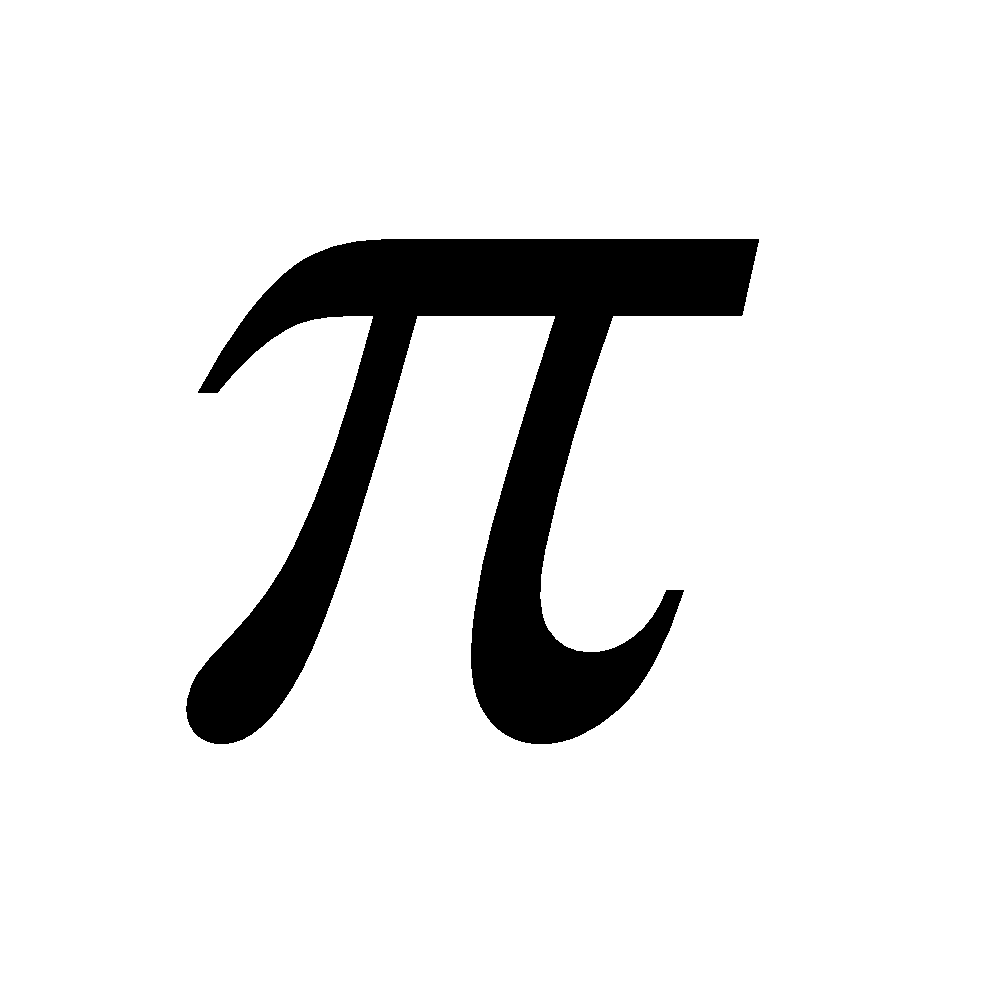 bond(s).
bond(s).
To have more efficient overlapping and to provide more symmetrical structure to the molecule the atomic orbitals on the same atom interact to provide hybrid atomic orbitals and the interaction is known as hybridisation. The hybrid atomic orbitals have enhanced electron density.
HYBRIDISATION OF CARBON
The ground state electronic configuration of carbon is 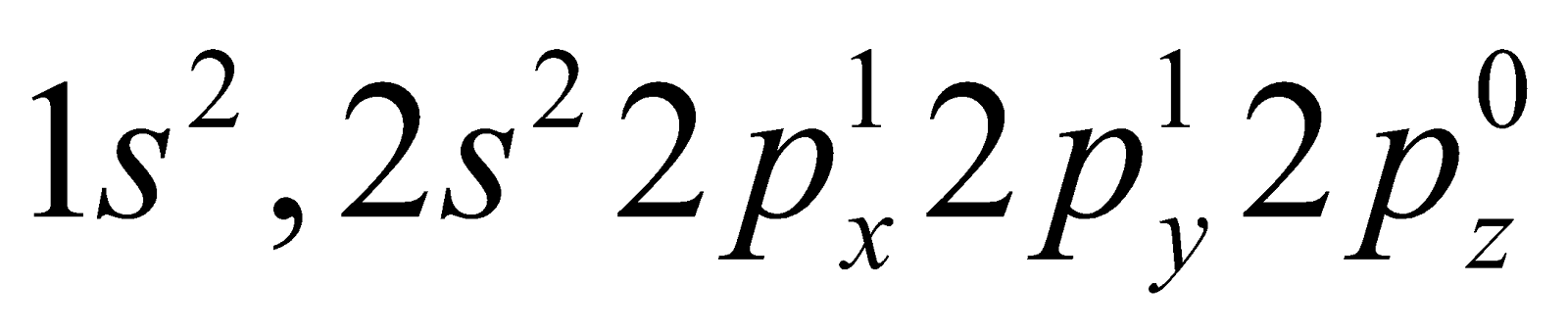 . The electronic configuration of carbon in excited state is
. The electronic configuration of carbon in excited state is 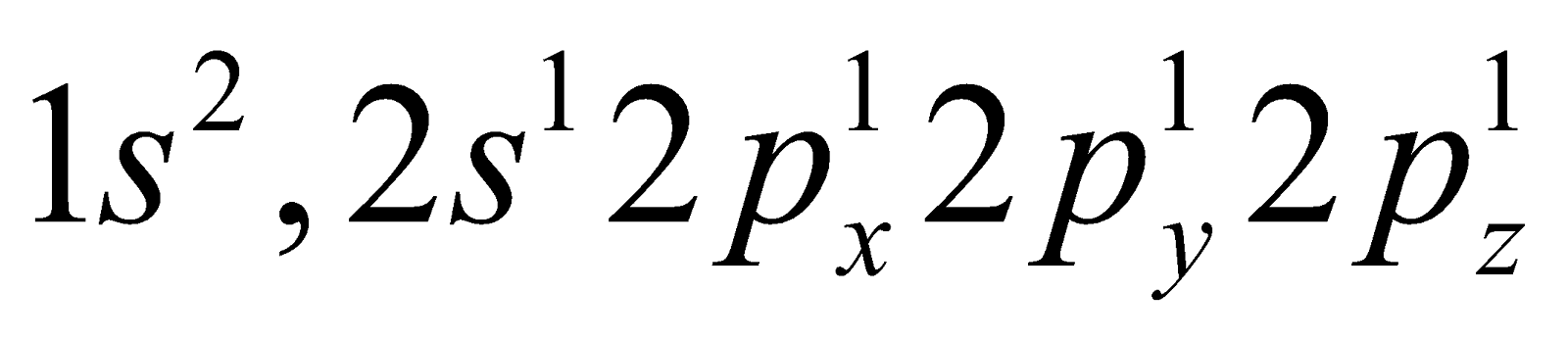 .
.
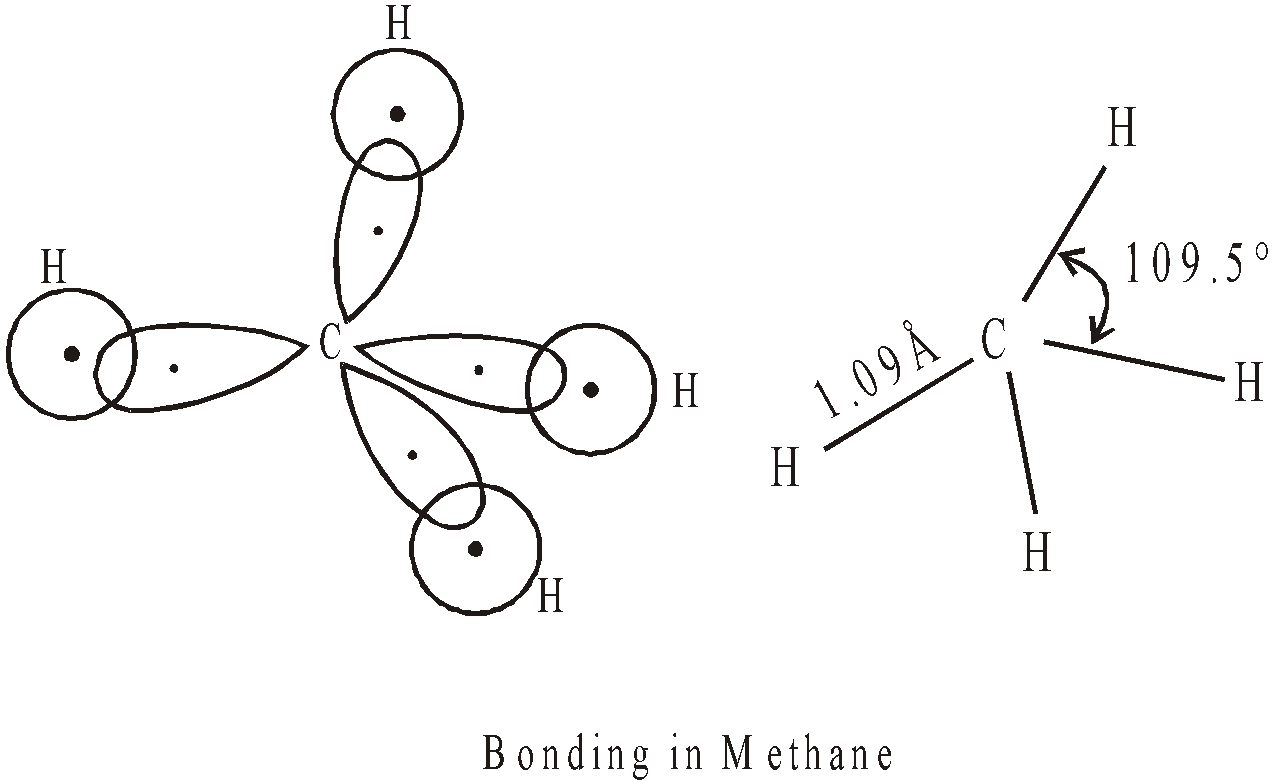
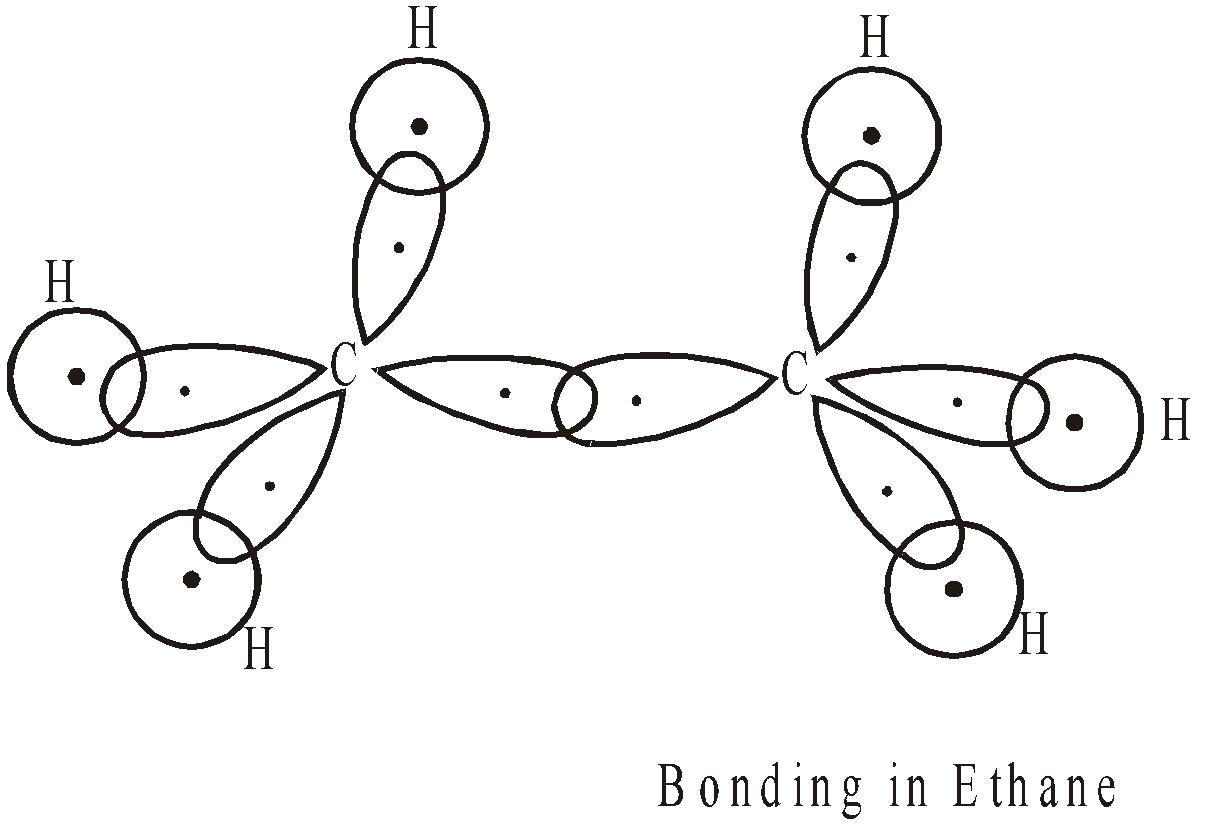
sp3 HYBRIDISATION
If we superimpose one s and three p atomic orbitals we get 4sp3 hybrid orbitals.

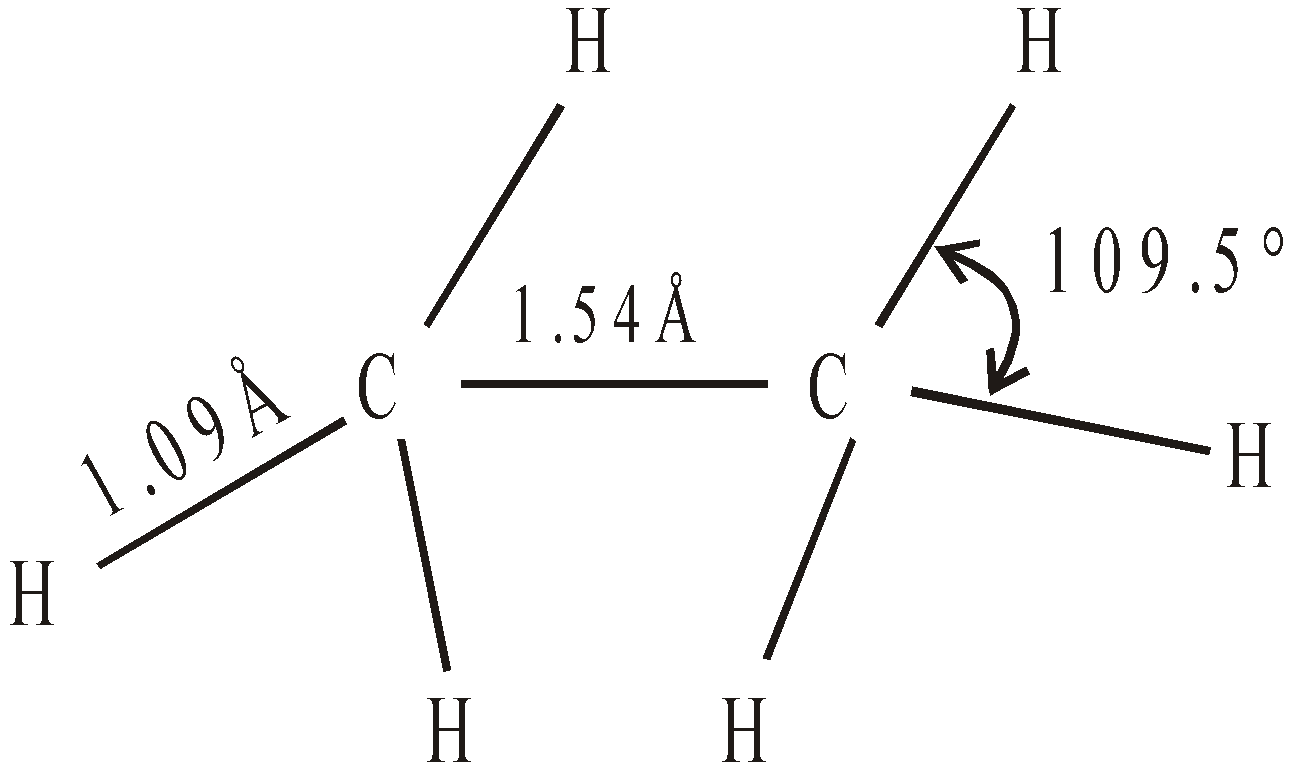
Each hybrid orbital contains single electron, has 25% s character and 75% p character. They are directed towards the four corners of a regular tetrahedron with the carbon located in the centre. The angle between any two sp3 hybrid orbitals is 109º 28′ (109.5º).
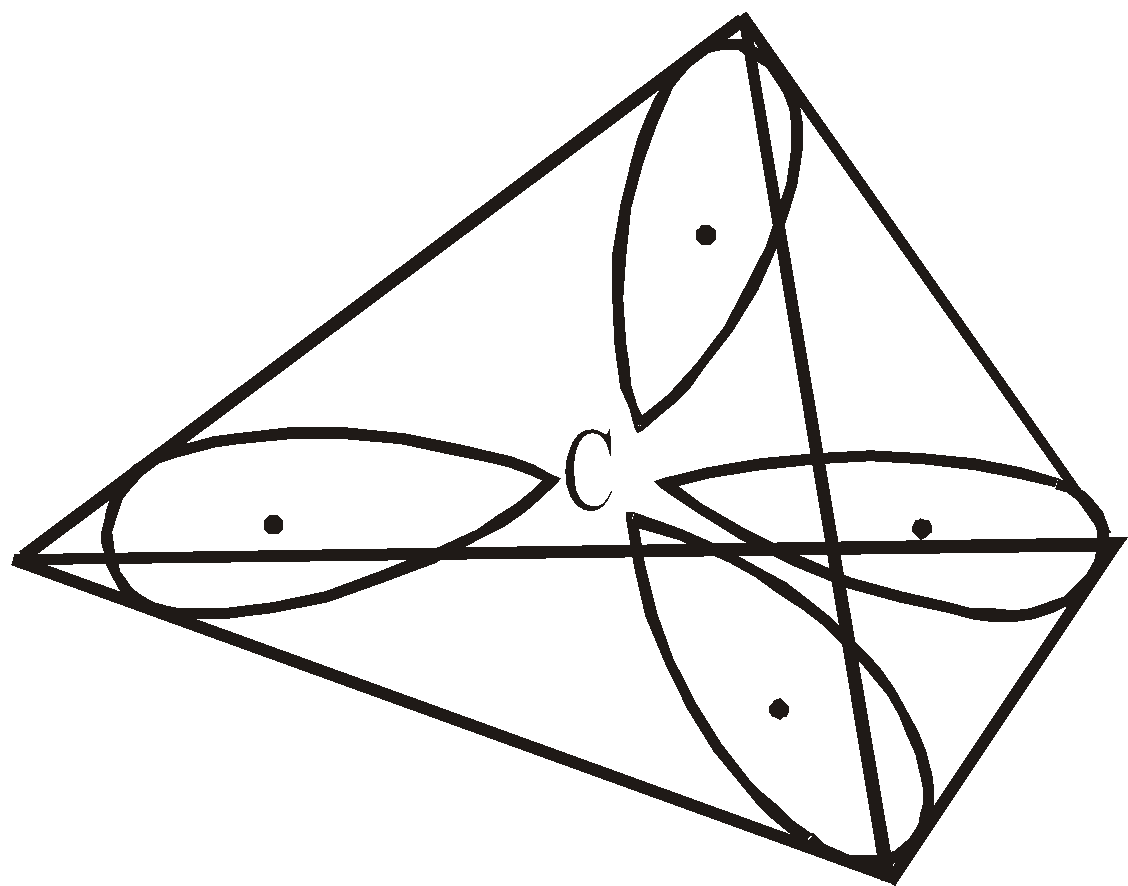
These hybrid orbitals can overlap with four s atomic orbitals provided by four hydrogen atoms to form methane molecule.
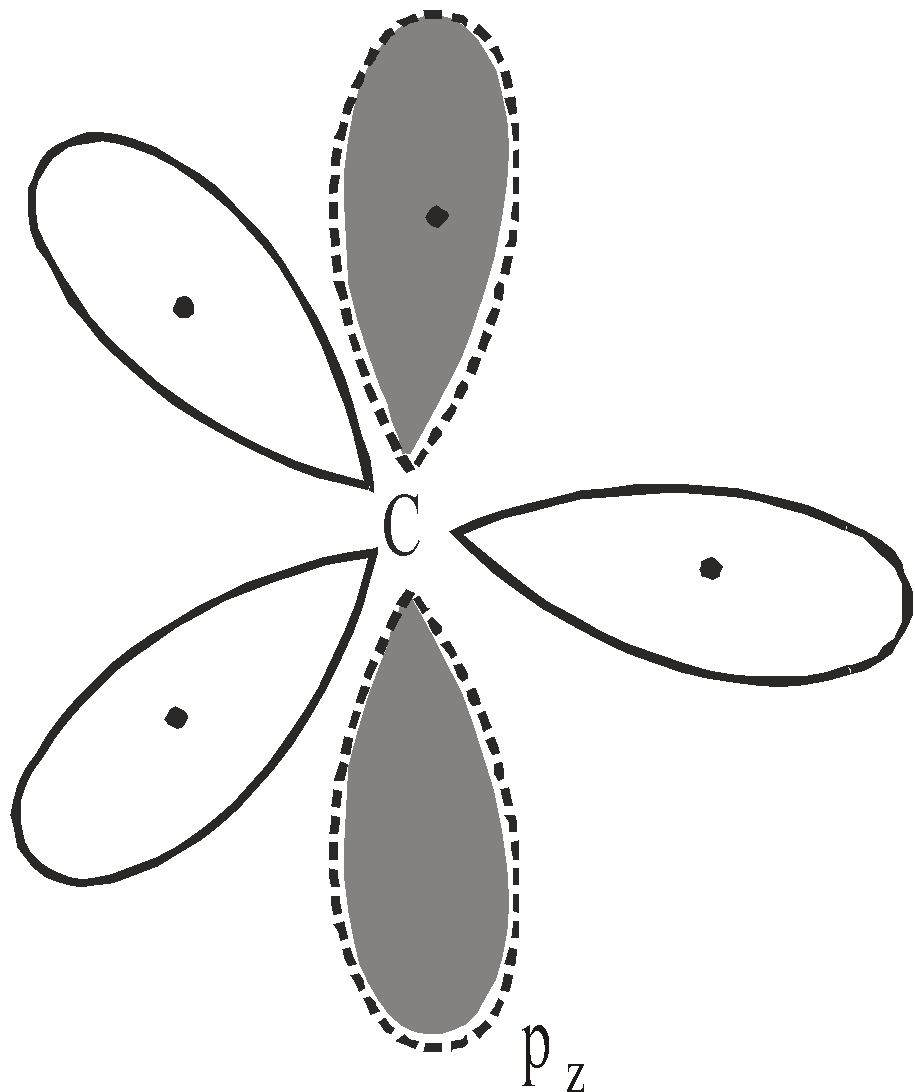
sp2 HYBRIDISATION
If we superimpose one s and two p atomic orbitals we get 3sp2 hybrid orbitals

Each sp2 hybrid orbital has 33% s character and 67% p character. They lie in the same plane with their axis directed towards the corner of an equilateral triangle and are 120º apart from each other. The unhybridized pz atomic orbital is perpendicular to the plane of sp2 hybrid orbitals.
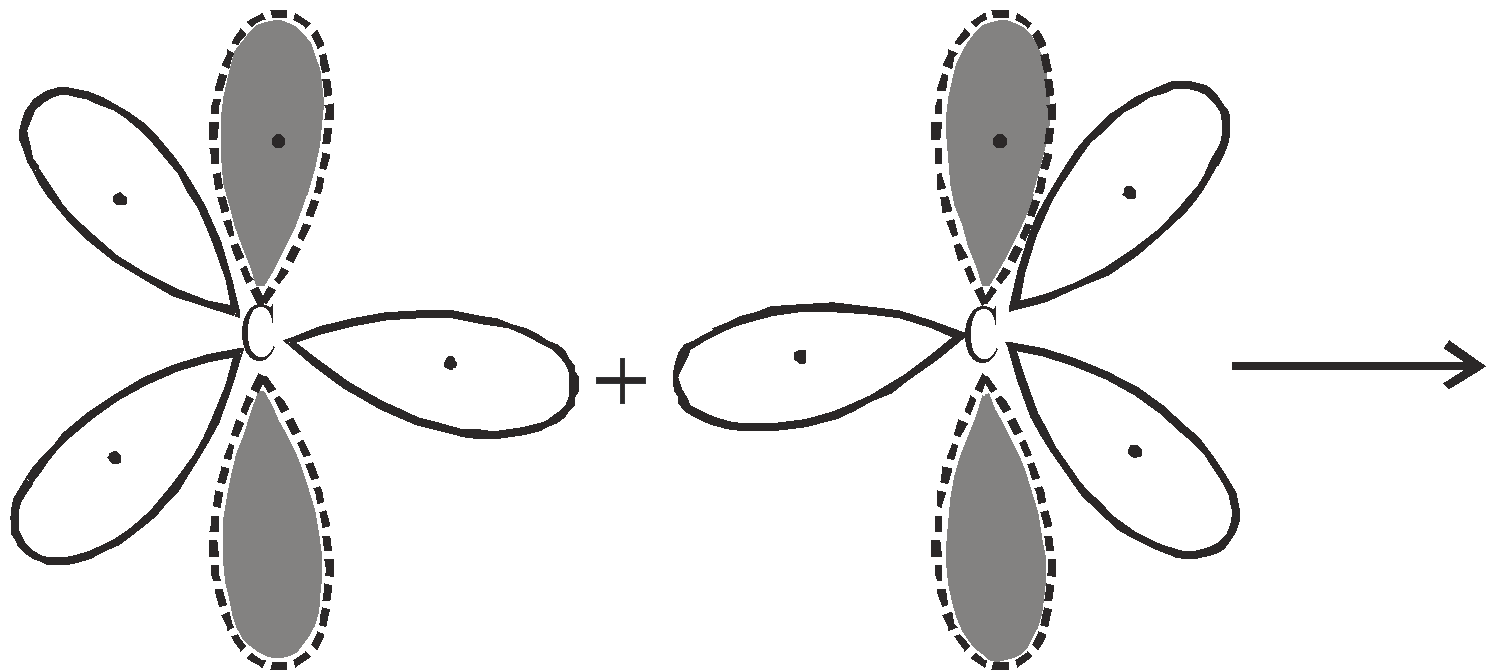
BONDING IN ETHYLENE
Consider two sp2 hybridised carbon atoms approaching to each other and four hydrogen atoms which provide four s atomic orbitals
sp HYBRIDISATION
If we superimpose one s and one p atomic orbitals we get 2sp hybrid orbitals.

Each sp hybrid orbital has 50% s character and 50% p character. They are diagonally present with their axis forming an angle of 180º. The unhybridized 2py and 2pz atomic orbitals are perpendicular to each other and perpendicular to hybrid orbitals also.
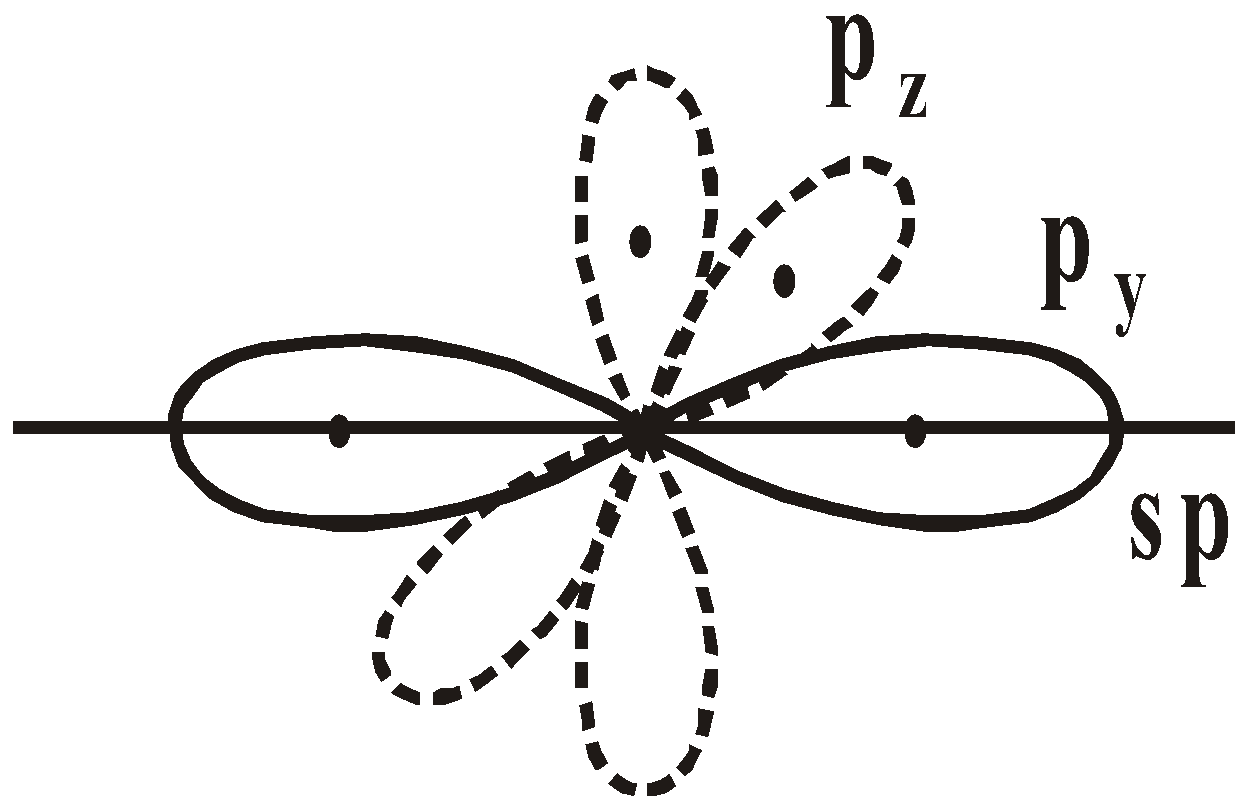
BONDING IN ACETYLENE
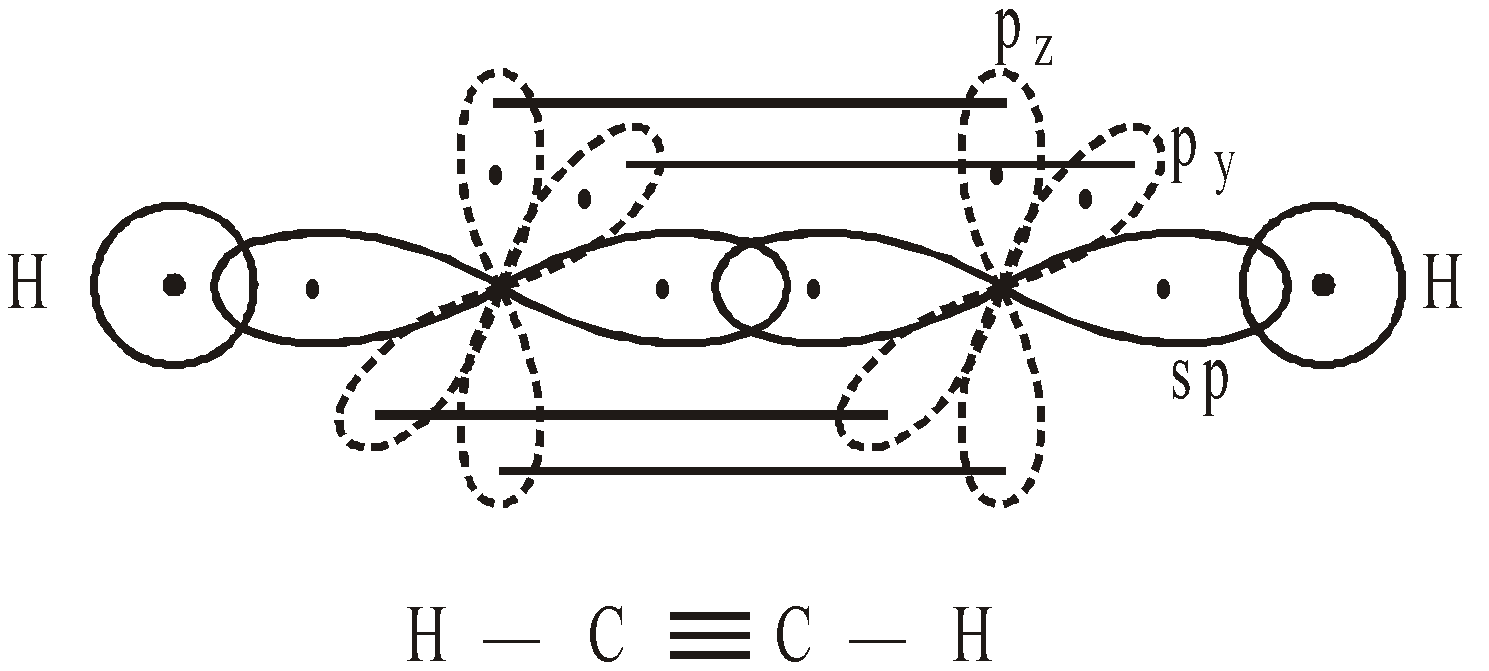
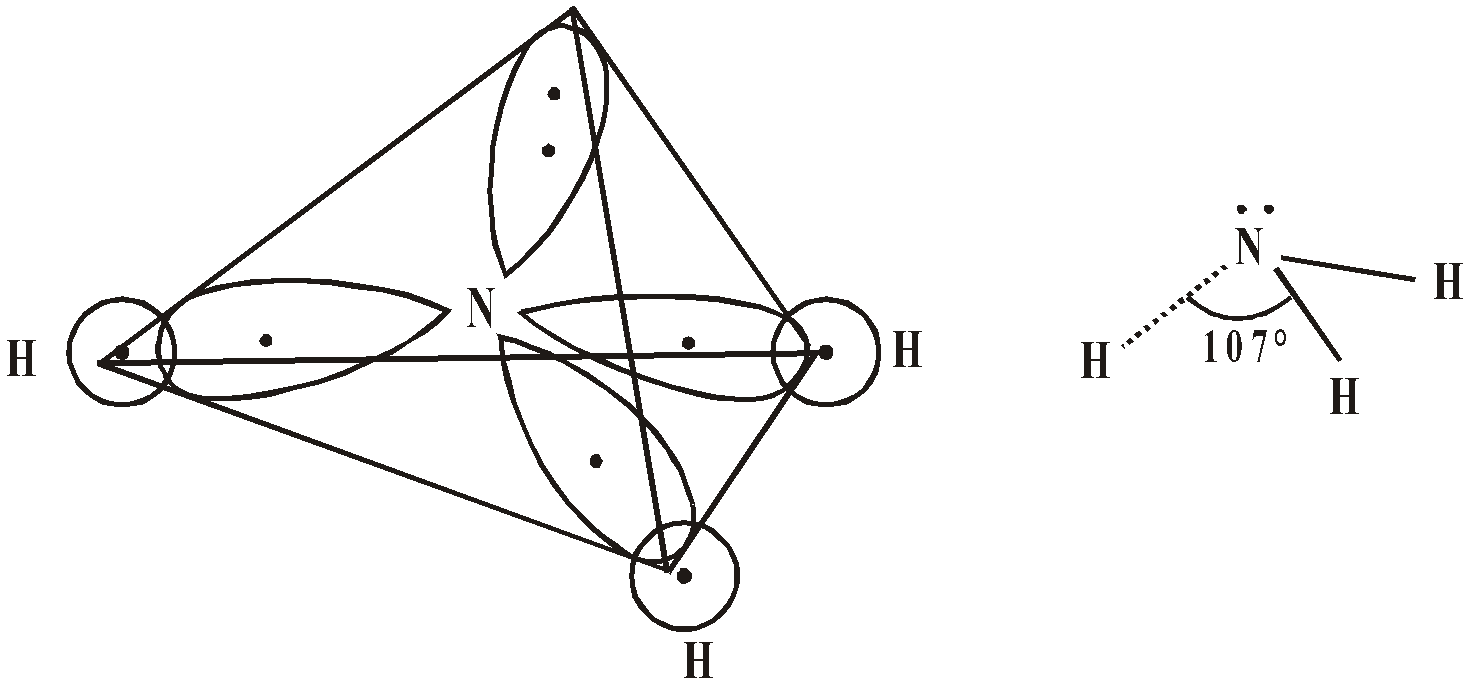
HYBRIDISATION OF NITROGEN
The ground state electronic configuration of nitrogen is
7N = 1s2, 2s2 2px1 2py1 pz1
One s and three p atomic orbitals superimpose and give 4sp3 hybrid orbitals. These are tetrahedrally present.
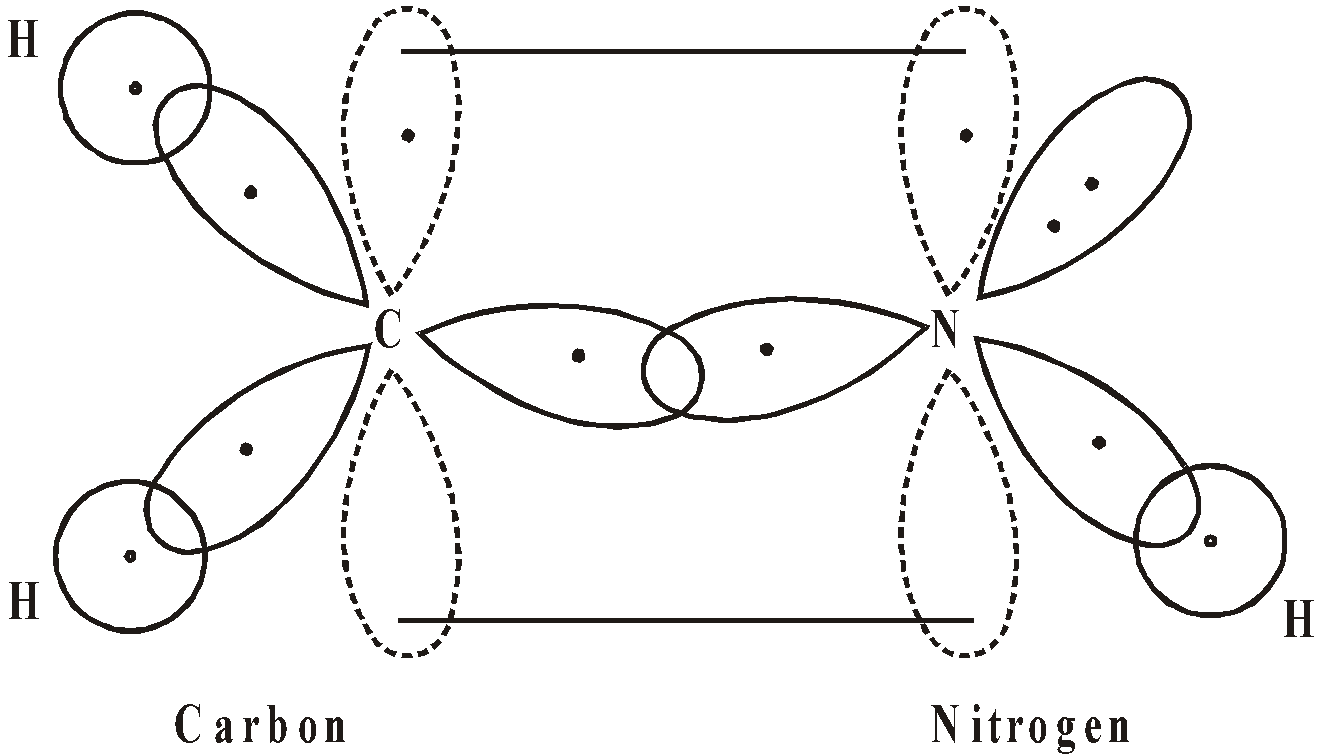
sp2 HYBRIDISATION
When nitrogen attaches itself to two other atoms it is present in the sp2 hybridised form. Consider the formation of methylimine CH2 = NH in which carbon and nitrogen both are in sp2 hybrid state
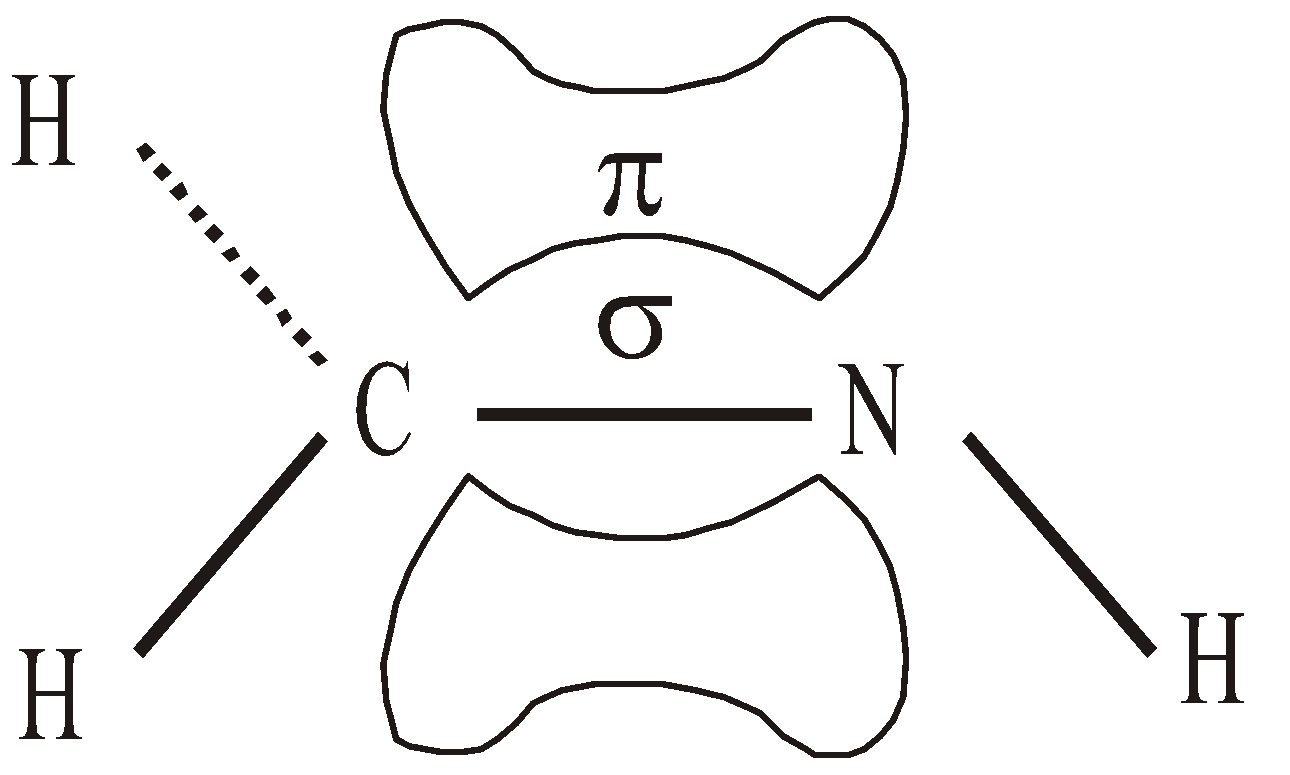
sp HYBRIDISATION
When nitrogen is attached to only one atom its hybridisation is sp. In both carbon and nitrogen are in sp hybridised form

HYBRIDISATION OF OXYGEN
The electronic configuration of oxygen is 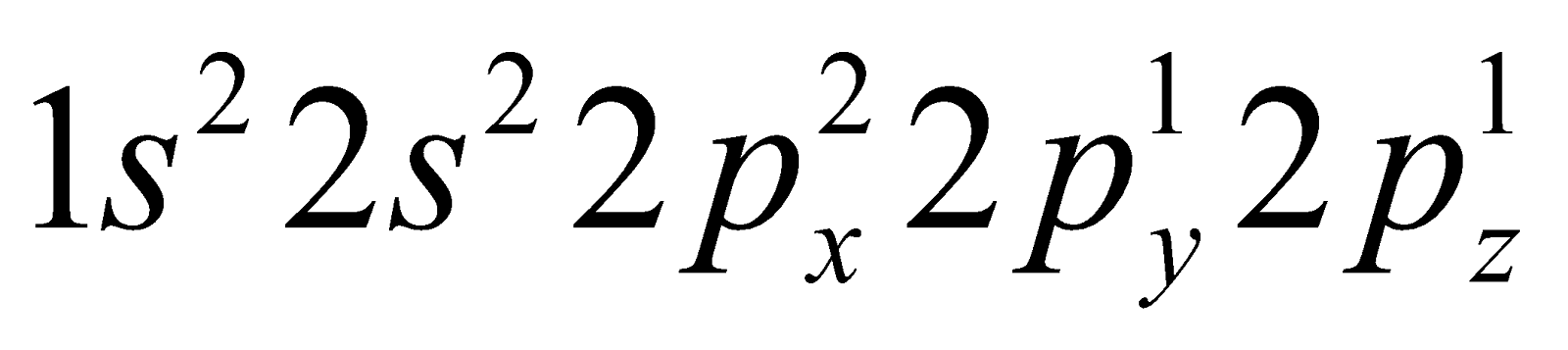 .
.
sp3 HYBRIDISATION
When oxygen is attached to two atoms the hybridisation is sp3.

sp2 HYBRIDISATION
When oxygen is attached to one atom as in case of aldehydes and ketones e.g. in Formaldehyde carbon and oxygen, both are in sp2 hybrid form.
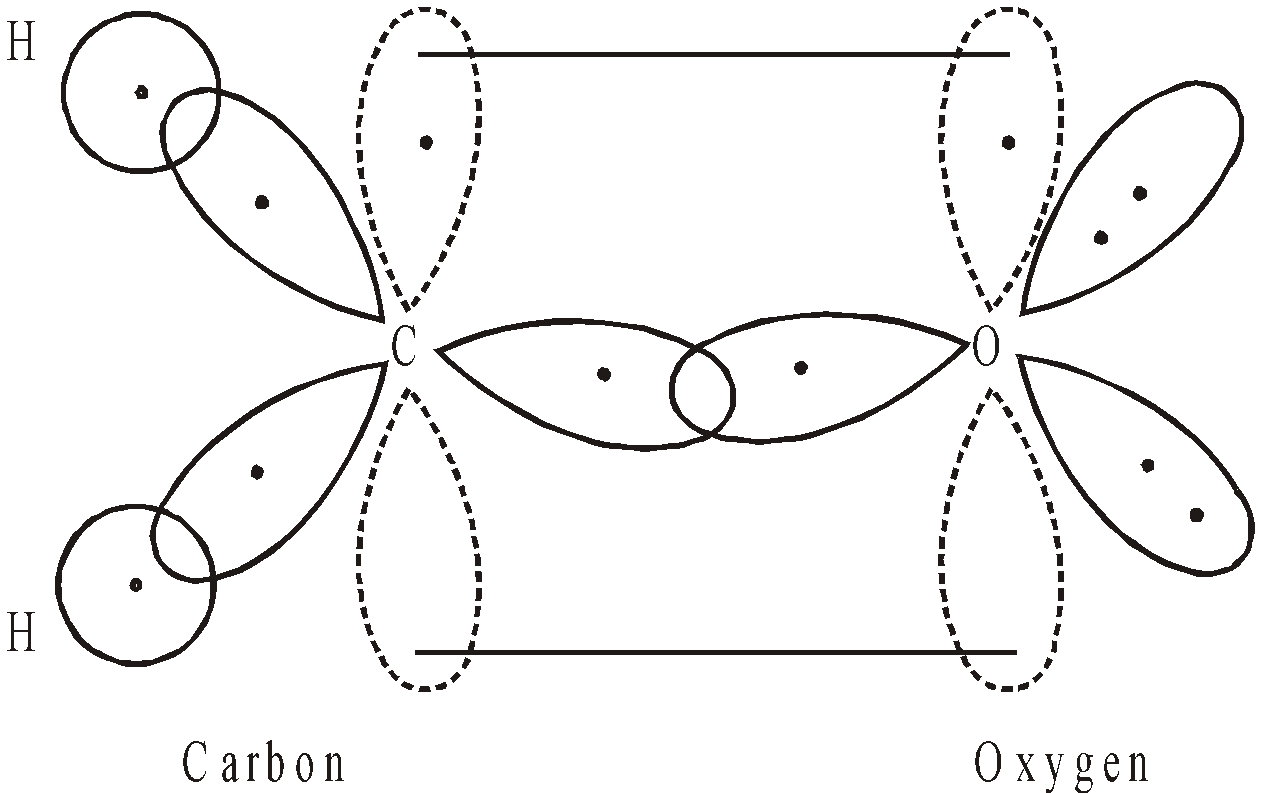
BOND LENGTHS
Some importants bond lengths are as follows
C–C sp3 – sp3 1.54 Å C–O sp3 – O 1.41 Å
sp3 – sp2 1.50 Å sp2 – O 1.34 Å
sp3 – sp 1.46 Å C=O sp2 – O 1.20 Å
sp2 – sp2 1.48 Å sp – O 1.16 Å
sp2 – sp 1.43 Å C–N sp3 – N 1.47 Å
sp – sp 1.38 Å sp2 – N 1.36 Å
C=C sp2 – sp2 1.34 Å C=N sp2 – N 1.28 Å
sp2 – sp 1.31 Å CºN sp – N 1.16 Å
sp – sp 1.28 Å
CºC sp – sp 1.21 Å
C–H sp3– H 1.11 Å
sp2 – H 1.10 Å
sp – H 1.08 Å
BOND ANGLES IN SELECTED MOLECULES
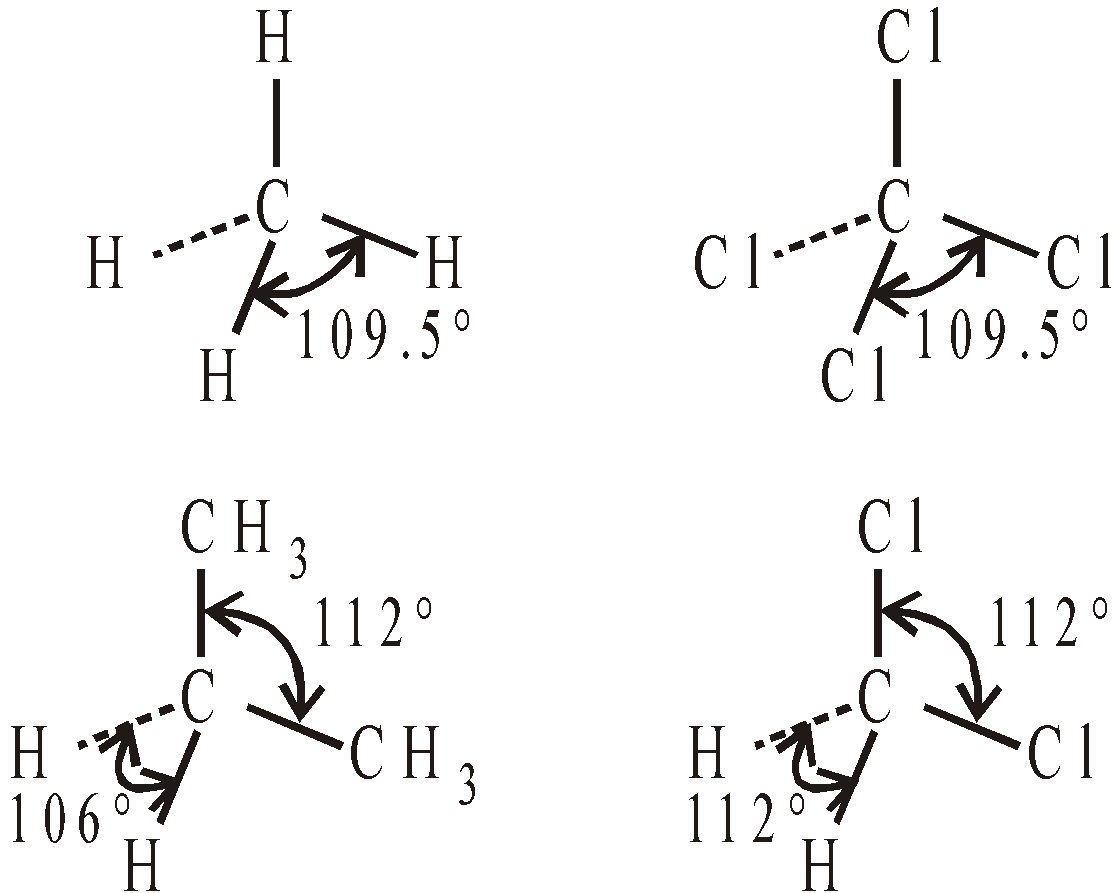
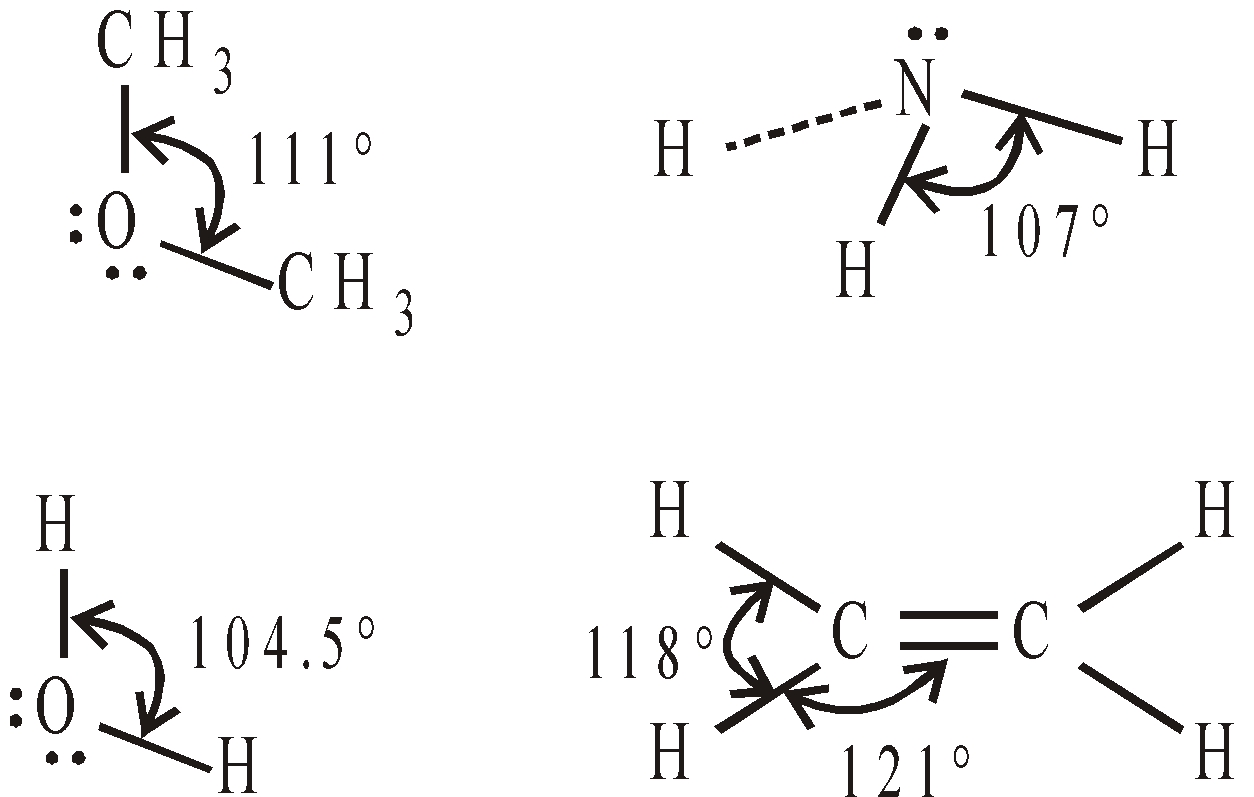
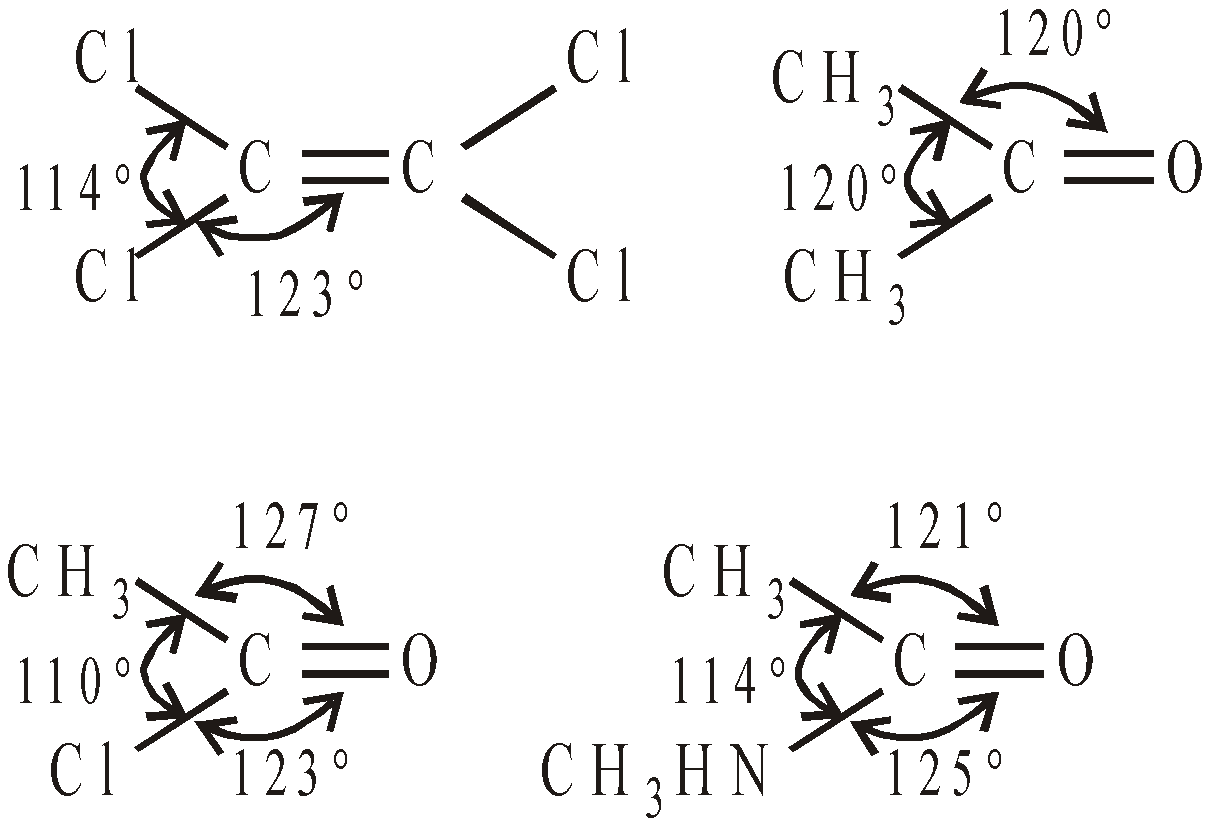
AROMATICITY AND AROMATIC COMPOUNDS
Aromatic indicates a stable system which undergoes substitution rather than addition, retaining the closed p-electron system. Many such systems contain only six p electrons, but generally they contain (4n+2) p electrons, where n is an integer.

Non-benzenoid heterocyclic compounds with 6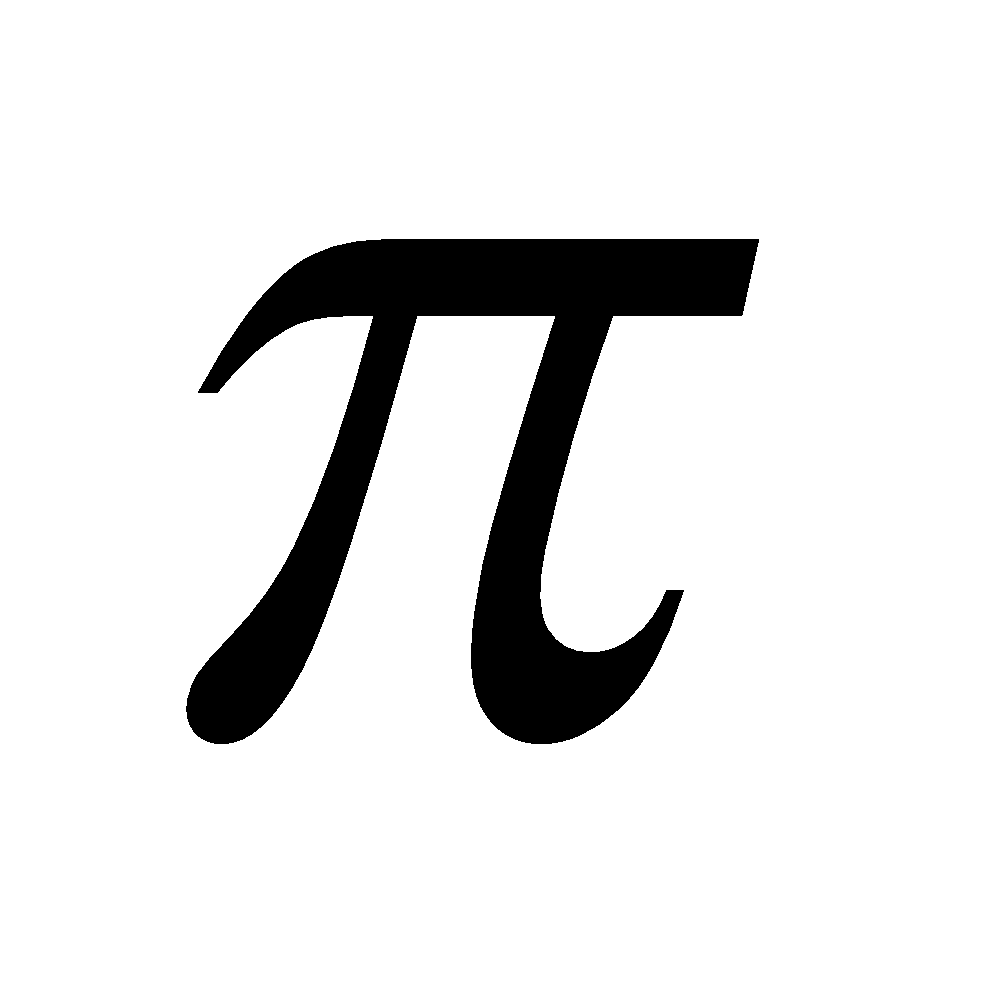 electrons are aromatics
electrons are aromatics
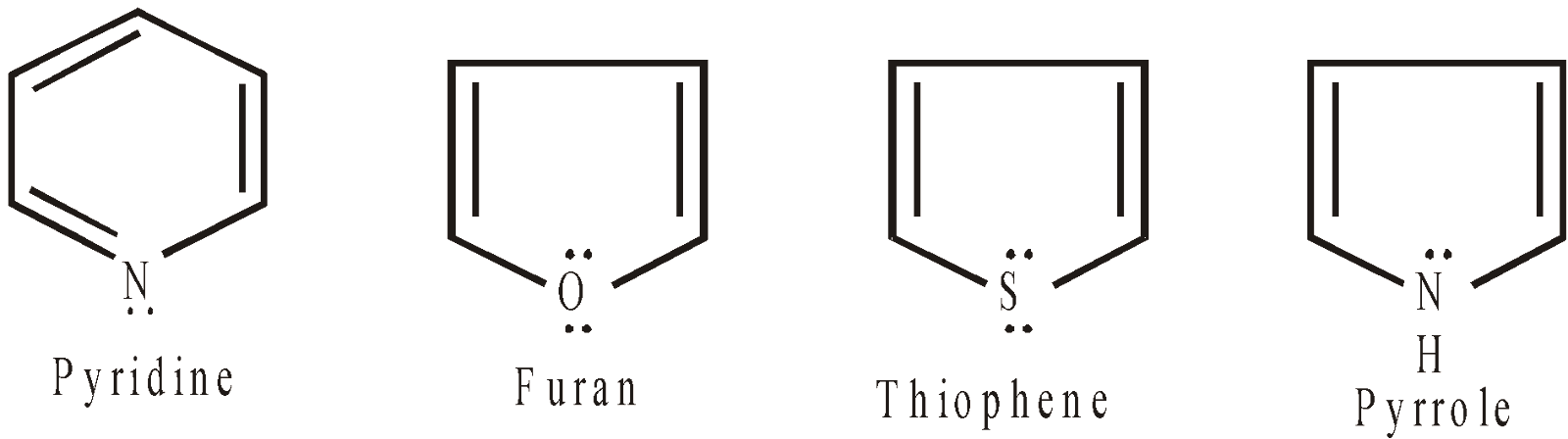
The hetero atom contributes to non bonded electrons, to complete the sextet.
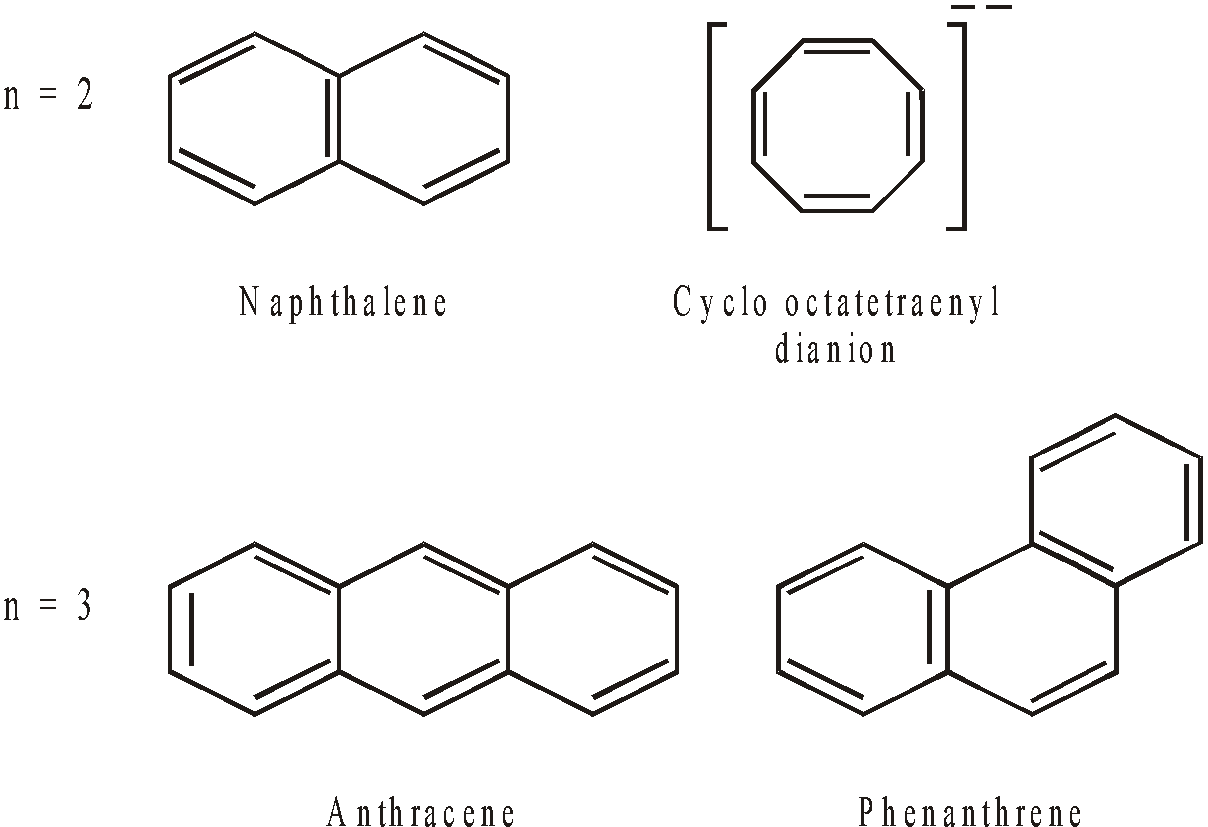
In general, higher polycyclic aromatic compounds are somewhat less stable than benzene.
n = 0 cyclopropenyl cation contains 2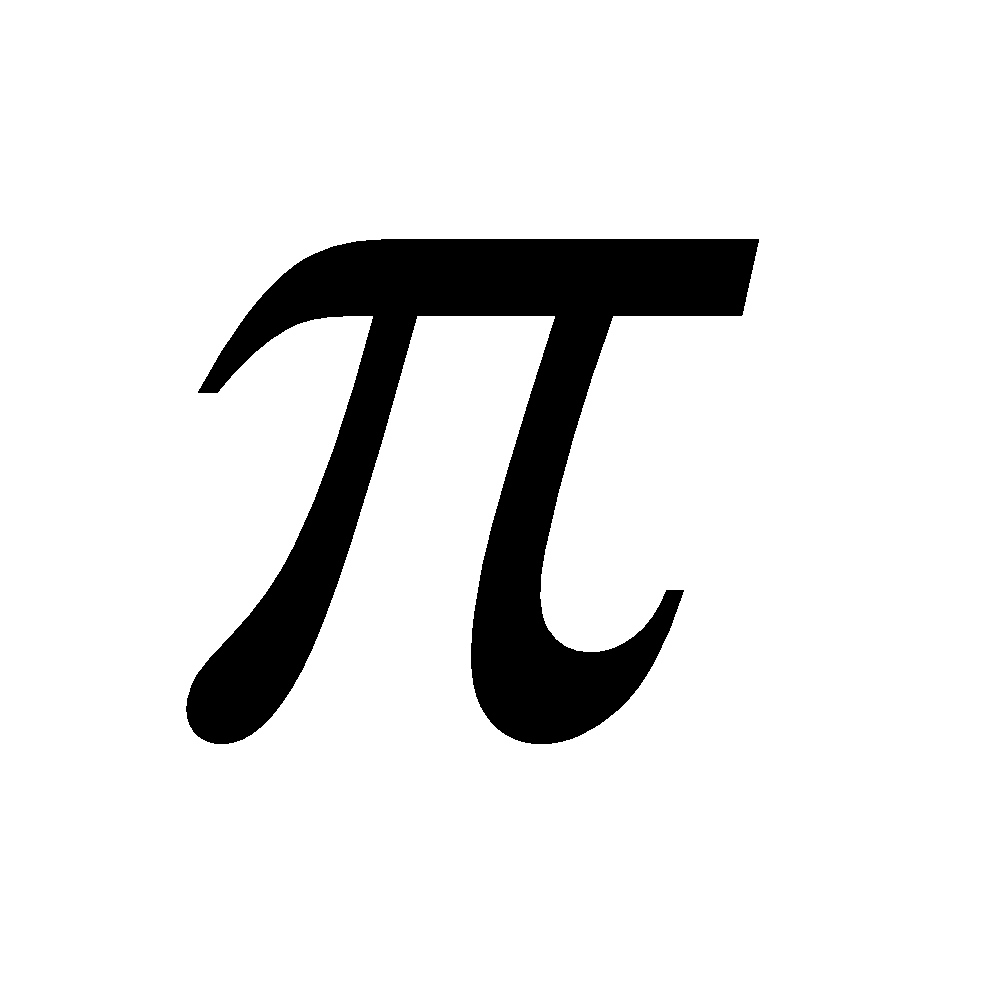 – electrons and is aromatic
– electrons and is aromatic
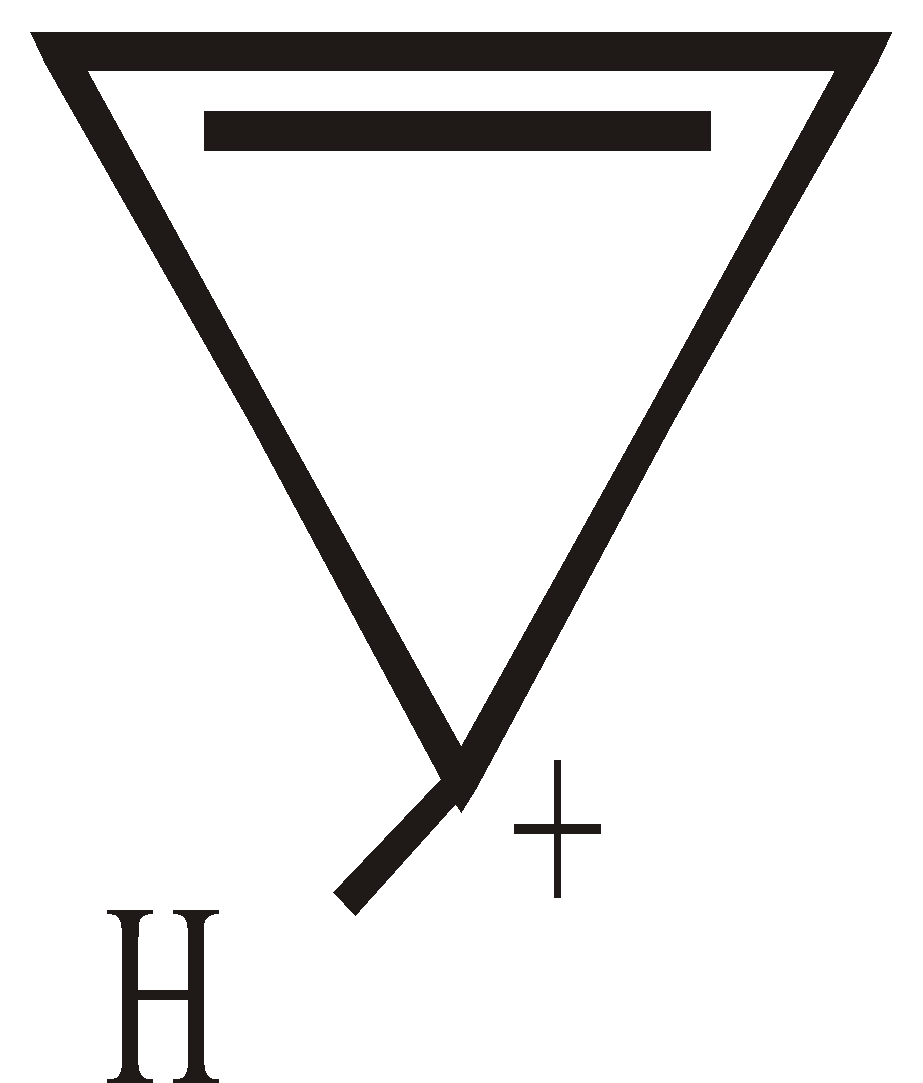
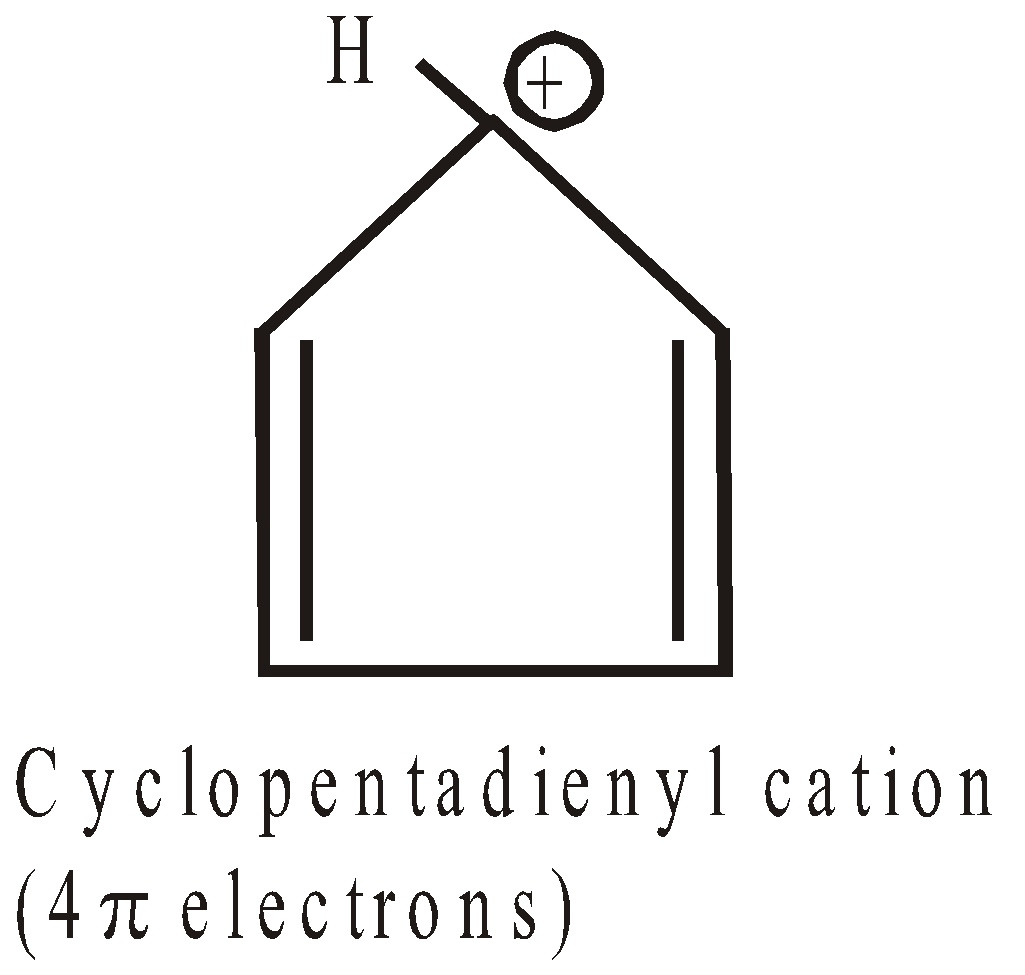
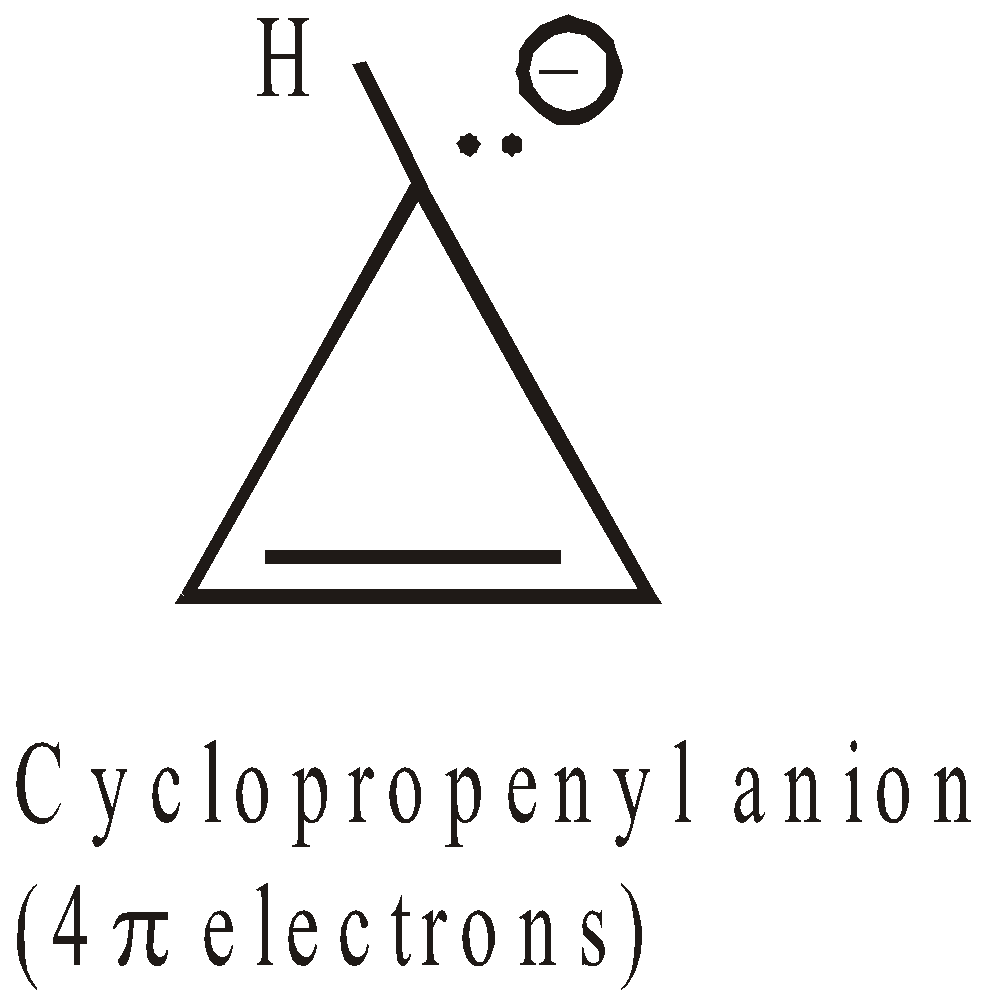
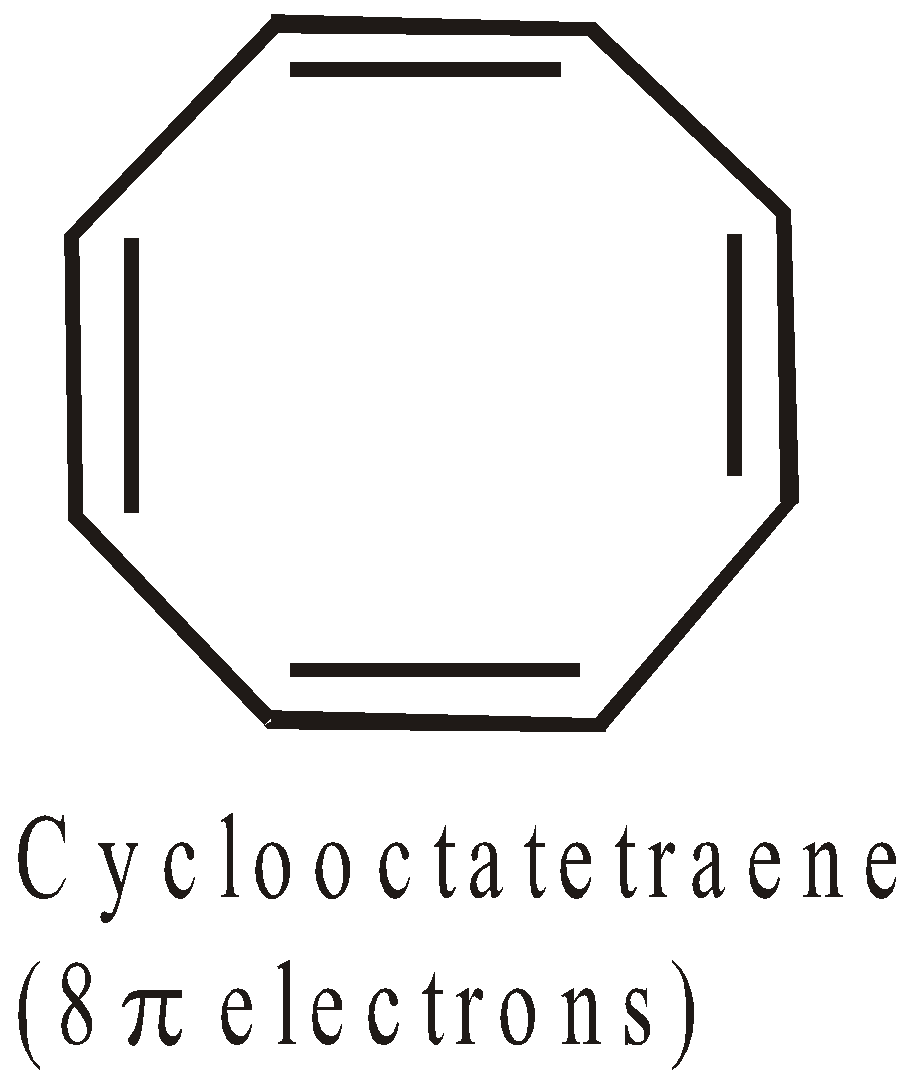
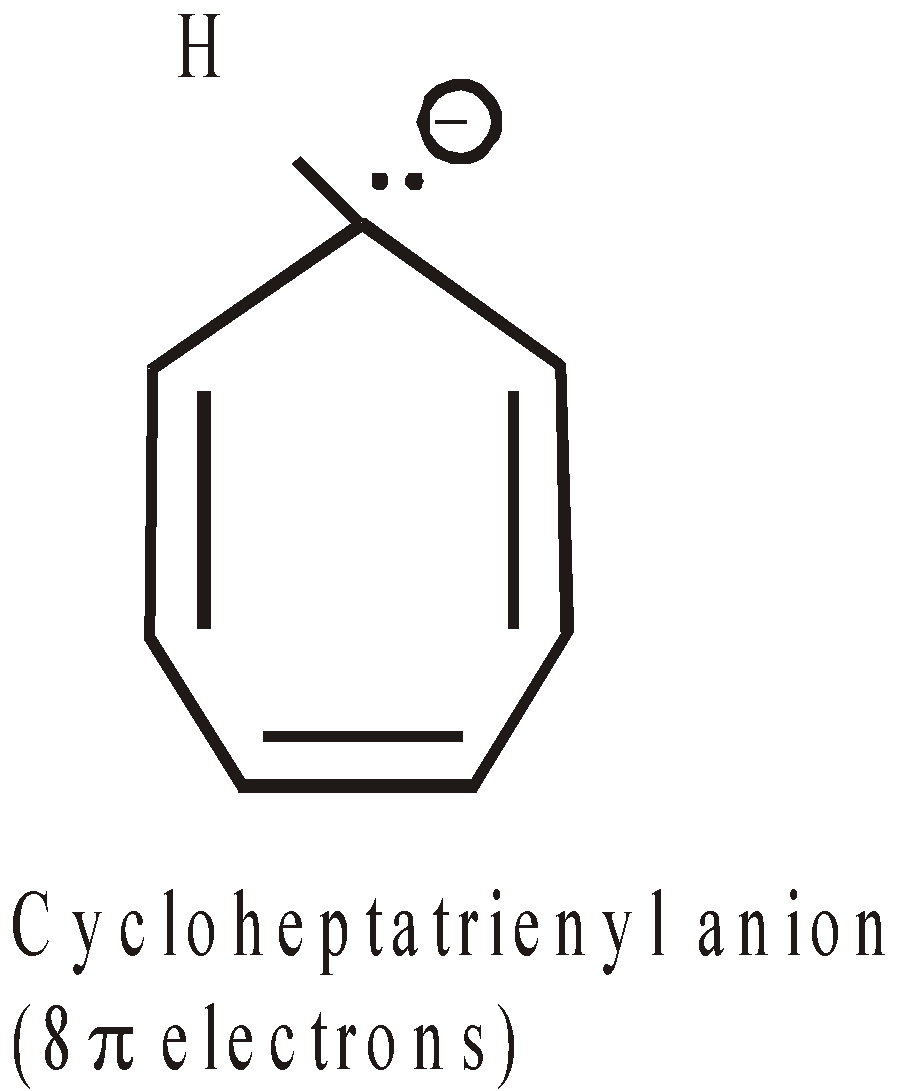
ANTIAROMATICITY
The less stability of monocyclic compounds containing (4n) 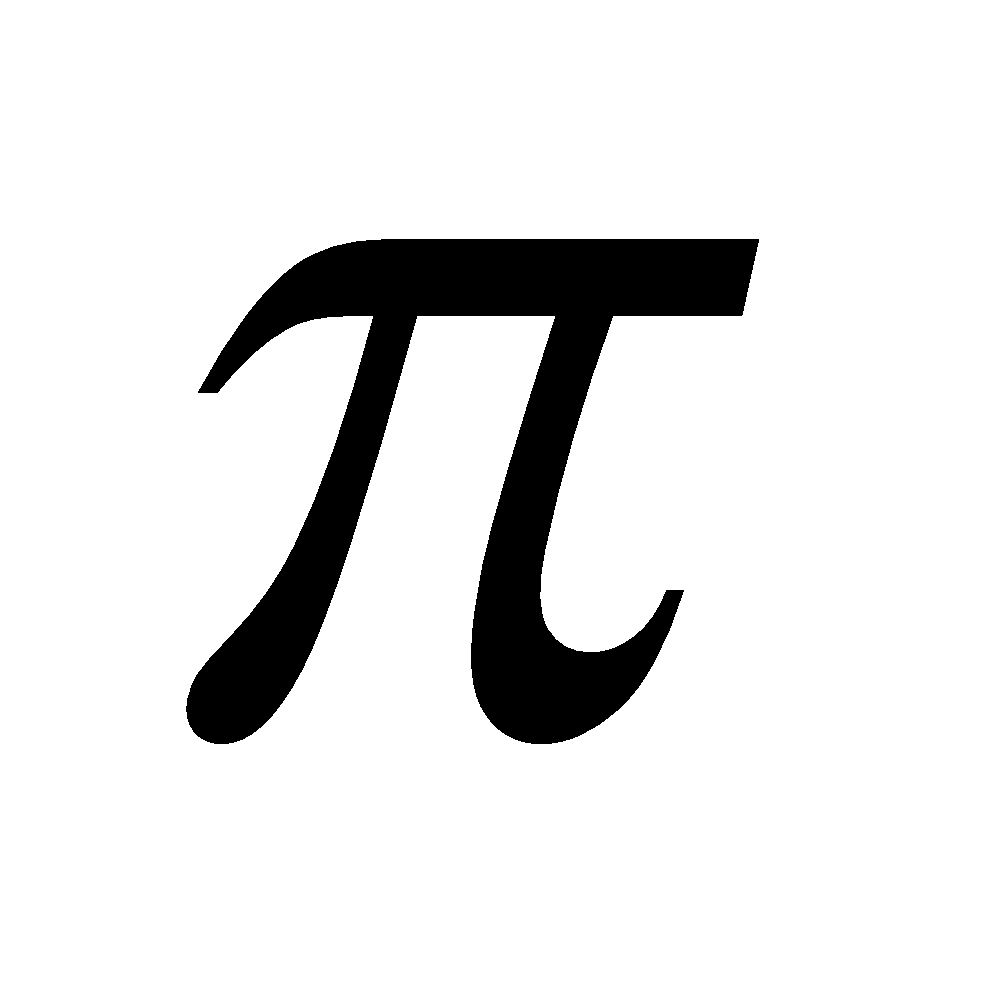 electrons than their acyclic analogues is called anti aromaticity. For example
electrons than their acyclic analogues is called anti aromaticity. For example
Cyclobutadiene  is less stable than 1,3-Butadiene
is less stable than 1,3-Butadiene
Here Resonance is the cause of destabilisation (hence the concept of antiaromaticity)
More examples of antiaromatic compounds
The electronic configuration of carbon in excited state is .