About this unit
Group 15 elements: General introduction, electronic configuration, occurrence, oxidation states, trends in physical and chemical properties; preparation and properties of ammonia and nitric acid, oxides of nitrogen (structure only); Phosphorous- allotropic forms; compounds of phosphorous: preparation and properties of phosphine, halides (PCI3 , PCI5 ) and oxoacids (elementary idea only).
Group 16 elements: General introduction, electronic configuration, oxidation states, occurrence, trends in physical and chemical properties; dioxygen: preparation, properties and uses; classification of oxides; ozone. Sulphur – allotropic forms; compounds of sulphur: preparation, preparation, properties and uses of sulphur dioxide; sulphuric acid: industrial process of manufacture, properties and uses, oxoacids of sulphur (structures only).
Group 17 elements: General introduction, electronic configuration, oxidation states, occurrence, trends in physical and chemical properties; compounds of halogens: preparation, properties and uses of chlorine and hydrochloric acid, interhalogen compounds oxoacids of halogens (structures only).
Group 18 elements: General introduction, electronic configuration, occurrence, trends in physical and chemical properties, uses.
| p-Block Elements – Nitrogen Family |
| p-Block Elements – Oxygen Family |
| p-Block Elements – Halogens |
p-BLOCK ELEMENTS – HALOGENS
GENERAL CHARACTERISTICS
ELECTRONIC CONFIGURATION
Element | Symbol | At No. | Valence shell electronic configuration |
Fluorine | F | 9 | |
Chlorine | Cl | 17 | |
Bromine | Br | 35 | |
Iodine | I | 53 | |
Astatine | At | 85 |
PHYSICAL STATE
ATOMICITY
ABUNDANCE
COLOUR
METALLIC CHARACTER
OXIDATION STATE
BOND ENERGY AND BOND LENGTH
F-F | Cl-Cl | Br-Br | I-I | |
Bond length (Å) | 1.42 | 1.99 | 2.28 | 2.67 |
Bond energy (kJ mol-1) | 158.8 | 242.6 | 192.8 | 151.1 |
Due to small size the interelectronic repulsions between non bonding electrons are high in case of fluorine which results in weakening of F-F bond.
DENSITY
- Atomic radii
- Ionic radii
- Atomic volume
- Density
- Electronegativity
- Oxidising power
- Reactivity
- Affinity for hydrogen
- Reduction potential
- Solubility, all follow the above given order
IONISATION POTENTIAL
ELECTRON AFFINITY
SOLUBILITY
PROPERTIES OF HALIDE IONS (X–)
NATURE OF BONDS WITH OTHER ELEMENTS
COMPOUNDS OF HALOGENS
HYDRACIDS (HX)
Itching of glass : Glass contains silica which reacts with HF.
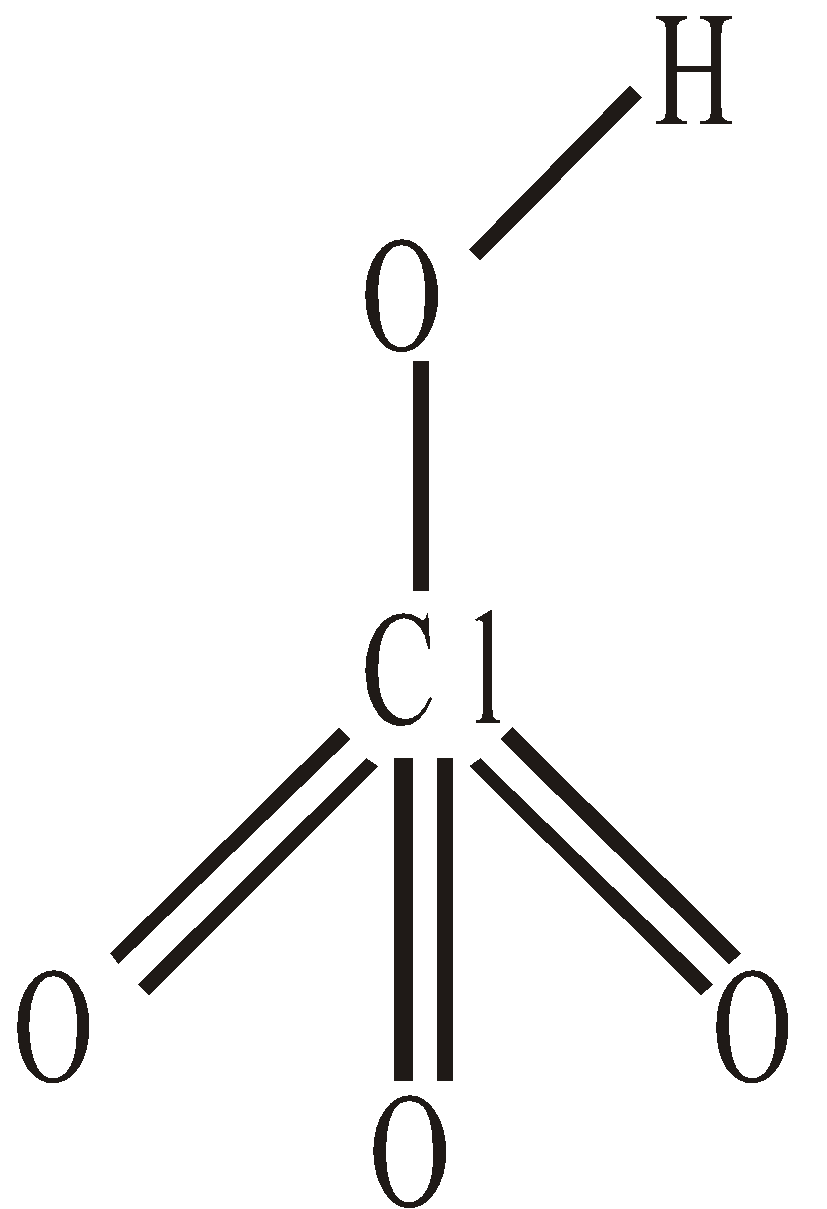
OXY ACIDS
Name | O. N. of X | Fluorine | Chlorine | Bromine | Iodine | Salt’s name |
Hypohalous acid, HXO | +1 | HOF | HOCl | HOBr | HOI | Hypohalite |
Halous acid, HXO2 | +3 | – | HClO2 | – | – | Halite |
Halic acid, HXO3 | +5 | – | HClO3 | HBrO3 | HIO3 | Halate |
Perhalic acid, HXO4 | +7 | – | HClO4 | HBrO4 | HIO4 | Perhalate |
Perhalates are obtained by electrolytic oxidation of halates
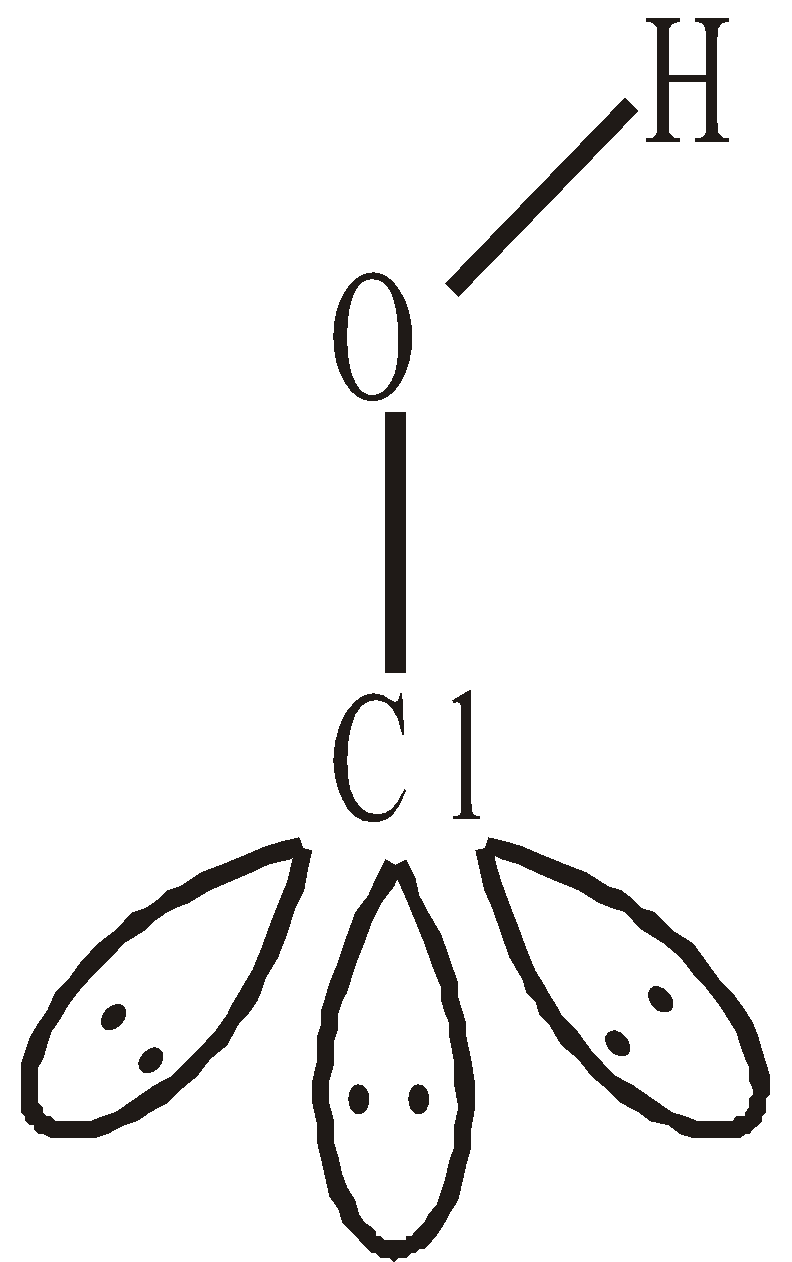
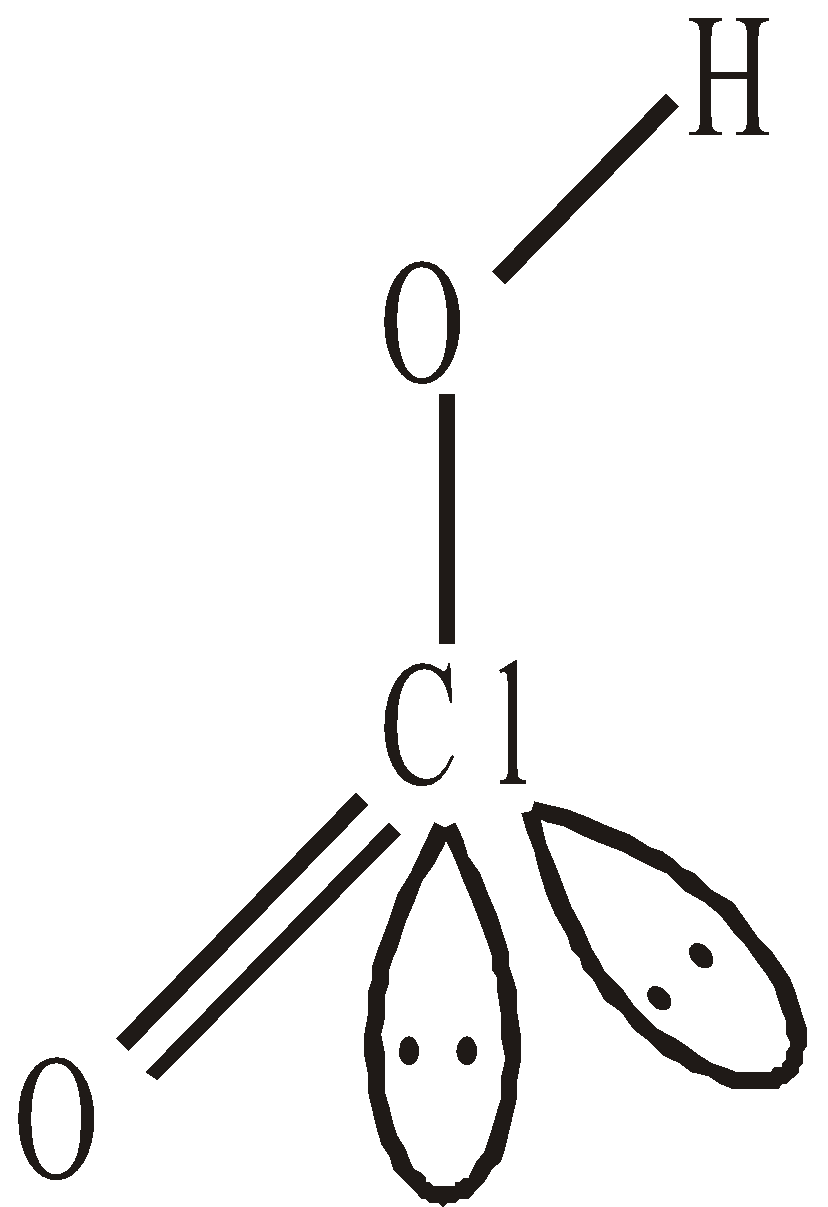
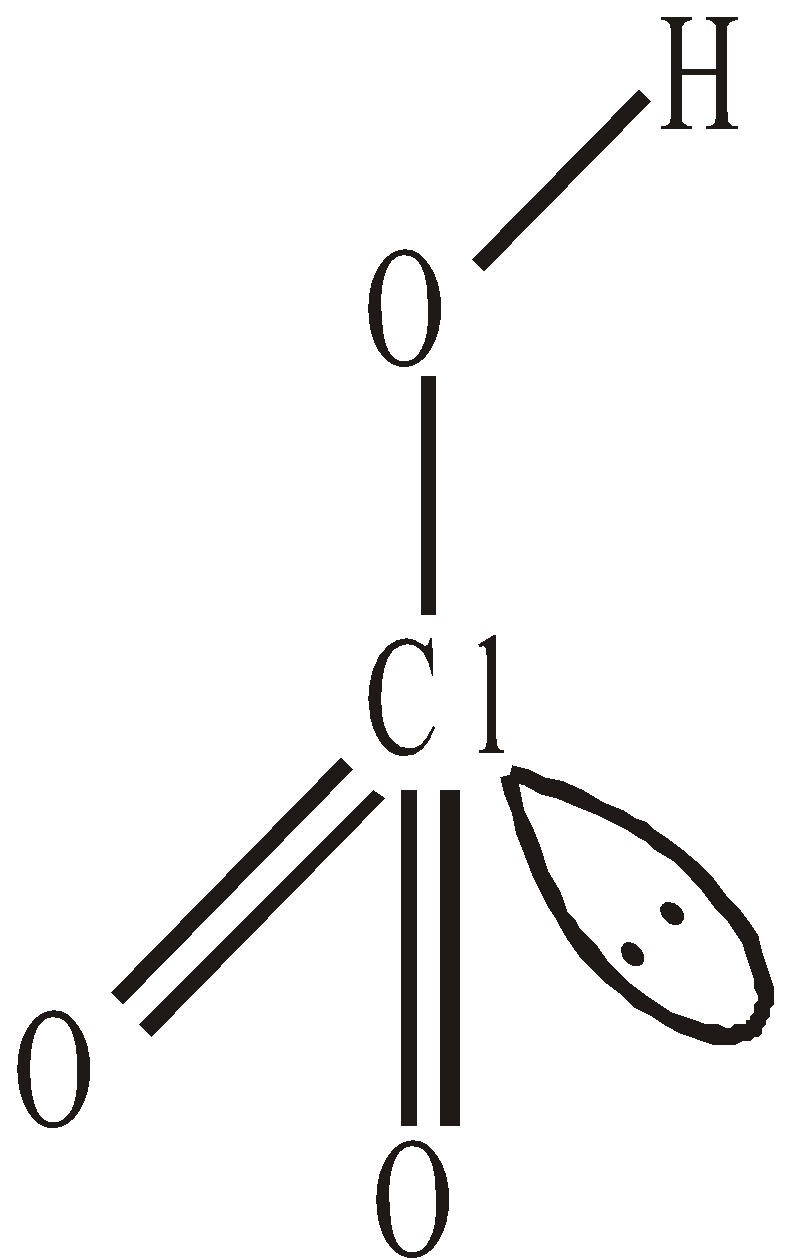
OXIDES
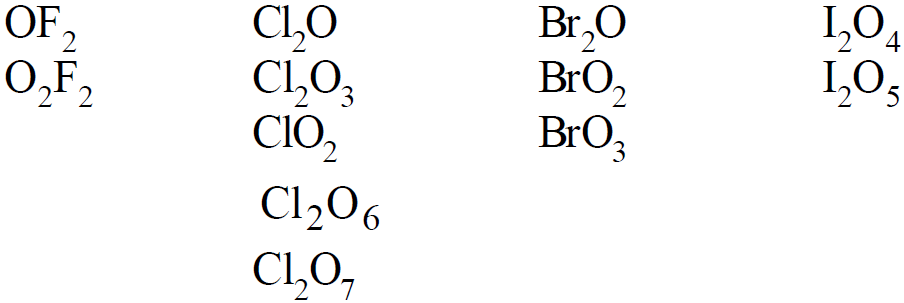
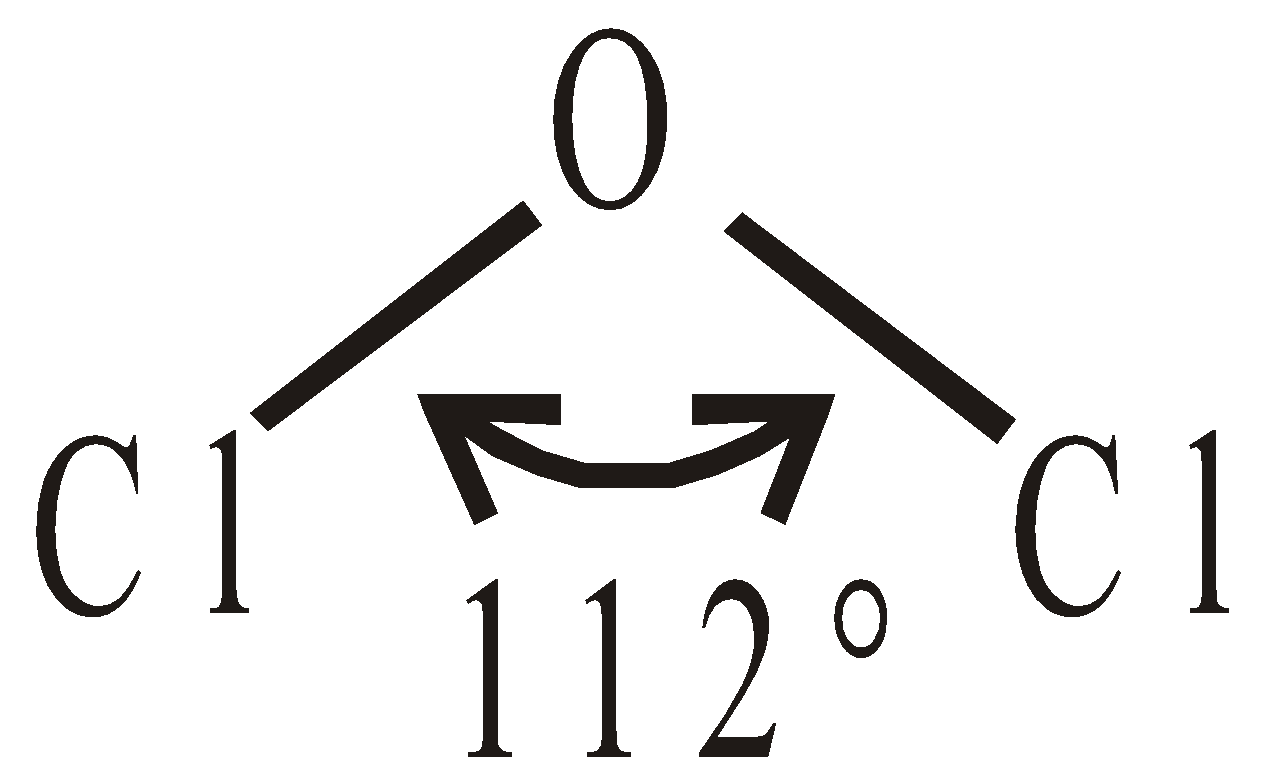
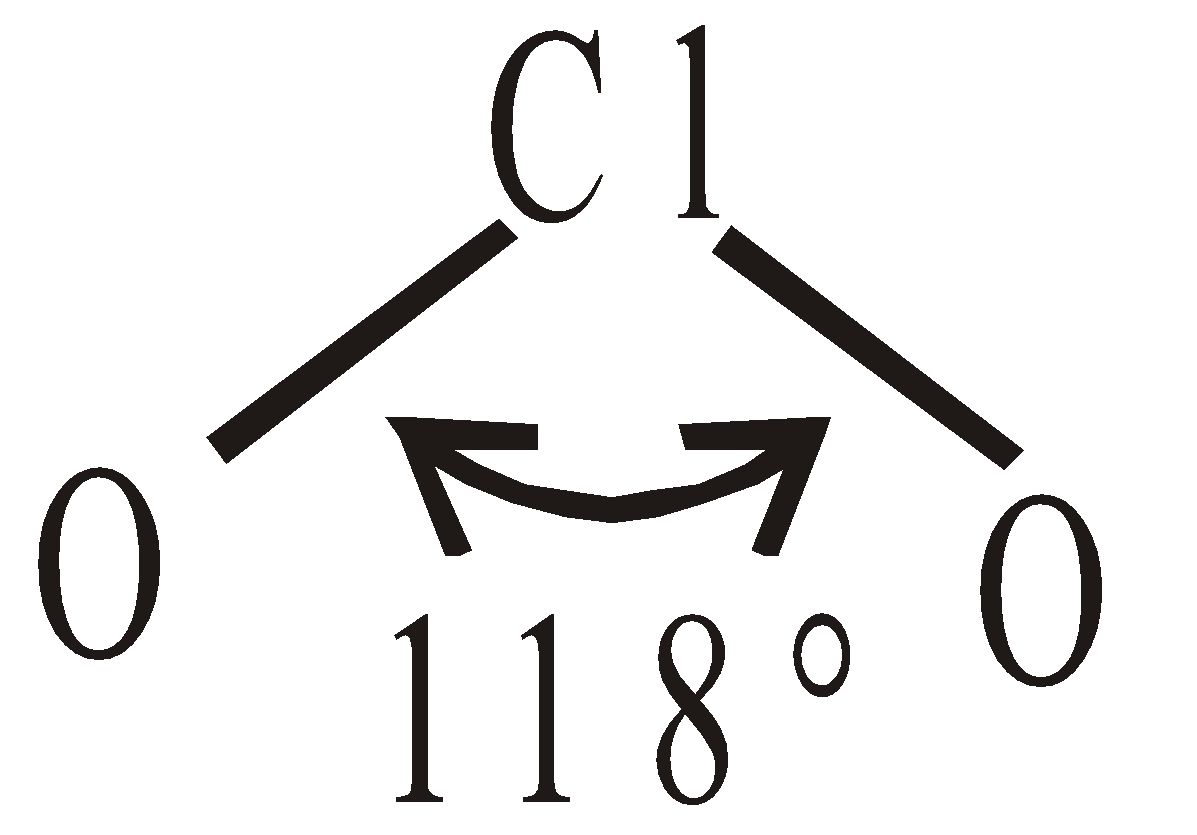
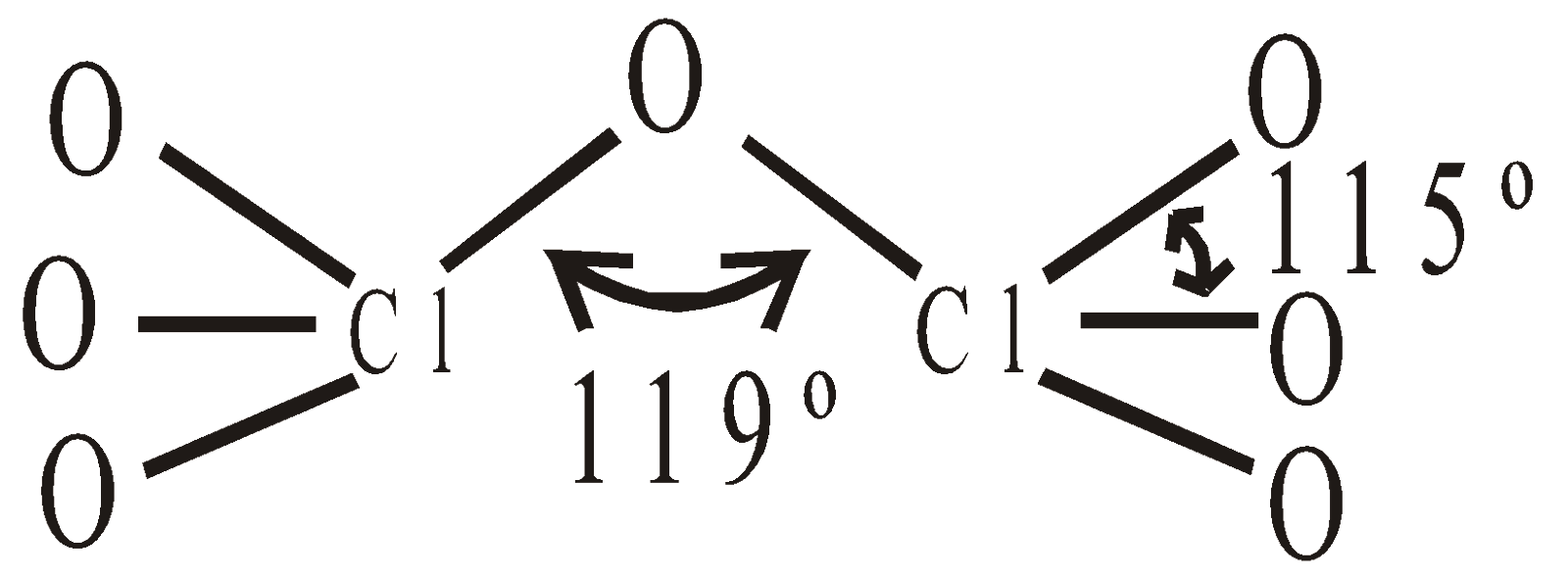
INTERHALOGEN COMPOUNDS
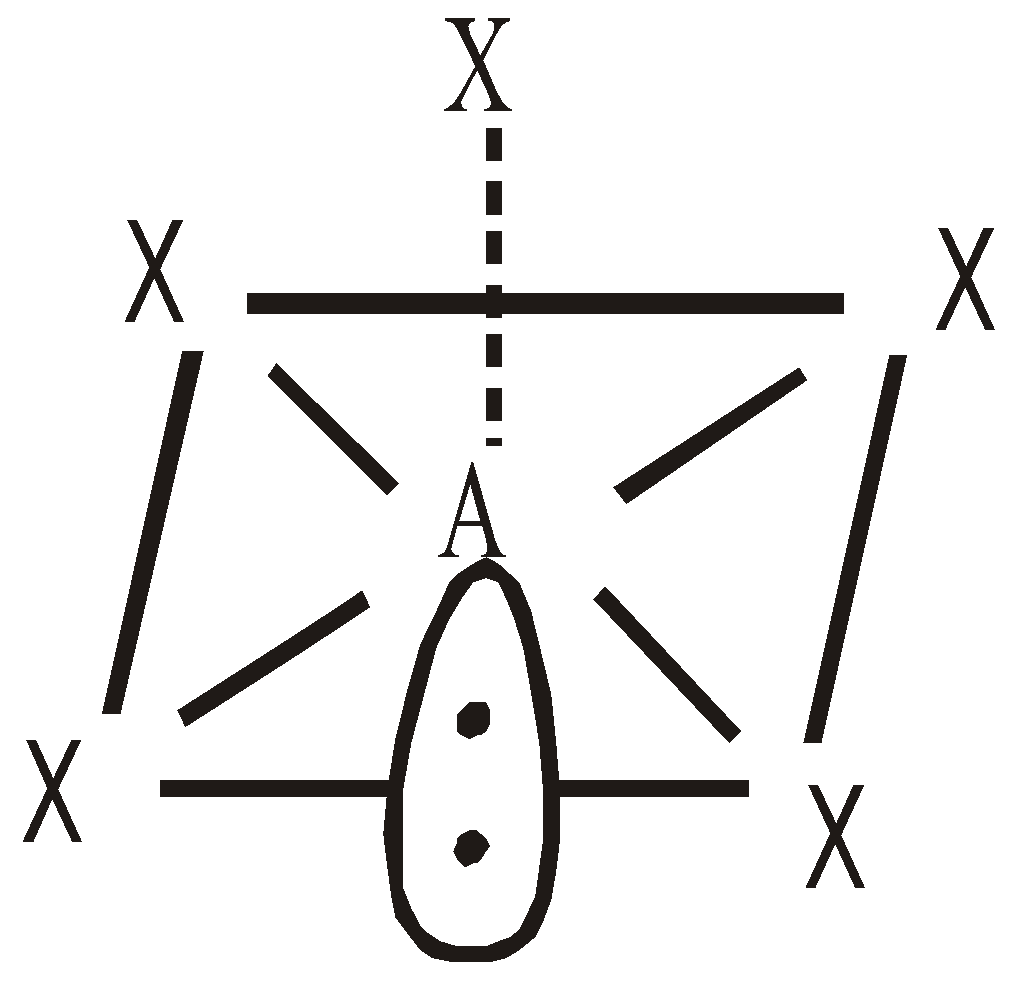
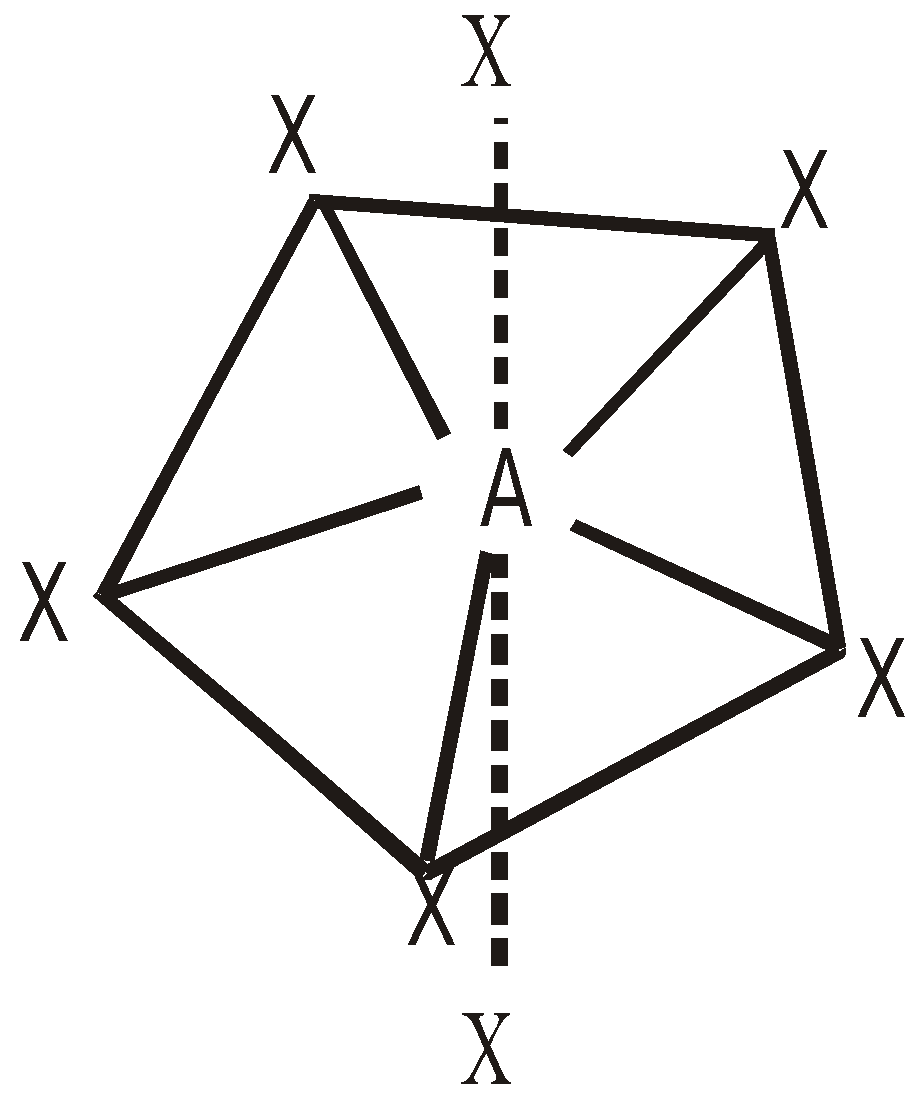
By direct combination of halogens
FLUORINE (SUPER HALOGEN)
OCCURRENCE
- Fluorspar CaF2
- Cryolite Na3AlF6
- Fluorapatite CaF2.3Ca3(PO4)2
PREPARATION
- Dennis method – By electrolysis of fused sodium or potassium hydrogen fluoride (dry) between graphite electrodes . (KHF2 is known as Fremy salt)
- Whytlaw Gray’s method – Electrolysis of fused KHF2 is carried out in Cu cell (electrically heated) which serves the purpose of cathode, Anode is of graphite
- Modern method – Electrolysis of fused mixture of KF and HF is carried out in steel vessel (cathode). Anode is of graphite
PROPERTIES
- It is pale greenish yellow gas can be condensed to pale yellow liquid and then pale yellow solid. It is very reactive.
- Most active – It directly combines with metals and non metals eg Al, Mg, C, P, S, As, Sb, Br2, I2 etc to from fluorides, Cu and Hg form a protective coating of fluoride.
- Reaction with Xe – With Xenon it forms three definite halides
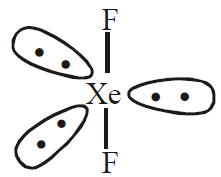 Linear symmetrical
Linear symmetrical 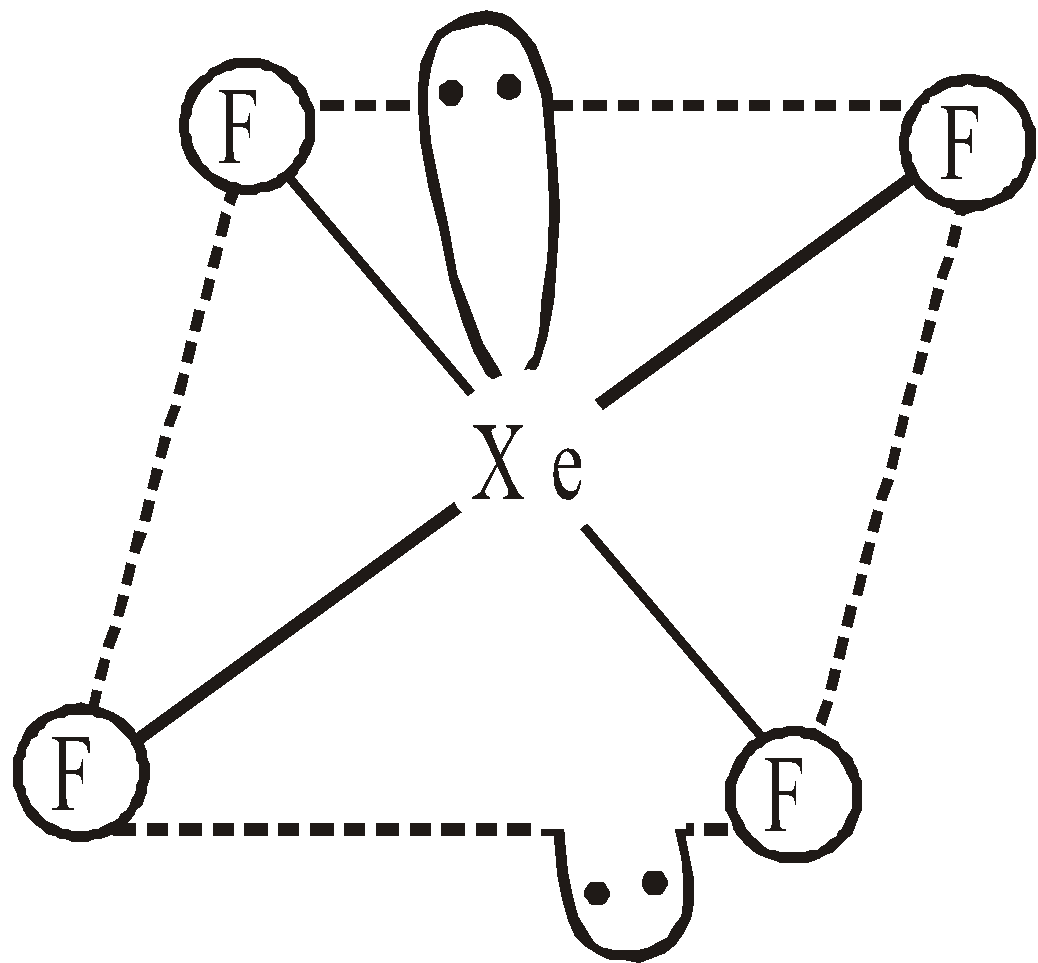 Square planar
Square planar 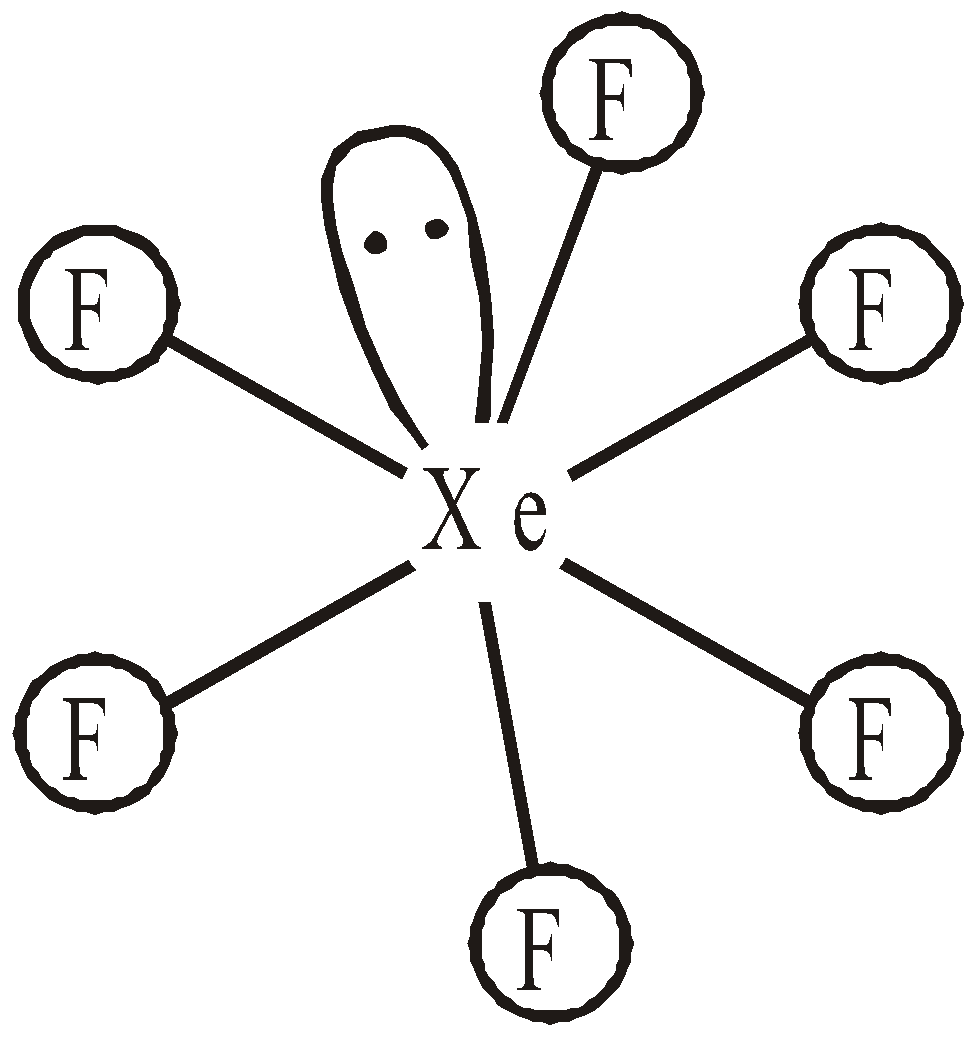 Distorted octahedral
Distorted octahedral - With hydrogen even in dark –
- With water
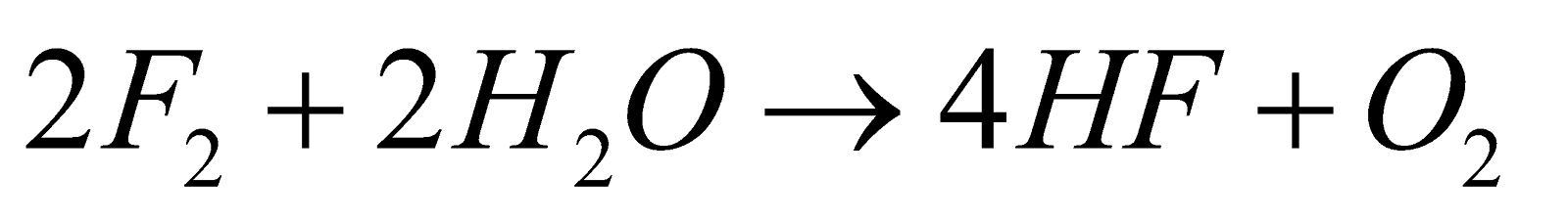
- Oxidising nature
- With alkali
Dil.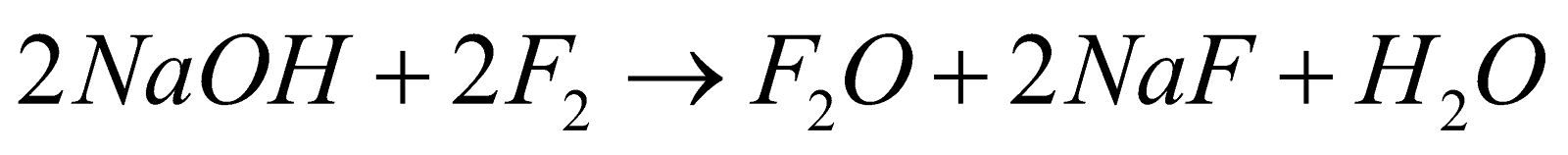
- With NH3
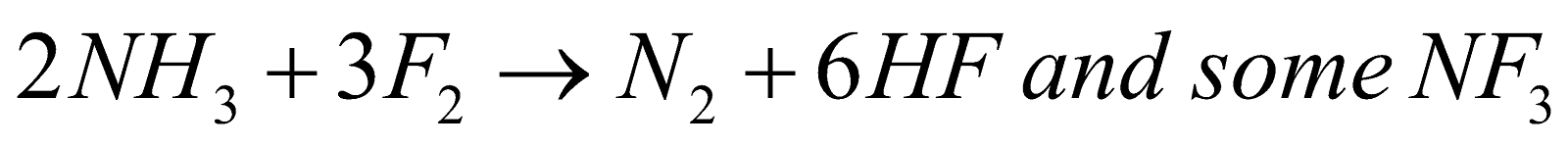 (not explosive)
(not explosive) - With H2S
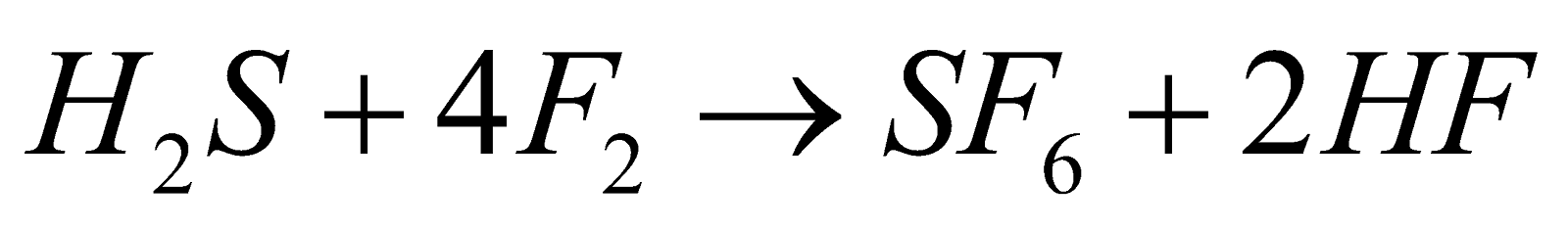
- With hydrocarbons – It reacts with hydrocarbons violently eg. CH4
USES
- Fluoro derivatives are solvents, lubricants, refrigerants, fire extinguishers, fungicides, germicides, dyes and plastics etc.
- For separation of U235 by forming UF6 from natural uranium
- Preparation of Teflon (C2F4)n
- FREONS – Chlorofluoro compounds of methane and ethane are known as freons. They are extremely, unreactive, non corrosive, easily liquefiable compounds. Freon-12 (CCl2F2) is most common and prepared as
- Magic acid – FSO3H SbF5 is strongest acid and known as magic acid.
CHLORINE
OCCURRENCE
PREPARATION
MANUFACTURE
- Weldon’s process – By heating pyrolusite with Conc HCl
- Deacon’s process – In this process HCl is oxidised by O2 in presence of CuCl2 as catalyst at 400° C
- Electrolytic process – By the electrolysis of brine solution in Nelson cell
- Pure Chlorine – By heating AuCl3 or PtCl4 in hard glass tube
PROPERTIES
- It is yellowish green gas. Collected by upward displacement of air, poisonous in nature, soluble in water. It’s aqueous solution is known as chlorine water which on careful cooling gives chlorine hydrate Cl2.8H2O
- Action of water
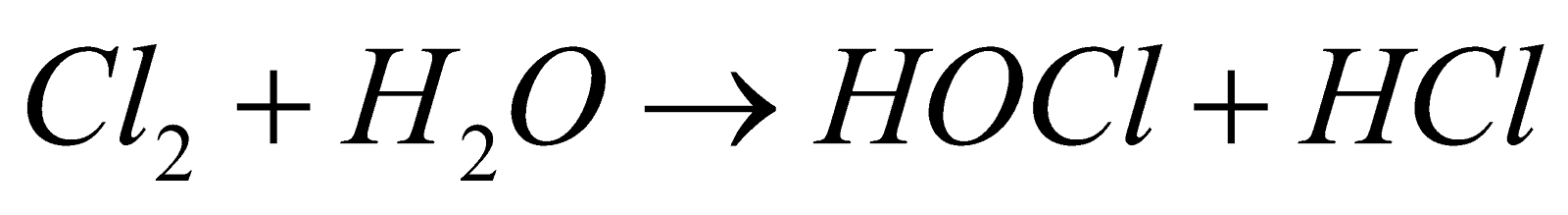
- Action of Hydrogen
- Displacement reactions
- Action of NaOH Cold
- Action of H2S
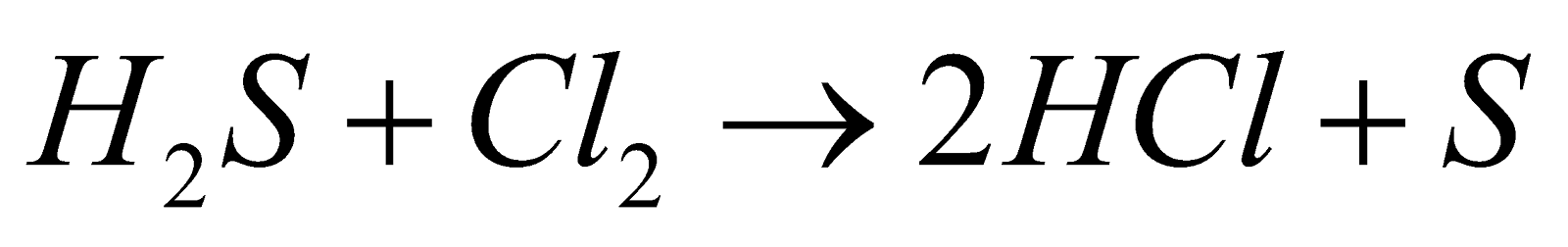
- Action of dry SO2
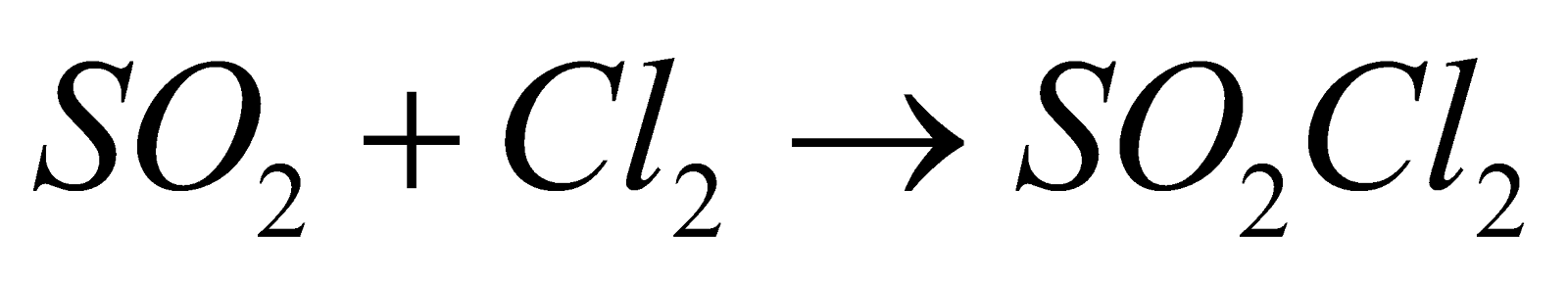
- Action of CO
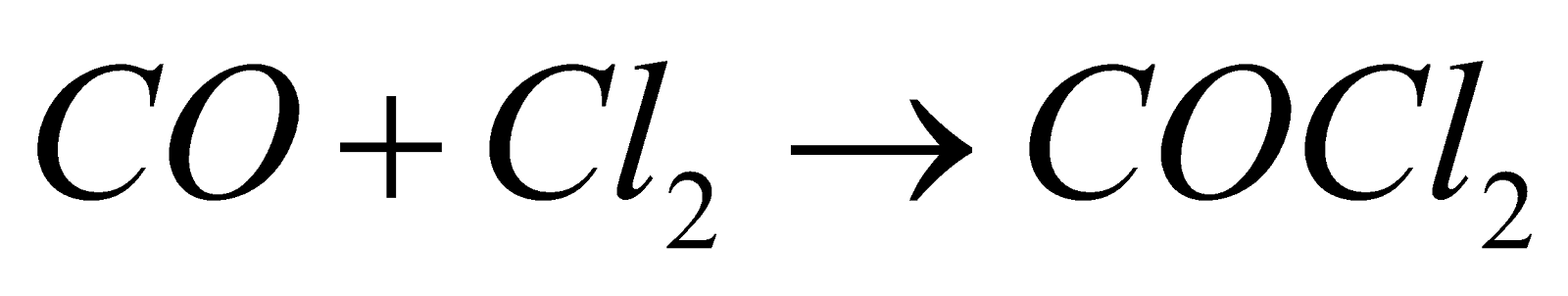
- Oxidising properties
In aqueous solution Cl2 acts as oxidising agent
- Reaction with ammonia
USES
- bleaching agent
- disinfectant
- in the manufacture of CHCl3, CCl4, DDT anti knock compounds, bleaching powder, poisonous gas phosgene (COCl2), tear gas CCl3NO2 and mustard gas ClC2H4SC2H4Cl.
It is a mixture of chlorine and chlorine dioxide and obtained by heating KClO3 with conc. HCl
BROMINE
OCCURRENCE
PREPARATION
- By heating mixture of potassium bromide manganese dioxide and conc H2SO4
- By passing chlorine through solution of a bromide
MANUFACTURE
- From carnallite – The mother liquor left after the crystallisation of chlorides from carnallite (KCl.MgCl2.6H2O) contains bromides of Na, K and Mg and is known as bittern.
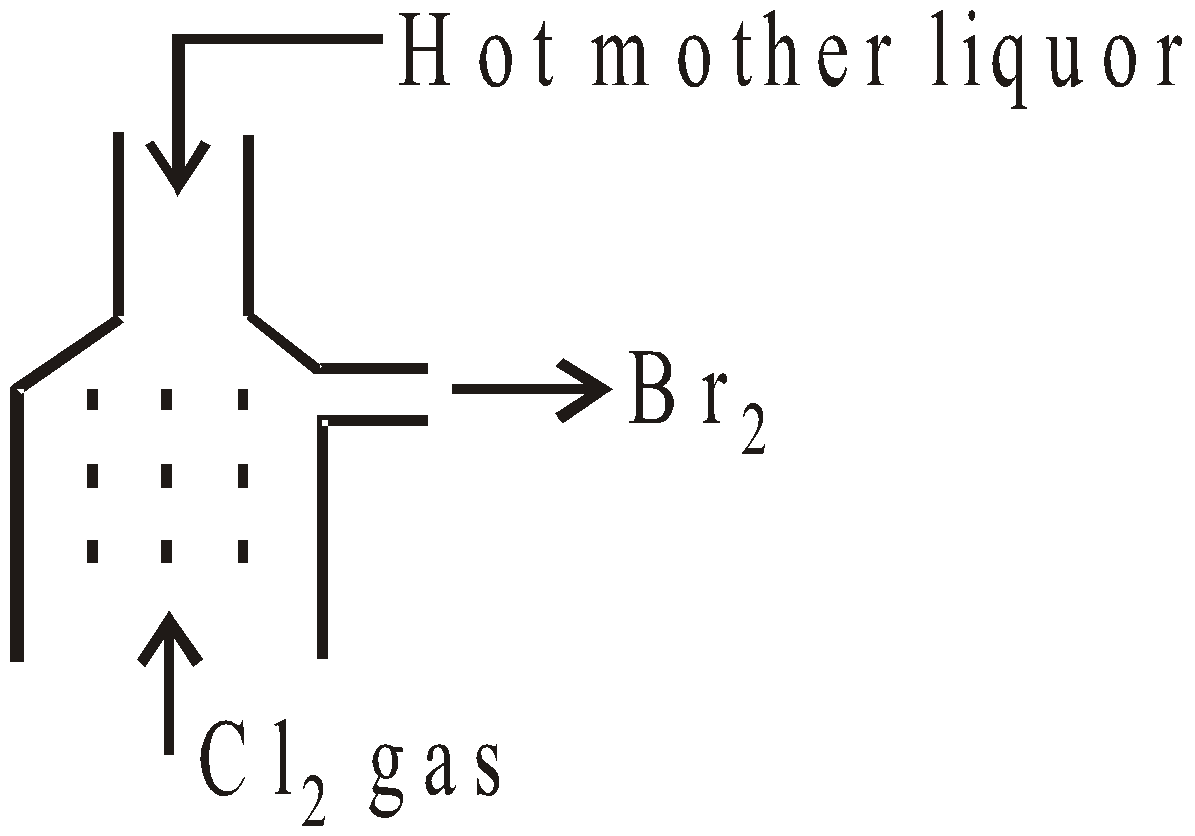
- From sea water – The sea water is concentrated when salts separate as crystals. The mother liquor contains MgBr2 and is treated as above to get bromine.
PHYSICAL PROPERTIES
CHEMICAL PROPERTIES
- Combination with hydrogen
- Oxidising nature
Under ordinary conditions it does not react with water but in presence of an oxidisable substance it gives HBr.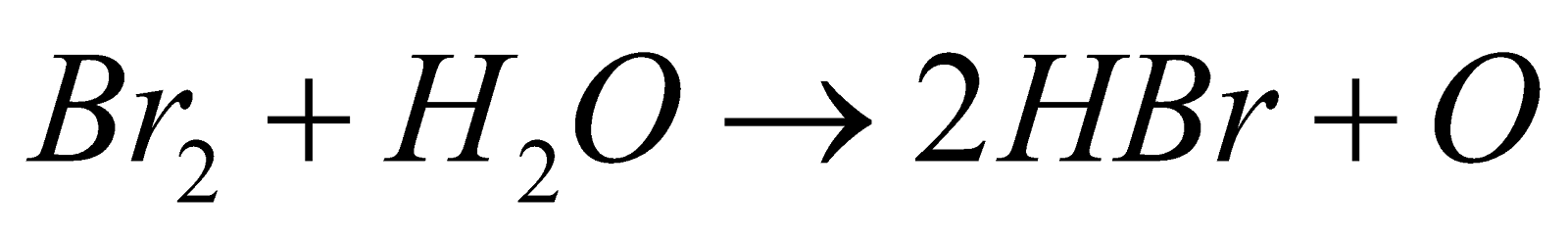
- Reaction with alkali
- Displacement reaction
- Action of ammonia
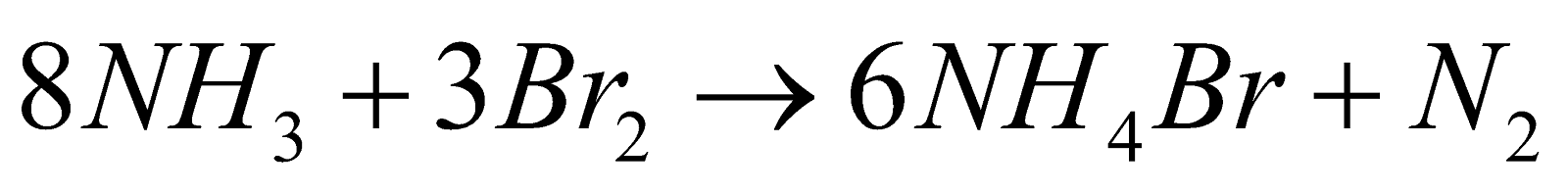
- Action of organic Compounds
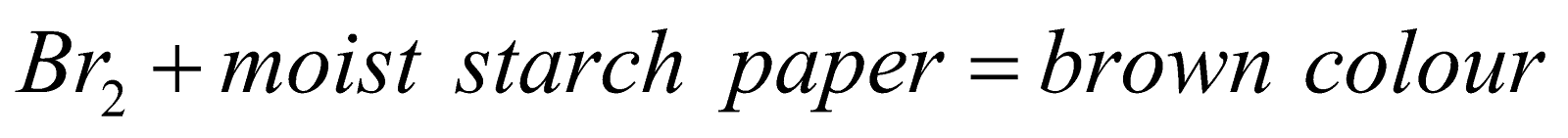 or Br2 + moist starch KI paper = violet
or Br2 + moist starch KI paper = violet
USES
- In the manufacture of tetraethyl lead an important antiknock compound
- NaBr and KBr are used as sedatives
- AgBr is used in photography
- Ethylene bromide increases efficiency of TEL
- NaBr and KBr are used as sedatives
IODINE
OCCURRENCE
PREPARATION
MANUFACTURE
- From sea weeds
- From Caliche
PURIFICATION
PHYSICAL PROPERTIES
CHEMICAL PROPERTIES
- Solubility
It is slightly soluble in water but dissolves in presence of KI
- Combination with elements
- Displacement reactions
- Reaction with alkalies
- Action of ammonia
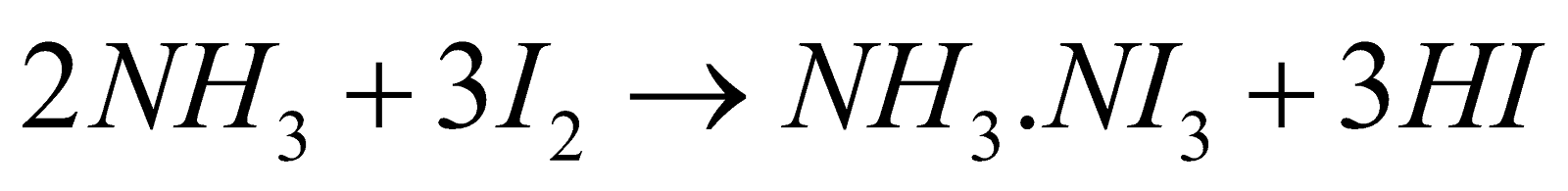
- Reaction with hypo
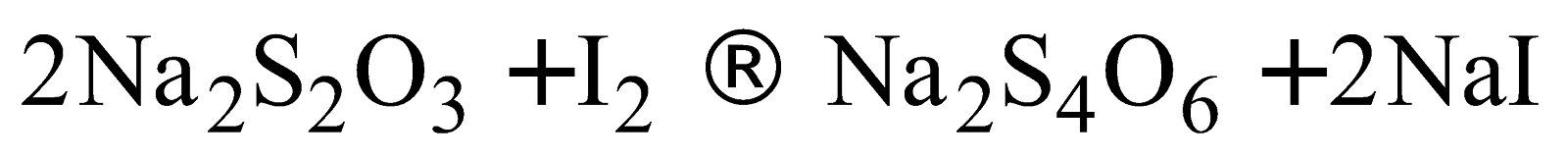
- Action of strong oxidising agents
eg conc HNO3, O3 and Cl2 They produce iodic acid.
USES
- In medicines eg as tincture of iodine, iodex, iodoform
- Solution of KI and I2 used in the treatment of goitre.
Bleaching powder (CaOCl2.H2O)
MANUFACTURE
PROPERTIES
- By the action of dilute acids or carbon dioxide it loses its chlorine
- It give O2 in presence of cobalt chloride solution
- In presence of slight amount of dilute acid it loses oxygen.
- It reacts with ethyl alcohol or acetone to form chloroform
- It does not form clear solution with water and aqueous solution contains
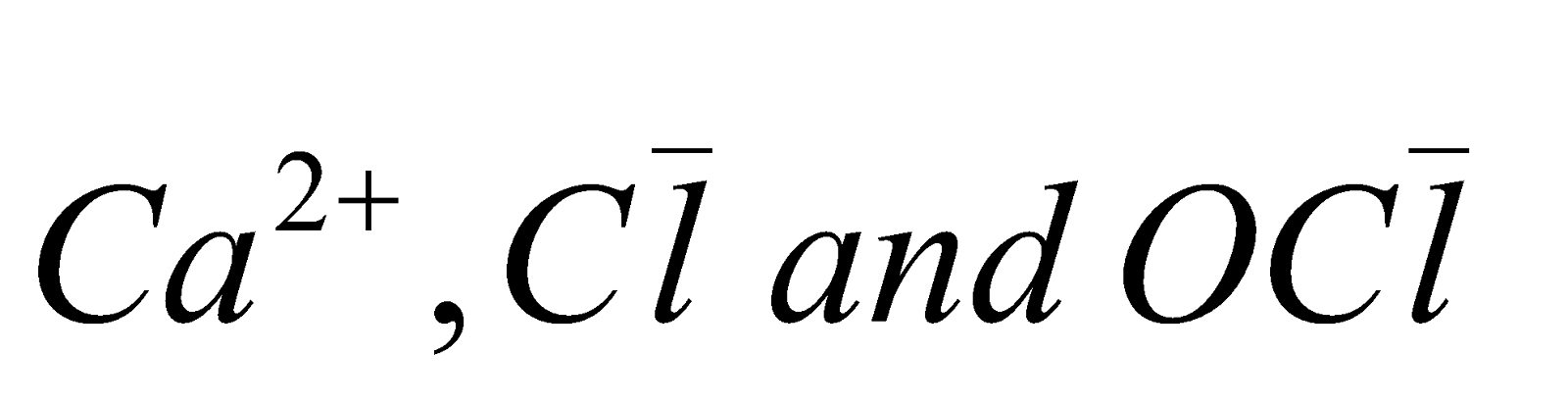 ions.
ions.
USES
- It is used as disinfectant and germicide
- For the manufacture chloroform
- For the sterilization of drinking water
- For making unshrinkable wool
- For bleaching cotton, wood pulp
STRUCTURE
- Due to Odling
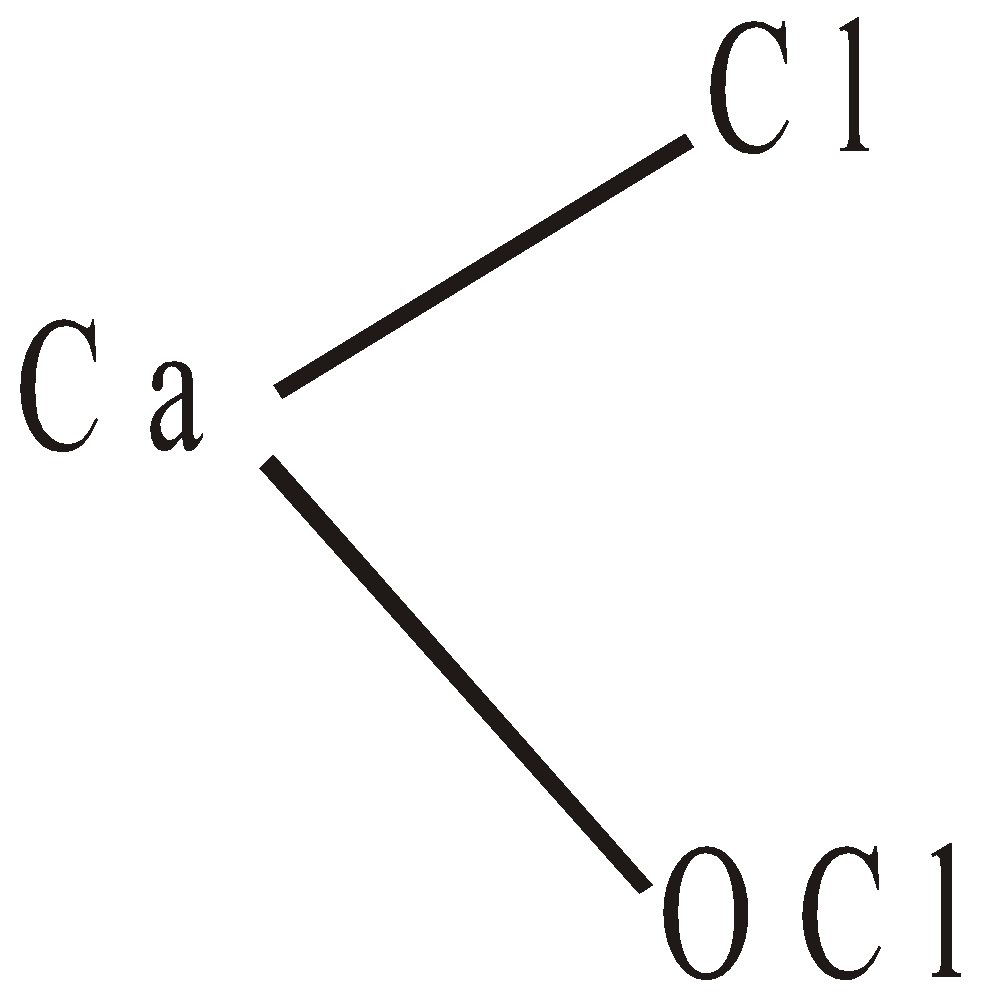
- According to Bunn, Clark and Clifford, it is a mixture of calcium hypochlorite
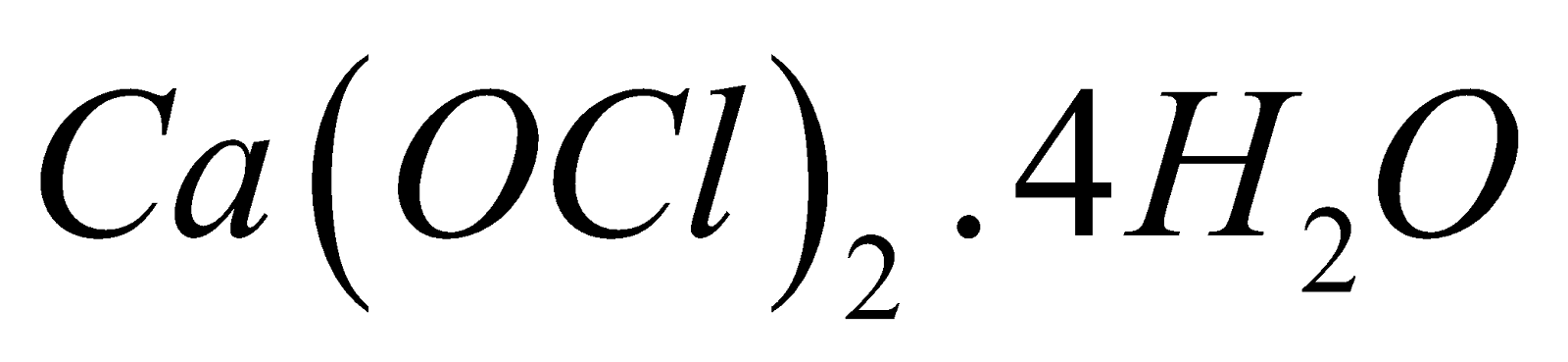 and basic calcium chloride
and basic calcium chloride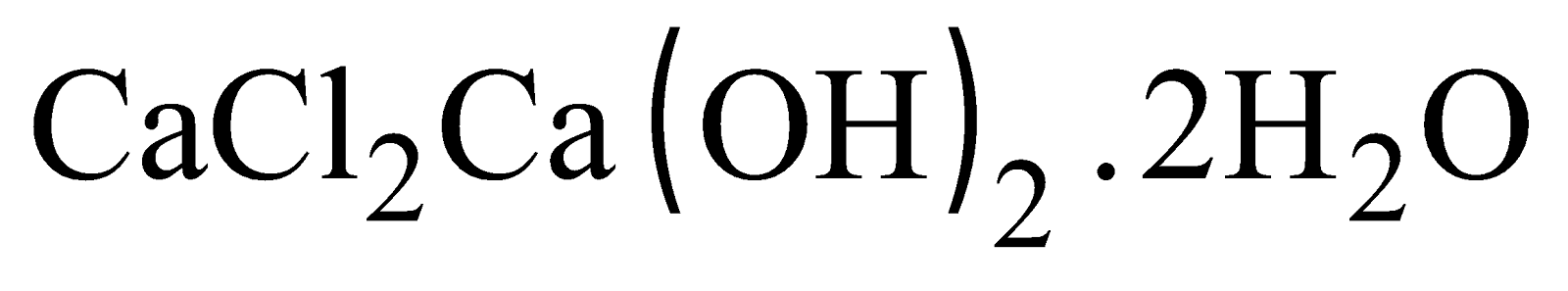
ABNORMAL PROPERTIES OF HYDROFLUORIC ACID
- It is highly poisonous and has corrosive action on skin
- It is exist as H2F2 even in gaseous state and forms two series of salts KHF2 (FREMY’S SALT) and K2F2.
- It is not oxidised even by strong oxidising agents.
- It reacts with silica and glass
- On heating with MnO2 and H2SO4 it does not give F2 while other hydrogen halides (HX) give X2
- AgF and PbF2 are soluble in water while chlorides, bromides and iodides of silver (Ag) and lead (Pb) are insoluble in water.
- CaF2 and SrF2 are insoluble in water while chlorides, bromides and iodides af Ca and Sr are soluble.
- Azeotropes of hydracids
Hydrogen | Halide Composition | Boiling point ºC |
H2F2 | 36% | 120 |
HCl | 20.4% | 110 |
HBr | 47.0% | 126 |
HI | 57.0% | 127 |
