| Redox Reactions NEET and AIIMS Special |
| Redox Reactions Refresher Course |
| Redox Reactions : Master File |
| Redox Reactions : Quick Revision Notes |
| Redox Reactions : Concepts File |
| Redox Reactions : Brain Map |
| Redox Reactions Reference Book |
About this unit
Equilibrium in physical and chemical processes, dynamic nature of equilibrium, law of chemical equilibrium, equilibrium constant, factors affecting equilibrium-Le Chatelier’s principle; ionic equilibrium- ionization of acids and bases, strong and weak electrolytes, degree of ionization, ionization of polybasic acids, acid strength, concept of PH., Hydrolysis of salts (elementary idea), buffer solutions, Henderson equation, solubility product, common ion effect (with illustrative examples).
REDOX REACTION
OXIDATION
Oxidation may be defined in any of the following terms
- Addition of oxygen.
2 Mg + O2  2MgO
2MgO
- Removal of hydrogen
3O2 + 4NH3  2N2 + 6H2O
2N2 + 6H2O
- Addition of electronegative portion
Cu + Cl2  CuCl2
CuCl2
- Removal or decrease in the electropositive portion
H2S + Cl2  2 HCl + S
2 HCl + S
- De – electronation
REDUCTION
Reduction may be defined in any of the following terms
- Addition of hydrogen
N2 + 3H2 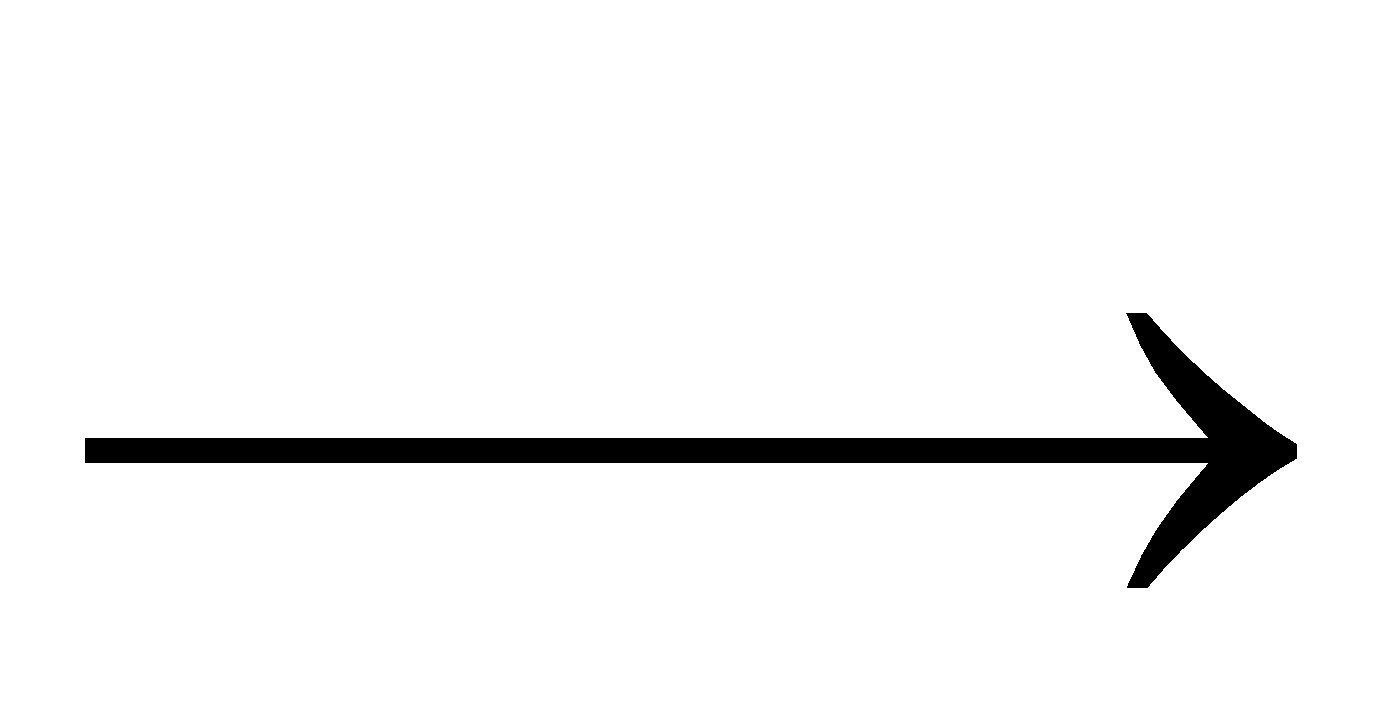 2NH3
2NH3
- Addition of electropositive portion
CuCl2 + Cu 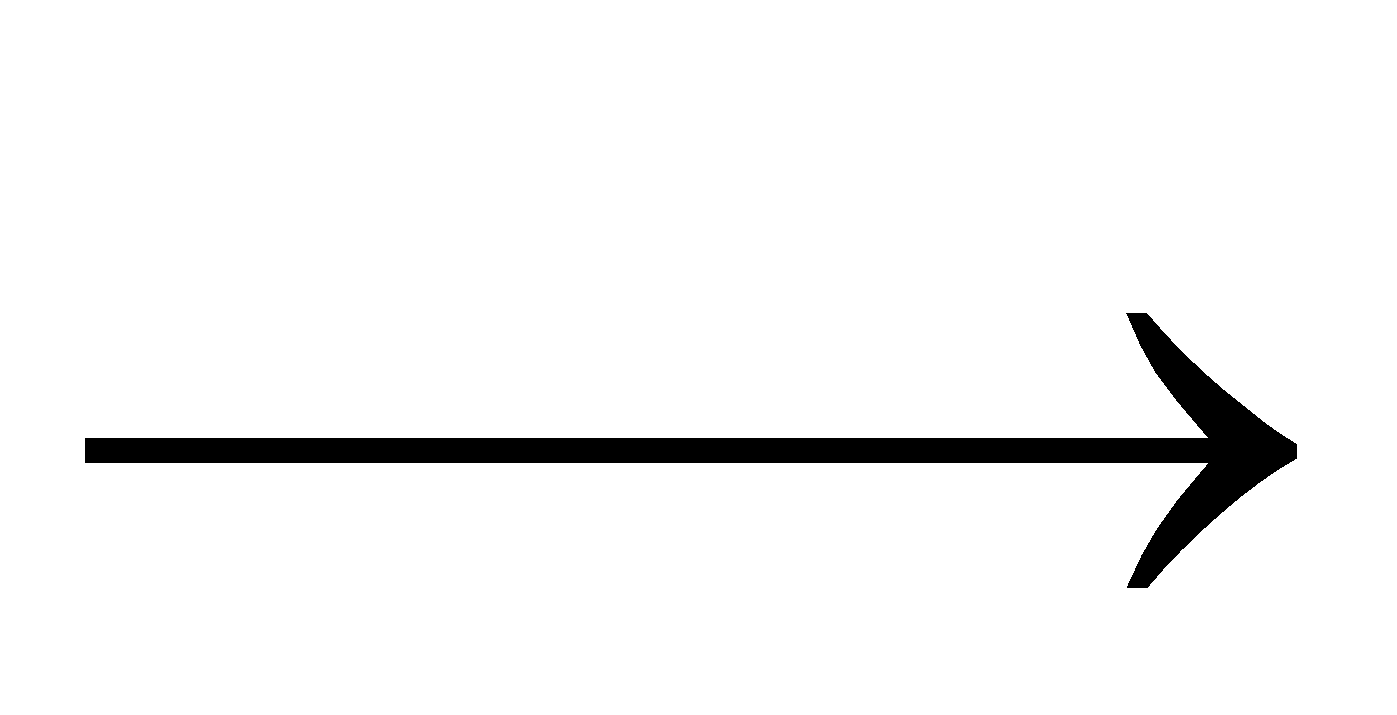 Cu2Cl2
Cu2Cl2
- Removal of oxygen
CuO + H2 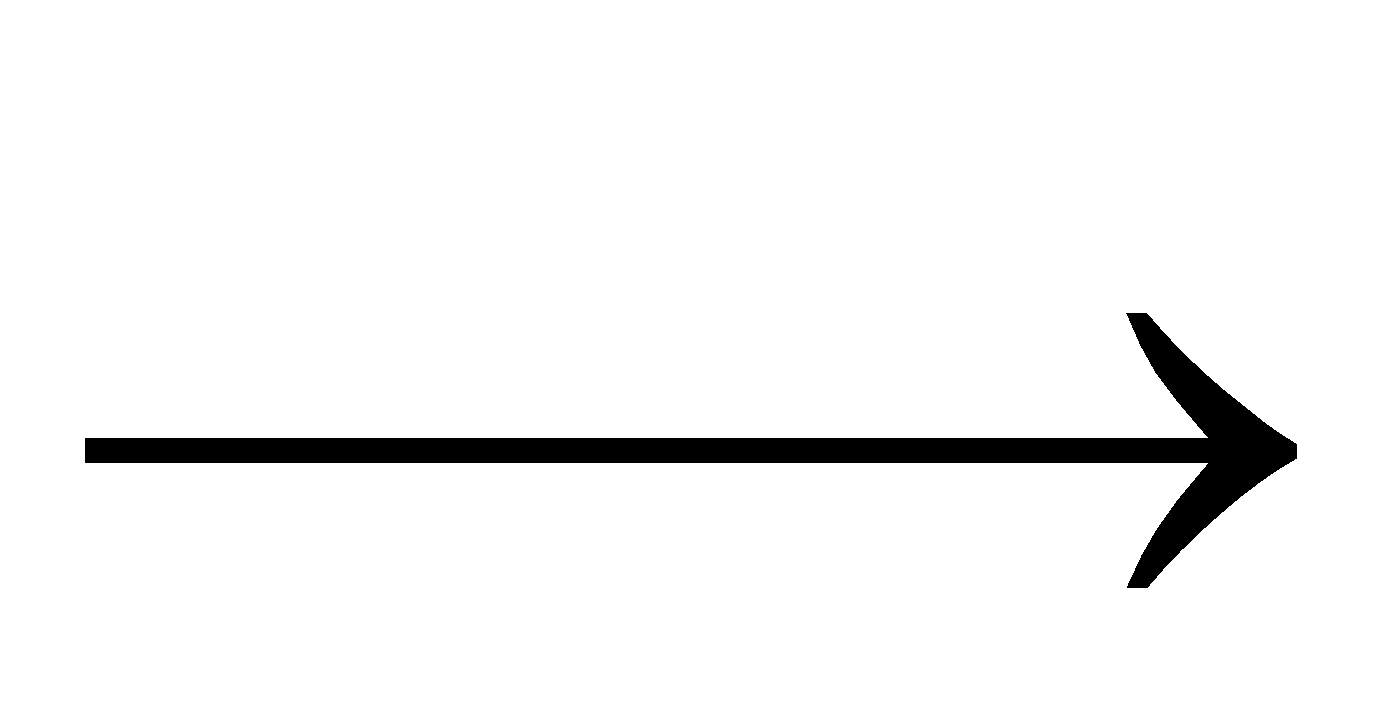 Cu + H2O
Cu + H2O
- Removal or decrease in the electronegative portion
2HgCl2 + SnCl2 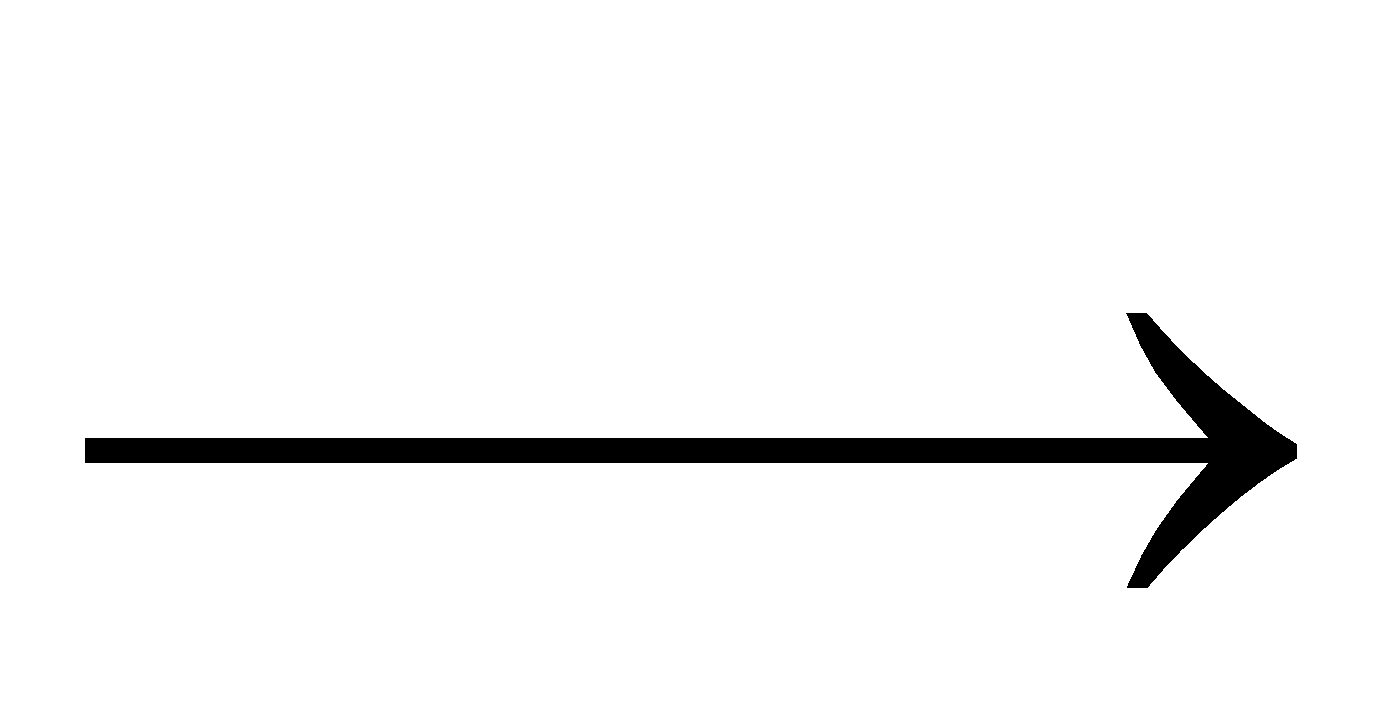 Hg2Cl2 + SnCl4
Hg2Cl2 + SnCl4
- Electronation
M + ne– Mn–
Mn–
OXIDANT OR OXIDISING AGENT
As stated above the oxidising agent may be defined as a substance supplying oxygen or electronegative element, removing hydrogen or electropositive element and can accept electrons. They show decrease in oxidation number
Examples – K2Cr2O7, KMnO4, H2O2, Cl2, Br2, KClO3, FeCl3 etc.
REDUCTANT OR REDUCING AGENT
A substance supplying hydrogen or electropositive element, removing oxygen or electronegative element and can donate electrons. They show increase in oxidation number.
Examples – SnCl2, H2, H2S, Mg, FeSO4, H2C2O4, H2SO3.
REDOX REACTIONS
Reactions comprising of simultaneous oxidation and reduction and called oxidation – reduction or redox reactions.
SnCl2 + 2HgCl2  SnCl4 + Hg2Cl2
SnCl4 + Hg2Cl2
TYPES OF REDOX REACTIONS
- Intermolecular redox reactions – In this case one substance is oxidised and another is reduced.
4 HCl + MnO2  MnCl2 + Cl2 + 2H2O
MnCl2 + Cl2 + 2H2O
Here HCl is oxidised and MnO2 is reduced.
- Disproportionation – In this case the same substance is oxidised and reduced eg.
- Intramolecular redox reactions – In this case one element of the compound is reduced while another element of the same compound is oxidised
Cr is reduced and N is oxidized
OXIDATION NUMBER
It is the number of electrons lost or gained by an
element during its change from free state in a particular compound.
element during its change from free state in a particular compound.
Or
It is defined as the formal charge present on an atom in a particular compound determined by certain arbitrary rules.
RULES FOR DETERMINING OXIDATION NUMBER
- O.N. of elements in free state is zero eg
- O.N. of hydrogen is always +1 except in ionic metal hydrides where it is – 1.
- O.N. of oxygen is –2 except in OF2 where it is + 2 and in peroxides where it is – 1.
- O.N. of metals is always +ve. For IA group elements it is +1 and for IIA group elements it is +2.
- O.N. of halogens is –1 in metal halides.
- O.N. of ion or radical is the number of electrons it must gain or lose to acquire neutrality i.e. it is equal to the electric charge for
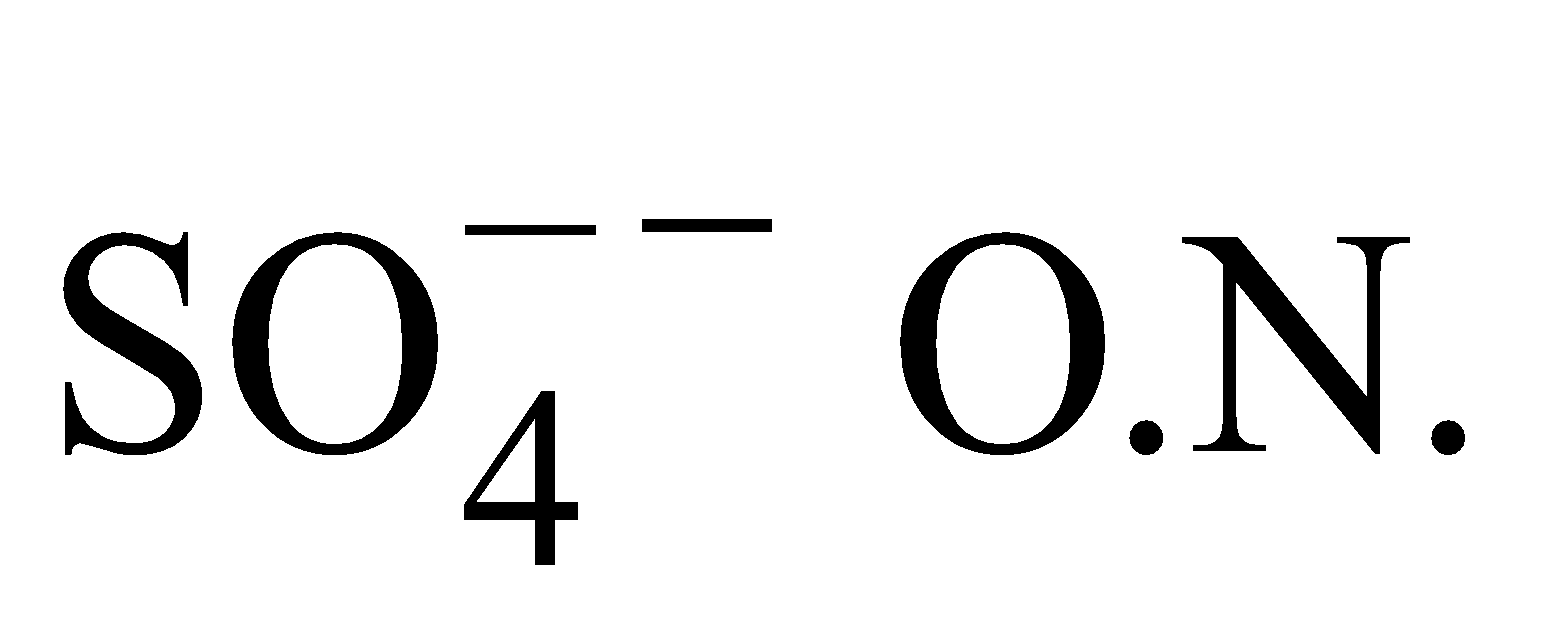 is –2.
is –2. - O.N. of an atom within compound can be +ve, –ve integer, zero or fraction.
- The algebraic sum of all the O.N. of elements is equal to zero.
- The algebraic sum of all the O.N. of elements in an ion is equal to net charge on the ion.
- Maximum O.N. of an element is equal to number of valence electrons i.e. group number.
- Minimum O.N. of an element (except metals) = (8 – group number).
- In metal corbonyl, and amalgams, O.N. of metals is zero.
COVALENCY AND OXIDATION STATE
- Covalency : It is the number of hydrogen atoms which can combine with a given atom
or
It is the number of single bonds which an atom can form.
or
It is the number of electrons an atom can share. Valency is always a whole number.
- Oxidation state : It is defined as the O.N. per atom.
STOCK NOTATION
Representation of oxidation state of element by Roman numerals within parenthesis is known as stock notation eg FeCl3 is written as Iron(III) chloride and FeSO4 as Iron (II) sulphate.
CHEMICAL BONDING METHOD FOR DETERMINATION OF OXIDATION NUMBER
Sometimes wrong results are obtained when the O.N. is determined by applying the above mentioned simple rules. In such cases applying the chemical bonding method is very useful. The rules are
- For one covalent bond assign one unit negative charge to electronegative atom and one unit positive charge to less electronegative atom e.g. (electronegativity A> B).
- No charge when the covalent bond is between like atoms.
- In case of coordinate bond assign two unit negative charge to acceptor atom and two unit positive charge to donor atom e.g. (electronegativity A > B).
- No charge when donor in coordinate bond is more electronegative than acceptor eg (electronegativity A > B).
- When coordinate bond is between similar atoms assign two unit negative charge to acceptor and two unit positive charge to donor e.g. (electronegativity same).
CALCULATION/ DETERMINATION OF OXIDATION NUMBER OF UNDERLINED ELEMENT IN SOME COMPOUNDS
Let the O.N. of Cr be x then
2 × (+1) + 2 × (x) + 7 × (–2) = 0
2 + 2x –14 = 0 x = +6
- KMnO4
Let the O.N. of Mn be x then
1 × (+1) + 1 × (x) + 4 × (–2) = 0
1 + 1x – 8 = 0 x = +7
- H2SO4
Let the O.N. of S be x then
2 × (+1) + 1 × (x) + 4 × (–2) = 0
2 + x –8 = 0 x = +6
- NH4NO3
Split into two ions NH4+ and NO3–
Let O.N. of N be x in ion then
1 × (x) + 4 × (+1) = +1
x + 4 = +1 x = –3
Let the O.N. of N be x in ion then
1 × (x) + 3 × (–2) = –1
x – 6 = –1 x = +5
Let the O. N. of P be x then
1 × (x) + 4 × (–2) = –3
x – 8 = –3 x = +5
- HNO3
Let the O.N. of N be x then
1 × (+1) + 1 × (x) + 3 × (–2) = 0
1 + x – 6 = 0 x = 5
- KI3
Let the O.N. of I be x then
1 × (+1) + 3 × (x) = 0
1 + 3x = 0 x = –1/3
- NaO2
It is super oxide.
Let O.N. of O be x then
1 × (+1) + 2 × (x) = 0 1 + 2x = 0 x = –1/2
- Fe3O4
It is mixed oxide FeO.Fe2O3 and Fe has O.N. +2 and +3 respectively. 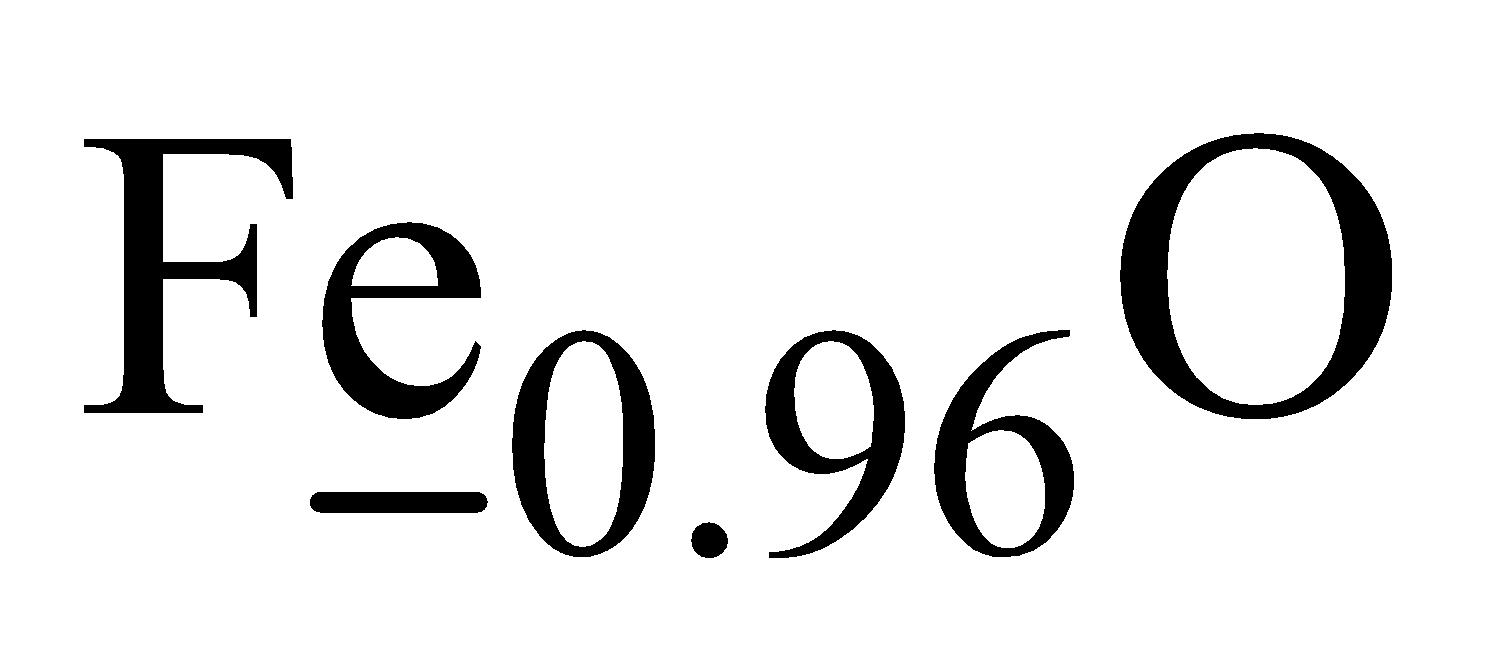
Let O.N. of Fe be x then 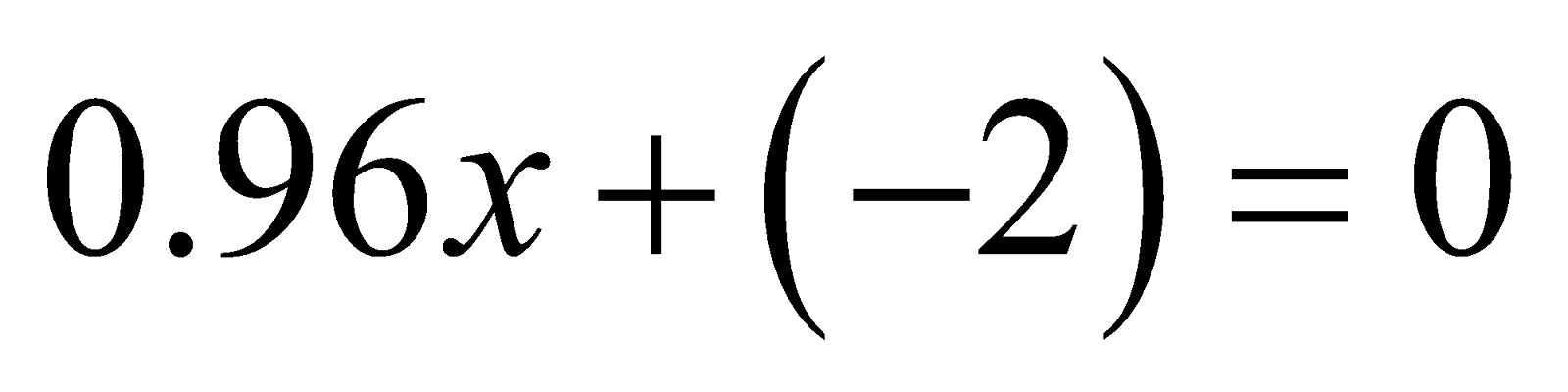
- N3H (hydrazoic acid)
Let O.N. of N be x then
3 × (x) + 1 × (+1) = 0
3x + 1 = 0 x = –1/3
- K4[Fe(CN)6]
Let the O.N. of Fe be x then
4 × (+1) + 1 × (x) + 6 × (–1) = 0
(sum of O.N. of CN– = –1)
4 + x – 6 = 0 x = 2
Determination of oxidation number by chemical bonding method
- CrO5
Let the O.N. of Cr be x then
1 × (x) + 5 × (–2) = 0
x – 10 = 0 x = 10 (wrong)
Apply chemical bond method
x + 1 × (–2) + 4 × (–1) = 0
(for Cr) (one=O) (four O – O )
x = +6
- HCN
Its structure is H – C N
for H – C bond H = +1, C = –1
for C N bond C = +3, N = –3
sum of O.N. of H = +1, C = +2 and N = –3
- H – N ≡ C
for H – N bond H = +1, N = –1
for N C, N = –2 and C = +2 (for two covalent bonds) No contribution of NC bond since N more electronegative than C
O.N. of different atoms H = +1, C = +2, N = –3
- H2SO5 (Caro acid) – Write structure and apply chemical bond method
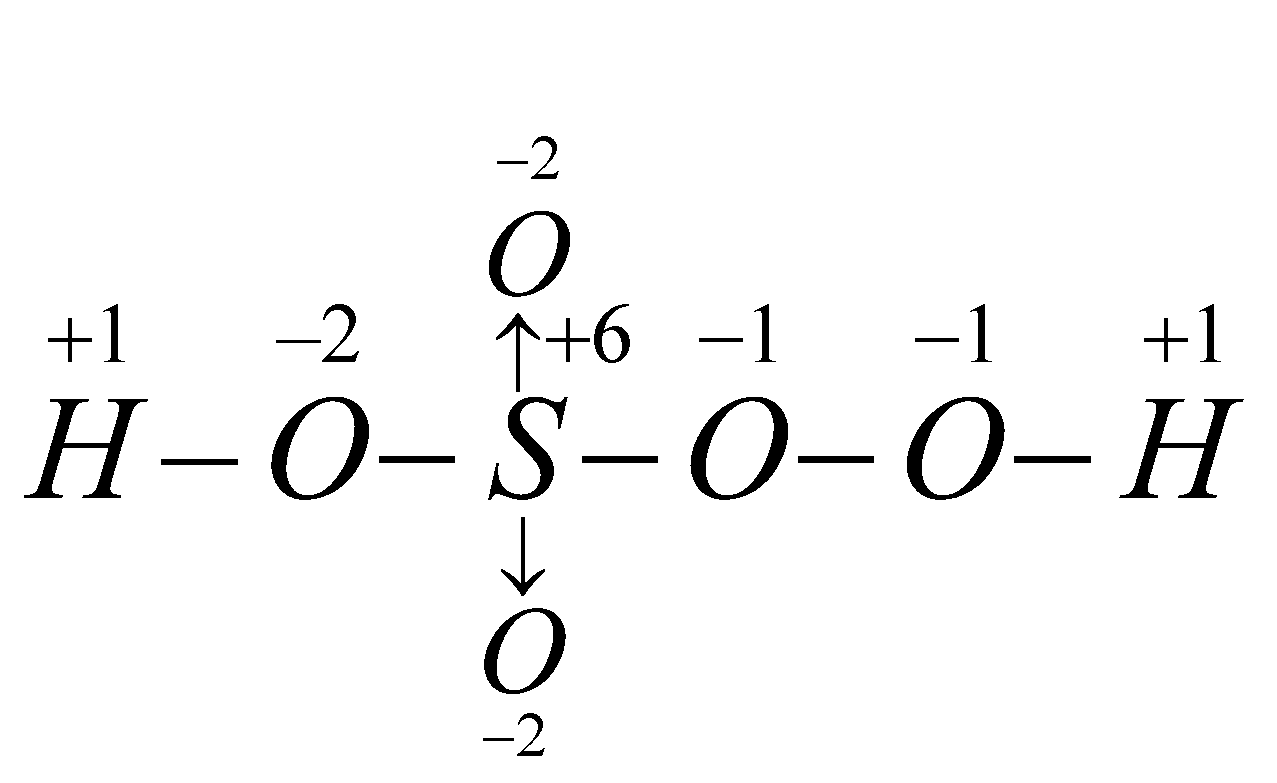
2 × (+1) + x + 3 × (–2) + 2 (–1) = 0
for H for S for O for O – O x = +6
- Na2S2O3
(Structure I) 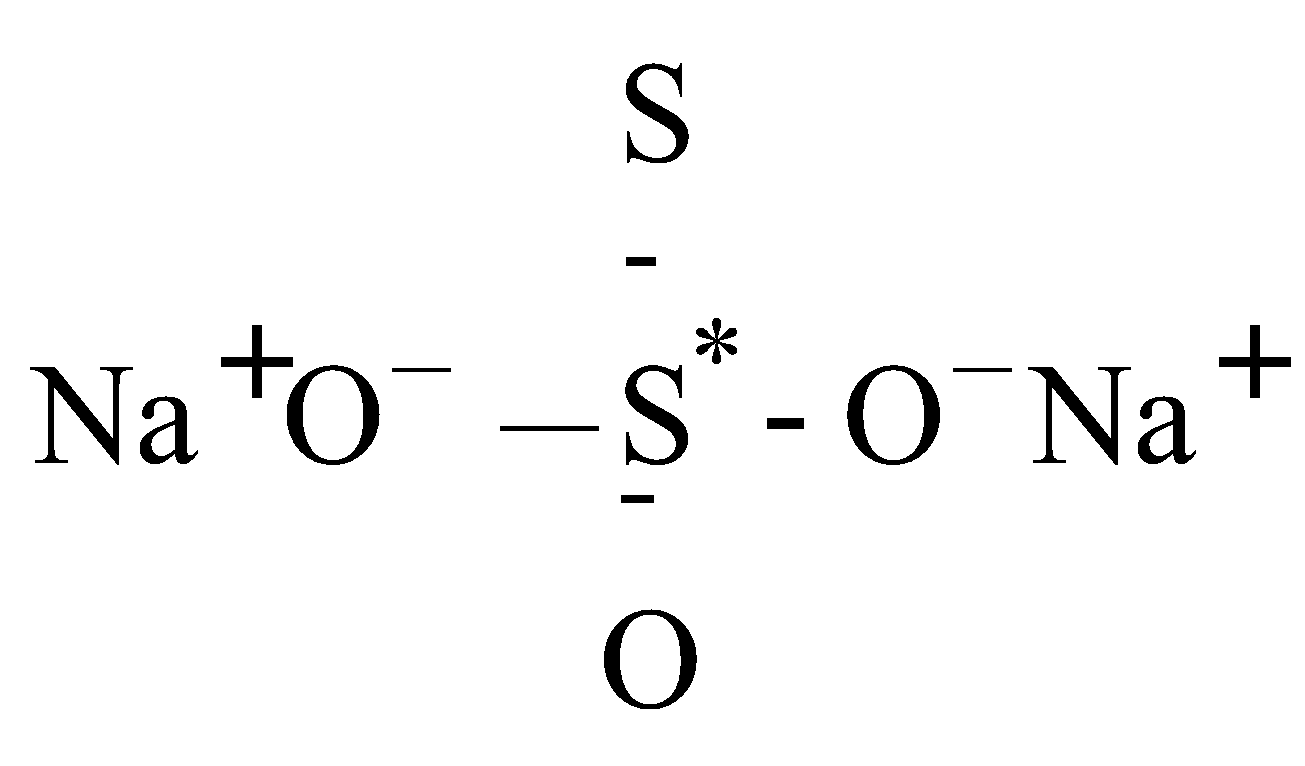
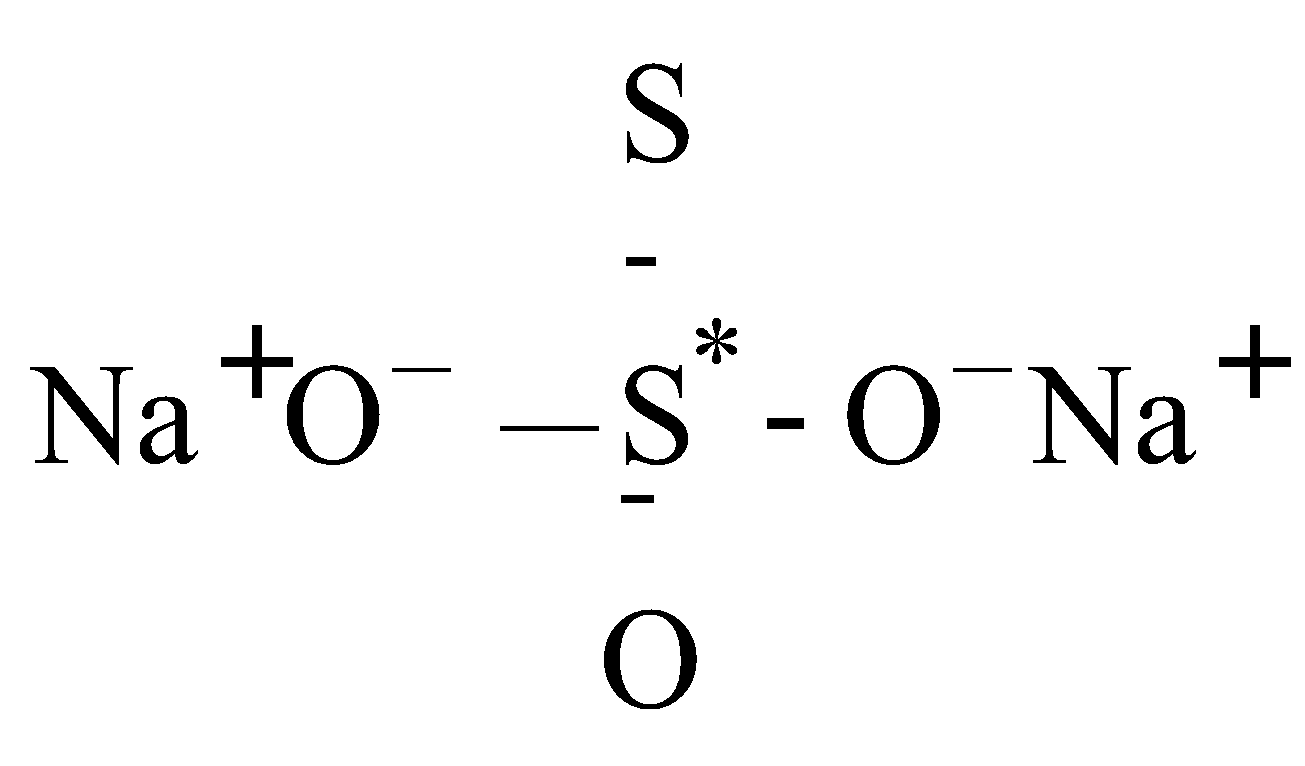
2 × (+1) + x + 1 × (–2) + 3 × (–2) = 0
for Na for S* for S for O
O.N. of S* = +6 and another S = –2
(Structure II) 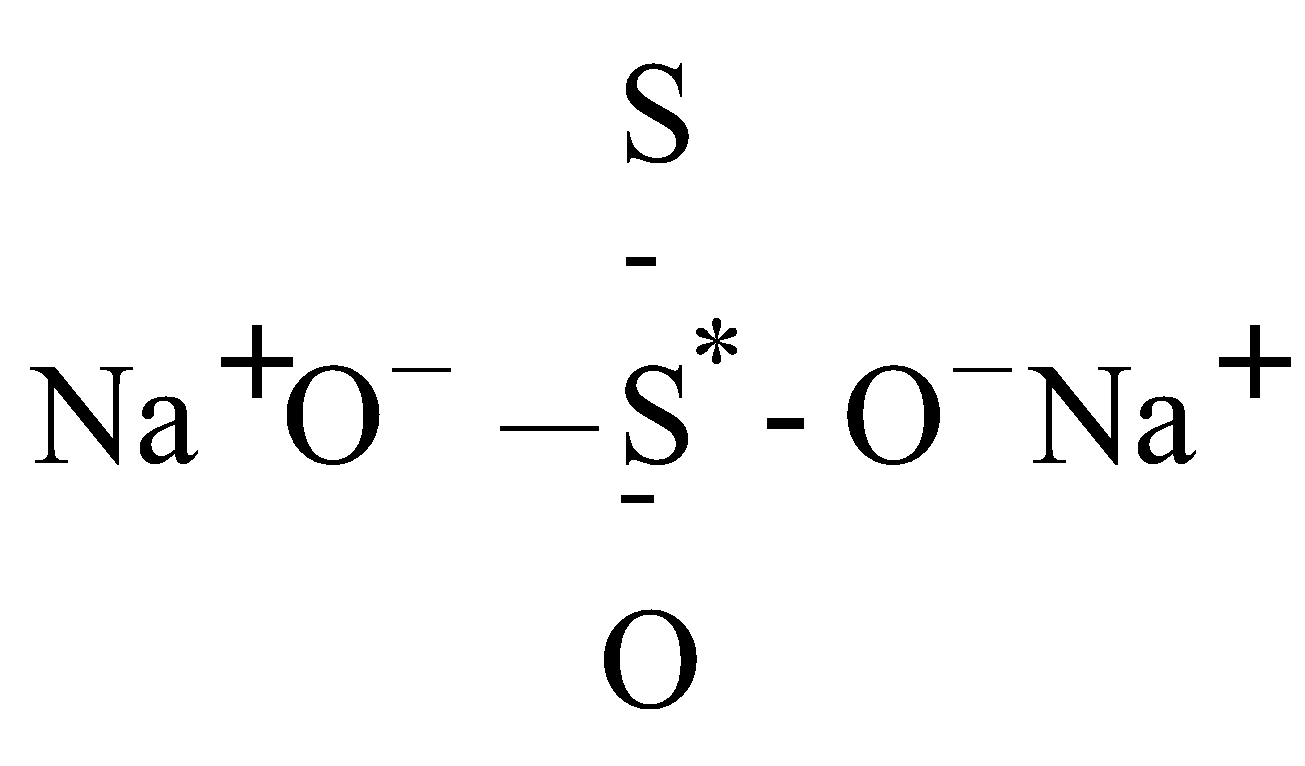
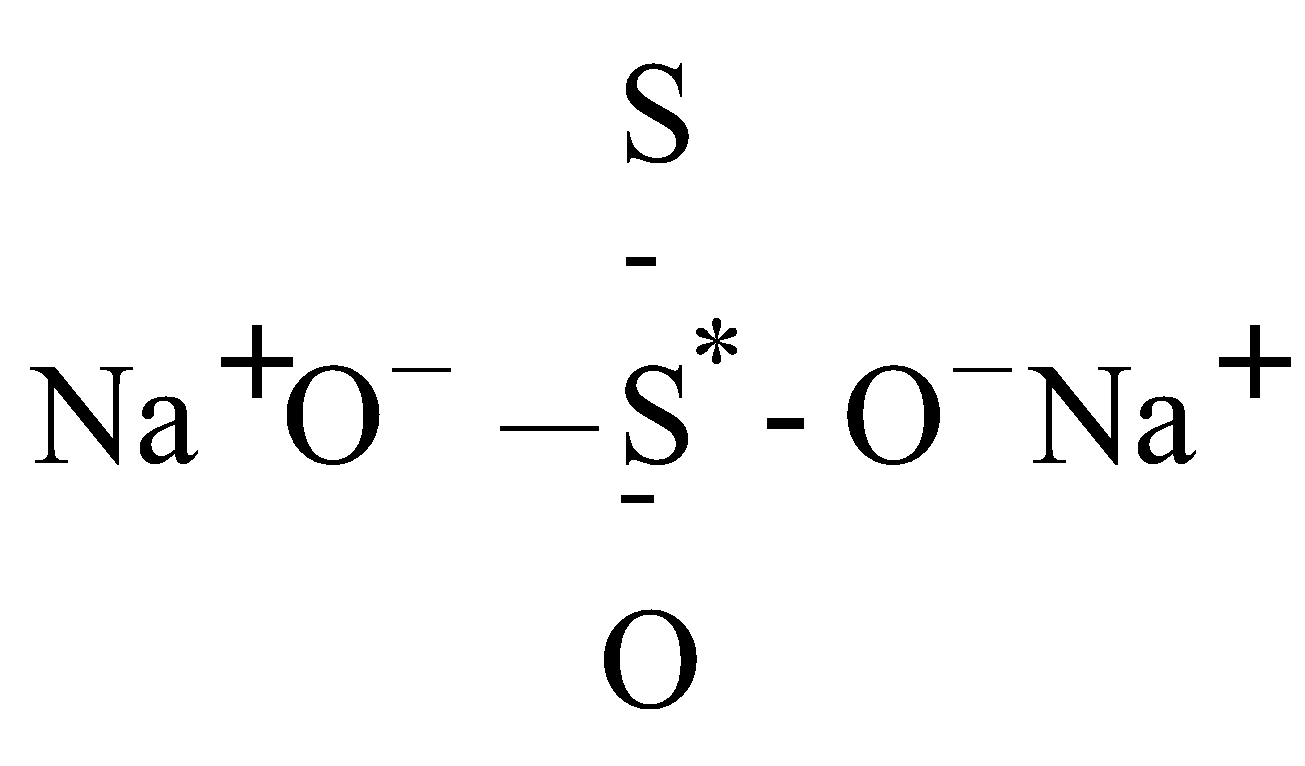
2 × (+1) + 1 × (–1) + 1 × (x) + 3 × (–2) = 0
for Na for S for S* for O
2 – 1 + x – 6 = 0 x = +5
O.N. of S* = +5 and another S = –1
- Na2S4O6
Sodium tetra thionate – its structure is as follows
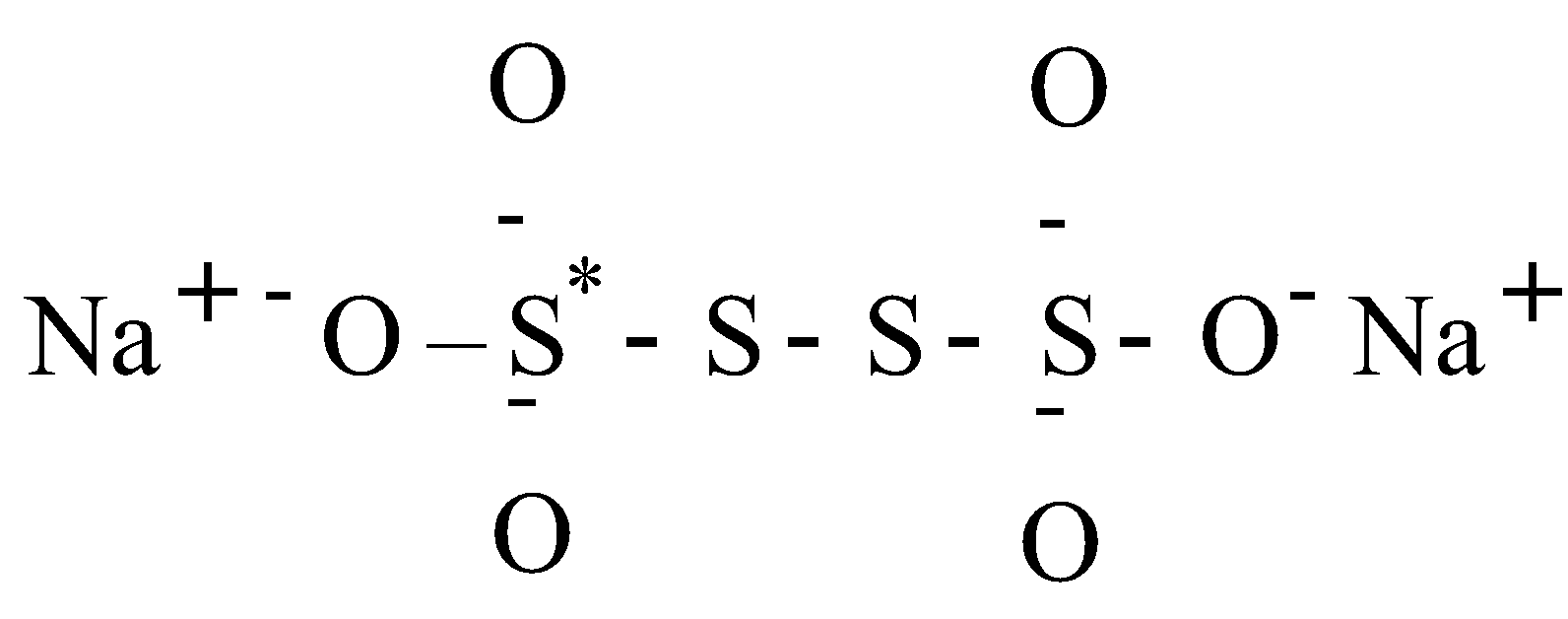
Let the O.N. of S* be x then
2 × (+1) + 6 × (–2) + 2 × (0) + 2 × (x) = 0
for Na for O for middle S
2 – 12 + 0 + 2x = 0
x = 5
- CaOCl2
Its structure is
O.N. of Cl is –1 and +1.
- O3
The structure of O3 is
O.N. of O in ozone is + 1 and –1
OXIDATION NUMBER CONCEPT OF OXIDANT (OXIDISING AGENT) AND REDUCTANT (REDUCING AGENT)
- Oxidising agent : A substance can act as oxidising agent if the oxidation number of one of its element is maximum eg HNO3 (O.N. of N = 5 which is maximum value)
The more the electronegativity of element and the more is O.N., the more is the oxidising power eg KClO4, KBrO4. KMnO4, K2Cr2O7, HClO4, HNO3 etc. Oxyanions are stronger oxidising agents in acidic solution than in basic or neutral solution.
- Reducing agent : A substance can act as reducing agent if the oxidation number of one of its element is minimum eg SnCl2 (O.N. of Sn = 2 which is minimum value), FeSO4, Na2S2O3, H2S, H2C2O4 Electronegative elements I–, Br–, N3– are powerfully reducing in nature.
- Reducing as well as oxidising agent : A substance that can act as both, reducing as well as oxidising agent if O.N. of one of its element is in between the maximum and the minimum value eg HNO2 (O.N. of N = +3 which is intermediate of +5 and 0).
OXIDATION NUMBER AND ACID STRENGTH
The greater the O.N. of the element in oxyacids, the greater is the acid strength.
EQUIVALENT WEIGHT OF AN OXIDISING AGENT
It can be obtained by dividing the molecular weight by the number of electrons gained represented in a chemical balanced equation eg.
Equivalent wt. of KMnO4 in acid medium
Eq. wt. of K2Cr2O7 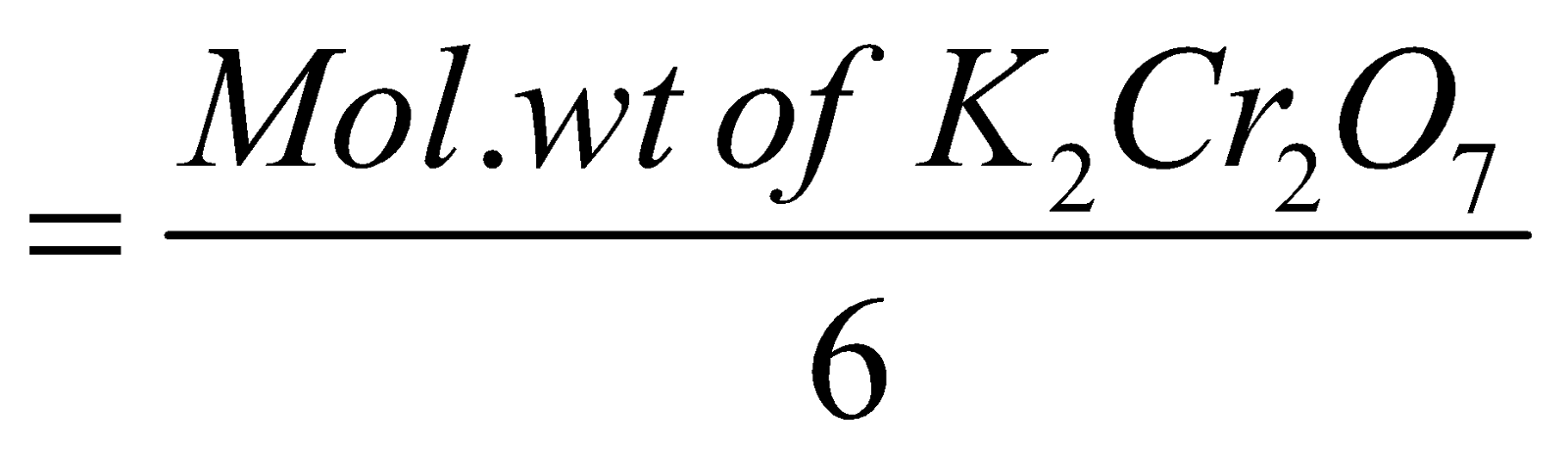
EQUIVALENT WEIGHT OF REDUCING AGENT
It can be obtained by dividing the molecular weight by the number of electrons lost as represented by a chemical balanced equation
The change in O.N. of two atoms of carbon is +2. Hence
Equivalent weight of oxalic acid 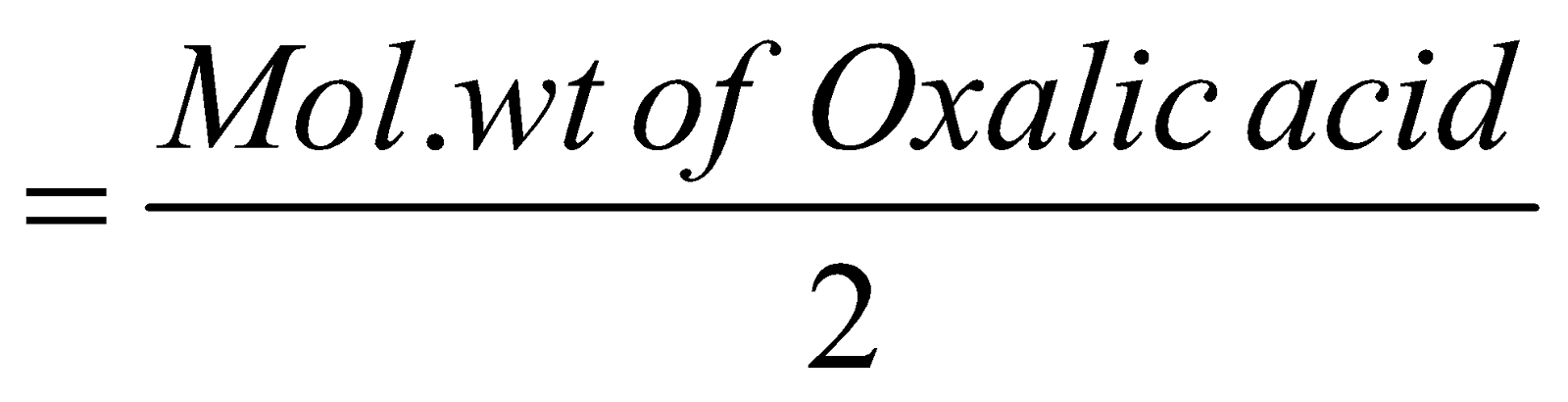
BALANCING OF CHEMICAL EQUATIONS
For balancing a chemical equation the two important methods are:-
OXIDATION NUMBER METHOD
The certain rules are as follows
- Assign oxidation number to the atoms showing a change in oxidation state.
- Balance the total number of atoms undergoing change in oxidation state.
- Balance the number of electrons gained and lost.
- Balance [O] on both sides by adding H2O.
- Balance H atoms by adding H+ ions
- If the reaction proceeds in basic solution add sufficient number of OH– ions on both sides
ION ELECTRON METHOD
The rules are as follows:
- Split up the reaction into two half reactions showing oxidation and reduction separately.
- Balance number of atoms undergoing the change of oxidation state.
- Balance O on both sides by adding H2O.
- Balance H atoms by adding H+ ions.
- Balance charge by adding required number of electrons
- Make the number of electrons equal in two half reactions by multiplying with suitable coefficient.
- Add the two half reactions
