About this unit
Adsorption-physisorption and chemisorption; factors affecting adsorption of gases on solids, catalysis homogeneous and heterogeneous, activity and selectivity: enzyme catalysis; colloidal state: distinction between true solutions, colloids and suspensions; lyophillic, lyophobic multimolecular and macromolecular colloids; properties of colloids; Tyndall effect, Brownian movement, electrophoresis, coagulation; emulsions- types of emulsions.
SURFACE CHEMISTRY
Thomas Graham divided the solids into two types.
CRYSTALLOIDS
The substances whose solution readily diffuse through a parchment membrane eg sugars, salts, acid and base.
COLLOIDS
The substance whose solution diffused at very slow rate through a parchment membrane eg glue, gelatin, gum arabic etc.
Later on it was found that crystalloids and colloids differ only in their size of particles. They can be interconverted, hence colloidal is a state.
On the basis of the size of particles we have

COLLOIDAL SYSTEM
Finely divided particles of any substance with diameters lying between 10Å – 2000Å dispersed in any medium constitute a colloidal system. It is heterogeneous system consisting of two phases.
DISPERSED PHASE
Discontinuous phase of the colloidal system is known as dispersed phase.
DISPERSION MEDIUM
The continuous phase of the colloidal system is known as dispersion medium.
TYPES OF COLLOIDAL SYSTEMS
Dispersed phase | Dispersion medium | Name | Examples |
Gas | Liquid | Foam | Shaving Cream, Soda Water froth |
Gas | Solid | Solid foam | Pumice stone, corck, foam rubber |
Liquid | Gas | Aerosol | Mist , cloud, fog |
Liquid | Liquid | Emulsion | Hair cream, milk |
Liquid | Solid | Gel | Cheese, butter |
Solid | Gas | Smoke | Occluded gases, dust soot in air |
Solid | Liquid | Sol | Colloidal gold |
Solid | Solid | Solid sol | Alloys |
HYDROSOLS OR AQUASOLS
For such sols the dispersion medium is water.
SOLVOSOLS
Depending upon the nature of the dispersion medium sols may be named as alcosols (in alcohol), benzosols (in benzene), aerosols (in air) and in general solvosols.
LYOPHILIC SOLS ( SOLVENT LOVING)
When dispersed phase has certain affinity for the dispersion medium it is known as lyophilic sol. e.g.- sols of gum, starch etc.
LYOPHOBIC SOLS (SOLVENT HATING)
When dispersed phase has no affinity for dispersion medium it is known as lyophobic sol. e.g.- sols of gold, iron and sulphur etc.
CHARACTERISTICS OF LYOPHILIC AND LYOPHOBIC SOLS
Property | Lyophilic Sol | Lyophobic Sol |
Preparation | Can be prepared easily | Prepared by special methods |
Charge | May or may not carry any charge | Always carry positive or negative charge. |
Solvation | Sol particles are heavily hydrated | Not solvated. |
Precipitation | High concentration of the electrolytes required to cause precipitation | Precipitated by low concentration of electrolytes. |
Viscosity | Very high | Same as that of dispersion medium. |
Surface tension | Low | Same as that of dispersion medium. |
Reversibility | Reversible | Irreversible |
Migration in electric field | May or may not migrate | Always migrate in electric field. |
Tyndall effect | No | Yes |
Stability | More stable | Less stable |
CLASSIFICATION ON THE BASIS OF SIZE OF SOL PARTICLES
MULTIMOLECULAR COLLOIDS
In this case the colloidal particles consists of aggregates of atoms or small molecules with diameters of less than 1nm e.g.- gold sol, sulphur sol.
MACROMOLECULAR COLLOIDS
In this case dispersed particles are of colloidal size and are called macromolecules, usually polymers such as starch, cellulose, proteins or synthetic polymers etc.
ASSOCIATED COLLOIDS
At low concentrations they behave as electrolytes and at higher concentrations as colloids. They are also known as micelles. Soaps and detergents are the examples. The micelles may contain about 100 molecules or more.
PREPARATION OF COLLOIDAL SOLUTIONS
Lyophobic sols and lyophilic sols are prepared by different methods.
Preparation of lyophobic sols – The methods employed are :
- Condensation methods
- Dispersion methods
CONDENSATION METHODS
In these methods small ions or molecules are induced to combine together to form aggregates of colloidal size and chemical or physical methods may be employed.
PHYSICAL METHOD
- Change of solvent – When ethanolic solution of sulphur is added to an excess of water, the sol of sulphur is obtained. This is physical method.
CHEMICAL METHODS
- Double decomposition – An arsenic sulphide (As2S3) sol is prepared by passing H2S through cold solution of As2O3 till yellow colour deepens to its maximum
- Oxidation – A sol of sulphur is prepared by passing H2S into solution of SO2
- Reduction – Sols of gold, platinum and silver are prepared by reduction of their compounds in water using, formaldehyde or hydrazine or tannic acid
- Hydrolysis – Ferric hydroxide sol is prepared by pouring dilute solution of ferric chloride into boiling water
Sols of chromium and aluminium can also be prepared by this method.
DISPERSION METHODS
Here lumps of the substances broken down to colloidal size in presence of dispersion medium and suitable stabilizer.
- Mechanical dispersion – In this method colloid mill, ball mill or ultrasonic disintegrator are used.
- Bredig’s arc method (electrical disintegration). An arc is struck between two metal electrodes of silver, gold or platinum held at the surface of cold water containing traces of alkali when sol of metal is obtained.
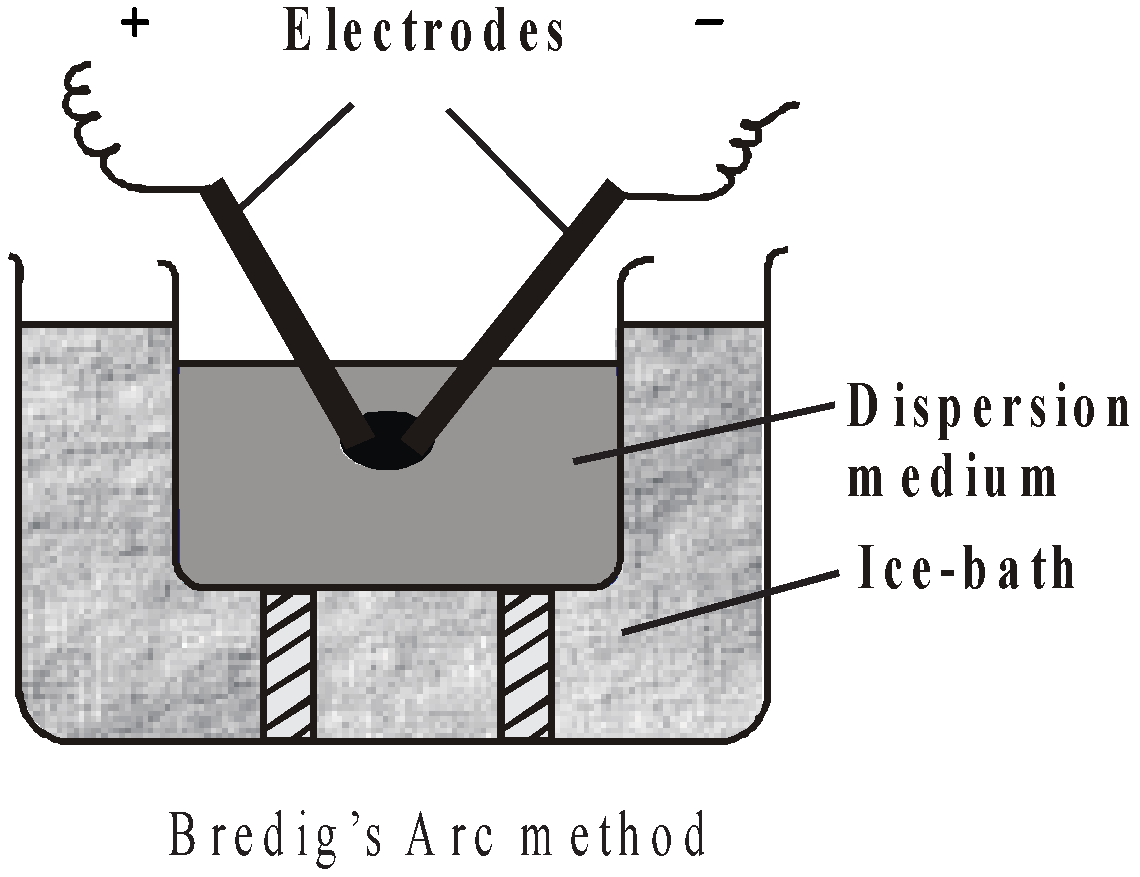
- By peptization – The dispersal of freshly precipitated substance into colloidal solution by the addition of some electrolyte having one ion common is known as peptization. The electrolyte used is called peptizing agent e.g.
- Ferric hydroxide Fe(OH)3 is peptized by ferric chloride giving sol. of [Fe(OH)3]Fe3+
- Silver chloride AgCl is peptized by HCl giving negative sol of [AgCl]Cl–
- Cadmium sulphide CdS is peptized by H2S giving negative sol of [CdS]S– –
- Cellulose nitrate is peptized by a mixture of ethanol and water. The product obtained is called “collodion”.
PURIFICATION OF COLLOIDAL SOLUTIONS
The removal of the particles of electrolytes from sols is known as purification of sols. It can be achieved by any of the following methods.
- Dialysis
- Electrodialysis
- Ultrafiltration
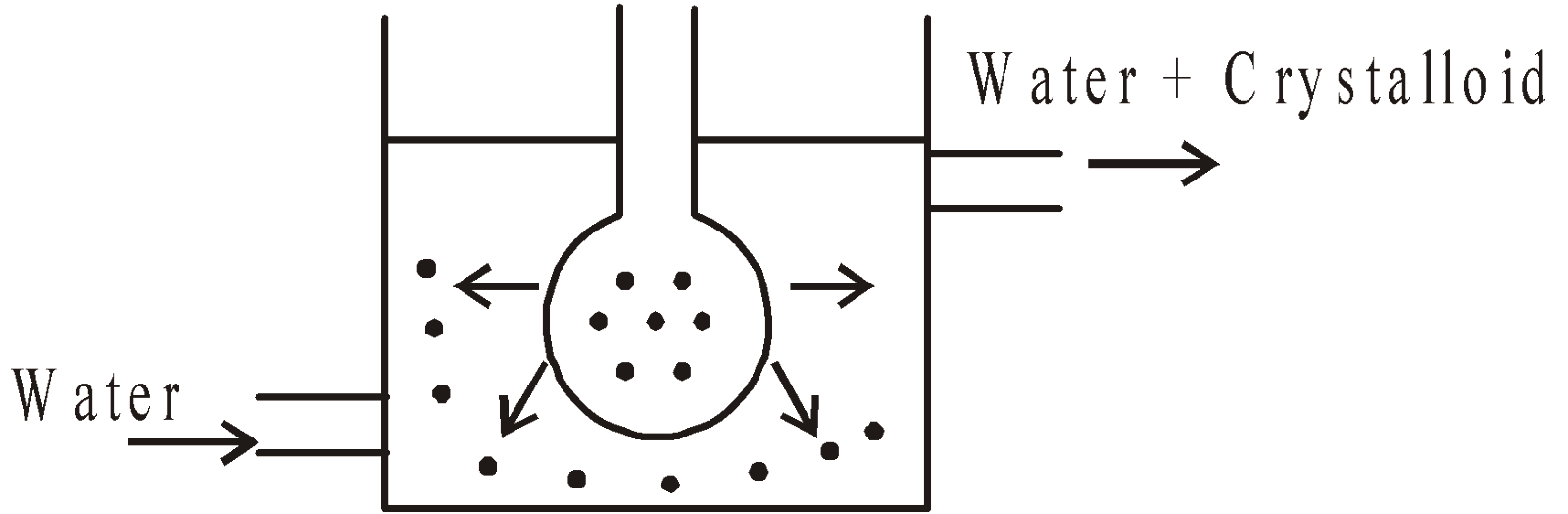
DIALYSIS
A mixture containing colloidal particles and particles of true solution is placed in a parchment bag. The bag is hanged in water vessel through which it is continuously flowing. The particles of true solution come out of membrane leaving behind colloidal solution.
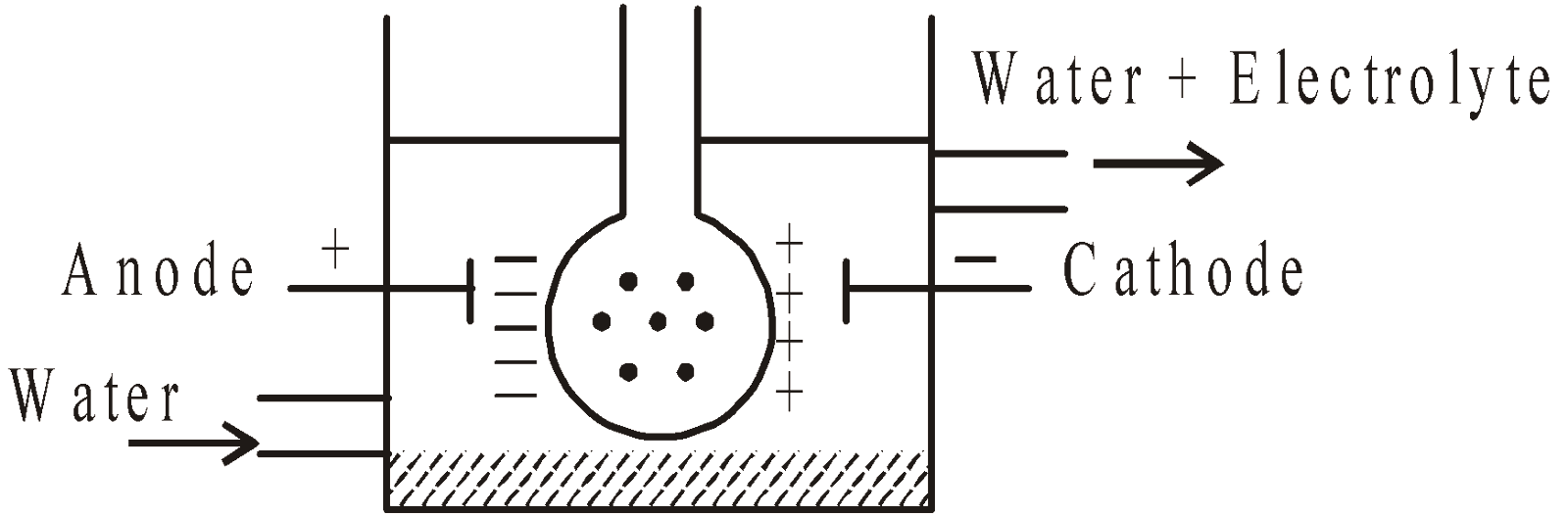
ELECTRODIALYSIS
The process is the same as above. The vessel is fitted with electrodes which make the removal of electrolytes quick. On applying the EMF ions of electrolyte migrate out to the oppositely charged electrodes while the colloidal particles are held back.
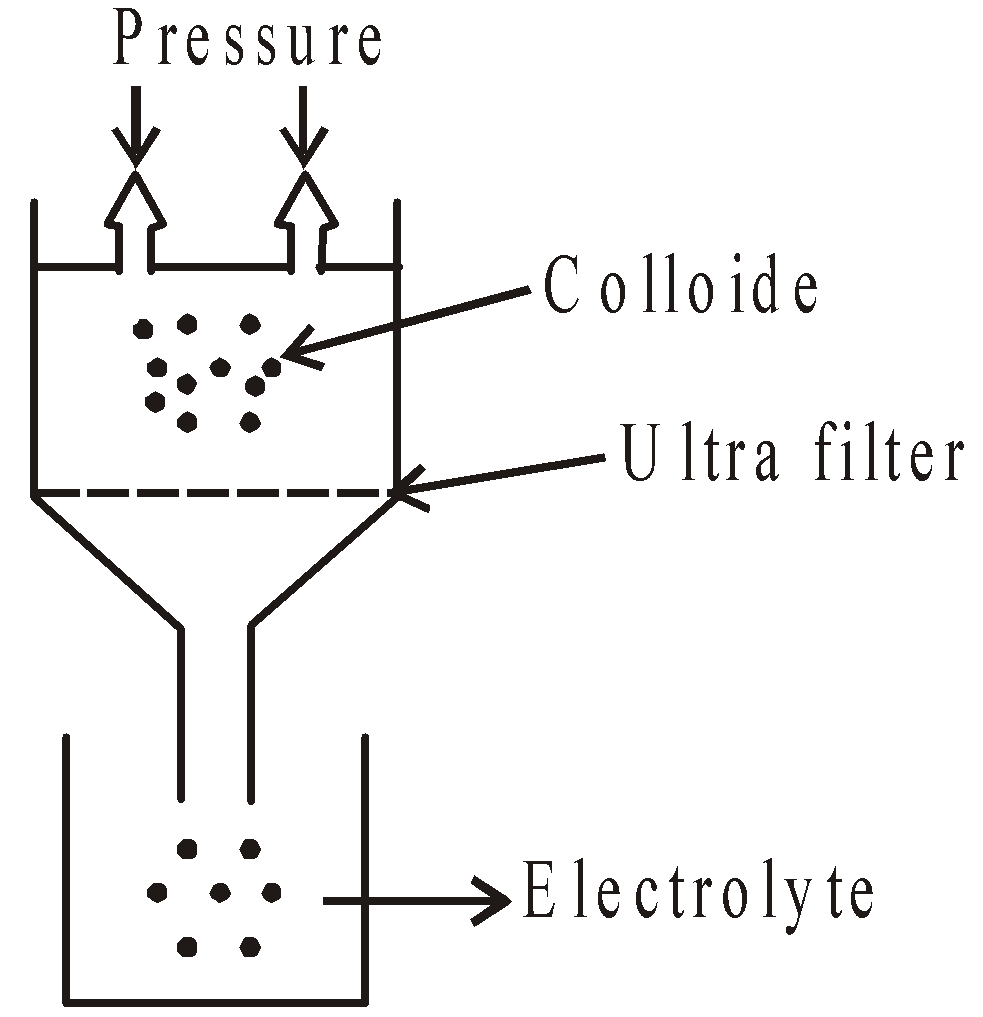
ULTRAFILTRATION
The pores of ordinary filter paper are made smaller in size by treating with solution of gelatin or collodion (4% solution of nitrocellulose in mixture of alcohol and ether) hardening by formaldehyde and drying. The colloidal particles left on the filter paper are then stirred with fresh dispersion medium to get pure colloidal solution.
PROPERTIES OF SOLS
The following are the properties given by sols
- Physical
- Colligative
- Optical
- Electrical
- Kinetic
PHYSICAL PROPERTIES
COLLIGATIVE PROPERTIES
Except osmotic pressure other colligative properties are little affected.
OPTICAL PROPERTIES
Tyndal effect – The phenomenon of the scattering of light by sol particles in all possible directions is called Tyndal effect.
The colloidal particles absorb the incident light energy, become self-luminious, and scatter the light in all directions. Blue colour of sky and sea water, visibility of projector path etc are due to this effect.
ELECTRICAL PROPERTIES
PRESENCE OF CHARGE
All the particles of dispersed phase carry a positive or negative charge and dispersion medium carry the opposite charge. Sol as a whole is neutral. The origin of the charge may be due to-
- Preferential adsorption of the common ion at the surface of sol particles
In Bredig’s Arc method the negative charge on the metal sol is due to adsorption of hydroxyl ions furnished by alkali.
- Ionisation of surface groups
- Charge on protein sols – Protein sols may be negative, positive or neutral depending upon the pH value of the solution
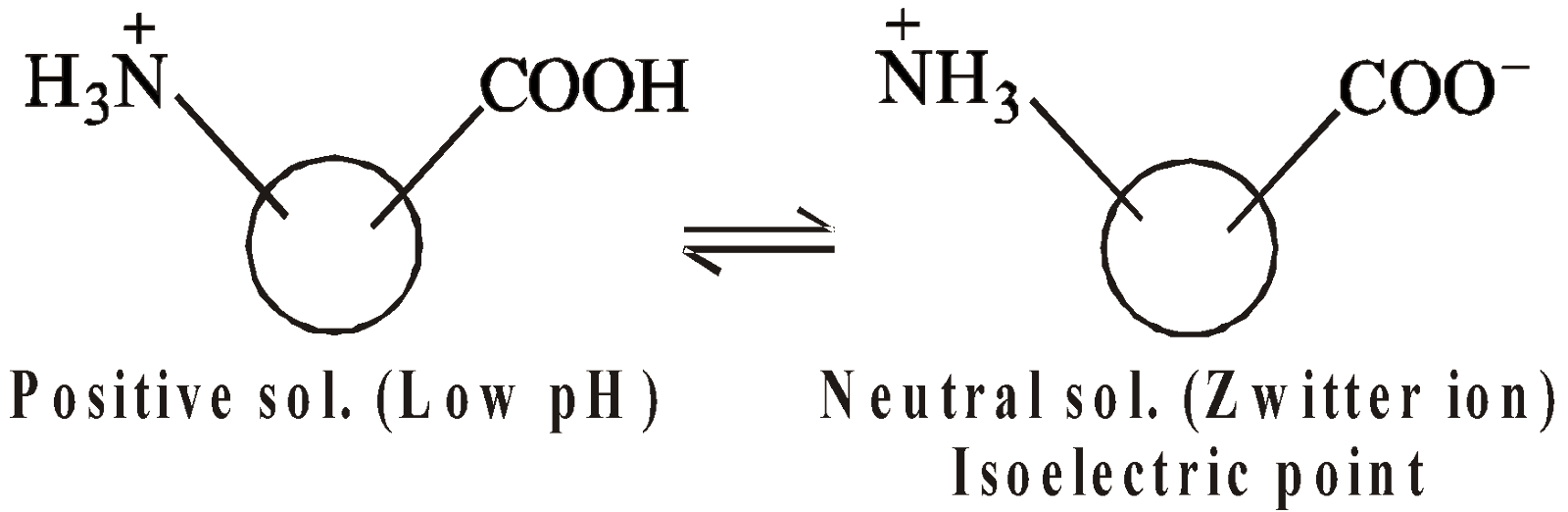
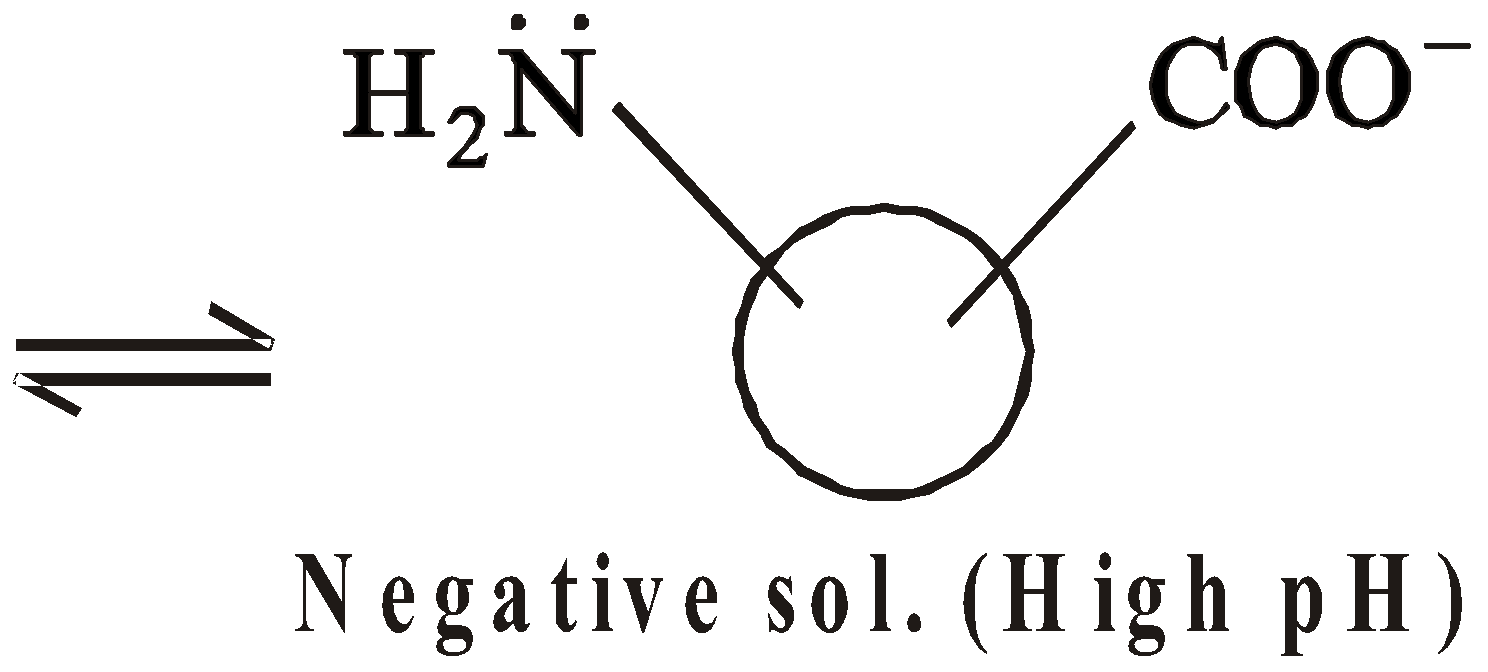
At low pH, concentration of H+ is large, COOH group is not ionised and NH2 group get protonated, hence positive sol.
At high pH, concentration of OH– is large, COOH group is ionised and NH2 group is not protonated, hence negative sol.
At intermediate pH, protein molecule exist as zwitterion, hence no charge.
- Charge on soaps and detergents – In soaps
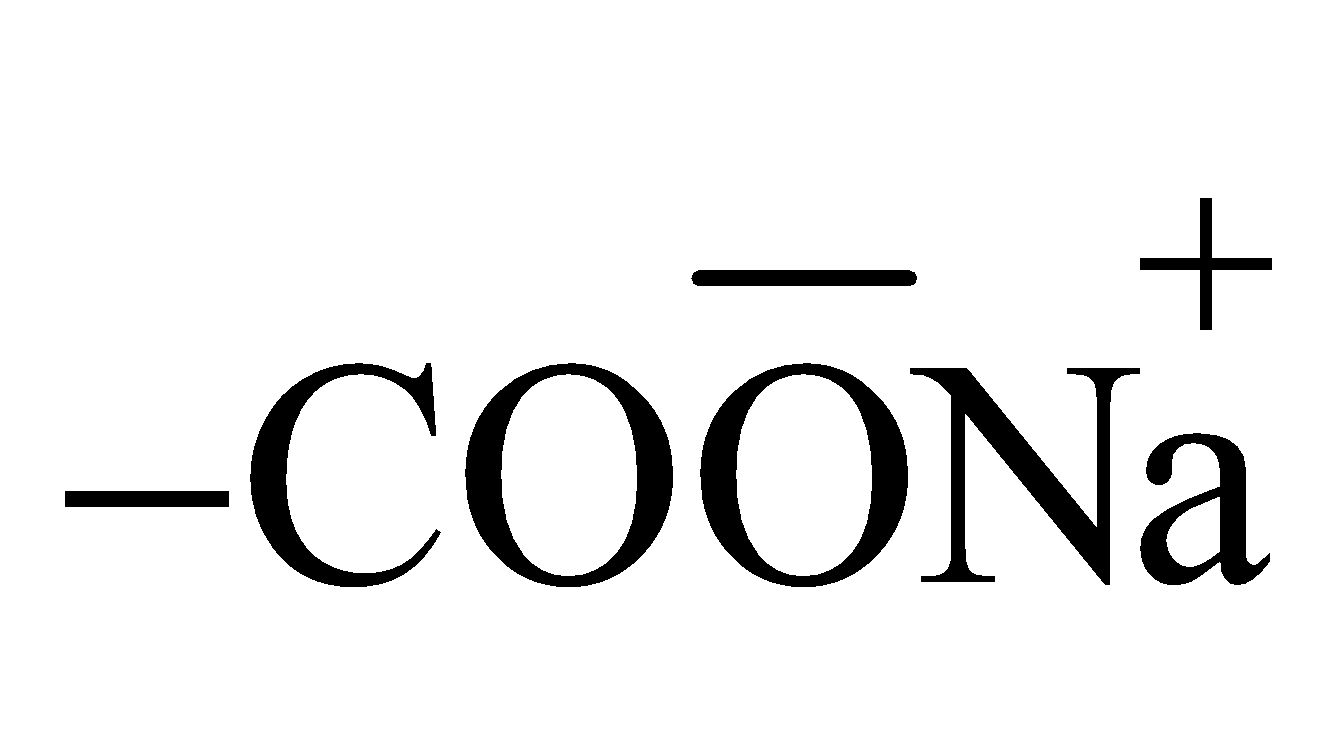 and in detergents
and in detergents 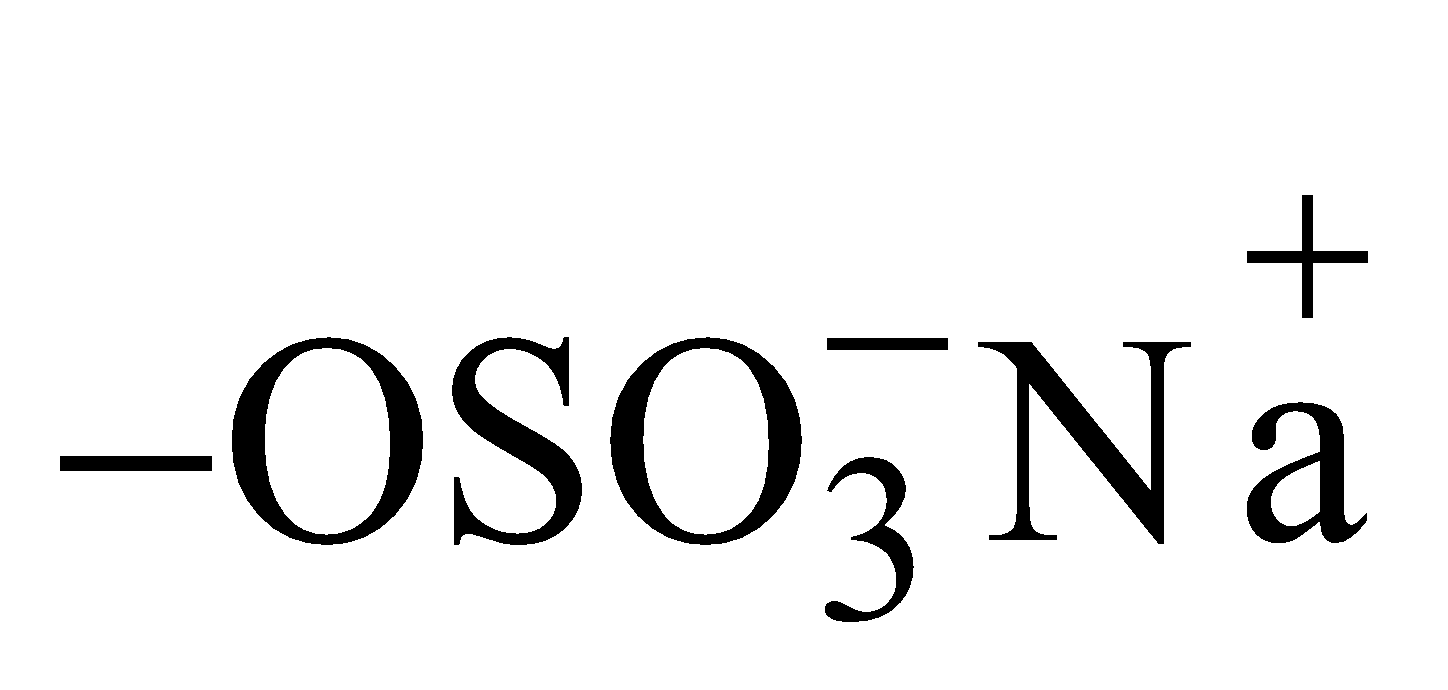 constitute the surface. Thus due to their ionisation soaps and detergents acquire negative charge.
constitute the surface. Thus due to their ionisation soaps and detergents acquire negative charge. - Charge on basic and acidic dyes – An acidic dye ionises to give H+ ions and acquire negative charge and a basic dye ionises to give OH– ions and acquire positive charge.
- Frictional electrification
It is due to rubbing of particles of dispersed phase with particles of dispersion medium.
HELMHOLTZ ELECTRICAL DOUBLE LAYER
Each sol particle is surrounded by either positive or negative ions in the form of fixed layer or compact layer. The second layer is diffuse or mobile layer consisting of ions of both the signs but net charge being equal and opposite to the fixed layer. This is known as Helmholtz electrical double layer.
Zeta potential – The potential difference developed between the two layers is known as zeta potential or electric kinetic potential.

ELECTROPHORESIS
The movement of sol particles under an applied electric potential is called electrophoresis or cataphoresis.
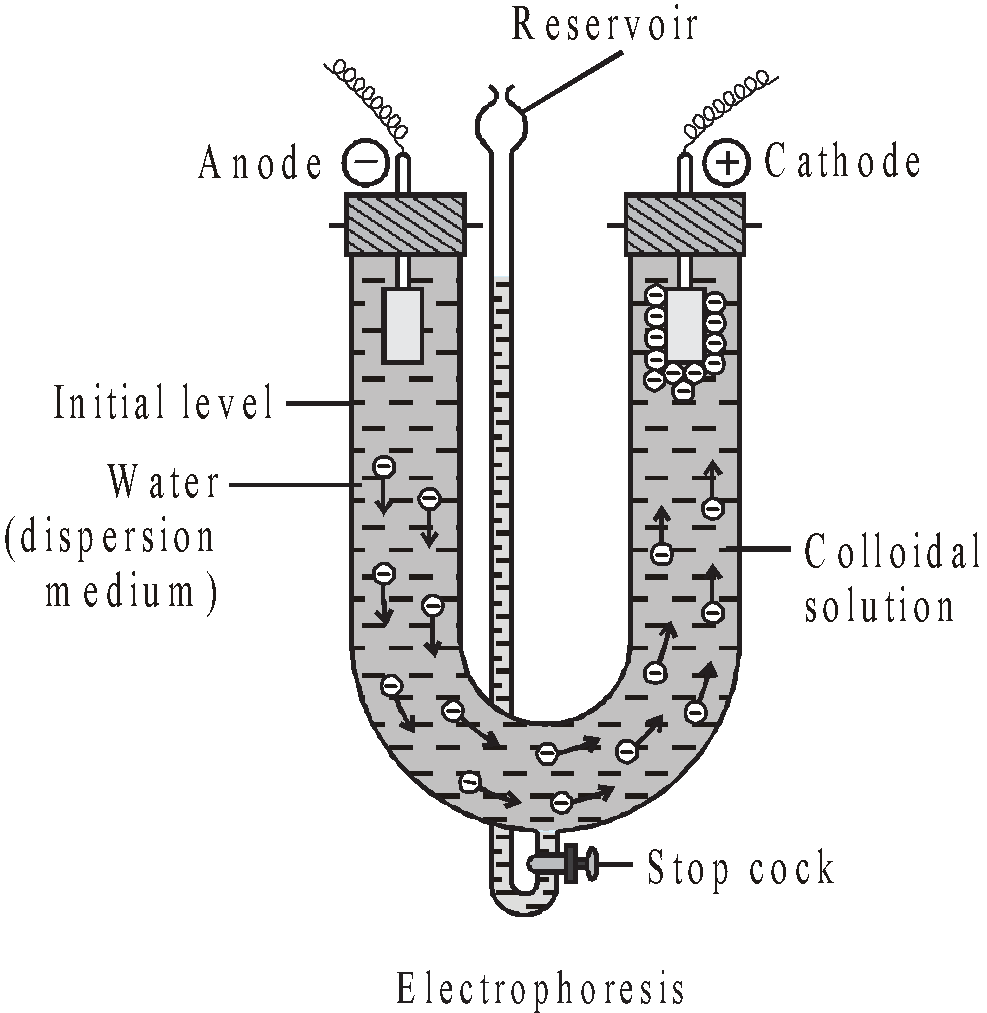
The phenomenon helps in-
- removing suspended impurities
- removing smoke from chimney gases
- electroplating of rubber
- painting metals with colloidal pigments
- coagulation of sols
- determination of charge
ELECTRO-OSMOSIS
The movement of the dispersion medium under the influence of applied electric potential is known as electro osmosis. The phenomenon helps in-
- Removing water from peat
- Dewatering of moist clay
- Drying dye pastes
KINETIC PROPERTIES
BROWNIAN MOVEMENT
The zigzag movement of colloidal particles in the dispersion medium is called Brownian movement. Brownian movement is due to the bombardment of the particles of the dispersion medium on the particles of dispersed phase.
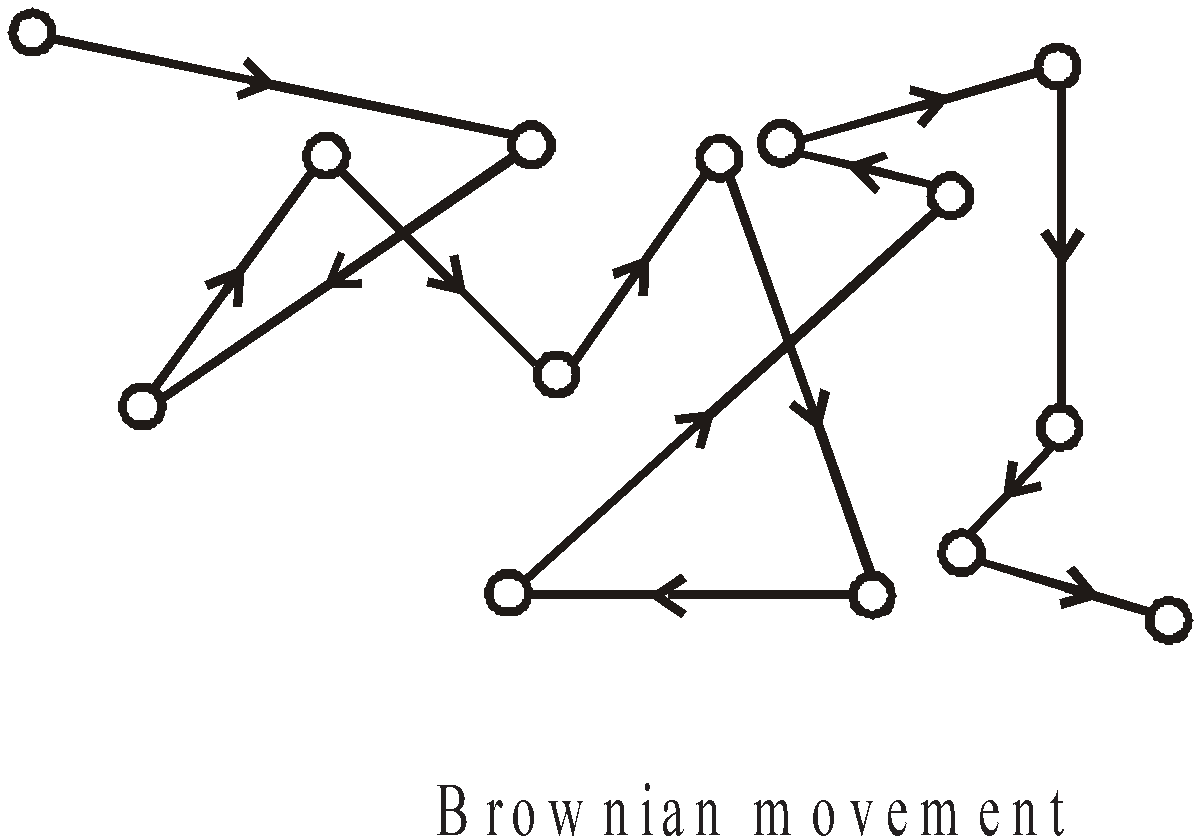
Factors affecting Brownian movement
- Size of particles – Small particles execute more rapid, brisk and vigorous motion than larger particles.
- Temperature – It is increases with temperature.
Importance
- Stability of colloidal solution
- Confirmation of kinetic theory
- Determination of avogadro number
COAGULATION, PRECIPITATION OR FLOCCULATION
The settling down of the discharged sol particles is called coagulation or precipitation of the sol. It can be achieved by
- Electrophoresis
- Addition of electrolytes
- By boiling
- Mixing two sols of opposite charge
- By persistent dialysis
HARDY SCHULZE RULE
It states that the precipitating effect of an ion increases with the valency of the ion.
For precipitating a negative sol the precipitating power of cations follow the order
For precipitating a positive sol the precipitating power of anions follow the order
Blood is positively charged sol (pH=7.4) and is coagulated by alum, Al2(SO4)3 or FeCl3. These salts lower the pH and denaturate globular proteins.
FLOCCULATION VALUE
The minimum concentration in millimoles per litre required to cause the precipitation of a sol in 2 hours. The smaller the flocculation value, the higher is the precipitating power of an ion.
PROTECTIVE ACTION OF LYOPHILIC SOLS
The lyophobic sols are less stable than lyophilic sols. The lyophilic sols are thus used to protect the lyophobic sols. This property of lyophilic sols is known as protective action of lyophilic sols. A little gelatin stabilises As2S3 sol.
GOLD NUMBER
The number of milligrams of a hydrophilic colloid that will just prevent the precipitation of 10 ml of a gold sol on the addition of 1 ml of 10% sodium chloride solution is known as gold number. The smaller the value of gold number of lyophilic sol, the greater is the protective action.
Gold number of some lyophilic sols.
Gelatin 0.005 – 0.01
Caseinate 0.01
Haemoglobin 0.03
Gum arabic 0.15
Sodium Oleate 0.4
Potato starch 25
STABILITY OF SOLS
It is mainly due to two factors
- Presence of like charge on sol particles. It prevents them from aggregating and settling down under the influence of gravity.
- Solvation of sol particles – In case of lyophilic sols a protective layer of solvent is formed around sol particles in addition to charge. Hence they are more stable than lyophobic sols.
EMULSIONS
Emulsion is a liquid liquid system. They are of two types
- Oil dispersed in water (O/W type) eg. milk and vanishing cream
- Water dispersed in oil (W/O type) eg. butter and cream.
The dispersed phase is always in the form of small droplets which increases the surface area and makes the emulsion unstable. Preparation of an emulsion is called Emulsification.
Emulsions are generally unstable and are stabilized by the use of emulsifying agents (emulsifiers). For O/W emulsions the emulsifiers are proteins, gums, natural and synthetic soaps. For W/O emulsions the emulsifiers are heavy metal salts of fatty acids, and long chain alcohols etc. The emulsifier concentrates at the interface, reduces surface tension on the side of one liquid.
PROPERTIES OF EMULSIONS
- They show Tyndal effect and Brownian movement.
- They can be demulsified (broken) by heating, adding electrolytes, freezing and centrifuging
- They can be diluted with dispersion medium.
GELS
The liquid solid system is called gel. They are of two types
- Elastic gels – They can be temporarily deformed by applying force eg. Gelatin, starch and soaps.
- Non elastic gels – They are rigid eg. silica gel.
PROPERTIES OF GELS
- Syneresis – Shrinkage of gels on standing by exudation of solvent is known as syneresis
- Thixotropy – Certain gels when shaken form a sol and on standing are converted into the form of gel They are known as thixotropic gels and sol – gel transformation is known as thixotropy.
- Swelling or imbibition of gels – The property of adsorbing definite amount of water and causing the volume of gel to increase is known as swelling or imbibition.
SOME USEFUL DEFINITIONS
- Streaming potential – When liquid is forced through a porous material or a capillary tube, a potential difference is set up between the two sides. This is called streaming potential.
- Sedimentation potential or dorn effect – When a particle is forced to move in a resting liquid a potential difference is set up which is known as sedimentation potential or dorn effect.
- Isoelectric point – The concentration at which the colloidal particles have no charge is known as the isoelectric point. The isoelectric point of gelatin is at pH 4.7.
- U-numbers – The number of mgs of a hydrophilic sol which is sufficient to produce the colour change from red to blue in 10 cc of gold sol.
- Congo-rubin number – The number of mgs of protective colloid which prevents the colour change in 100 ml of 0.01 % congo rubin dye to which 0.16 g equivalent of KCl is added
ASSOCIATED COLLOIDS
The molecules of certain substances are smaller than colloidal particles but aggregate spontaneously in a given solvent to form particles of colloidal size. The aggregates are known as micelles and the substances as associated colloids. The soaps and detergents are the examples.
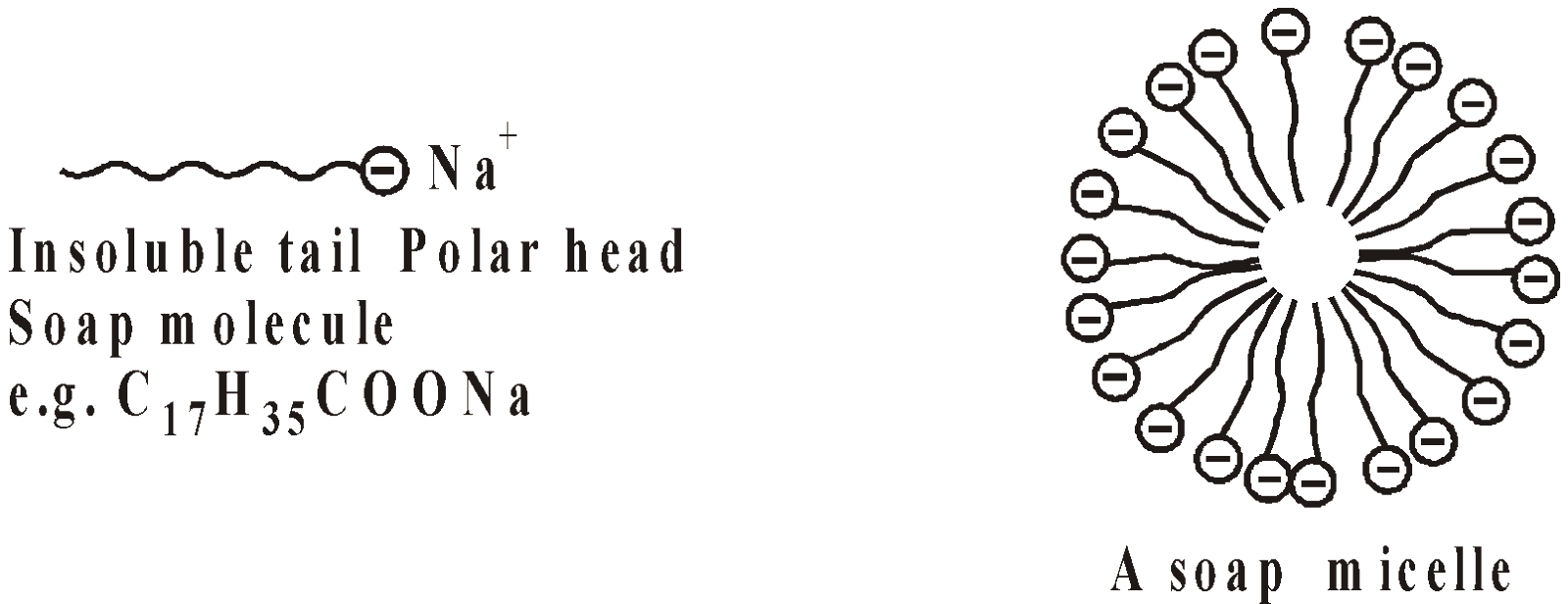
The tails being insoluble in water are directed towards the centre and soluble polar heads are on the surface in contact with water. The charge stabilises the micelle. The minimum concentration required for the micelle formation is called critical micellization concentration (CMC).
CLEANSING ACTION OF SOAP
It may be due the following two factors
- Solubilisation of grease into the micelle
- Emulsification of grease
When soap is applied on to a fabric, the tails of the soap anions are pegged into the grease stains and polar head form a charged layer around it. By mutual repulsion the grease droplets are suspended in water (formation of emulsion) and are washed away with water.
APPLICATIONS OF COLLOIDS
- Industrial applications
- Purification of drinking water – By adding alum, the suspended impurities are coagulated and removed.
- Electrical precipitation of smoke – Smoke carry negative charge and is removed by the principle of electrophoresis in cottrell’s precipitator.
- Sewage disposal – It is passed through big tanks fitted with electrodes. The colloidal particles lose their charge and settled down and removed.
- Electroplating of rubber – Latex is colloidal suspension of negatively charged rubber particles in water and can be deposited on metals by electrophoresis.
- Artificial rains – Clouds are aerosols (water dispersed in air). Aggregates of particles of water cause the rain fall which can be artificially achieved by throwing electrified sand or AgI on clouds and cause the artificial rain. AgI has similar crystal structure as that of ice.
- Leather tanning – Skin of animals is positively charged colloidal system. Extract of barks, wood leaves is negatively charged colloidal solution of tannin. When latter is applied on the surface of skin (leather) it becomes hard and does not putrefy.
- In warfare – Animal charcoal is used in gas masks to adsorb poisonous gases. Smoke screens are titanium oxide particles dispersed in air.
- In everyday life – Blood, milk, butter, cheese, clothes, shoes all are colloidal system.
- In medicines – Colloidal medicines are easily adsorbed and assimilated, hence are widely used. Colloidal antimony is effective medicine for kala azar. Blood is coagulated by FeCl3. Colloidal sols of Ag (argyrol and protargol) are used as eye lotions.
- In nature – Blue colour of sky, tails of comets are due to scattering of light.
- Formation of deltas in rivers is due to coagulation of negatively charged sand particles by Na+, Mg2+ etc present in sea water.
CATALYSIS
CATALYST
A substance which when added in very small amount to a chemical reaction change the speed of that reaction without itself undergoing any chemical change is known as catalyst and the phenomenon is known as catalysis.
TYPES OF CATALYSIS
The catalysis may be of two types
HOMOGENEOUS CATALYSIS
When catalyst, reactants and the products all are present in one phase, the process is known as homogeneous catalysis eg.
- Oxidation of sulphur dioxide to sulphur trioxide with nitric oxide
- Combination of hydrogen and chlorine in presence of steam
- Hydrolysis of an ester in presence of acid or alkali
HETEROGENEOUS CATALYSIS
When the catalyst is in a different phase than that of reactants and products the process is known as heterogeneous catalysis eg.
- Formation of ammonia by Haber’s process
- Formation of sulphur trioxide (contact process for sulphuric acid)
- In solution decomposition of hydrogen peroxide by MnO2 or Pt
CLASSIFICATION OF CATALYSTS
According to their mode of action they have been classified into four groups.
POSITIVE CATALYSTS
They always accelerate the speed of a chemical reaction eg.
- Decomposition of potassium chlorate in presence of manganese dioxide
- Manufacture of ammonia in presence of iron
NEGATIVE CATALYSTS
They always retard the speed of chemical reaction eg.
- Oxidation of chloroform in presence of ethanol
- Decomposition of hydrogen peroxide in presence of dilute acids or glycerol
AUTO CATALYST
In this case one of the products act as a catalyst eg.
Oxidation of oxalic acid by acidic permanganate
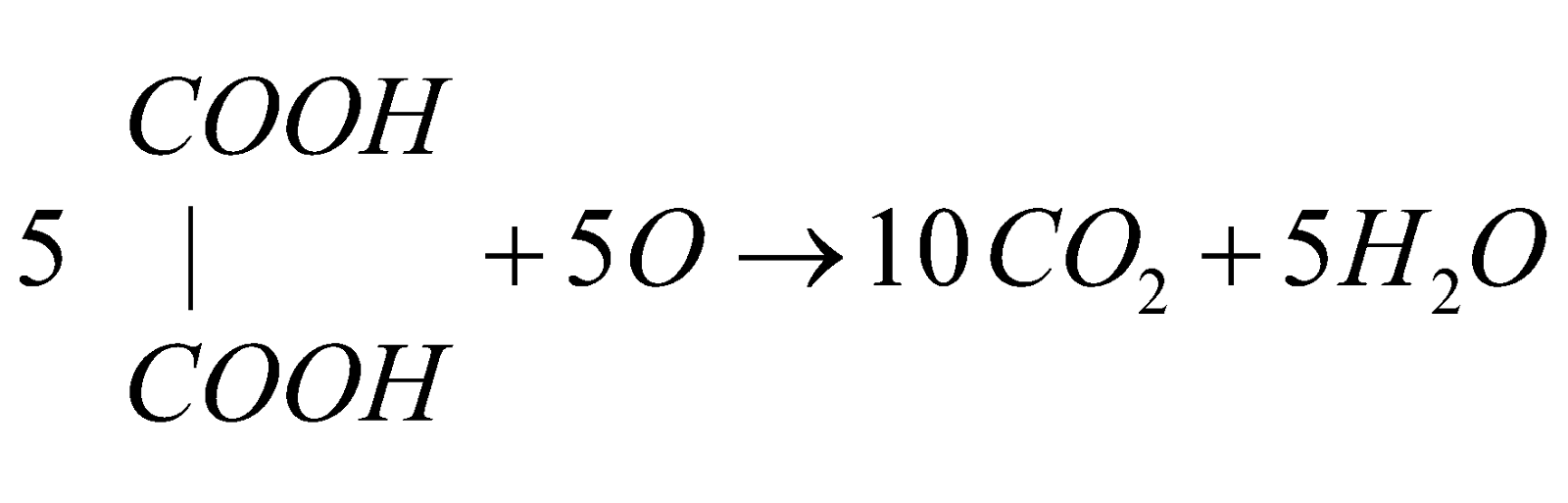
The speed of the reaction is slow in the beginning but increases rapidly due to the formation of Mn++ ions which act as auto catalyst.
INDUCED CATALYST
A substance which induces the similar reaction in another which is otherwise is not possible act an induced catalyst eg sodium sulphite solution oxidises in air to sodium sulphate but sodium arsenite is not oxidised by air. In presence of sodium sulphite it is also oxidised.
Therefore sodium sulphite is induced catalyst.
CATALYST PROMOTERS
The substances which increase the activity of catalyst are known as catalyst promoters eg Molybdenum (Mo) or aluminium oxide (Al2O3) increase the activity of iron catalyst in Haber’s process for the manufacture of ammonia
CATALYST POISONING
The substance which destroys the activity of a catalyst is called a poison and process is called catalytic poisoning eg.
CHARACTERISTICS OF CATALYST
The following are some important features for a catalyst or catalysis.
- Small quantity – Generally small amount of a catalyst is required but sometimes the large quantity of catalyst is effective eg anhydrous AlCl3 in Friedel Crafts reaction is needed upto 30%.
- Unchangeability – Although there is no change in the chemical composition and mass, there may be a change in physical state of catalyst eg granular MnO2 changes to fine powder in decomposition of potassium chlorate
- Specific action of catalyst – From the same reactants the different products are obtained with different catalysts eg.
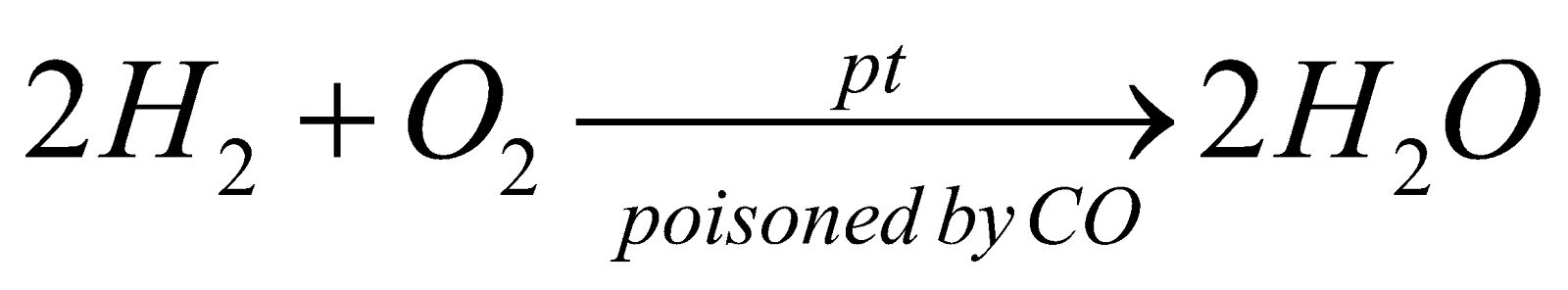 (Dehydration)
(Dehydration)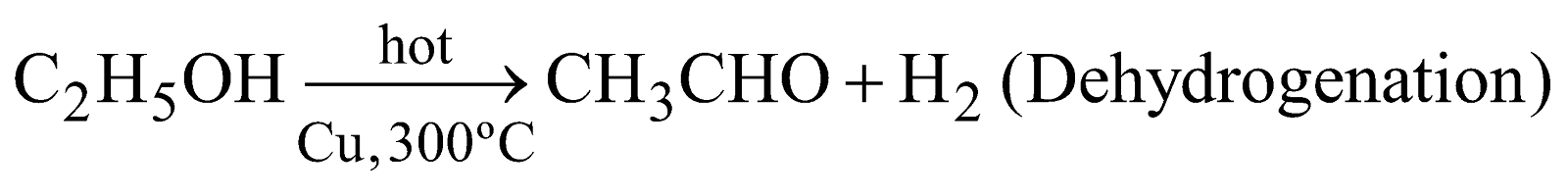
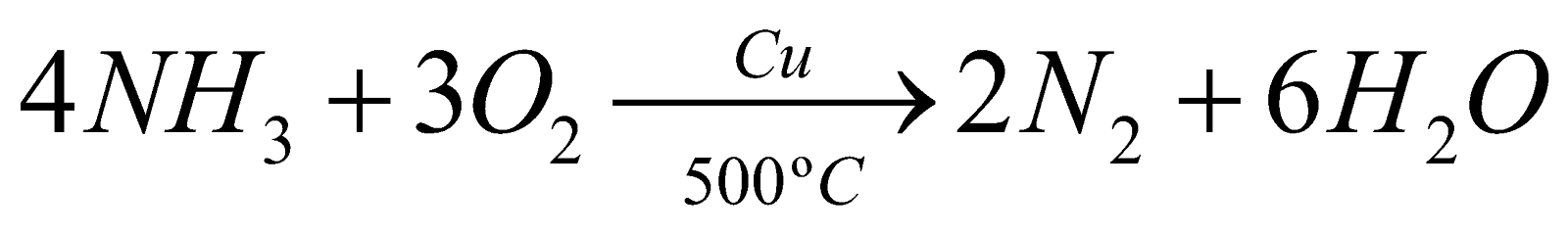
- Initiation of reaction – In general a catalyst does not start a chemical reaction, but there are reactions initiated by catalysts eg.
- Equilibrium – A catalyst does not affect the state of equilibrium, it simply helps to attain it quickly.
- Optimum temperature – The rate of reaction is maximum at a particular temperature which is fixed for every reaction.
- Physical state of catalyst – Finely divided catalyst gives better yield in a short period than lumps.
- Activation energy – A positive catalyst lowers the activation energy of a chemical reaction.
THEORY OF CATALYSIS
There are following main theories
- Intermediate theory
- Adsorption theory
- Modern theory
INTERMEDIATE THEORY
A catalyst combines with one of the reactants and forms the unstable intermediate compound which in turn combines with another reactant and generates the catalyst eg. consider the oxidation of SO2 by O2 in presence of NO as catalyst
Intermediate mechanism
Catalytic oxidation of hydrochloric acid by atmospheric oxygen in presence of cupric chloride (Deacon’s process)
Mechanism – (2CuCl2Cu2Cl2 + Cl2) × 2
Limitations of intermediate theory
- Action of promoters and poisons
- Participation of catalyst in chemical reaction
ADSORPTION THEORY
By the adsorption of the reactants at the surface of a catalyst, their concentrations are increased. The rate being directly proportional to concentration, is increased.
MODERN THEORY
It is the combination of intermediate theory and adsorption theory. At the surface of a catalyst there are residual or free valencies and reactant molecules are retained at the surface in distorted or strained positions. These molecules then react vigorously to give products and make space for other reactant molecules. The greater the surface area of the catalyst, the more is its activity. Further catalysts with rough surface, having greater number of corners, peaks, cracks have more active centres and more effective e.g. finely divided nickel or platinum
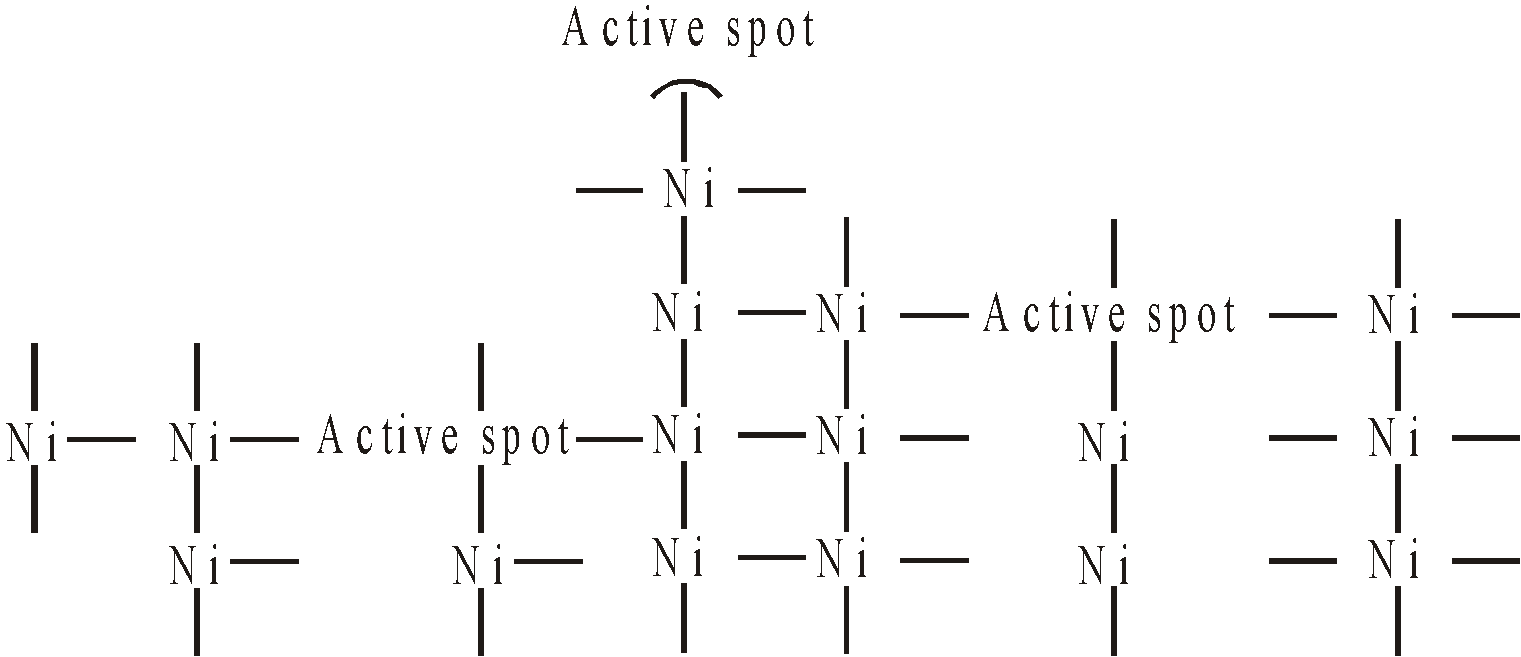
ACTION OF PROMOTERS
It can be assumed that a loose compound is formed between the catalyst and the promoter which possesses an increased capacity of adsorption.
ACTION OF CATALYTIC POISON
It may be due to the preferential adsorption of poison on the active spots of the catalyst thus reducing the number of free active spots for reacting molecules.
ACID BASE CATALYSIS
A large number of reactions catalysed by acids or bases are known eg.
- Hydrolysis of an ester in presence of an acid
- Cyanides are hydrolysed by acids or alkalis
Such reactions are called acid base catalysis and H+ or OH– ions act as catalyst.
MODERN CONCEPT OF ACID BASE CATALYSIS
According to this concept
- A reaction which is catalysed by an acid is also catalysed by all substances having tendency to lose protons (H+)
- A reaction which is catalysed by a base is also catalysed by all substances having a tendency to gain protons.
ENZYMES OR BIOLOGICAL CATALYSTS
Many complex chemical reactions like oxidation, reduction or hydrolysis take place in presence of enzymes which are highly complex, nitrogenous non living organic substances. They are highly specific in nature eg.
- Starch is catalysed by diastase maltose is formed and then maltose by maltase
- Urea is converted into ammonium carbonate by Urease
MECHANISM OF ENZYME ACTION
Enzyme can increase the rate of biochemical reactions by 106 to 1012 times. They are highly selective and specific and act on certain molecules called substrates and leave the rest of the system unaffected (LOCK AND KEY THEORY). Michaelis and Menten proposed the following mechanism for enzyme catalysis
CHARACTERISTICS OF ENZYME CATALYSIS
- Enzymes are most efficient catalysts.
- Enzymes are very selective and specific.
- Enzymes require optimum condition of temperature and pH of the medium.
- Enzymes are easily poisoned by addition of other substances particularly heavy metal ions (Hg2+ or Ag+)
- Activity of enzymes is increased by activators (certain metal ions Co2+, Mn2+, Na+, Cu++ etc) or coenzymes.
ADSOPRTION
Adsorption is the phenomenon of higher concentration of molecular species at the surface than in the bulk of a solid or liquid. Forces involved are Van der Waal’s forces.
The unbalanced or residual forces acting along the surface of a solid or liquid attract the molecules of other species when brought into their contact. These molecules remain at surface and do not go into bulk, hence making the concentration more at surface.
ADSORBATE
The substance that concentrates at the surface is called adsorbate.
ADSORBENT
Adsorbent is the solid or liquid at whose surface the concentration occurs.
ABSORPTION
Absorption is the phenomenon of uniform distribution of a substance throughout the body of a solid or liquid. For eg water vapour is absorbed by a sponge or anhydrous CaCl2 while it is adsorbed by silicagel. The pressure of gases (SO2, Cl2, NH3) pressure decreases when treated with powdered charcoal in a closed vessel due to adsorption of gas on charcoal surface.
SORPTION
Adsorption and absorption go hand in hand thus making it tough to distinguish between them. The general term sorption was introduced by McBain to include both processes.
CHARACTERISTICS OF ADSORPTION
- It is a surface phenomenon.
- It is a spontaneous process.
- Adsorption is accompanied by evolution of heat as the residual forces acting along the surface of adsorbent decrease. Heat evolved on adsorption of 1 mole of a gas is molar heat of adsorption.
- Adsorption is accompanied by decrease in enthalpy. i.e. H = -ve
- Adsorption is accompanied by decrease in entropy i.e. T
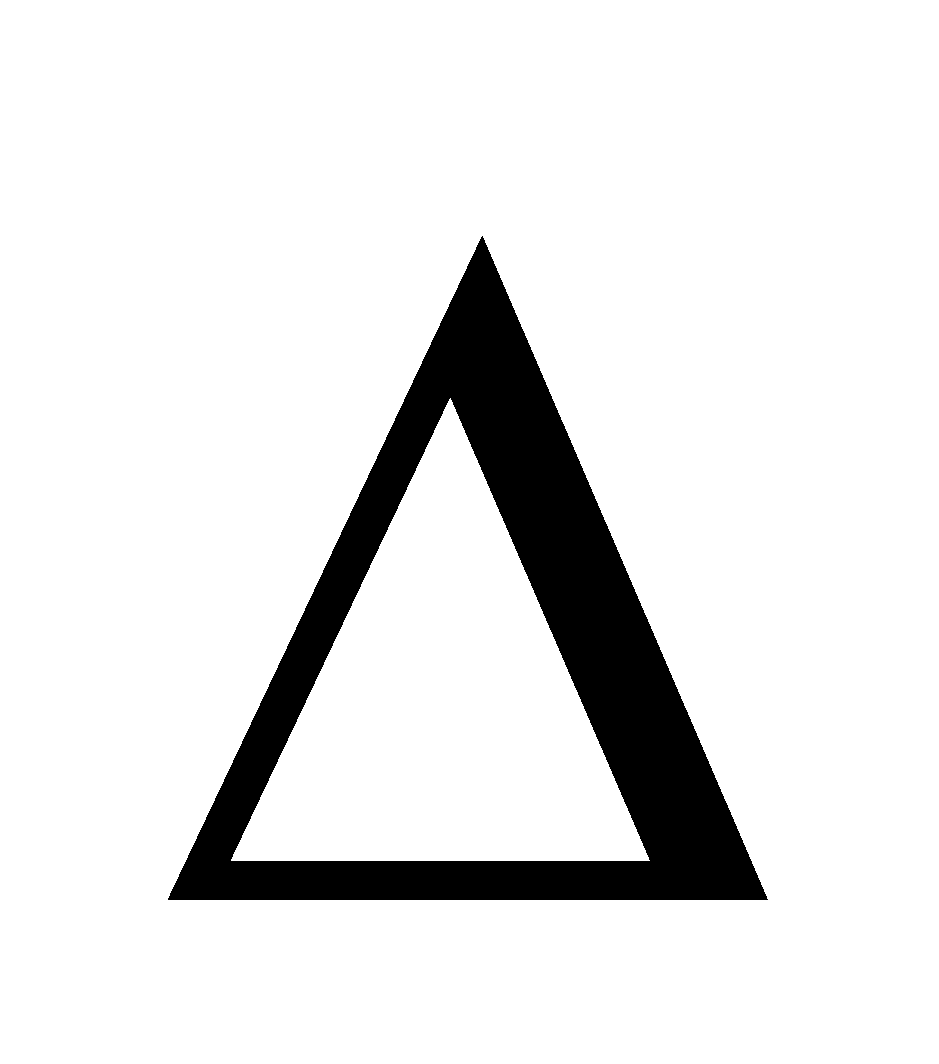 G is negative.
G is negative.
Also,  G =
G = 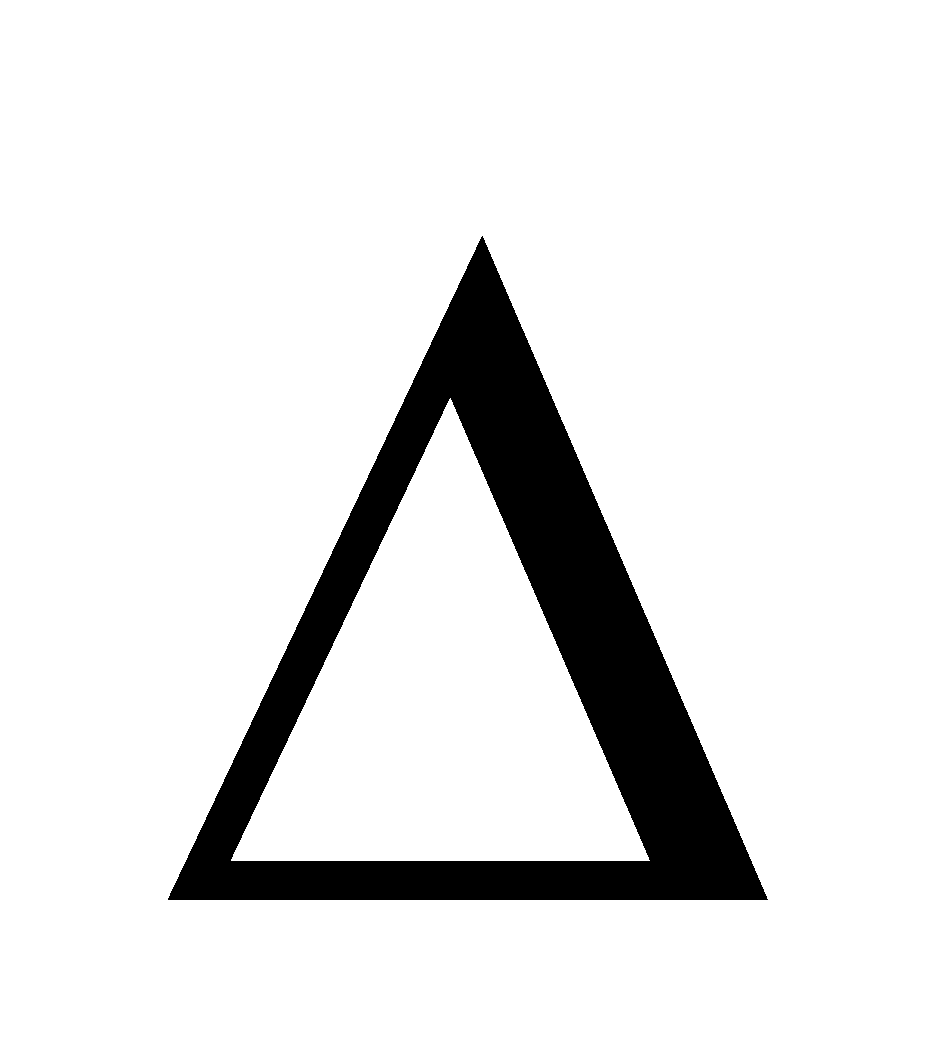 H – T
H – T S. As H is negative and so is
S. As H is negative and so is S,
S,
 G is negative. But experimentally
G is negative. But experimentally H becomes less negative as the adsorption progresses and ultimately becomes zero as the equilibrium is reached.
H becomes less negative as the adsorption progresses and ultimately becomes zero as the equilibrium is reached.
Adsorption of gases at the surface of metals like Ni, Pt, Pd etc is called Occlusion.
PHYSICAL ADSORPTION
It is also called Van der Waal’s adsorption which involves weak forces, physical in nature, with small heat of adsorption. For example adsorption of H2 or O2 on charcoal.
CHEMICAL ADSORPTION
Forces of attraction between adsorbate and adsorbent being of the same strength as chemical bonds, given the name chemisorption. It may involve covalent or ionic bonds. e.g. H2 is chemisorbed on Ni. Hydrogen is first adsorbed by Van der Waal’s forces and then dissociates. Hence hydrogen atoms are chemisorbed on Ni.
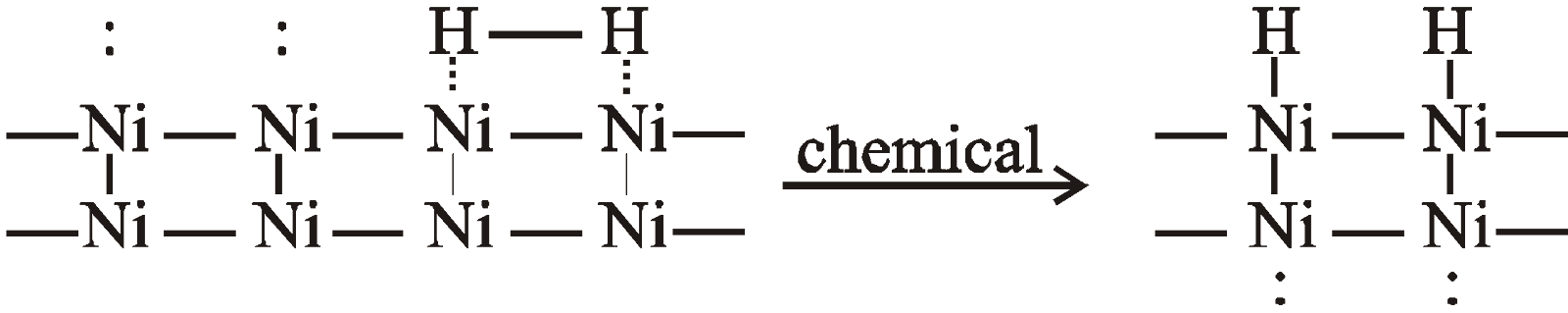
Often adsorption involves combination of two types of adsorption
FACTORS AFFECTING ADSORPTION OF GASES BY SOLIDS
- Nature of gas – Easily liquefiable gases get adsorbed readily. Thus 1g of activated charcoal adsorbs 380 ml of SO2 (critical temp. 157ºC). 16 ml. of methane (critical temp. = –83º ), 4.5 ml of H2 (critical temp. –20º C)
But chemisorption is more specific and occurs if there is a possibility of reaction between gas adsorbed and the solid.
- Nature of adsorbent – Different solids adsorb same gas at given temperature and pressure to different extent. Adsorbing power depends upon chemical nature, surface area cleanliness of surface, distribution of capillaries or forces acting on surface. Finely divided metals (Ni, Pt) and porous substances (charcoal, silica gel) are best solid adsorbents as they provide large surface area.
- Effect of pressure – Extent of adsorption increases with pressure as adsorption is a reversible process and is accompanied by decrease of pressure x/m is the extent of adsorption where m is the mass of adsorbent and x that of adsorbate at equilibrium.
The relation 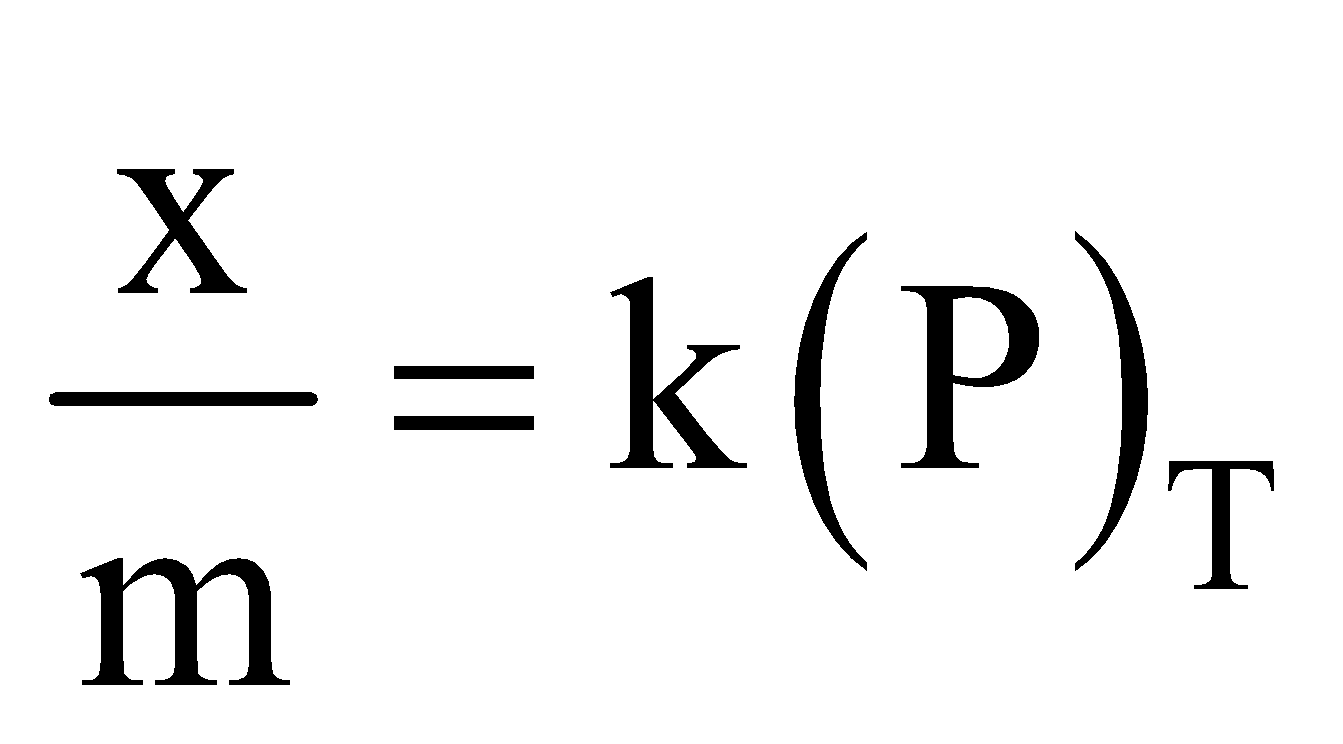 solid, gas is called adsorption isotherm. Such isotherms are obtained when adsorbate forms a unimolecular layer on surface of adsorbent.
solid, gas is called adsorption isotherm. Such isotherms are obtained when adsorbate forms a unimolecular layer on surface of adsorbent.
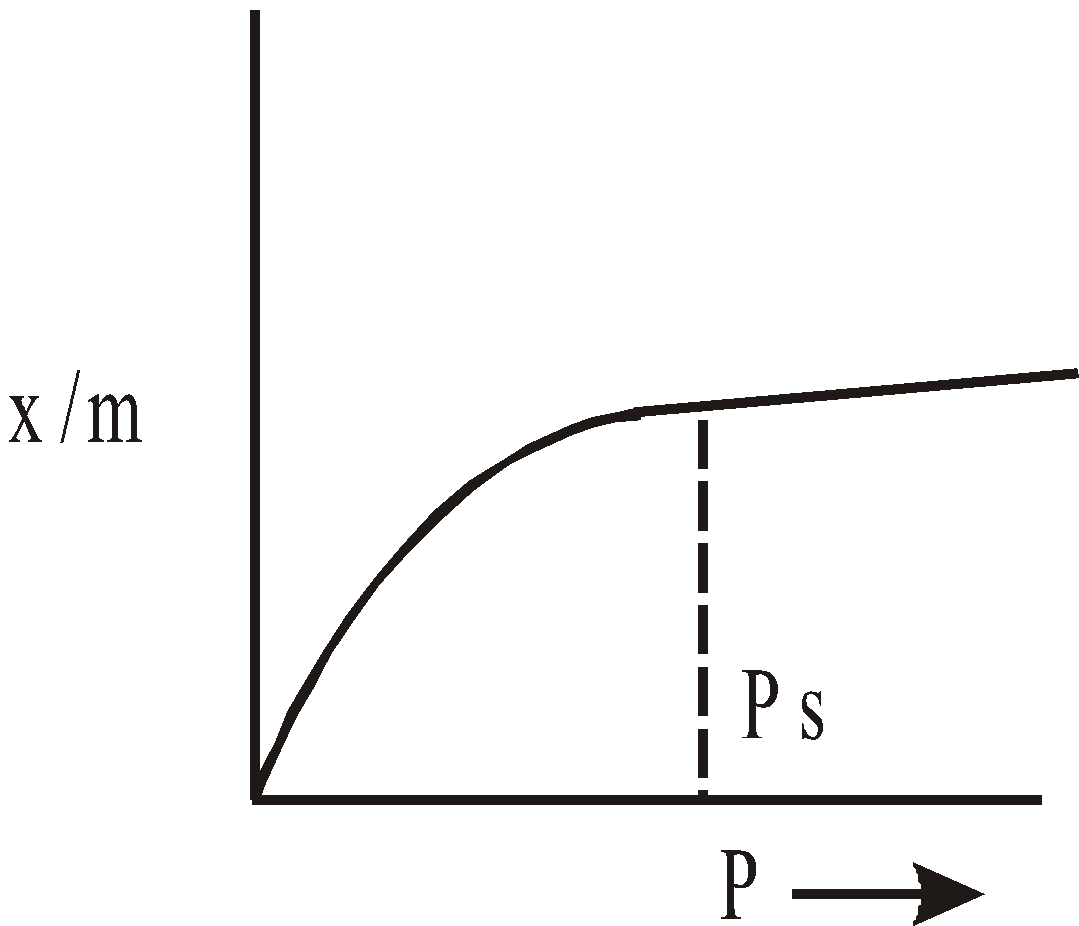
At low pressure, x/m p1 or x/m = kp1 where
k = constant
k = constant
At high pressure, beyond saturation x/m is independent of p and x/m 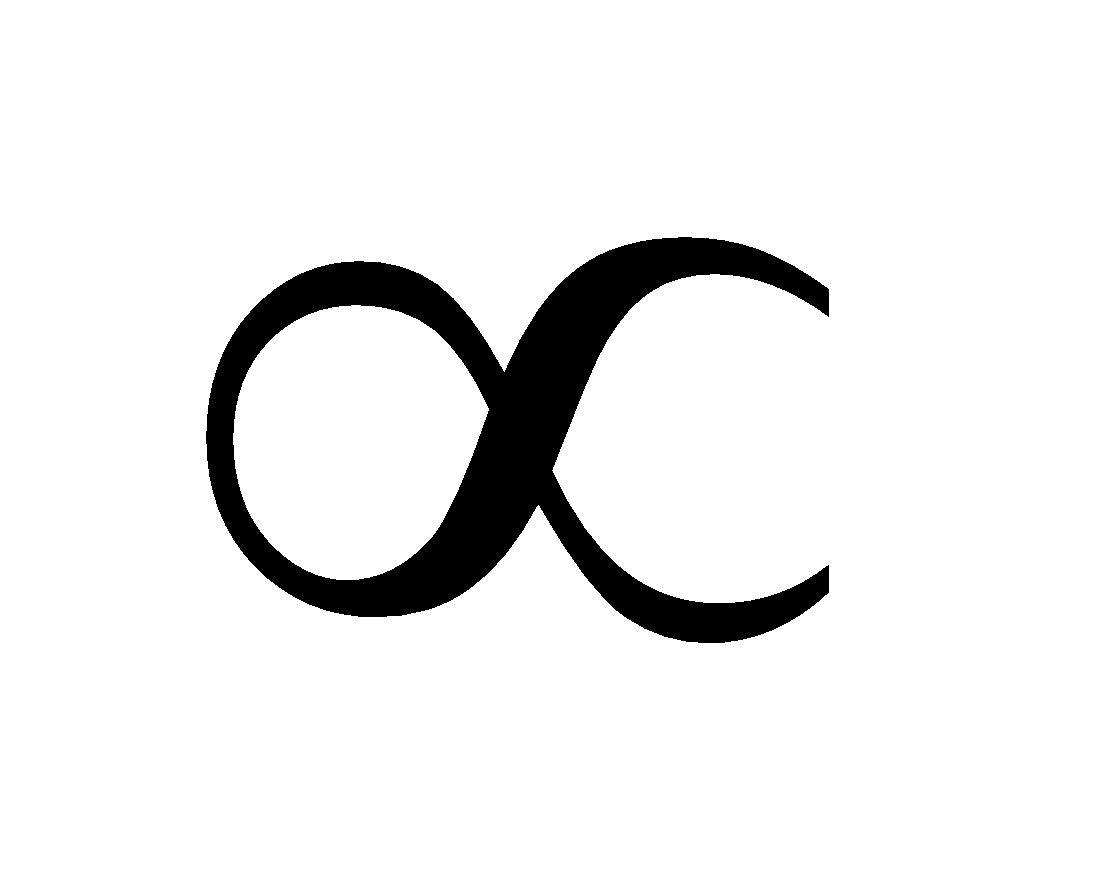 pº.
pº.
At intermediate pressures, x/m 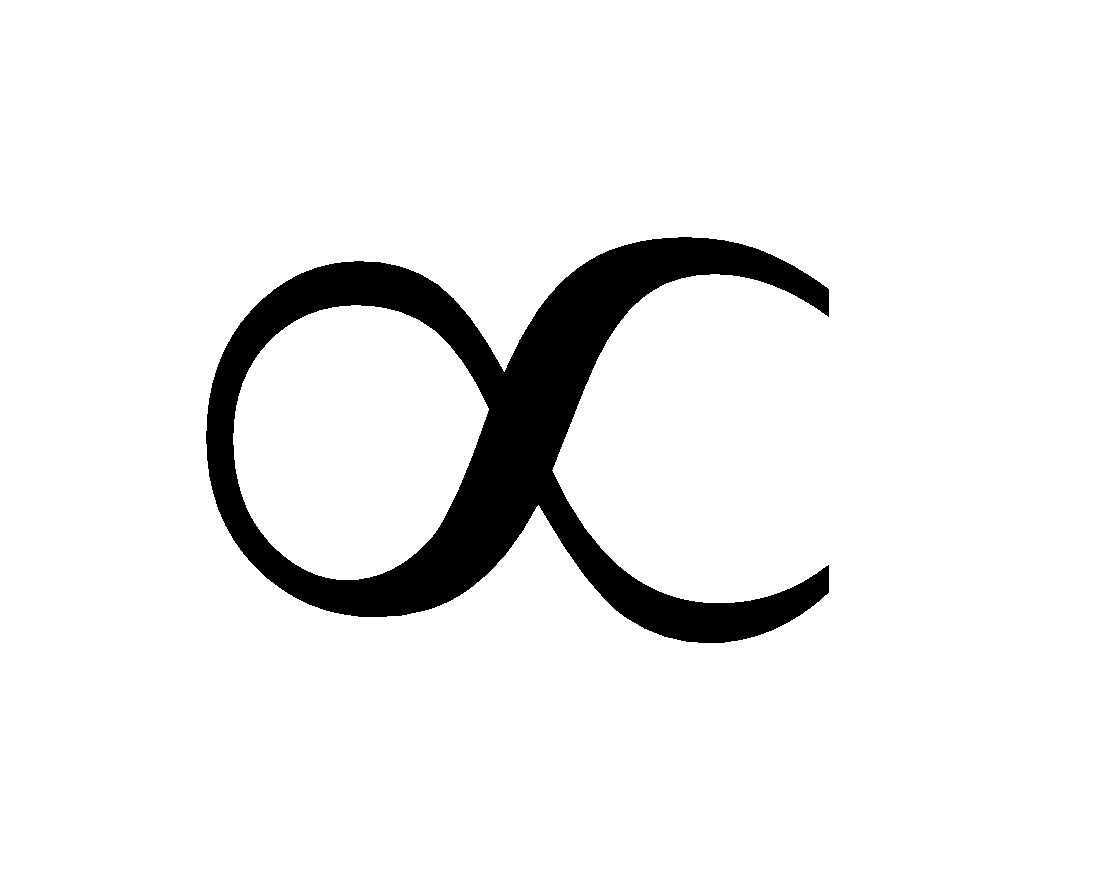 p1/n where n > 1
p1/n where n > 1
or log 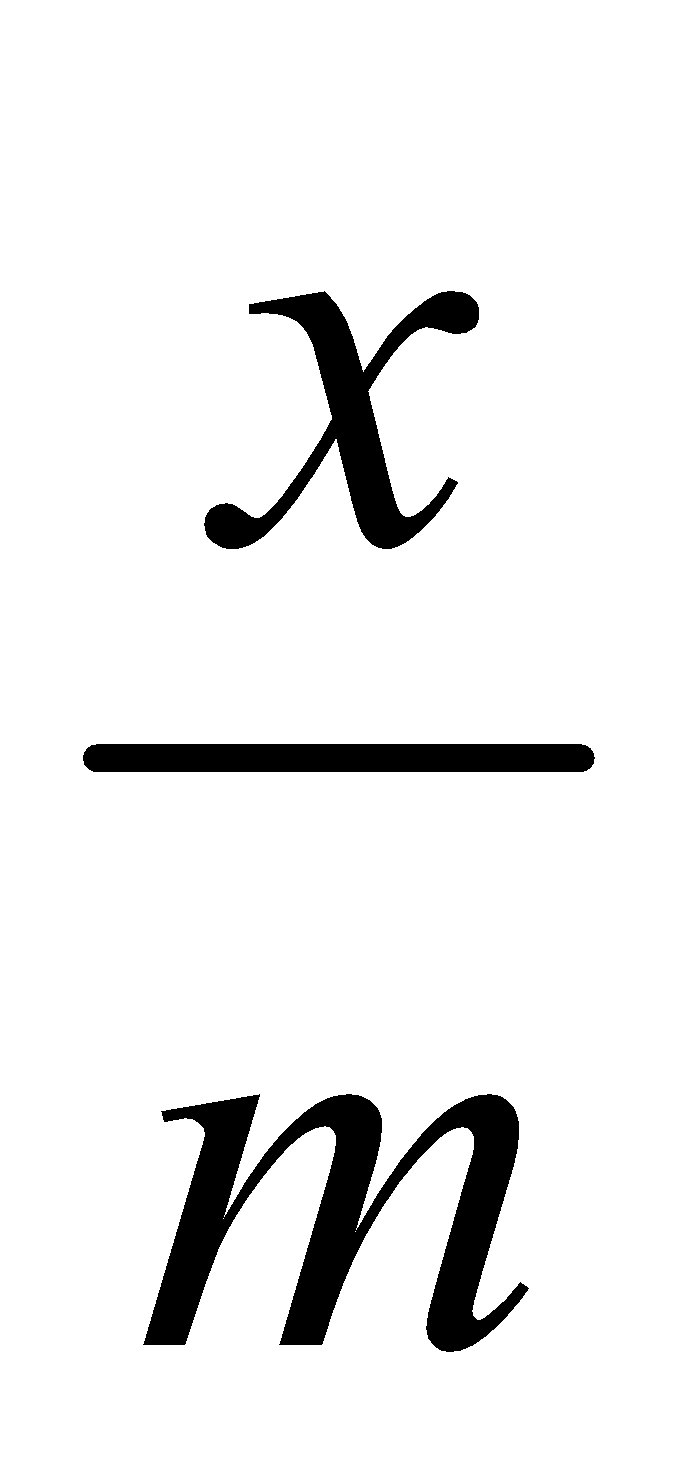 = log k +
= log k + 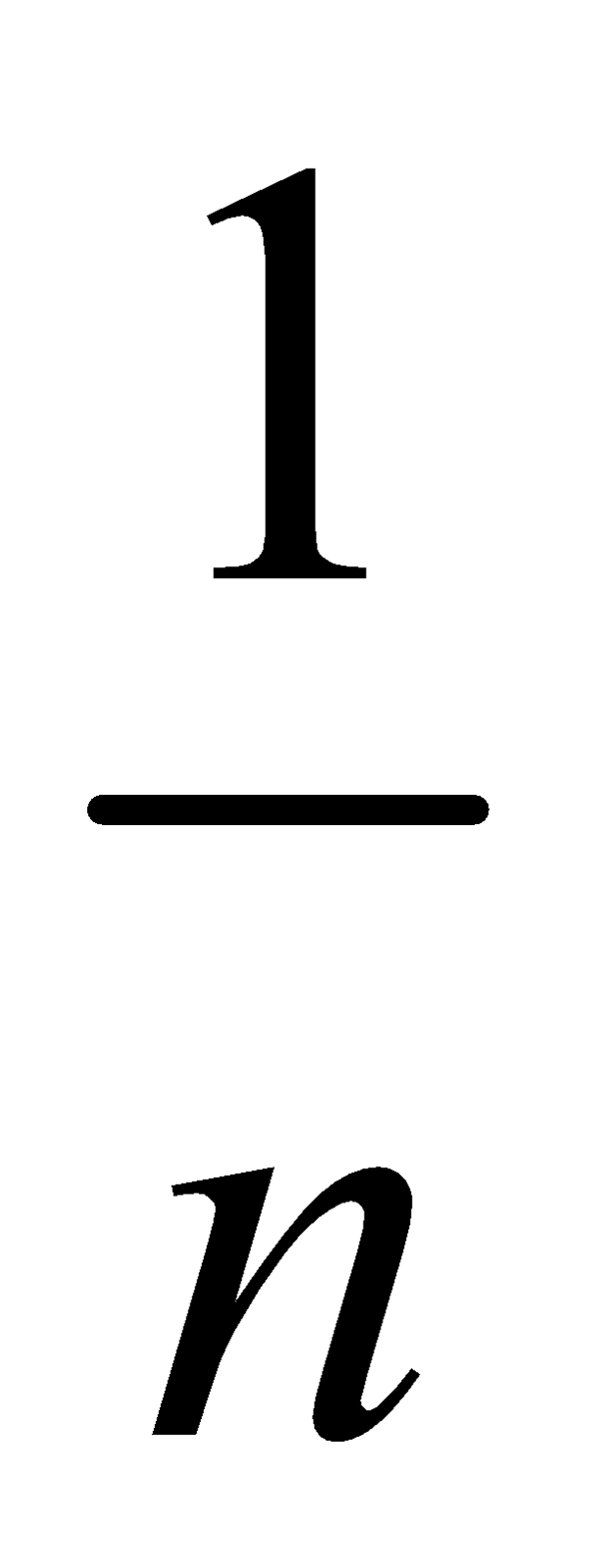 log p where k = constant.
log p where k = constant.
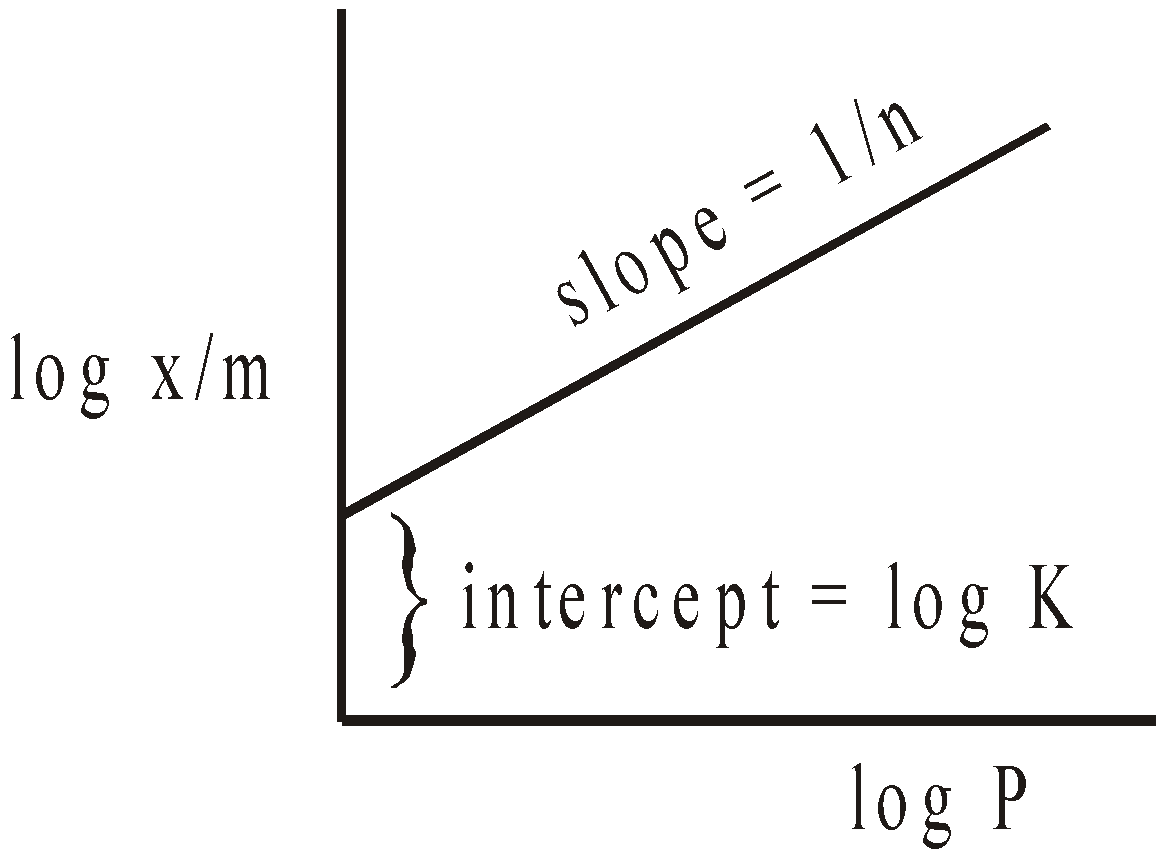
Above equation is called FREUNDLICH ISOTHERM
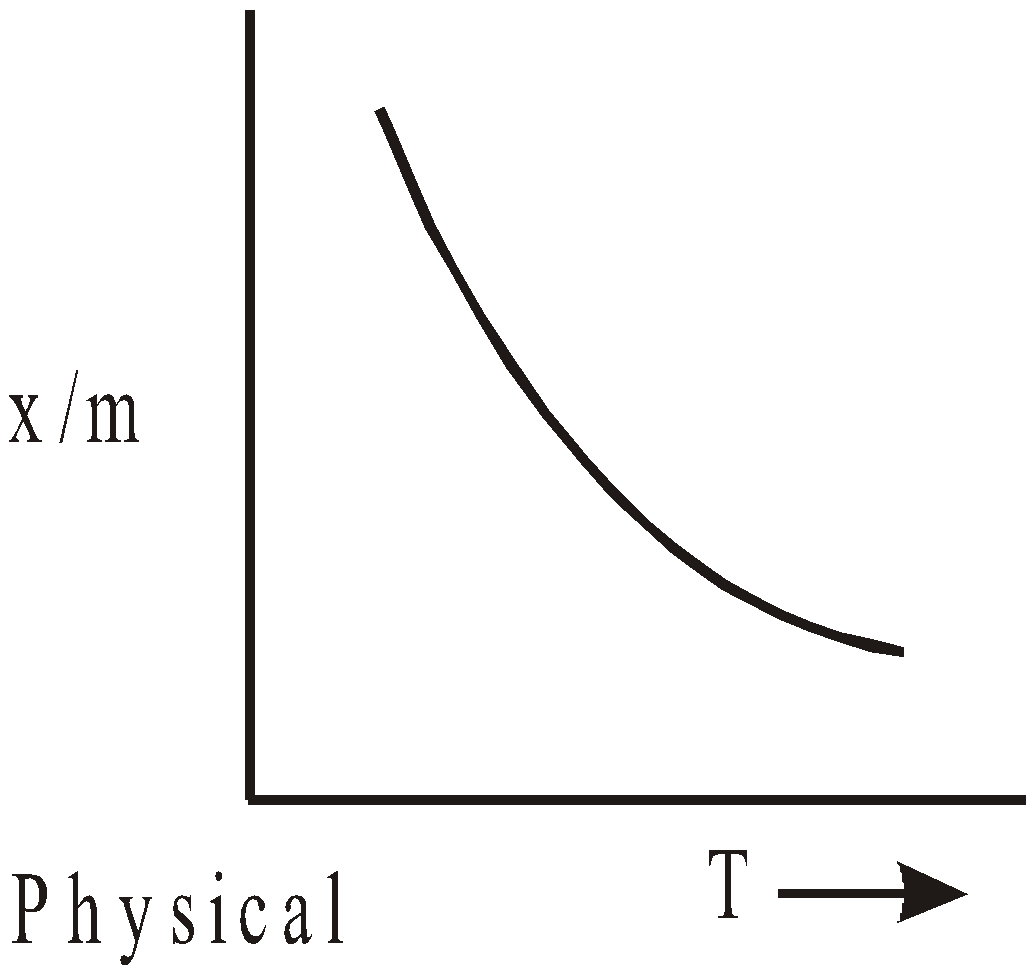
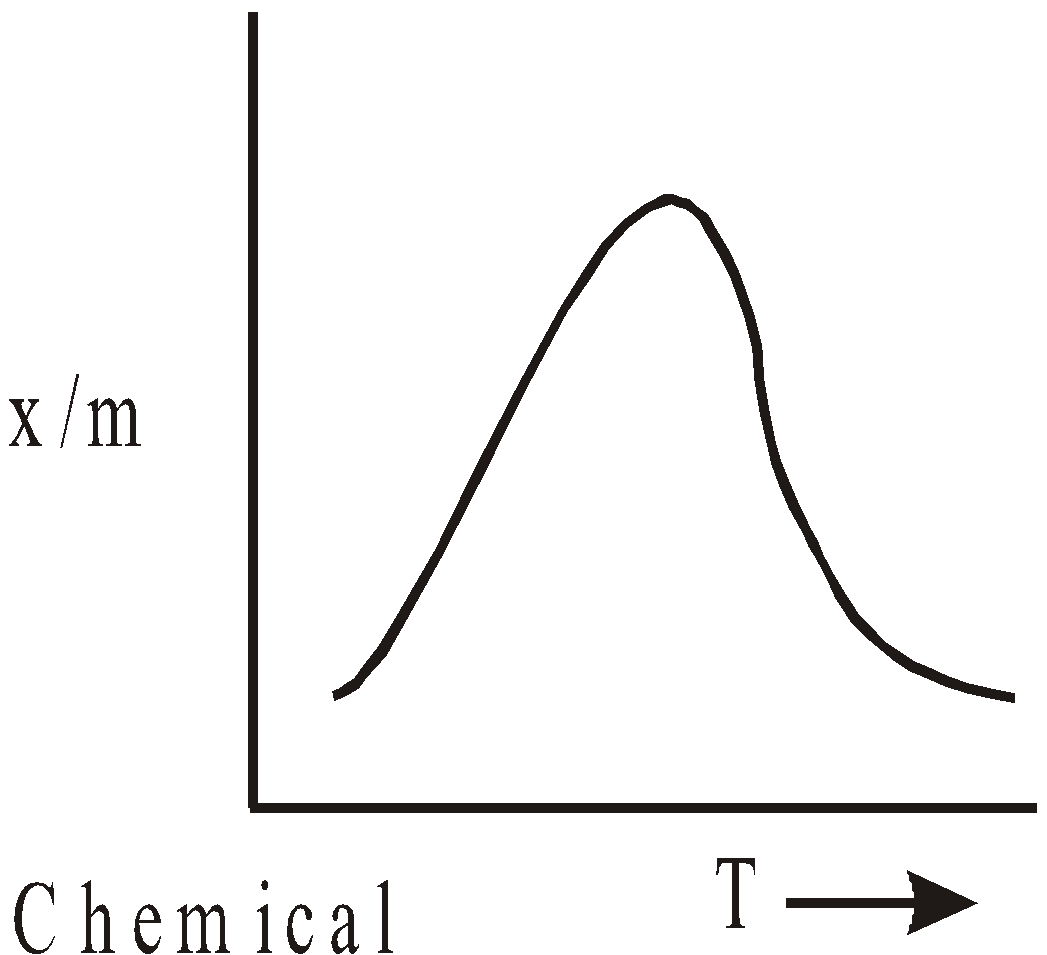
- Effect of temperature – According to Le chatelier’s principle, physical adsorption occurs rapidly and lower temperature and decreases with increasing temperature. Chemisorption increases with temperature. Rise of temperature might cause physical adsorption to change to chemical adsorption e.g. N2 is physically adsorbed on Fe at 190ºC but chemisorbed to form nitride at 500ºC.
The relation 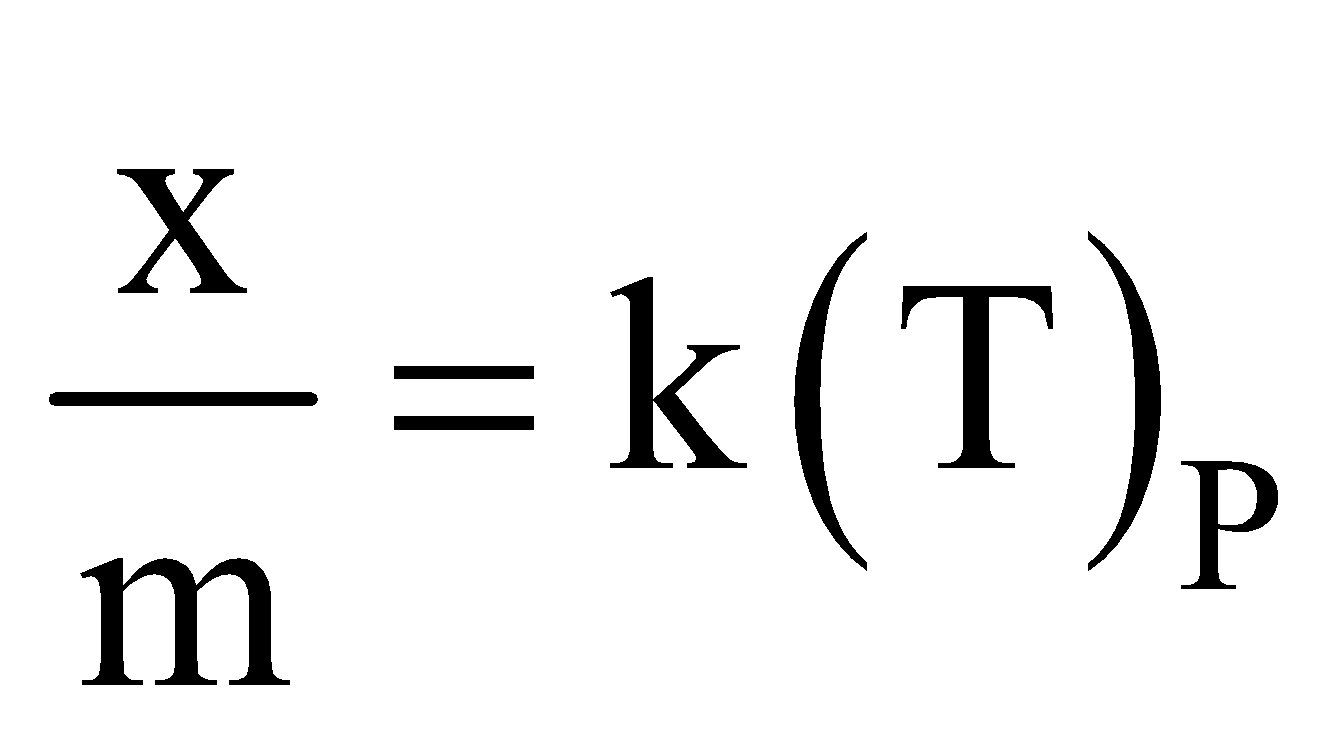 liquid, solid is called adsorption isobar.
liquid, solid is called adsorption isobar.
- Surface area – Larger specific area (surface area per g of adsorbent) means greater adsorption.
- Heat of adsorption – Energy released when 1g mole of a gas is adsorbed on solid surface is called heat of adsorption. Adsorption is an exothermic process similar to condensation. Heat of adsorption is small as attractive forces between gas molecules and solid surface are due to weak Van der Waal’s forces.
ADSORPTION OF SOLUTES FROM SOLUTIONS
Porous and finely divided solid substances can also adsorb dissolved substances from solution eg activated charcoal adsorbs dye stuffs. When solution of acetic acid is shaken with activated charcoal, part of acid is removed by adsorption and concentration of the solution is decreased. Some precipitates act as adsorbents. For e.g. Mg(OH)2 when precipitated in the presence of dyestuff magnesium forms a blue lake. Adsorption from solution follows same principles as that for adsorption of gases by solids. Thus,
- Some adsorbents specifically adsorb some solute more effectively than others
- Increase in surface area causes increase in adsorption
- Adsorption increases with temperature
- It involves establishment of equilibrium between the amount adsorbed and the concentration of solute in solution
Freundlich isotherm using concentration can be written as:
where x = mass of solute adsorbed.
m = mass of adsorbent
c = equilibrium concentration of solution.
log 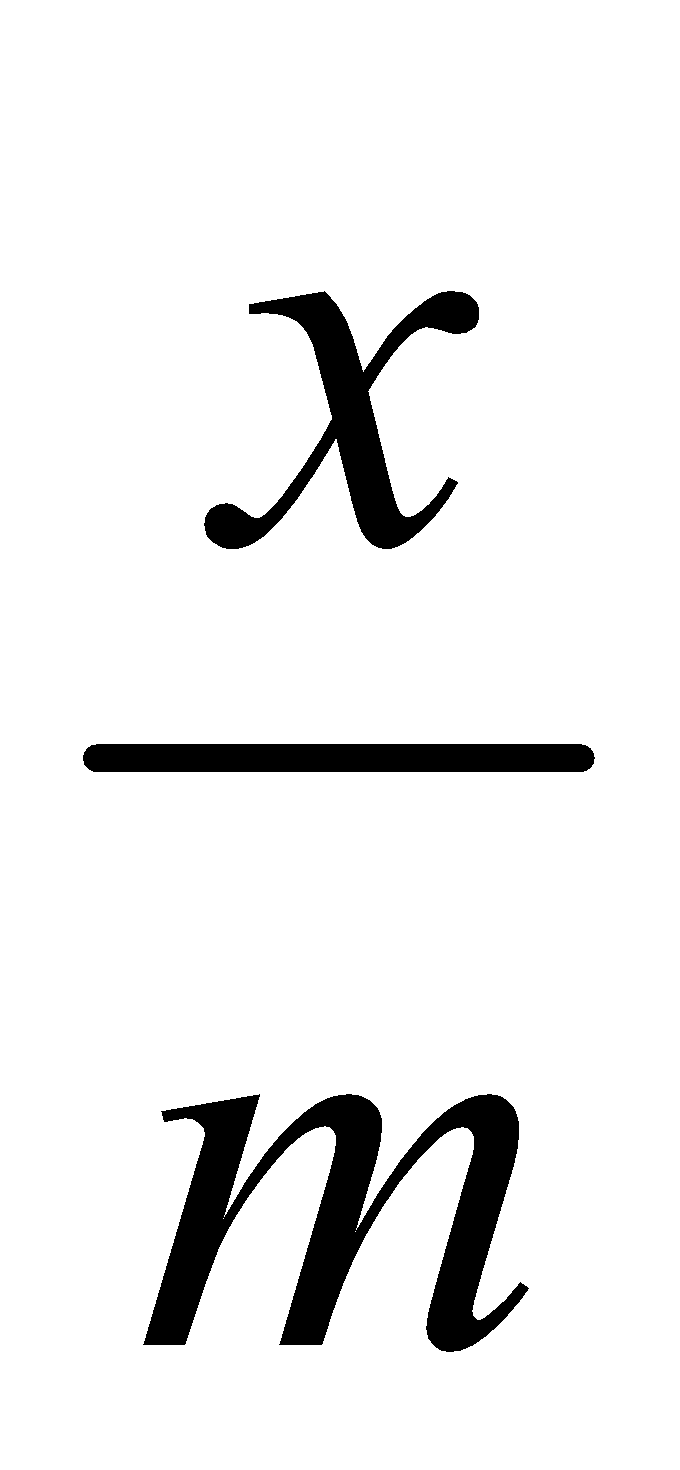 = log k +
= log k + 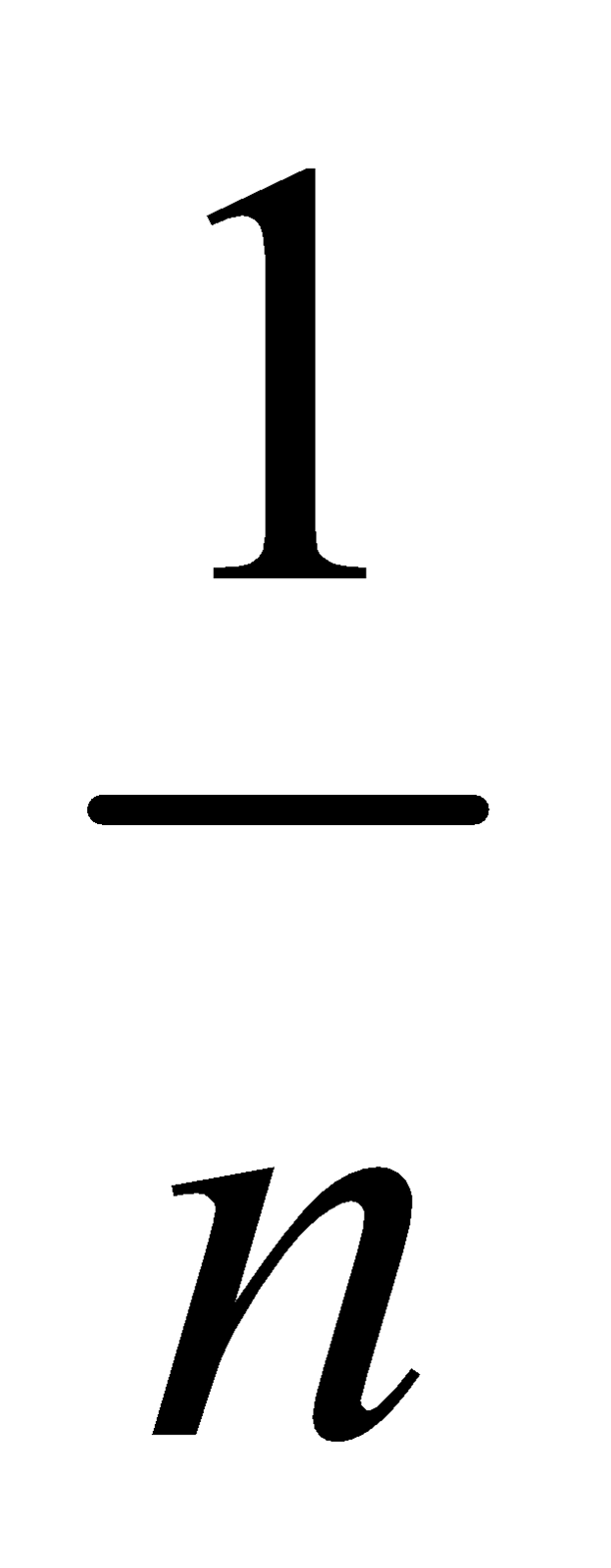 log c is a straight line.
log c is a straight line.
LANGMUIR’S ADSORPTION ISOTHERM
Langmuir exploited theoretical considerations based on kinetic theory of gases to derive an adsorption isotherm. If w is mass of gas adsorbed per unit mass of adsorbent and p is the pressure of the gas then
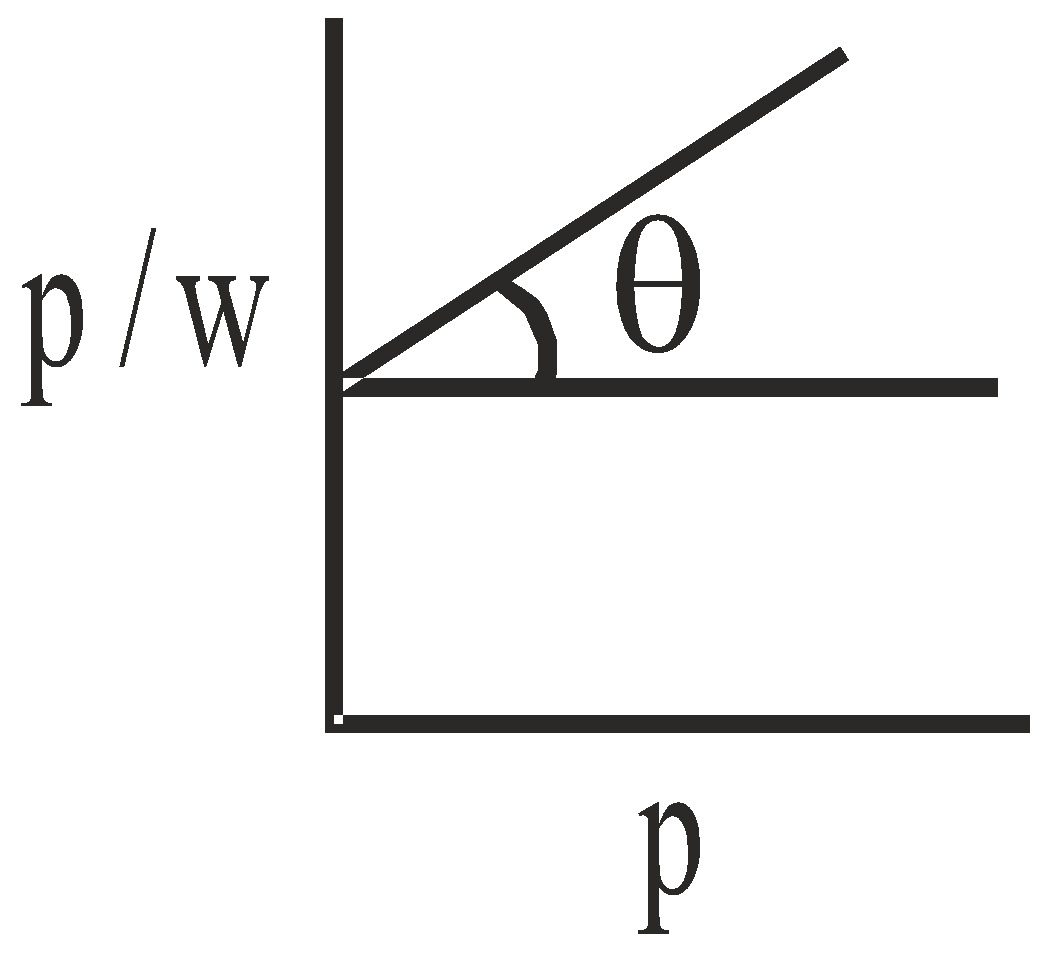 slope =
slope = COMPETING ADSORPTION
Different adsorbates compete among themselves to adsorb on the adsorbent. Strongly adsorbable substance can displace a weakly adsorbable substance. For e.g. Cl2 and NH3 can replace O2 and N2 already adsorbed on charcoal. Moisture is also strongly adsorbed by silica though it is present in small proportion in air and charcoal adsorbs polluting gases present in air in small amounts.
ACTIVATION OF ADSORBENT
Activation or increasing the power of adsorption can be achieved in different ways.
- by increasing surface area
- by making surface rough
Small size of particles makes the interparticle space too small to allow penetration of gas molecules and thus may decrease the extent of adsorption.
APPLICATIONS
- High vacuum can be generated if a partially evacuated vessel is connected to a container of activated charcoal cooled with liquid air. This is due to adsorption of gas molecules in vessel.
- Gas masks contain activated charcoal or adsorbents to remove poisonous gases and thus purify the air.
- Heterogeneous catalysis (see chapter on catalysis)
- Chromatographic analysis is based on principle of selective adsorption. Mixture of gases can also be separated by selective adsorption of gases by liquids (gas chromatography).
- Colouring matter can be removed from solutions by animal charcoal which adsorbs the colouring matter.
- Froth floatation process which involves adsorption of mineral particles wetted with oil is used to free low grade sulphide ores (PbS, ZnS, Cu2S) from silica.
DISTRIBUTIVE LAW
NERNST DISTRIBUTION LAW OR PARTITION LAW
When a solute is shaken with two immiscible liquids, having solubility in both, the solute distributes itself between the two liquids in such a way that the ratio of its concentrations in two liquids is constant at a given temperature, provided the molecular state of the solute remains the same in both the liquids.
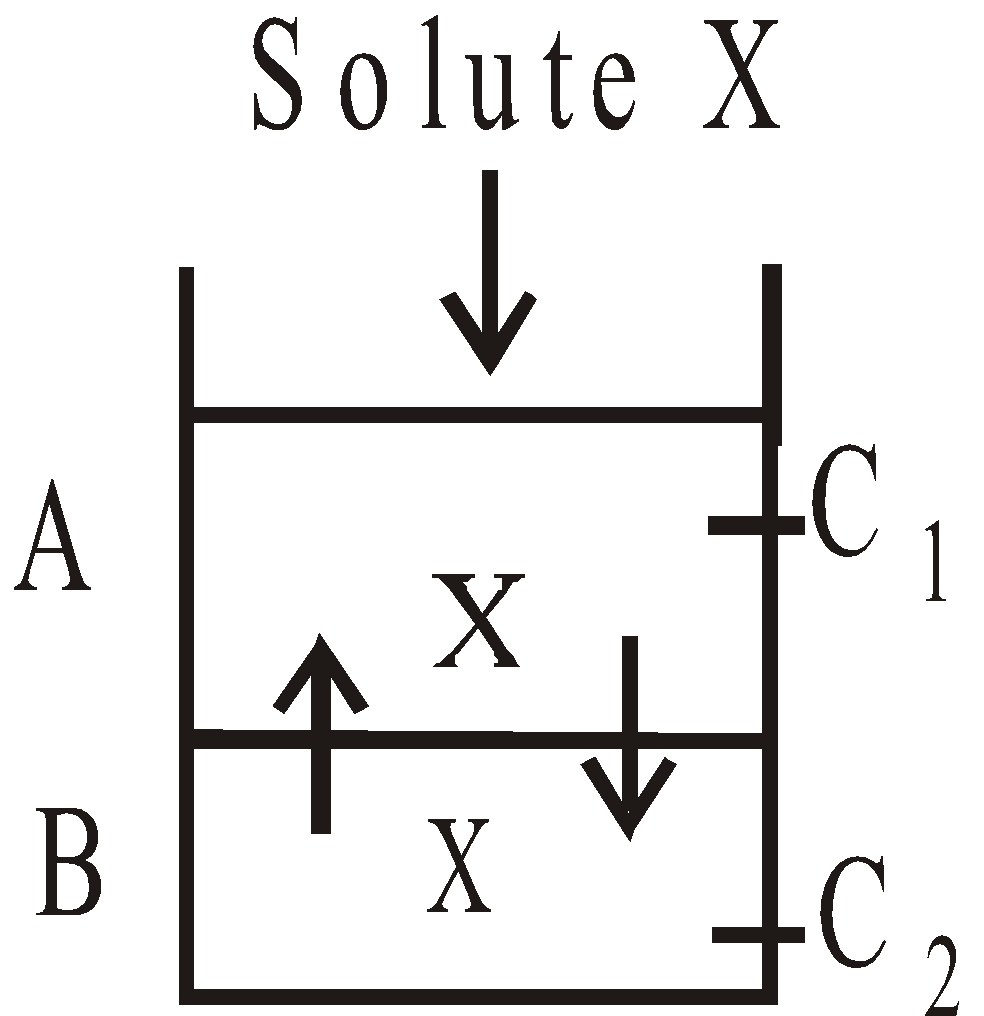
(Where KD = distribution coefficient or partition coefficient or distribution ratio)
Distribution of Succinic acid between ether and water illustrates the consistency of the ratio of concentrations in each layer
C1 in ether C2 in water C1 /C2
0.013 0.069 0.188
0.022 0.119 0.184
Since the solubility also represents concentration, the distribution law can also be written as
Where S1 and S2 are the solubilities of the solute in two solvents.
LIMITATIONS OF DISTRIBUTION LAW
The law holds good when the following conditions are fulfilled.
- Molecular State : The molecular state of the solute must remain the same in both the solvents i.e. there should be no association or dissociation of solute molecules.
- Constant temperature : The temperature is kept constant
- Non-miscibility of solvents : The two solvents must be immiscible and the non miscibility must not change on addition of solute.
- Dilute solutions : The law is applicable to dilute solutions only
- Equilibrium concentrations : The C1 and C2 are the equilibrium concentrations
MODIFICATIONS
The following modifications are applied under different conditions-
- When solute associates
Apply the following formula
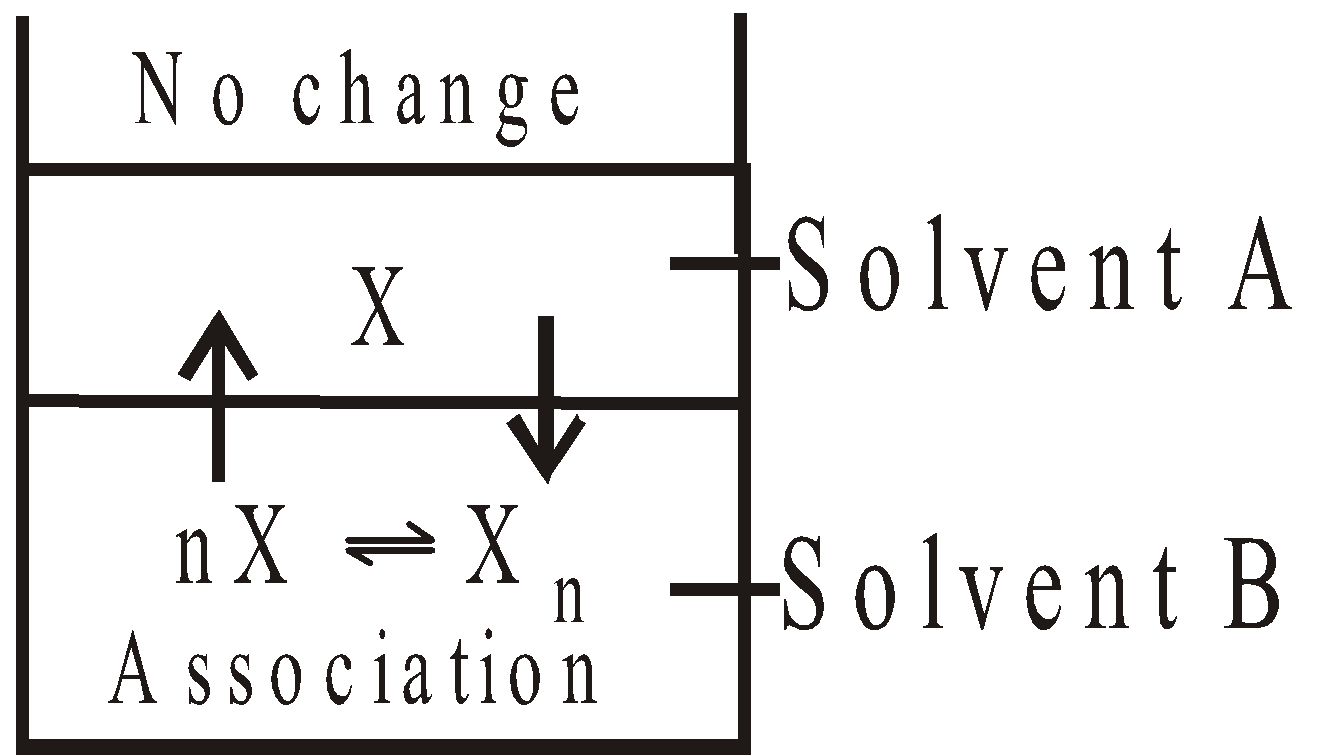
Where n is the number of molecules undergoing association
- When solute dissociates
Apply the following formula
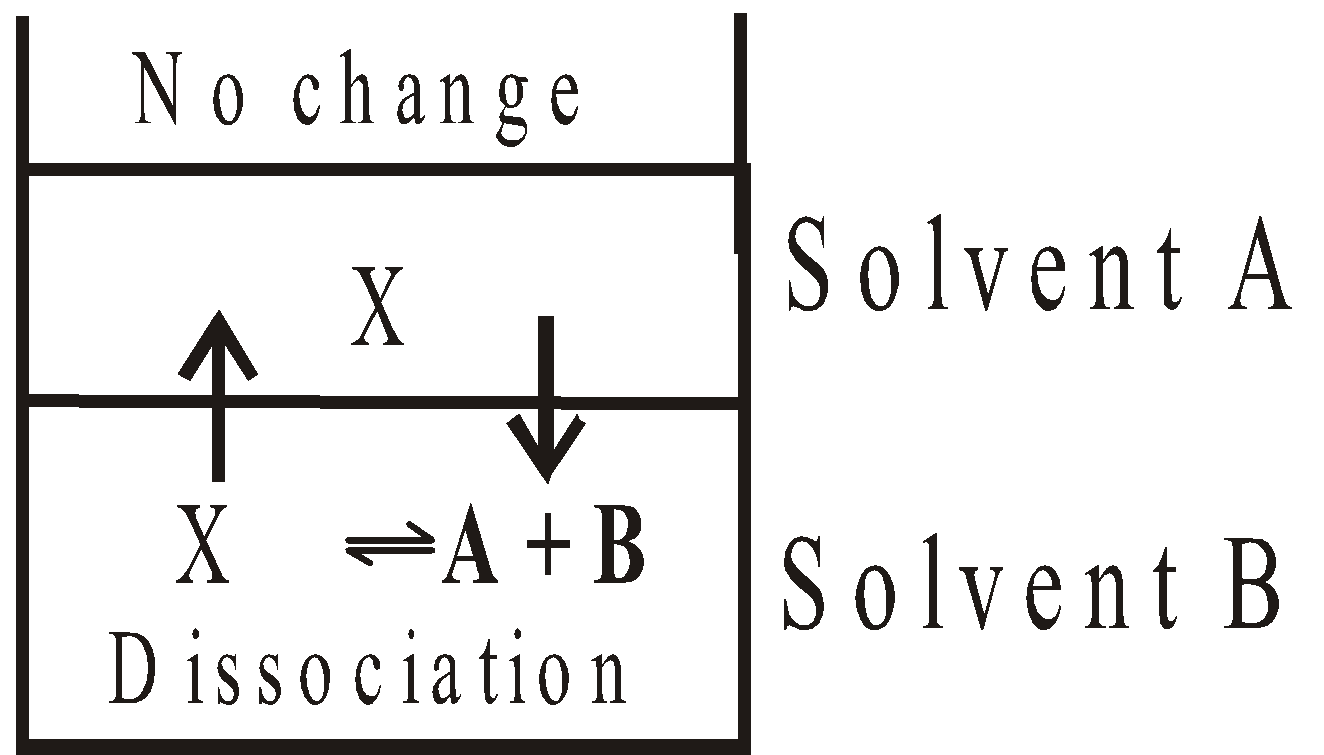
Where a is the degree of dissociation
- When temperature varies
Variation of distribution coefficient with change of temperature is given by following relation
HENRY’S LAW
It states that at a constant temperature the solubility of a gas in a liquid is directly proportional to the pressure of the gas above it.
C 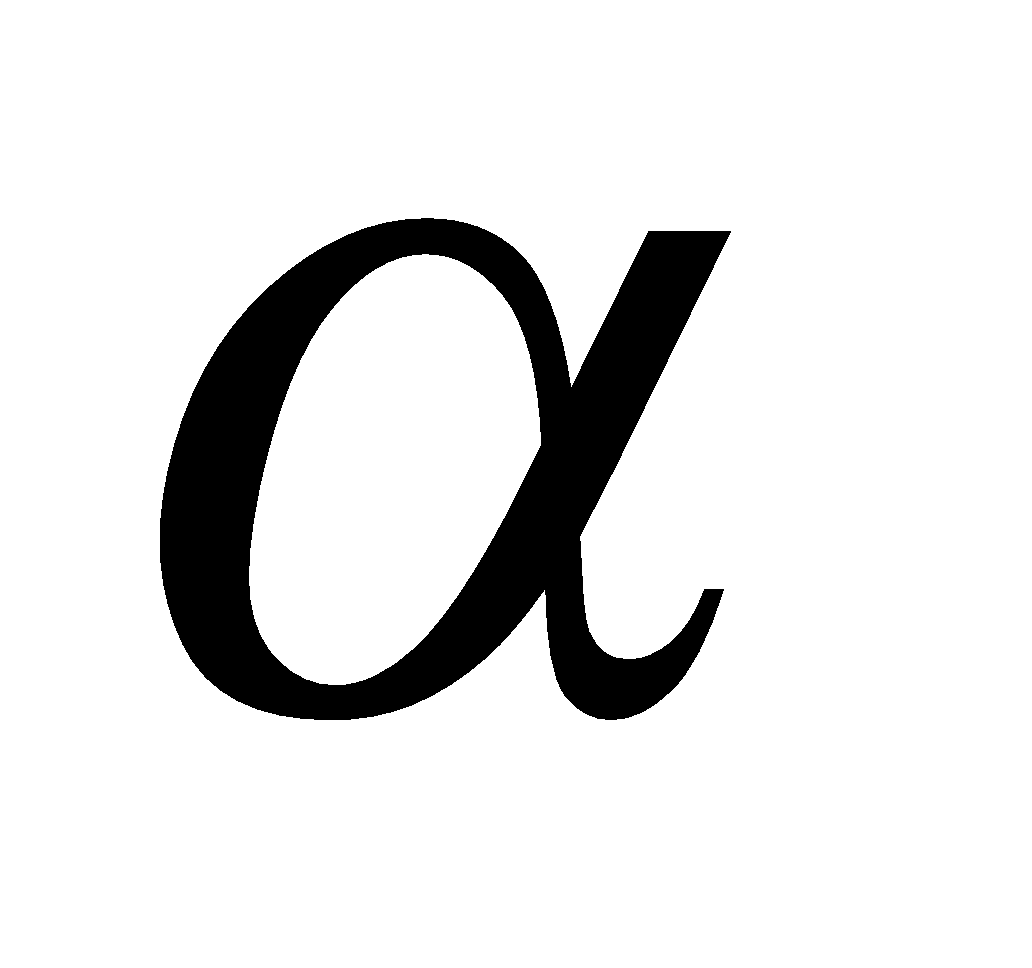 P or C = KHP
P or C = KHP
Where C is the solubility or concentration of the gas, KH is a constant known as Henry’s constant and p is the pressure of the gas.
- The law holds good for dilute solutions of gases which do not react with the solvent.
- When mixture of gases is there, the solubility of each gas is proportional to its partial pressure.
APPLICATIONS OF DISTRIBUTION LAW
- Solvent extraction : The separation of organic substances from aqueous solutions by the use of organic solvent generally ether is based on partition law. The aqueous solution of organic substance is shaken with ether. The organic substance distributes itself in both the liquids which are separated by separating funnel. The solvent layer on evaporation leaves behind organic substance.
For more recovery of the substance, the number of operations should be more. The amount of organic substance left in aqueous solution is given by
n = Number of operations
K = Distribution constant
V= Volume of aqueous solution
v = Volume of organic solvent
A = Amount of solute
- Determination of degree of association
- Determination of degree of dissociation
- Determination of solubility
- Purification of organic substances by chromatography
- Desilverisation of lead (Parke’s process)
- Layer test for Bromide and Iodide
- Study of complex formation
- Distribution Indicators
- Determination of molecular weight in different solvents
