Question-1 :2021-may-Chemistry_paper_1__TZ2_SL
Topic:
Givesn : $0.20 \mathrm{~mol}$ of magnesium is mixed with $0.10 \mathrm{~mol}$ of hydrochloric acid.
$$
\mathrm{Mg}(\mathrm{s})+2 \mathrm{HCl}(\mathrm{aq}) \rightarrow \mathrm{MgCl}_2(\mathrm{aq})+\mathrm{H}_2(\mathrm{~g})
$$
Discuss: Which is correct?
Answer/Explanation
Solution:
To determine the limiting reagent, we need to compare the amount of moles of each reactant and their stoichiometric ratio in the balanced chemical equation.
From the equation, we can see that 1 mole of $\mathrm{Mg}$ reacts with 2 moles of $\mathrm{HCl}$ to produce 1 mole of $\mathrm{H} _2$.
For Mg: $0.20 \mathrm{~mol}$
For $\mathrm{HCl}: 0.10 \mathrm{~mol}$
So, if all the $\mathrm{HCl}$ reacts, it will require $0.05 \mathrm{~mol}$ of $\mathrm{Mg}(0.10 \mathrm{~mol}~~ \mathrm{HCl} / 2 \mathrm{~mol} ~~\mathrm{HCl}$ per $1 \mathrm{~mol}$ $\mathrm{Mg}=0.05 \mathrm{~mol}~~ \mathrm{Mg})$
Since we have more $\mathrm{Mg}$ than the required amount $(0.20 \mathrm{~mol}>0.05 \mathrm{~mol}), \mathrm{Mg}$ is not the limiting reagent. Therefore, $\mathrm{HCl}$ is the limiting reagent.
The maximum yield of $\mathrm{H} _2$ will be determined by the limiting reagent, which is $\mathrm{HCl}$ in this case.
The balanced chemical equation shows that 2 moles of $\mathrm{HCl}$ are required to produce 1 mole of $\mathrm{H}_ 2$. The amount of $\mathrm{HCl}$ available is $0.10 \mathrm{~mol}$, which means that the maximum amount of $\mathrm{H} _2$ that can be produced is:
$0.10 \mathrm{~mol} ~\mathrm{HCl} \times\frac{1 \mathrm{~mol}~ \mathrm{H}_ 2 }{ 2 \mathrm{~mol}~ \mathrm{HCl}}=0.05 \mathrm{~mol} ~\mathrm{H}_ 2$
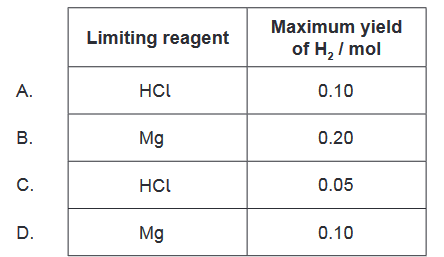
Therefore, the correct answer is $\mathrm{C}$, which shows that the limiting reagent is $\mathrm{HCl}$, and the maximum yield of $\mathrm{H} _2$ is 0.05 mol.
Question-2 :2021-may-Chemistry_paper_1__TZ2_SL
Topic:
Discuss: Which amount, in mol, of sodium chloride is needed to make $250 \mathrm{~cm}^3$ of $0.10 \mathrm{~mol}^{-3}$ solution?
A. $4.0 \times 10^{-4}$
B. $0.025$
C. $0.40$
D. $25$
Answer/Explanation
Solution:
To calculate the amount of sodium chloride needed to make a $0.10 \mathrm{~mol} / \mathrm{L}$ solution in $250 \mathrm{~mL}$ of solvent, we can use the formula:
moles of solute $=$ concentration $x$ volume
where concentration is in $\mathrm{mol} / \mathrm{L}$, and volume is in $\mathrm{L}$. Since the volume is given in $\mathrm{cm}^3$, we need to convert it to $L$ by dividing it by 1000 :
moles of solute $=0.10 \mathrm{~mol} / \mathrm{L} \times 250 \mathrm{~cm}^3 / 1000=0.025 \mathrm{~mol}$
Therefore, the correct answer is $B$, which shows that 0.025 mol of sodium chloride is needed to make a $0.10 \mathrm{~mol} / \mathrm{L}$ solution in $250 \mathrm{~cm}^3$ of solvent.
Question-3 :2021-may-Chemistry_paper_1__TZ2_SL
Topic:
Discuss: Which molecule has the same empirical formula as molecular formula?
A. $\mathrm{CH}_3 \mathrm{COOH}$
B. $\mathrm{C}_2 \mathrm{H}_5 \mathrm{OH}$
C. $\mathrm{C}_2 \mathrm{H}_4$
D. $\mathrm{C}_4 \mathrm{H}_{10}$
Answer/Explanation
Solution:
The molecule that has the same empirical formula as its molecular formula is actually option B, $\mathrm{C}_2 \mathrm{H}_5 \mathrm{OH}$, which is ethanol.
The molecular formula of ethanol is $\mathrm{C}_2 \mathrm{H}_5 \mathrm{OH}$, and its empirical formula can be determined by dividing each subscript by the greatest common factor. In this case, the greatest common factor is 1, so the empirical formula is also $\mathrm{C}_2 \mathrm{H}_5 \mathrm{OH}$. Therefore, option B is the correct answer.
Question-4 :2021-may-Chemistry_paper_1__TZ2_SL
Topic:
Calculate: What is the sum of the coefficients when the equation is balanced with whole numbers?
$$
_{–} \mathrm{Sn}(\mathrm{OH})_4(\mathrm{aq})+_{–} \mathrm{NaOH}(\mathrm{aq}) \rightarrow{ }_{–} \mathrm{Na}_2 \mathrm{SnO}_3(\mathrm{aq})+{ }_{–} \mathrm{H}_2 \mathrm{O}(\mathrm{l})
$$
A. $4$
B. $5$
C. $6$
D. $7$
Answer/Explanation
Solution:
First, we need to balance the equation by making sure that the number of atoms of each element is the same on both sides of the equation.
The equation can be balanced by adding coefficients in front of the reactants and products as follows:
$1\mathrm{Sn}(\mathrm{OH})_4(\mathrm{aq})+2\mathrm{NaOH}(\mathrm{aq}) \rightarrow 1\mathrm{Na}_2\mathrm{SnO}_3(\mathrm{aq})+3\mathrm{H}_2\mathrm{O}(\mathrm{l})$
Now we can see that the sum of the coefficients is $2 + 1 + 1 + 3 = 7$.
Question-5 :2021-may-Chemistry_paper_1__TZ2_SL
Topic:
Discuss: What is represented by “$2^{-}$” in ${ }_\mathrm{Z}^\mathrm{A}\mathrm{ X}^{2-}$ ?
A. loss of electron
B. gain of electron
C. loss of proton
D. gain of proton
Answer/Explanation
Solution:
In the notation ${ }_\mathrm{Z}^\mathrm{A}\mathrm{X}^{n-}$, the superscript “-n” represents the charge of the ion.
In the case of ${ }_\mathrm{Z}^\mathrm{A}\mathrm{X}^{2-}$, the superscript “-2” indicates that the ion X has a charge of minus two. This means that the ion has gained two electrons, and the correct answer is B, gain of electron.