Question-1 :2022-nov-Chemistry_paper_1__TZ0_HL
Topic:
Discuss: How many oxygen atoms are present in $0.0500 \mathrm{~mol} ~\mathrm{Ba}(\mathrm{OH})_2 \cdot 8 \mathrm{H}_2 \mathrm{O}$ ?
$$
N_{\mathrm{A}}=6.02 \times 10^{23}
$$
A. $3.01 \times 10^{23}$
B. $6.02 \times 10^{23}$
C. $3.01 \times 10^{24}$
D. $6.02 \times 10^{24}$
Answer/Explanation
Solution:
The compound $\mathrm{Ba}(\mathrm{OH})_2 \cdot 8 \mathrm{H}_2 \mathrm{O}$ contains 10 oxygen atoms, two from $\mathrm{OH}$ group, and eight oxygen atoms in each water molecule, for a total of $2+8=10$ oxygen atoms.
To find the number of oxygen atoms in $0.0500 \mathrm{~mol}$ of the compound, we can use Avogadro’s number ($N_{\mathrm{A}}$) to convert from moles to number of atoms, and then multiply by the number of oxygen atoms present in formula.
The calculation is as follows:
\begin{align*}
\text{Total no. of atoms} &= \frac{0.0500 \mathrm{~mol}}{1} \times \frac{N_{\mathrm{A}}}{1 \mathrm{~mol}}\\
&= 0.0500 \times N_{\mathrm{A}}
\end{align*}
Then, the total number of oxygen atoms in $0.0500 \mathrm{~mol}$ of the compound is:
\begin{align*}
\text{Number of oxygen atoms} &= \text{Total no. of atoms } \times \text{Number of oxygen atoms}\\
&= 0.0500 \times N_{\mathrm{A}} \times 10\\
&= 3.01 \times 10^{23}
\end{align*}
$\colorbox{yellow}{Correct Option: (A) $3.01 \times 10^{23}$ oxygen atoms.}$
Question-2 :2022-nov-Chemistry_paper_1__TZ0_HL
Topic:
Discuss: What is the change of state for a gas to a solid?
A. Condensation
B. Deposition
C. Freezing
D. Sublimation
Answer/Explanation
Solution:
The change of state for a gas to a solid is called “Deposition”. During deposition, the gas molecules lose energy and come together to form a solid without passing through the liquid phase. This is the opposite process of sublimation, which is the change of state from a solid directly to a gas. Condensation is the change of state from a gas to a liquid, while freezing is the change of state from a liquid to a solid.
Question-3 :2022-nov-Chemistry_paper_1__TZ0_HL
Topic:
Discuss: How many moles of carbon dioxide are produced by the complete combustion of $7.0 \mathrm{~g}$ of ethene, $\mathrm{C}_2 \mathrm{H}_4(\mathrm{~g})$ ?
$$
M_{\mathrm{r}}=28
$$
A. $0.25$
B. $0.5$
C. $0.75$
D. $1.0$
Answer/Explanation
Solution:
The balanced equation for the combustion of ethene is:
$\mathrm{C}_2 \mathrm{H}_4+3 \mathrm{O}_2 \rightarrow 2 \mathrm{CO}_2+2 \mathrm{H}_2 \mathrm{O}$
From the balanced equation, we see that 2 moles of $\mathrm{CO}_2$ are produced for every 1 mole of ethene consumed.
First, we need to calculate the number of moles of ethene in 7.0 g:
$n\left(\mathrm{C}_2 \mathrm{H}_4\right)=\frac{m}{M_{\mathrm{r}}}=\frac{7.0 \mathrm{~g}}{28 \mathrm{~g} / \mathrm{mol}}=0.25 \mathrm{~mol}$
Since 2 moles of $\mathrm{CO}_2$ are produced for every 1 mole of ethene consumed, the number of moles of $\mathrm{CO}_2$ produced will be:
$n\left(\mathrm{CO}_2\right)=2 \times n\left(\mathrm{C}_2 \mathrm{H}_4\right)=2 \times 0.25 \mathrm{~mol}=0.5 \mathrm{~mol}$
$\colorbox{yellow}{Correct Option : B}$
Question-4 :2022-nov-Chemistry_paper_1__TZ0_HL
Topic:
Given: Successive ionization energies of an element, $\mathbf{X}$, are shown.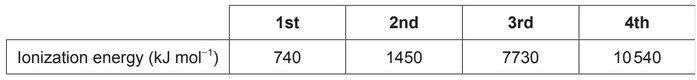
Calculate: What energy, in $\mathrm{kJ} \mathrm{mol}^{-1}$, is required for element $\mathbf{X}$ to reach its most stable oxidation state in ionic compounds?
A. $740$
B. $1450$
C. $2190$
D. $7730$
Answer/Explanation
Solution:
Looking at the successive ionization energies, we can see that the third ionization energy is much larger than the first and second ionization energies, indicating that it is much more difficult to remove the third electron from the cation. Therefore, the most stable oxidation state for element X would be a cation with a $+2$ charge, which means it needs to lose two electrons.
The energy required to remove the first two electrons is:
$\mathrm{IE}_1+\mathrm{IE}_2=740+1450=2190 \mathrm{~kJ} / \mathrm{mol}$
$\colorbox{yellow}{Correct Option : C}$
Question-5 :2022-nov-Chemistry_paper_1__TZ0_HL
Topic:
Discuss: Which quantities are different between two species represented by the notation ${ }_{52}^{128} \mathrm{Te}$ and ${ }_{53}^{128} \mathrm{I}^{-}$?
A. The number of protons only
B. The number of protons and electrons only
C. The number of protons and neutrons only
D. The number of protons, neutrons and electrons
Answer/Explanation
Solution:
The notation ${ }{52}^{128} \mathrm{Te}$ represents the isotope of tellurium with 52 protons, 76 neutrons, and 52 electrons, while the notation ${ }{53}^{128} \mathrm{I}^{-}$ represents the iodide ion, which has 53 protons, 75 neutrons, and 54 electrons.
Therefore, the quantities that are different between these two species are:
- The number of protons (52 vs 53)
- The number of neutrons (76 vs 75)
- The number of electrons (52 vs 54)
The number of protons, neutrons and electrons are different between the two species.
$\colorbox{yellow}{Correct Option : D}$