Reaction Rates
- Spontaneous reactions: reactions that will happen but we cant tell how fast
- Kinetics: the study of the rates of a chemical reaction
- Can determine the coeffiecents of the chemical equation when given graph of concentration vs time by doing –.
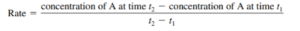
- Ex:
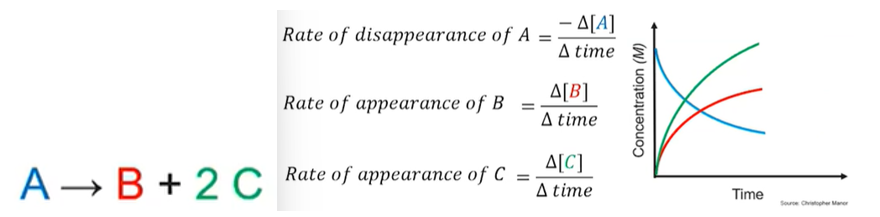
- The rate of disappearance of A is equal to the rate of appearance of B and ½ the rate of appearance of C
Factors that Affect Reaction Rates
- According to the collision theory, the rate of a reaction is influenced by anything that affects the number or force of collisions
- Increase collisions = increase rate
- Reactant concentration
- Increasing concentration causes an increase in the frequency of collisions which increases reaction rates (except for zero-order reactions)
- Increasing temperature: increasing the temp increases the frequency of collisions and the number of particles that have enough kinetic energy to collide and overcome the AE and form products
- Increase temperature = increases number of successful collisions
- Pressure: For reactions involving gases, increasing pressure increases the collisions between reactants,
- Particle size and increasing surface area
- The smaller the particle size with more sides, the larger the surface area for a given mass of particle
- Increase surface area → more collisions
- Catalysts: A catalyst will change the value of K because the activation energy changes
- The value of the rate constant is also dependent on the activation energy
- Inhibitor: a substance that decreases the rate of a reaction by increasing Ea