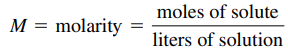Representation of Solution
- Molarity (M): K.A Concentration
- Ex: A solution that is 1.0 molar (written as 1.0 M) contains 1.0 mole of solute per liter of solution.
- Note: brackets around something it means the “molarity of what is inside”
- Dilution: water is added to achieve the molarity desired for a particular solution
- Does not change the amount of moles present
- Mass percent (weight percent):
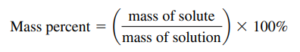
- Molality:
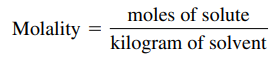
- Normality (N): Molarity x number of equivalents (definition of an equivalent depends on the reaction taking place in the solution)
- Acid–base reaction → number of protons = equivalents
- Oxidation–reduction reactions → number of e- in half-reaction = equivalents
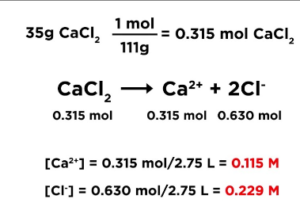
Calculating Concentration of Ions
- Write out balanced formula for dissolution reaction
- Ex: 35g CaCl2 dissolved in 2.75L
Mole Fraction, Mass Percent, & Density Questions
- Find moles → mass percent / molar mass
- Molarity = (MP as a decimal )(1000)(density/molar mass)
- Remember that Density = g/cm3 (mL)
- With water (d =1g/cm3) can just convert mL to grams
Steps in Solution Formation
- Overcoming solute-solute interactions (endothermic) (ΔH₁)
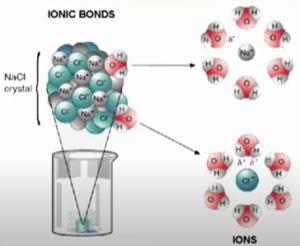
- Breaking ionic compound= breaking ionic bonds; breaking covalent solute = breaking interMF
- Expanding the solvent (endothermic) (overcoming interMF) to make room for the solute (ΔH₂)
- Allowing the solute and solvent to interact to form bonds and a solution (exothermic) (ΔH₃)
- Enthalpy (heat) of solution (ΔHsoln): is the sum of the ΔH values for the steps of solution formation
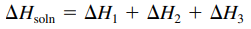
- Might have a positive sign (energy absorbed) or a negative sign (energy released)
- Enthalpy (heat) of solution (ΔHsoln): is the sum of the ΔH values for the steps of solution formation