Acid-Base Titrations
- Neutralization reaction: acid base reaction; produces salt and water
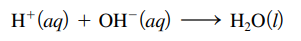
- Neutralized: when just enough base is added to react exactly with the acid in a solution
- Titrant: solution of known concentration used in titration (in buret)
- Analyte: substance of unknown concentration (is being analyzed; in the flask)
- Endpoint: the indicator changes color so you can tell that the equivalence point has been reached
- Can tell that a chemical change has occurred by a change in color or if solution feels warm (heat often associated with acid-base reaction)
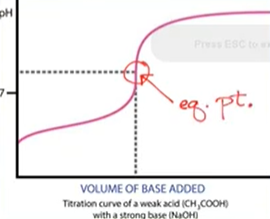
- Goal of titration is to reach equivalence (stoichiometric) point: enough titrant added to react exactly with the analyte
- Equivalence point on graph = most vertical point on graph where the pH change is the greatest
- “Neutralization “ means at eq. point
- Equivalence point on graph = most vertical point on graph where the pH change is the greatest
Redox Titration
- Some might not need an indicator; eq point can be detected by observing a faint pink color persisting in the analyte
Steps to Titration Calculation Questions
- Substance with molarity and volume will be the titrant → determine moles of titrant
- Substance with only volume given will be analyte
- Use balanced equation to determine moles of analyte reacted
- Determine the molarity of the analyte by using moles/liters