Reaction Mechanisms
- A chemical equation does not tell us how reactants become products
- Simply a summary of the overall process
- Elementary steps: series of steps by which a chemical reaction occurs
- Cancel out identical species on opposite sides of the arrows in elementary steps to find overall balanced reaction
- Elementary reactions involving the simultaneous collision of three or more particles are rare
- Bcuz is unlikely that the collision will have sufficient energy and orientation for reaction to occur
- Molecularity: refers to the number of moles that are reacting
- Intermediate: something that is a product first in one elementary step and becomes a reactant in another
- Catalyst: something that is a reactant first in one elementary step and becomes a product in another
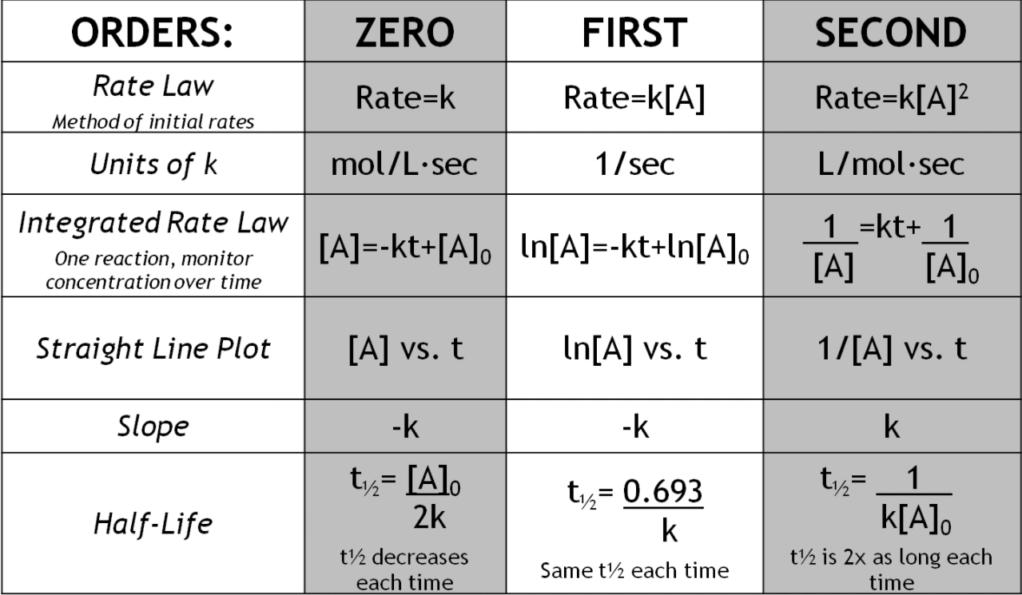
- Writing Rate Law Trick: can use the coefficient from the elementary step and turn it into the exponent for the rate law
- Rate laws can only be determined in this manner for elementary steps!
- The rate law for the slow rate determining step is the rate law for the entire overall balanced reaction
- As long as the slow step is the first elementary step!