Heat Transfer and Thermal Equilibrium
- Temperature: average kinetic energy of a substance
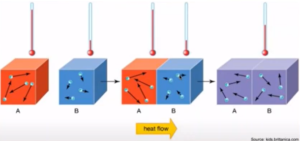
- Reflects random motions of particles
- A change in temperature indicates an energy change
- Heat: involves the transfer of energy between two objects due to a temperature difference
- Heat flows from hot (higher KE) to cold (lower KE)
- When two objects at different temperatures are in contact, they will eventually reach the same intermediate temperature/thermal equilibrium: particles will have the same avg KE & temp
- Decrease temperature = decrease avg KE of particles = decrease avg speed/velocity of particles