Introduction to Equilibrium
- Chemical equilibrium: when the rates of the forward reaction and the rates of the reverse reaction are equal (there is no net change in the concentrations of reactants and products)
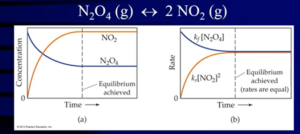
- Notes:
- Although the rate of the forward reaction equals the rate of the reverse reaction, this does not mean that the amounts of products equals the amount of reactants
- The reaction has not stopped, but the their concentrations are changing at a constant rate
- Notes:
- Heterogeneous equilibria: equilibria that involve more than one phase
- Homogeneous equilibria: systems in the gas phase, where all reactants and products are gases