Reaction Quotient and Le Chateilier’s Principle
- A disturbance to a system at equilibrium causes Q to differ from K
- The reaction will “shift” to bring Q back into agreement with K
Effects of Changes to a System
1. Pressure (only affects gasses); there are three ways to change the pressure of a system with gas
- Changing pressure may alter the equilibrium position, but it does not alter the equilibrium constant (K)
a. Effect of changing Pressure:
- Increase pressure, the equilibrium will shift to the side with less moles of gas
- Pressure decreases, equilibrium will shift to side to side with more moles of gas
b. Addition of an inert (unreactive) gas:
- Increases the total pressure but has no effect on the equilibrium of the system or the concentrations or partial pressures of the reactants or products
c. Effect of changing Volume:
- Increase volume: shift to side with more moles of gas
- Decrease volume: shift to side with less moles of gas
d. Changing the volume of the system affects pressure (Boyle’s Law) → See letter a…
- V increases → P decreases; V decreases → P increases
- When volume of the container decreases, the system responds by reducing its own volume by decreasing the total number of gaseous molecules
- V increases → P decreases; V decreases → P increases
2. Temperature: K will change depending on temp (treat energy as either a reactant or product)
- Ex: Endothermic

- Treat heat as reactant
- Effect on K: Adding heat will shift in forward direction so K > 1
- Forward direction → high temp & LP; Reverse direction → low temp & HP
- Ex: Exothermic:

- Treat heat as a product
- Effect on K: Adding heat will shift in reverse reaction so K < 1
- Forward direction → low temp & HP; Reverse direction → high temp and LP
3. Concentration: the system will shift away from the added component (or toward the removed component)
- If question adds something in reaction, added component will likely react with smthn in the reaction
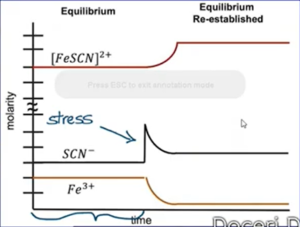
- If add something that forms a precipitate (often OH-) → are taking reactant out of reaction
- What if dilute the solution? (ex: add water vapor)
- Dilute → all molarities (products and reactants) will decrease
- Diluting will always cause a shift toward more aqueous species
- If there are more reactants (denominator) than products → Q > K
- If there are more products (numerator) than reactants → Q < K
- What if increase the concentration of the solution?
- Concentrate → all molarities (products and reactants) will increase
- Concentrating will always cause a shift toward less aqueous species!
- If there are more reactants (denominator) than products → Q < K
- If there are more products (numerator) than reactants → Q > K
- Changing the amount of liquid/solid or adding a catalyst will have NO SHIFT