Common-Ion Effect
- Common-Ion Effect: when you try to dissolve the solid in a solution with either the cation or anion already present less solid will dissolve/more solid will be produced → the solubility of that salt is going to be less that it was in pure water
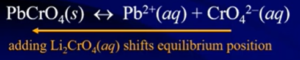
- Q > Ksp → reaction will shift to consuming more ions and producing more reactant (shift left) (more solid forming)
Calculations
- Asked to calculate the solubility of solid (s) in a solution of another compound
- Write balanced equation [dissolution/ions on product side] and ICE table
- Nothing in the Solid column, difference is that will have initial value for the ion that is present in solution
- Write balanced equation [dissolution/ions on product side] and ICE table