pH and Solubility
- The solubility of some salts is affected by the pH of the environment
Basic Solutions
- High pH (more basic) → lots of OH- ions
- Ex:

- Increasing pH (adding OH-) → Q >Ksp → decreases solubility of the salt → solubility is less in a basic environment that in pure water
- Ex:
Acidic Solutions
- Low pH (more acidic) environment → lots of hydronium ions (H3O+)
- Ex:

- Decreasing pH (adding H+) → Q < Ksp → increased solubility
- Ex:
- General Rule: If the anion X- is an effective base (HX is a weak acid) the salt MX will show increased solubility in an acidic solution
- Common anions that are effective bases
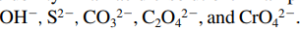
- Salts containing these anions are much more soluble in an acidic solution than in pure water
- Common anions that are effective bases
- Remember: if you have an equilibrium situation where adding more of something that isn’t in eq. Equation then likely that added thing will react with smthn in eq. equation
- Ex:

- Ex:
Reaction as a result of adding hydronium ![]() → shift in forward direction
→ shift in forward direction