Acid-Base Reactions and Buffers
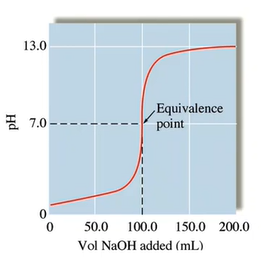
- Equivalence point: enough titrant has been added to react exactly with the solution being analyzed (analyte)
- In acid-base reactions: moles acid = moles base
- In acid-base titrations, are dealing with neutralization reactions (NmN → water is not reactant)
- N: neutralization
- M: moles
- N: numbers
- In ice table, subtract number of mmols of limiting reactant
- Millimoles:
- Ex: 40 mL x 0.2 M = 8 mmol
- Halfway point/halfway equivalence point: is halfway to eq point (half of analyte is neutralized)
- At halfway point pH = pKa;
- Before eq point → have left over H+; after eq point → have left over OH-
Strong Acid-Strong Base Titrations
- They both dissociate completely
- Strong acids: molarity of acid = M of H+
- Strong Base: molarity of base = molarity of OH-
- The net ionic reaction for a strong acid–strong base titration is:
- Do the stoichiometry and there is no equilibrium
- pH is equal to 7 at the eq point (only strong + strong)
Weak Acid-Strong Base Titration
- The weaker the acid being titrated, the smaller the vertical area around the equivalence point
- At halfway point, the molarity of weak acid and conjugate base is equal
- At 0 mL base added: use WMX ICE table, use Ka to determine pH
- pH at equivalence is above 7
- Weak acid before eq point:
- NmN ICE table first
- Then Henderson-Hasselbalch
- At eq point: Have to do double ICE tables only at the eq point!
- NmN ICE table first
- Then, WMS ICE table, use Kb to find [OH-]
- pH after equivalence:
- NmN ICE table first → then do mmol/mL to find moles of [OH-] → find pOH- → find pH
Weak Base-Strong Acid Titration
- Process is practically the same with some differences
- pH at equivalence is below 7
- At 0 mL acid added:
- WMX ICE table, use Kb to determine pOH, then pH
- Weak base before equivalence point:
- NmN ICE table first → then henderson-Hasselbalch
- At equivalence point:
- NmN ICE table first → then WMX ICE table → use Ka to find [H+]
- pH after equivalence:
- NmN ICE table first → leftover strong acid → do mmol/mL to find moles of [H+] → find pH
Titration Curves
Strong Acid Strong Base Titration
- Some base has started to be added but before the equivalence point:
- Have left over strong acid (H+) which is why the pH is below 7
- Everywhere beyond equivalence point: have excess OH- which is why the pH is above 7
- At eq point, for Strong + strong the pH at eq point is exact 7
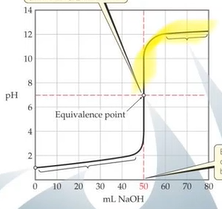
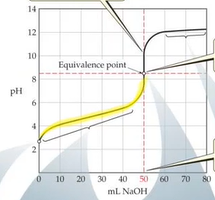
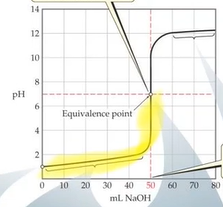
Weak Acid-Strong Base Titration
- After base has started to be added but before eq point → what was left was buffer solution → buffer zone 25mL is halfway point and is where the buffer that is created is the most effective
- At eq point only the conjugate base is present which is why the eq point is above 8
- This reaction
 takes place
takes place
- This reaction

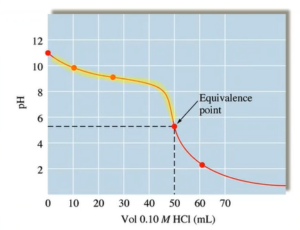
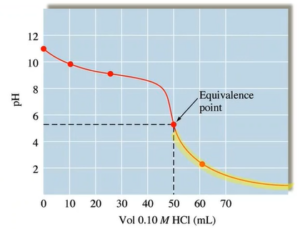
Weak Base-Strong Acid Titration
- In buffer zone 25mL is halfway point pH = pKa
- Will have left over conjugate acid at eq point → pH will be below 7
- Justify: because this reaction takes place

- Justify: because this reaction takes place
- Beyond eq point:
Acid-Base Titration Problems
- How to know whether to use Ka or Kb? Look at equation → is species accepting or donating H+
Sketch a Titration Curve
- Need to plot 3 data point:
- Starting PH
- pH at equivalence
- pH at the halfway point
- If given pkB, can solve for pKa to find pH
Solve for what volume of titrant (acid or base) needed to reach equivalence point
- Write out major species → write net ionic equation → use stoichiometry