pH and pKa
- Based on the pH of the environment, you can tell the relative ratio of conjugate base to acid
- If pH < pKa, then the acid form has the higher concentration
- Weak base & conj acid → conj acid has higher concentration
- If pH > pKa, then the conjugate base form has the higher concentration
- Weak base & conj acid → weak base has higher concentration
Acid-Base Indicators
- Used in titrations to indicate when we have passed the equivalence point
- Are weak acids that change color when they become bases
- Endpoint: when the indicator changes color → tell us we have reached the eq point
- Indicators have a “useful range” → have kA and pKa values
- Useful indicator range =
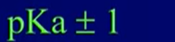
- Best indicator to choose for a particular titration will be the one whose useful range includes or is closest to the pH at equivalence for that particular titration