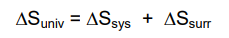Introduction to Entropy
- Thermodynamics let us predict whether a process will occur but does not tell us about the amount of time required
- A spontaneous or “thermodynamically favored” process is one that occurs without intervention; may be fast or slow
- Ex: a ball rolls down a hill but never spontaneously rolls back up
Entropy
- Entropy (S): measure of the randomness/disorder of a system
- More disorder = greater entropy
- Trends in entropy:
- Ssolid < Sliquid << Sgas
- More complex molecules have higher entropy
- Weaker forces of attraction = higher S
- Greater mass = higher S
| Sign of ΔS | ΔS = + | ΔS = – |
| Meaning | Things are becoming more disordered = thermodynamically favored | Things are becoming more organized |
| Example | Decomposition reaction (one reactant becoming two products) Dissolving Endothermic reactions Products have more moles of gas | Synthesis reactions (two reactants become one product) Exothermic reactions Molecules dispersed equally → move to single chamber Products have less moles of gas |
Second Law of Thermodynamics
- In any thermodynamically favored process (energy transfer or transformation), there is usually an increase in the entropy of the universe
- Since energy never flows spontaneously in the other direction, the entropy of the universe is always increasing
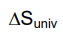 > 0 → usually a favored process
> 0 → usually a favored process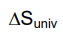 < 0 → usually NOT a favored process (favored in the opposite direction)
< 0 → usually NOT a favored process (favored in the opposite direction)