Electrolysis and Faraday’s Law
- Electrolysis: the process of creating an electrolyte cell
- Used for electroplating
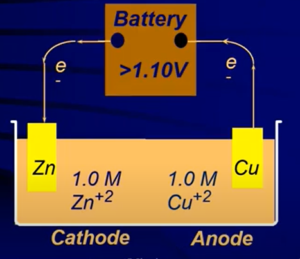
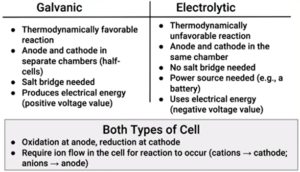
- Electrolyte cell: runs by running a galvanic cell backwards → the electrons are flowing in the opposite direction
- In galvanic cells, electron flow is a spontaneous process; in electrolytic cells it is nonspontaneous
- For electrolysis, the total cell potential (E) will be negative → ΔG° = + (Unfavorable process)
Electrolytic Cell (Electrolysis)
Stoichiometry of Electrolysis
- Shortcut:
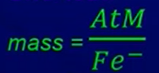
- A = time in seconds
- M = molar mass
- Fe- = Faraday’s constant
Faraday’s Law
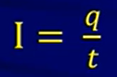 (on RFS) → the amount of charge that flows thru an electrolytic cell is a factor of the current and time that its running
(on RFS) → the amount of charge that flows thru an electrolytic cell is a factor of the current and time that its running- I = current
- Q = charge (in coulombs)
- T = time
- One Faraday = one mole of electrons “pushed” thru the wire