Cell Potential and Free Energy
- Cell potential or electromotive force: the “pull” or driving force on the electrons
- Substance being reduced “pulls” the electrons thru the wire
- Substance being oxidized “pushes” the electrons
- Ecell is measured in Volts (V) using a voltmeter: measures the voltage (electricity)→ how much electricity is flowing thru the wire
- The total cell potential is the sum of the potential at each electrode
- Ex:

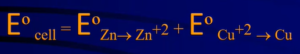
- “Ecell = Oxidation – Reduction”
- One of the reactions must be reversed, so the sign must be changed
- How to know which half-reaction should be reversed: For a galvanic (voltaic) cell, the overall cell potential will be POSITIVE → have to flip the one with more negative E° (the oxidation-half reaction)
- Key word = voltaic → E° must be (+)
Standard Hydrogen Electrode
- E° = 0
- When changing the amount of electrons by multiplying an integer, DO NOT change/multiply E°
- Substance with most favorable reduction potential (stronger reducing power/agent) = more negative E° red
- Substance with the most favorable oxidation potential (stronger oxidizing power/agent) = more positive E° red
Description of a Galvanic Cell
3 things for a complete description:
- Cell potential (+) and balanced cell reaction
- Direction of electron flow
- Designation of anode and cathode
- Whichever half-reaction that flipped/reversed = anode
Favorability of a Galvanic Cell
- The reaction always run spontaneously in the direction that produces a positive (total) cell potential
- If E° < 0, then ΔG° > 0 → nonspontaneous
- If E° > 0, then ΔG° < 0 → spontaneous
Free Energy and Cell Potential
- ΔG° = –nFE° (on RFS)
- N = number of moles = coefficient of the number of electrons that you canceled out
- F = Faraday’s constant = 96, 485 coulombs per mole of electrons