Electric Charge
- Charge is a fundamental property of objects, like mass.
- Units of charge: Coulombs (C).
- There are two types of charge, positive charge (due to protons) and electrons (caused by electrons).
- Protons and electrons have equal but opposite charges. The magnitude of the charge of a proton or electron is the fundamental charge is equal to 1.60×10−19 bC. Any net charge on an object is due to an unequal amount of protons/electrons and the net charge is a multiple of the fundamental charge.
- Classically demonstrating that there are two types of charge:
When rubbing a glass rod with silk, electrons are transferred from the glass rod to the silk. The glass rod loses electrons and becomes positively charged.
When rubbing a rubber rod with fur, the rod gains electrons and becomes negatively charge.
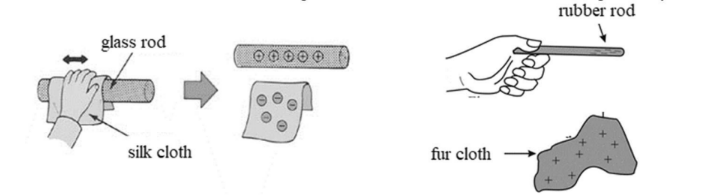
*Charge is always transferred by the flow of electrons. A Positive charge means an electron deficit.
Atomic Structure
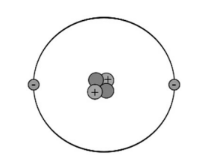
- The atomic nucleus contains protons and neutrons. Protons have positive charge while neutrons have no charge. Almost all the mass of an atom is in the nucleus.
- Protons and neutrons are held together by the strong force.
- Electrons exist far outside the nucleus of an item. They have little mass, but have a negative charge, equal to the positive charge of protons. Electrons are scattered out over region of space outside the nucleus (an orbital). You can’t precisely state where an electron is at a given moment, but can state where it is likely to be found (electron locations are given by probability).
Conductors vs. Insulators
- Conductors – Materials with free electrons that can move easily. As a result, charge is easily transferred due to the flow of electrons.
- Most metals are good conductors
- The atomic structure of metals causes electrons to move as far away from each other as possible. Free electrons in metal are what causes conductivity from metal.
- Insulators – Material in which electrons are tightly bound to nucleus and thus charge is not transferred.
- Glass, rubber, and plastic are examples of non-conductors (insulators)
- Electrons in insulators are tightly bound to the nucleus, so there are no free electrons to transfer.
The Electroscope
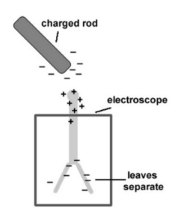
- The electroscope – Electroscopes are used to detect charge. An electroscope consists of two “leaves” made of foil. When the electroscope is charged, the leaves repel each other and diverge. When the charge has more magnitude, the leaves divege more and more.
Charging by Conduction
- Both insulators and conductors can be charged by direct contact in a process known as conduction.
- When a charged rod touches an uncharged electroscope charge from the rod is transferred to electroscope.
- A negatively charged rod will transfer electrons to the electroscope. A positively charged rod will cause electrons form the electroscope to transfer to the rod.
*when the rod is remvoed, the electroscope retains a charge that is the same sign as the rod.
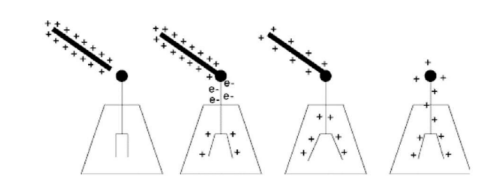
Charging by Induction
- In induction, conductors are charged without coming into direct contact with another charged object
- To charge an electroscope by induction:
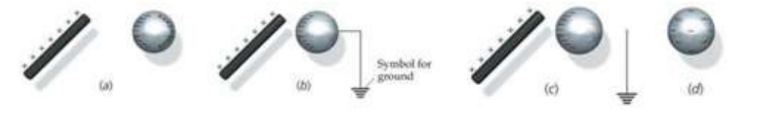
(a) Bring a charged object close to the knob of the electroscope, but do not touch it.
*When a conductor is close to a charged object, free electrons on the conductor will be attracted to the charged object.
(b) Ground the electroscope by touching it.
*In electricity, “ground” means to make an electrical connection between the earth and the object being “grounded”. When an object is grounded, free electrons between the object and earth. Because earth is large, it can make an object charge neutral by either absorbing or supplying an essentially limitless amount of electrons.
(c) Break the connection with ground.
*with the grond connection broken, there will be no way for electrons to be transferred.
(d) Remove the charged object.
*the charge on the conductor will redistribute itself evenly on the object as soon as the charged rod is removed leaving it with a charge opposite the rod.
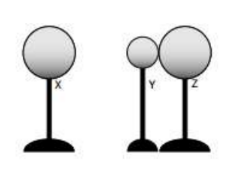
Example A: Three metal spheres rest on insulating stands as shown in the diagram at right. Sphere Y is smaller than Spheres X and Z, which are the same size. Spheres Y and Z are initially neutral, and Sphere X is charged positively. Spheres Y and Z are placed in contact near Sphere X, though Sphere X does not touch the other spheres.
(a) Does sphere Y have a positive, negative, or neutral charge?
(b) Does sphere Z have a positive, negative, or neutral charge?
(c) Sphere X is moved away Spheres Y and Z are separated. Compare the magnitude of the charge on Spheres Y and Z.

(a) Y has a negative charge. X has a positive charge, so negative charge is attracted towards it. Therefore, electrons will flow from Z to Y.
(b) Z has a positive charge since it lost electrons to Y.
(c) Equal and opposite charge.
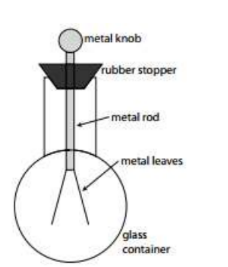
Example B: A student investigates how a negatively charged ebonite rod influences an
uncharged electroscope as shown in the diagram at right.
(a) Briefly describe how the ebonite rod can be given a negative charge.
(b) Write a procedure of how the negatively charged ebonite rod could produce a positive charge on the electroscope. Other materials can be used, but none can be charged, and none can alter the charge of the ebonite rod.
(c) Assume the student charges the electroscope. Describe how the negatively charged ebonite rod could be used
to test whether the electroscope is positively charged. Also explain what the student should expect to observe and why
(a) The ebonite rod can be rubbed with a fur cloth. Compared to fur, ebonite is a strong insulator, so electrons
from the fur will transfer to the ebonite, giving it excess electrons and a subsequent negative charge.
(b) Bring the ebonite rod close to, but not touching, the electroscope knob. Ground the electroscope. Break the ground connection. Move the rod away.
(c) Touch the rod to the scope. If the leaves collapse, they were positively charge. If they further diverge, they were negatively charge.
Rate your understanding: Electrostatics
