Question 1
(a) Describe and explain the variation in the solubilities of the hydroxides of the Group 2 elements.[4]
The table lists the standard enthalpy changes of formation, $\Delta H_f^{-}$, for some compounds and aqueous ions.
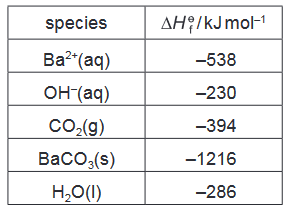
(b) (i) Reaction 1 occurs when $\mathrm{CO}_2(\mathrm{~g})$ is bubbled through an aqueous solution of $\mathrm{Ba}(\mathrm{OH})_2$.
Use the data in the table to calculate the standard enthalpy change for reaction $1, \Delta H_{\mathrm{r} 1}^{\circ}$.
$
\mathrm{Ba}(\mathrm{OH})_2(\mathrm{aq})+\mathrm{CO}_2(\mathrm{~g}) \rightarrow \mathrm{BaCO}_3(\mathrm{~s})+\mathrm{H}_2 \mathrm{O}(\mathrm{l}) \quad \text { reaction } 1
$
$
\Delta H_{r 1}^{\infty}=
$ $\mathrm{kJmol}^{-1}[2]$
If $\mathrm{CO}_2(\mathrm{~g})$ is bubbled through an aqueous solution of $\mathrm{Ba}(\mathrm{OH})_2$ for a long time, the precipitated $\mathrm{BaCO}_3(\mathrm{~s})$ dissolves, as shown in reaction 2.
$
\mathrm{BaCO}_3(\mathrm{~s})+\mathrm{CO}_2(\mathrm{~g})+\mathrm{H}_2 \mathrm{O}(\mathrm{l}) \rightarrow \mathrm{Ba}\left(\mathrm{HCO}_3\right)_2(\mathrm{aq}) \quad \text { reaction } 2
$
The standard enthalpy change for reaction $2, \Delta H_{12}^*=-26 \mathrm{~kJ} \mathrm{~mol}^{-1}$.
(ii) Use this information and the data in the table to calculate the standard enthalpy change of formation of the $\mathrm{HCO}_3^{-}(\mathrm{aq})$ ion.
If $\mathrm{CO}_2(\mathrm{~g})$ is bubbled through an aqueous solution of $\mathrm{Ba}(\mathrm{OH})_2$ for a long time, the precipitated $\mathrm{BaCO}_3(\mathrm{~s})$ dissolves, as shown in reaction 2.
$
\mathrm{BaCO}_3(\mathrm{~s})+\mathrm{CO}_2(\mathrm{~g})+\mathrm{H}_2 \mathrm{O}(\mathrm{l}) \rightarrow \mathrm{Ba}\left(\mathrm{HCO}_3\right)_2(\mathrm{aq}) \quad \text { reaction } 2
$
The standard enthalpy change for reaction $2, \Delta H_{12}^*=-26 \mathrm{~kJ} \mathrm{~mol}^{-1}$.
(ii) Use this information and the data in the table to calculate the standard enthalpy change of formation of the $\mathrm{HCO}_3^{-}(\mathrm{aq})$ ion.
(iii) The overall process is shown by reaction 3 .
Use your answer to (ii), and the data given in the table, to calculate the standard enthalpy change for reaction $3, \Delta H_{\mathrm{r} 3}^{\circ}$.
$
\mathrm{Ba}(\mathrm{OH})_2(\mathrm{aq})+2 \mathrm{CO}_2(\mathrm{~g}) \rightarrow \mathrm{Ba}\left(\mathrm{HCO}_3\right)_2(\mathrm{aq}) \quad \text { reaction } 3
$
(iii) The overall process is shown by reaction 3.
Use your answer to (ii), and the data given in the table, to calculate the standard enthalpy change for reaction $3, \Delta H_{r 3}^{\circ}$.
$
\mathrm{Ba}(\mathrm{OH})_2(\mathrm{aq})+2 \mathrm{CO}_2(\mathrm{~g}) \rightarrow \mathrm{Ba}\left(\mathrm{HCO}_3\right)_2(\mathrm{aq}) \quad \text { reaction } 3
$
(iv) How would the value of $\Delta H_{\mathrm{r} 3}^-$ compare with the value of $\Delta H_{\mathrm{r} 4}^-$ for the similar reaction with $\mathrm{Ca}(\mathrm{OH})_2(\mathrm{aq})$ as shown in reaction 4 ?
Explain your answer.
$
\mathrm{Ca}(\mathrm{OH})_2(\mathrm{aq})+2 \mathrm{CO}_2(\mathrm{~g}) \rightarrow \mathrm{Ca}\left(\mathrm{HCO}_3\right)_2(\mathrm{aq}) \quad \text { reaction } 4
$
(iv) How would the value of $\Delta H_{\mathrm{r} 3}^-$ compare with the value of $\Delta H_{\mathrm{r} 4}^{\text {- }}$ for the similar reaction with $\mathrm{Ca}(\mathrm{OH})_2(\mathrm{aq})$ as shown in reaction 4 ?
Explain your answer.
$
\mathrm{Ca}(\mathrm{OH})_2(\mathrm{aq})+2 \mathrm{CO}_2(\mathrm{~g}) \rightarrow \mathrm{Ca}\left(\mathrm{HCO}_3\right)_2(\mathrm{aq}) \quad \text { reaction } 4
$
(c) The standard entropy change for reaction 1 is $\Delta S_{\mathrm{r} 1}^-$.
Suggest, with a reason, how the standard entropy change for reaction 3 might compare with $\Delta S_{\mathrm{r} 1}^{-}$
▶️Answer/Explanation
Ans:
1(a) $\quad$ solubility increases down the group
$\Delta H_{\text {latt }}$ and $\Delta H_{\text {hyd }}$ both decrease
or $\Delta H_{\text {latt }}$ and $\Delta H_{\text {hyd }}$ both become less exothermic / more endothermic
$\Delta H_{\text {latt }}$ decreases / changes more (than $\Delta H_{\text {hyd }}$ as $\mathrm{OH}^{-}$being smaller than $\mathrm{M}^{2+}$ )
$\Delta H_{\mathrm{sol}}$ becomes more exothermic / more negative / less endothermic / less positive
1(b)(i) $\begin{aligned} & \Delta H_{\mathrm{r} 1}-(538+2 \times 230+394)=-(1216+286) \\ & \Delta H_{\mathrm{r} 1}-1392=-1502\end{aligned}$
$\Delta H_{\mathrm{r} 1}=-110$
1 (b)(ii) $\begin{aligned} & \text { let } \Delta H_1\left(\mathrm{HCO}_3{ }^{-}(\mathrm{aq})\right)=\mathrm{y} \\ & 2 \mathrm{y}-538=-1216-394-286-26 \\ & y=-692\end{aligned}$
1(b)(iii) $\begin{aligned} & \Delta H_{13}-538-2(230+394)=-538-2(692) \\ & \Delta H_{13}=-136\end{aligned}$
1(b)(iv) $\quad \Delta H r_{3}$ will be identical to $\Delta H_{14}, /$ unchanged
as the reaction is the same, or:
$
2 \mathrm{OH}^{-}(\mathrm{aq})+2 \mathrm{CO}_2(\mathrm{g}) \longrightarrow 2 \mathrm{HCO}_3{ }^{-}(\mathrm{aq}) \text { or }
$
metal ions stay in solution/metal ions are unchanged/ are spectators
1(c) $\quad$ more gaseous moles are being consumed (in reaction 3 ) or more $\mathrm{CO}_2$ moles are being consumed (in reaction 3 )
$\Delta S$ is therefore expected to be more negative/less positive for reaction 3 .
Question 2
(a) Describe the trend in the reactivity of the halogens $\mathrm{Cl}_2, \mathrm{Br}_2$ and $\mathrm{I}_2$ as oxidising agents. Explain this trend using values of $E^*\left(\mathrm{X}_2 / \mathrm{X}^{-}\right)$from the Data Booklet.[2]
(b) (i) Write an equation for the reaction between chlorine and water.[2]
(ii) Use standard electrode potential, $E^{\circ}$, data from the Data Booklet to calculate the $E_{\text {cell }}^{\circ}$ for the following reaction.
$
\mathrm{Cl}_2+2 \mathrm{OH}^{-} \rightleftharpoons \mathrm{Cl^{-}}+\mathrm{ClO}^{-}+\mathrm{H}_2 \mathrm{O}
$
(iii) The $\left[\mathrm{OH}^{-}\right]$was increased and the $E_{\text {cell }}$ was measured.
Indicate how the value of the $E_{\text {cell }}$ measured would compare to the $E_{\text {cell }}{\text {calculated in (ii) by }}$ placing one tick $(\checkmark)$ in the table.

Explain your answer. [2]
(c) A half-equation involving bromate( $(\mathrm{V})$ ions, $\mathrm{BrO}_3^{-}$, and bromide ions is shown.
$
\mathrm{BrO}_3^{-}(\mathrm{aq})+3 \mathrm{H}_2 \mathrm{O}(\mathrm{l})+6 \mathrm{e}^{-} \rightleftharpoons \mathrm{Br}^{-}(\mathrm{aq})+6 \mathrm{OH}^{-}(\mathrm{aq}) \quad E^{\circ}=+0.58 \mathrm{~V}
$
(i) An alkaline solution of chlorate(I), $\mathrm{ClO}^{-}$, can be used to oxidise bromide ions to bromate(V) ions.
Use the Data Booklet and the half-equation shown to write an equation for this reaction.[1]
(ii) Calculate the $E^-{\text {cell }}$for the reaction in (i).
(iii) When a concentrated solution of bromic(V) acid, $\mathrm{HBrO}_3$, is warmed, it decomposes to form bromine, oxygen and water only.
Write an equation for this reaction. The use of oxidation numbers may be helpful.[1]
▶️Answer/Explanation
Ans:
2(a) the $E^{\circ}$ for $X_2 / X^{-}$becomes less positive / decrease down the group so the halogens are less reactive (as oxidants) down the group
2(b)(i) $\quad \mathrm{Cl}_2+\mathrm{H}_2 \mathrm{O} \longrightarrow \mathrm{HCl}+\mathrm{HClO}$
2(b)(ii) $\begin{aligned} & \mathrm{Cl}_2 / \mathrm{C} l^-=+1.36 \mathrm{~V} \text { and } \mathrm{ClO}^{-} /\left(\mathrm{C} l+\mathrm{OH}^{-}\right)=+0.89 \mathrm{~V} \\ & \text { so } E_{\text {cell }}^o=1.36-0.89=(+) 0.47 \mathrm{~V}\end{aligned}$
2(b)(iii) $\quad$ box three ticked
Le Chatelier argument, more $\mathrm{OH}^{-} /$increase reactant concentration so equilibrium shifts right or an argument based on the half cell with $\mathrm{OH}^{-}$
2(c)(i) $\quad \mathrm{Br}^{-}+3 \mathrm{ClO}^{-} \longrightarrow \mathrm{BrO}_3^{-}+3 \mathrm{Cl}^{-}$
2(c)(ii) $\quad E_{\text {Cell }}=0.89-0.58=+0.31 \mathrm{~V}$
2 (c)(iii) $\quad 4 \mathrm{HBrO}_3 \longrightarrow 2 \mathrm{Br}_2+5 \mathrm{O}_2+2 \mathrm{H}_2 \mathrm{O}$
Question 3
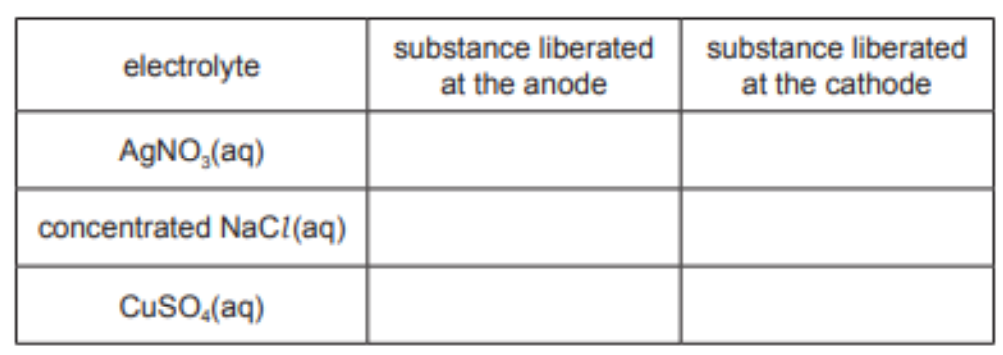
(a) Complete the table, identifying the substance liberated at each electrode during electrolysis with inert electrodes.[3]
(b) Molten calcium iodide, $\mathrm{CaI}_2$, is electrolysed in an inert atmosphere with inert electrodes.
(i) Write ionic equations for the reactions occurring at the electrodes.[2]
(ii) The electrolysis of molten $\mathrm{CaI}_2$ is a redox process.
Identify the ion that is oxidised and the ion that is reduced, explaining your answer by reference to oxidation numbers.[2]
(iii) Describe two visual observations that would be made during this electrolysis.$[1]$
1……………………………………………………………………………..
2……………………………………………………………………………
(c) An oxide of iron dissolved in an inert solvent is electrolysed for 2.00 hours using a current of $0.800 \mathrm{~A}$. The electrolysis products are iron and oxygen. The mass of iron produced is $1.11 \mathrm{~g}$.
Calculate the oxidation number of $\mathrm{Fe}$ in the oxide of iron. Show all your working.
▶️Answer/Explanation
Ans:
3(a)
$2 \mathrm{I}^{-} \rightarrow \mathrm{I}_2+2 \mathrm{e}^{-}$
$\mathrm{Ca}^{2+}+2 \mathrm{e}^{-} \rightarrow \mathrm{Ca}$
3(b)(iii)
- metal/grey/silvery
- $\quad$ purple AND vapour / gas / fumes
- amount of melt decreases
any 2 points for 1 mark
3(c)
$\begin{aligned} & 2 \times 60 \times 60 \times 0.8=5760 \mathrm{C} \\ & \text { AND } \\ & 5760 / 96500=0.060(0.0597) \mathrm{F}\end{aligned}$
$1.11 / 55.8=0.020(0.0199) \mathrm{mol}$ of $\mathrm{Fe}$
$0.06 / 0.02=3 \therefore \mathrm{Fe}^{3+}$ or +3 or 3
Question 4
(i) Complete the electronic configuration of a copper atom.$1 s^2 2 s^2 2 p^6…….$
(ii) Explain why most copper(II) salts are coloured.
Suggest why copper(I) salts are usually white.
(b) Brass is an alloy of copper and zinc. The following reaction can be used to determine the amount of copper in a sample of brass.
$
2 \mathrm{Cu}^{2+}(\mathrm{aq})+4 \mathrm{I}^{-}(\mathrm{aq}) \rightarrow 2 \mathrm{CuI}(\mathrm{s})+\mathrm{I}_2(\mathrm{aq})
$
The procedure was carried out using the following steps.
- A solution of $\mathrm{Cu}^{2+}(\mathrm{aq})$ was obtained by dissolving a $1.50 \mathrm{~g}$ sample of brass in concentrated sulfuric acid and diluting with water.
- An excess of $\mathrm{I}^{-}(\mathrm{aq})$ was added.
- The iodine produced was titrated against a $0.500 \mathrm{~mol} \mathrm{dm}^{-3}$ solution of thiosulfate ions, $\mathrm{S}_2 \mathrm{O}_3^{2-(a q)}$.
$
\mathrm{I}_2(\mathrm{aq})+2 \mathrm{~S}_2 \mathrm{O}_3^{2-(\mathrm{aq})} \rightarrow 2 \mathrm{I}-(\mathrm{aq})+\mathrm{S}_4 \mathrm{O}_6^2-(\mathrm{aq})
$
- The volume of $\mathrm{S}_2 \mathrm{O}_3^{2-}$ solution needed to reach the end-point was $28.35 \mathrm{~cm}^3$.
Calculate the percentage by mass of copper in the sample of brass.
(c) (i) Use standard electrode potential data from the Data Booklet to calculate $E_{\mathrm{cell}}^{\text {- }}$ for the reaction.
$
2 \mathrm{Cu}^{2+}(\mathrm{aq})+4 \mathrm{I}^{-}(\mathrm{aq}) \rightarrow 2 \mathrm{CuI}(\mathrm{s})+\mathrm{I}_2(\mathrm{aq})
$
(ii) Explain how the value of $E_{cell}^-$ calculated in (i) predicts that the reaction is not likely to occur.
In an experiment, a solution of $\mathrm{I}-(\mathrm{aq})$ is added to a solution of $\mathrm{Cu}^{2+}(\mathrm{aq})$. A reaction does occur and a precipitate of sparingly soluble $\mathrm{CuI}(\mathrm{s})$ is formed.
The concentration of $\mathrm{Cu}^{2+}(\mathrm{aq})$ remaining in the solution is $1.00 \mathrm{~mol} \mathrm{dm}^{-3}$. The concentration of $\mathrm{Cu}^{+}(\mathrm{aq})$ in a saturated solution of $\mathrm{CuI}$ is $1.3 \times 10^{-6} \mathrm{~mol} \mathrm{dm}^{-3}$.
(iii) Use the Nernst equation to calculate the electrode potential, E, for the $\mathrm{Cu}^{2+} / \mathrm{Cu}^{+}$half cell in this experiment.
(iv) Copper(I) chloride is also sparingly soluble in water.
Suggest why the following reaction does not occur.
$
2 \mathrm{Cu}^{2+}(\mathrm{aq})+4 \mathrm{Cl}^{-}(\mathrm{aq}) \longrightarrow 2 \mathrm{CuCl}(\mathrm{s})+\mathrm{Cl}_2(\mathrm{aq})
$
(d) When chloride ions are added to a solution containing $\mathrm{Cu}^{2+}(\mathrm{aq})$, the complex ion $\left[\mathrm{CuCl}_4\right]^{2-}(\mathrm{aq})$ is formed.
(i) State the colours of $\mathrm{Cu}^{2+}(\mathrm{aq})$ and $\left[\mathrm{CuCl}_4\right]^{2-}(\mathrm{aq})$.
$\mathrm{Cu}^{2+}(\mathrm{aq})$
$\left[\mathrm{CuCl}_4\right]^{2-}(\mathrm{aq})$
(ii) Name the type of reaction that occurs when $\left[\mathrm{CuCl}_4\right]^{2-}(\mathrm{aq})$ is formed from $\mathrm{Cu}^{2+}(\mathrm{aq})$.
(iii) Write an expression for the stability constant, $K_{\text {wab }}$, for $\left[\mathrm{CuCl}_4\right]^2-(\mathrm{aq})$. Include the units in your answer.
$K_{\mathrm{stab}}=$
▶️Answer/Explanation
Ans:
4(a)(i) $\left(1 s^2 2 s^2 2 p^6\right) 3 s^2 3 p^6 3 d^{10} 4 s^1$
4(a)(ii) M1 d orbitals / sub-shell split into two levels by repulsion of approaching ligands
M2 light absorbed and complementary colour observed
M3 (d) electron(s) promoted / excited OR (d) electron(s) moves to higher (d) orbital
M4 (in $\mathrm{Cu}(\mathrm{I})$ complexes) all the orbitals in $\mathrm{Cu}$ are full OR $\mathrm{Cu}(\mathrm{I})$ is $\mathrm{d}^{10}$
4(b) $
\mathrm{n}\left(\mathrm{S}_2 \mathrm{O}_{3^2}{ }^{-}\right)=28.35 \times 0.5 / 1000=0.0142(0.014175)
$
this also equals $n\left(\mathrm{Cu}^{2+}\right)$
mass of $\mathrm{Cu}=0.014175 \times 63.5=0.90 \mathrm{~g}$
$\%$ of $\mathrm{Cu}=100 \times 0.90 / 1.5=60 \%$
$4(\mathrm{c})(\mathrm{i}) \quad E^{\circ}$ cel $=0.15-0.54=-0.39(\mathrm{~V})$
4(c)(ii) $\quad$ since $E^{\circ}$ cell is negative (reaction is not likely to occur) OR since $E^{\circ}$ cell $<0$ (reaction is not feasible / not spontaneous)
4(c)(iii) $\begin{aligned} E & =E^{\circ}+(0.059 / 1) \log \left(1.0 / 1.3 \times 10^{-6}\right) \\ & =+0.15+0.059 \times 5.89 \\ & =+0.50 / 0.497 \mathrm{~V}\end{aligned}$
4 (c)(iv) $\quad E_{\text {cell }}$ is very negative OR calculation $\left(E_{\text {cell }}=0.15-1.36=\right)-1.21 \mathrm{~V}$
4(d)(i) $\mathrm{Cu}^{2+}(\mathrm{aq})$ is (light) blue AND $\left[\mathrm{CuCl}_4\right]^{2-}(\mathrm{aq})$ is yellow
4(d)(ii) ligand displacement/ replacement/ substitution/ exchange
4 (d)(iii) $\quad K_{\text {stab }}=[\mathrm{CuCl}]^{2-} /\left(\left[\mathrm{Cu}^{2+}\right][\mathrm{Cl}]^4\right)$
units: $\mathrm{mol}^{-4} \mathrm{dm}^{12}$
Question 5
(a) Benzene reacts with bromine in the presence of an aluminium bromide catalyst, $\mathrm{AlBr}_3$, to form bromobenzene. This is a substitution reaction. No addition reaction takes place.
(i) Explain why no addition reaction takes place.[1]
$\mathrm{AlBr}_3$ reacts with bromine to generate an electrophile, $\mathrm{Br}^{+}$.
(ii) Draw the mechanism of the reaction between benzene and $\mathrm{Br}^{+}$ions. Include all relevant arrows and charges.[3]
(iii) Write an equation to show how the $\mathrm{AlBr}_3$ catalyst is reformed. [1]
(b) Suggest why bromination of phenol occurs more readily than bromination of benzene.[2]
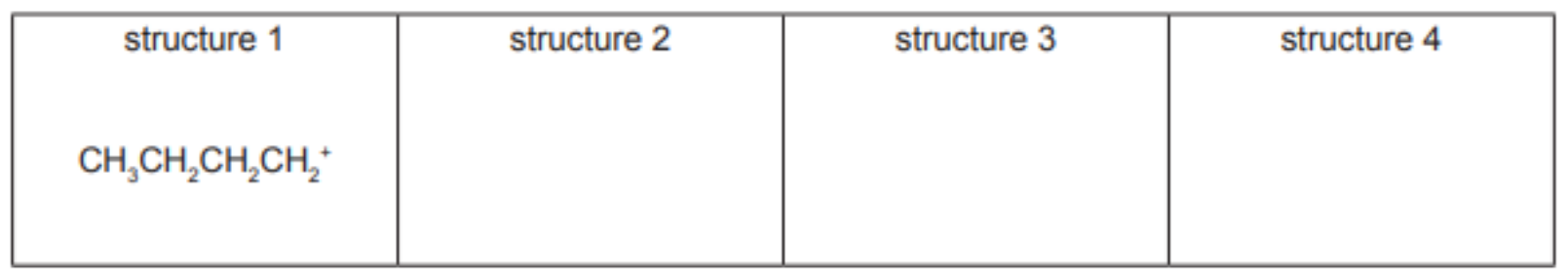
(c) (i) There are four different carbocations with the same formula, $\mathrm{C}_4 \mathrm{H}_9{ }^+$. One structure is given in the table.
Suggest the structural formulae of the three other carbocations.
[3]
(ii) Benzene reacts with each of these carbocations in separate Friedel-Crafts alkylation reactions.
In each reaction an organic compound with formula $\mathrm{C}_{10} \mathrm{H}_{14}$ is formed. The number of peaks observed in the carbon-13 NMR spectrum of each compound is given.
Suggest the structures for the three other compounds.
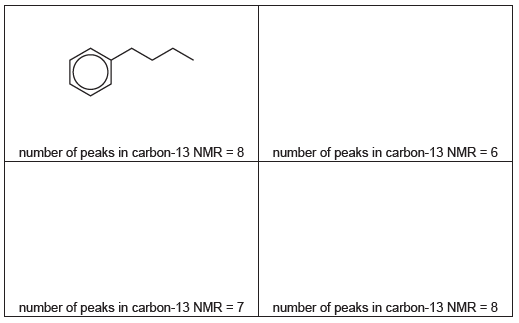
▶️Answer/Explanation
Ans:
5(a)(i) The substitution product is stabilised by delocalisation of $(6) \pi$-electrons OR
The addition product is not stabilised by delocalisation of $(6) \pi$-electrons [1]
5(a)(ii)
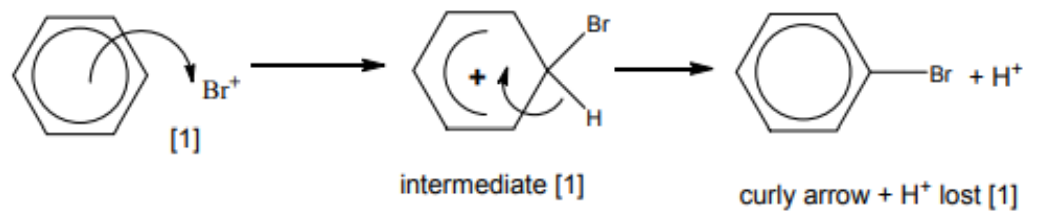
- first curly arrow
- intermediate
- $2^{\text {nd }}$ curly arrow, product and $\mathrm{H}^{+}$formed/lost
5 (a)(iii) $\quad \mathrm{AlBr}_4+\mathrm{H}^{+} \rightarrow \mathrm{AlBr}_3+\mathrm{HBr}$
5(b) lone pair of oxygen is delocalised into the ring
any one from:
- phenol has a higher electron density in the ring
- phenol can polarise/induce a dipole in $\mathrm{Br}_2$
5(c)(i) $\mathrm{CH}_3 \mathrm{CH}_2 \mathrm{CH}^{+} \mathrm{CH}_3 \quad\left(\mathrm{CH}_3\right)_2 \mathrm{CHCH}_2^{+} \quad\left(\mathrm{CH}_3\right)_3 \mathrm{C}^{+} \quad$ Each correct structure $=1$ mark
$5(c)(i i)$
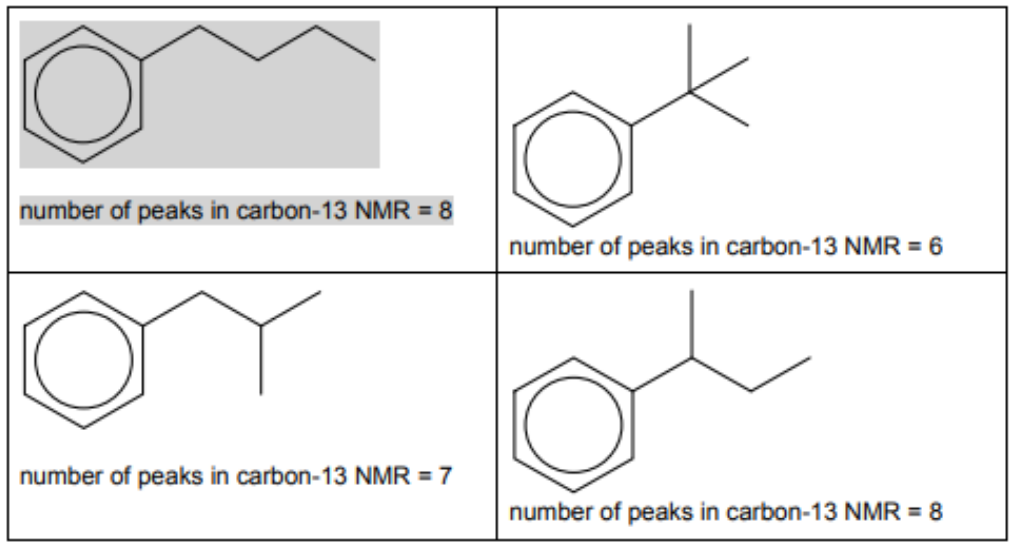
Two correct organic products $=1$ mark three correct organic products $=2$ marks
all products linked correctly to NMR $=2$ marks
Question
An excess of sodium iodide is added to a solution of copper(II) sulfate. lodine and a white precipitate of copper(I) iodide are formed.
(a) Write an equation for the reaction that occurs. [1]
(b) (i) Explain why the copper(II) sulfate solution is coloured.[4]
(ii) Suggest why the precipitate of copper(I) iodide is white. [1]
(c) Use suitable $E^-$ values from the Data Booklet to predict whether iodide ions can reduce $\mathrm{Cu}^{2+}$ to $\mathrm{Cu}^{+}$under standard conditions. Explain your answer.[2]
(d) An excess of sodium iodide is added to copper(II) sulfate solution. Copper(I) iodide forms as a precipitate. After precipitation, $\left[\mathrm{Cu}^{+}\right]$is much lower than $1.0 \mathrm{~mol} \mathrm{dm}^{-3}$.
Use this information and your answer to (c) to explain how the relevant electrode potentials change and hence why $\mathrm{I}^{-}$ions can reduce $\mathrm{Cu}^{2+}$ ions.
▶️Answer/Explanation
Ans:
$
6\left(\text { a) } \quad 2 \mathrm{Cu}^{2+}+4 \mathrm{I}^{-} \rightarrow 2 \mathrm{Cul}+\mathrm{I}_2 \quad \text { OR } 2 \mathrm{CuSO}_4+4 \mathrm{Nal} \rightarrow 2 \mathrm{CuI}+\mathrm{I}_2+2 \mathrm{Na}_2 \mathrm{SO}_4\right.
$
6(b)(i) M1: d-d orbital splitting occurs
M2: electron(s) promoted/ excited
M3: wavelength / frequency of light is absorbed
M4: colour seen is complementary
OR wavelength / frequency of light not absorbed is seen [1]
6(b)(ii) $\mid$ (for $\left.\mathrm{Cu}^{+}\right) \quad 3 d^{10}$ OR $3 d$ subshell full [1]
6(c) $\quad \mathrm{M} 1: \quad\left(\mathrm{Cu}^{2+} / \mathrm{Cu}^{+}\right) E^{-}=(+) 0.15 \mathrm{~V} \mathrm{AND}\left(\mathrm{I}_2 / \mathrm{I}^{-}\right) E^{-}=(+) 0.54 \mathrm{~V}$
M2: $\quad$ No, since $\left(E_{\text {cell }}\right)$ negative $/-0.39 \mathrm{~V}$
OR $\underline{\text { No, }}$, since $\left(\mathrm{I}_2 / \mathrm{I}^{-}\right)$is more positive than $\left(\mathrm{Cu}^{2+} / \mathrm{Cu}^{+}\right)$
OR $\underline{\text { No }}, \mathrm{I}_2$ is more easily reduced OR No, $I_2$ stronger oxidant ORA [1]
6(d) $M 1: \mathrm{Cu}^{2+} / \mathrm{Cu}^{+} E$ becomes more positive as equilibrium shifts to the right [1]
M2: The new $\mathrm{E}$ for $\mathrm{Cu}^{2+} / \mathrm{Cu}^{+}$is more positive than $0.54 / \mathrm{E}^{\circ}\left(\mathrm{I}_2 / \mathrm{I}^{-}\right)$ [1]
Question 7
Procaine is used as an anaesthetic in medicine. It can be synthesised from methylbenzene in five steps as shown in Fig. 7.1.
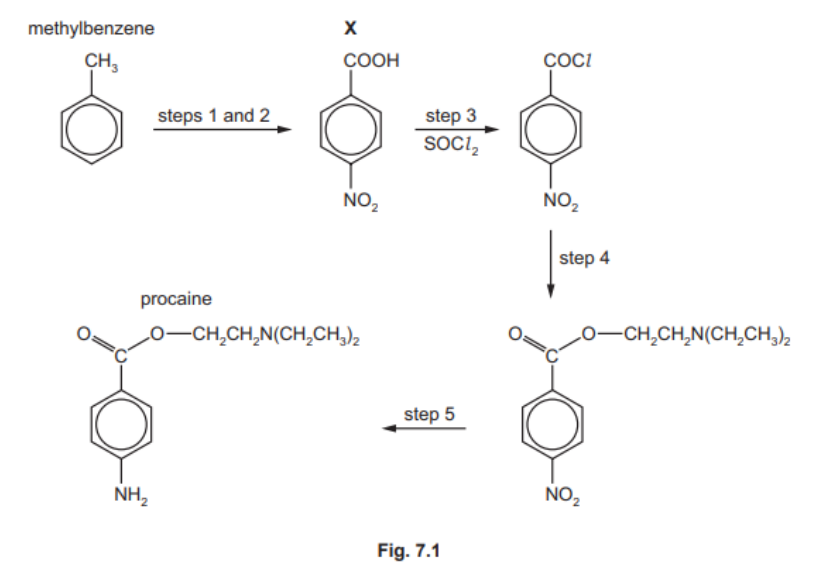
(a) (i) Name all the functional groups present in procaine. [1]
(ii) A molecule of procaine has 13 carbon atoms.
State the number of carbon atoms that are $\mathrm{sp}, \mathrm{sp}^2$ and $\mathrm{sp}^3$ hybridised in procaine.
sp carbons $=$ $\mathrm{sp}^2$ carbons $=$ $\mathrm{sp}^3$ carbons $=$[1]
(b) The proton ( $\left.{ }^1 \mathrm{H}\right)$ NMR spectrum of procaine dissolved in $\mathrm{D}_2 \mathrm{O}$ is recorded.
Predict the number of peaks observed.
(c) State why procaine can act as a base. [1]
(d) Compound $\mathbf{X}$ can be synthesised in two steps from methylbenzene.[1]
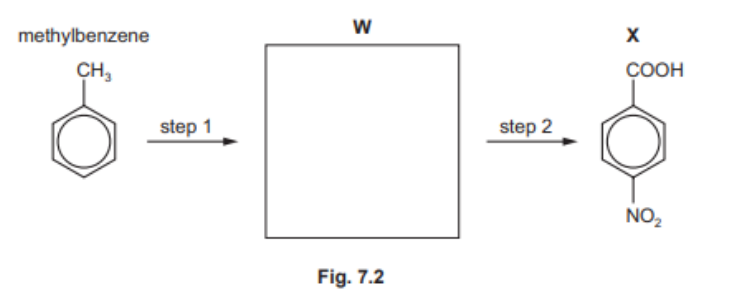
(i) Draw the structure of compound $\mathbf{W}$ in the box provided. [1]
(ii) State the reagents and conditions for step 1 and step 2. [2]
step 1
step 2
(e) Procaine is synthesised in three steps from $X$.
Suggest the reagents and conditions for step 4 and for step 5 in Fig. 7.1.[3]
step 4
step 5
(f) (i) Explain what is meant by partition coefficient, $K_{\mathrm{pc}}$.[2]
(ii) The partition coefficient of procaine between octan-1-ol and water is 1.77.
Octan-1-ol and water are immiscible. A solution containing $0.500 \mathrm{~g}$ of procaine in $75.0 \mathrm{~cm}^3$ of water is shaken with $50.0 \mathrm{~cm}^3$ of octan-1-ol.
Calculate the mass of procaine that is extracted into the octan-1-ol.
mass of procaine extracted $=$ ……………….$g$ [2] [Total: 14]
▶️Answer/Explanation
Ans:
7(a)(i) phenylamine AND amine AND ester
7(a)(ii) $s p$ carbons $=0, \mathrm{sp}^2$ carbons $=7, \mathrm{sp}^3$ carbons $=6$
7 (b) 6
7(c) lone pair on the $\mathrm{N}$ can accept a proton
7(d)(i)
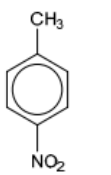
7(d)(ii) step $1 \quad$ M1 concentrated $\mathrm{HNO}_3$ and $\mathrm{H}_2 \mathrm{SO}_4$
step 2 M2 hot (alkaline) $\mathrm{KMnO}_4$ (followed by addition of $\mathrm{H}^*$ )
7(e) step $4 \quad \mathrm{M} 1 \mathrm{HOCH}_2 \mathrm{CH}_2 \mathrm{~N}\left(\mathrm{CH}_2 \mathrm{CH}_3\right)_2$
step 5 M2 Sn AND $\mathrm{HCl}$
M3 concentrated ( $\mathrm{HCl}$ ) AND heat/ reflux
7(f)(i) M1 ratio of the concentration of a solute in two solvents M2 at equilibrium
7(f)(ii) $\quad$ M1 $K_{\mathrm{pc}}=$ [procaine $]_{\text {oct }} /[\text { procaine }]_{\text {water }}$
$1.77=(\mathrm{x} / 50) /(0.5-\mathrm{x} / 75)$
M2 $1.77=1.5 x / 0.5-\mathrm{x}$
$0.885-1.77 x=1.5 x$
$x=0.271 \mathrm{~g} \min 2 \mathrm{sf}$
Question 8
Benzene, $\mathrm{C}_6 \mathrm{H}_{\mathrm{6}}$, is an aromatic molecule.
(a) State the $\mathrm{C}-\mathrm{C}-\mathrm{C}$ bond angle and the hybridisation shown by the carbon atoms in benzene. bond angle hybridisation $[1]$
(b) Benzene reacts with chloroethane in the presence of a catalyst. The reaction mechanism is called electrophilic substitution.
(i) The first step in the reaction is the generation of the ${ }^{+} \mathrm{CH}_2 \mathrm{CH}_3$ electrophile.
Write an equation for the reaction that generates this electrophile. [1]
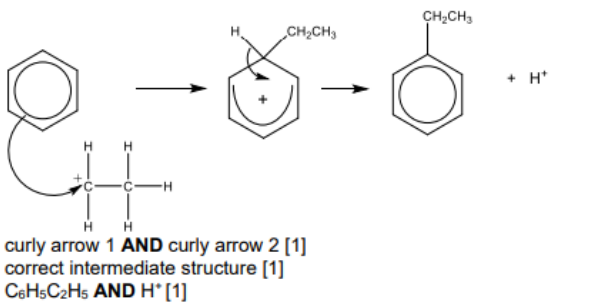
(ii) Describe the mechanism for the reaction between benzene and the ${ }^{+} \mathrm{CH}_2 \mathrm{CH}_3$ electrophile. Include all relevant curly arrows and charges.[3]
(c) Chlorobenzene and chloroethane have different reactivities in nucleophilic substitution reactions.
(i) Identify a suitable reagent to illustrate this difference in reactivity.
The reagent chosen should give visibly different results with chlorobenzene and chloroethane.[2]
(ii) Write equations to describe any reactions that occur.
(iii) Explain the difference in the reactivities of chlorobenzene and chloroethane in nucleophilic substitution reactions.[1]
▶️Answer/Explanation
Ans:
8(a) $\quad 120^{\circ}$ AND sp $^2$
B(i) $\mathrm{C}_2 \mathrm{H}_5 \mathrm{Cl}+\mathrm{AlC} l_3 \rightarrow \mathrm{CH}_3 \mathrm{CH}_2{ }^{+}+\mathrm{AlCl}_4^{-}$
8(b)(iii)
8(c)(i) $\quad$ (aqueous / alkaline) $\mathrm{AgNO}_3 /$ silver nitrate
$8(c)(i i)$
$
\begin{aligned}
& \mathrm{C}_2 \mathrm{H}_5 \mathrm{Cl}+\mathrm{H}_2 \mathrm{O} \rightarrow \mathrm{C}_2 \mathrm{H}_5 \mathrm{OH}+\mathrm{HCl} \\
& \quad \quad \quad \mathrm{C}_2 \mathrm{H}_5 \mathrm{Cl} l+\mathrm{NaOH} \rightarrow \mathrm{C}_2 \mathrm{H}_5 \mathrm{OH}+\mathrm{NaCl}
\end{aligned}
$
AND $\mathrm{Ag}^{+}+\mathrm{Cl}^{-} \rightarrow \mathrm{AgCl}$
AND NO equation shown for $\mathrm{C}_6 \mathrm{H}_5 \mathrm{Cl}$
AND $\mathrm{Ag}^{+}+\mathrm{Cl}^{-} \rightarrow \mathrm{AgCl}$
AND NO equation shown for $\mathrm{C}_6 \mathrm{H}_5 \mathrm{Cl}$
8(c)(iii) lone pair / p-orbital from $\mathbf{C l}$ overlaps with benzene ring
AND
stronger / partial double $\mathrm{C}-\mathrm{C} l$ bond OR difficult to break $\mathrm{C}-\mathrm{Cl}$ bond
Question 9
(a) (i) Explain what is meant by the following terms:
homogeneous catalyst
heterogeneous catalyst [1]
(ii) lodide ions react with peroxydisulfate ions.
$
2 \mathrm{I}^{-}+\mathrm{S}_2 \mathrm{O}_8^{2-} \rightarrow \mathrm{I}_2+2 \mathrm{SO}_4^{2-}
$
This reaction is slow, but it is catalysed by $\mathrm{Fe}^{2+}$ ions.
Write two equations to explain how this reaction is catalysed by $\mathrm{Fe}^{2+}$ ions.
1………………………………..
2………………………………… [2]
(iii) Suggest why the alternative route in the presence of $\mathrm{Fe}^{2+}$ ions has a lower activation energy than the route in the absence of a catalyst. [1]
(b) Nitrogen monoxide reacts with oxygen.
$
2 \mathrm{NO}(\mathrm{g})+\mathrm{O}_2(\mathrm{~g}) \rightarrow 2 \mathrm{NO}_2(\mathrm{~g})
$
This reaction is second order with respect to nitrogen monoxide and first order with respect to oxygen.
Under certain conditions the value of the rate constant, $k$, is $8.60 \times 10^6 \mathrm{~mol}^{-2} \mathrm{dm}^6 \mathrm{~s}^{-1}$.
(i) Construct the rate equation for this reaction.
$
\text { rate }=
$
(ii) Calculate the initial rate of the reaction under these conditions when the initial concentration of nitrogen monoxide is $7.20 \times 10^{-4} \mathrm{~mol} \mathrm{dm}^{-3}$ and the initial concentration of oxygen is $1.90 \times 10^{-3} \mathrm{~mol} \mathrm{dm}^{-3}$.
rate of reaction $=$ moldm $\mathrm{m}^{-3} \mathrm{~s}^{-1}[1]$
(c) The drug cisplatin, $\mathrm{Pt}\left(\mathrm{NH}_3\right)_2 \mathrm{Cl}_2$, hydrolyses in water.
$
\mathrm{Pt}\left(\mathrm{NH}_3\right)_2 \mathrm{Cl}_2+\mathrm{H}_2 \mathrm{O} \rightarrow\left[\mathrm{Pt}\left(\mathrm{NH}_3\right)_2 \mathrm{Cl}\left(\mathrm{H}_2 \mathrm{O}\right)\right]^{+}+\mathrm{Cl}^{-}
$
The rate equation is shown.
$
\text { rate }=k\left[\mathrm{Pt}\left(\mathrm{NH}_3\right)_2 \mathrm{Cl}_2\right]
$
The value of $k$ is $2.50 \times 10^{-5} \mathrm{~s}^{-1}$ under certain conditions.
(i) This reaction has a constant half-life.
Explain why this is the case.
(ii) Use the information in this question to show that the half-life of this reaction is $2.77 \times 10^4 \mathrm{~s}$. [1]
(iii) $8.00 \times 10^{-6}$ moles of $\mathrm{Pt}\left(\mathrm{NH}_3\right)_2 \mathrm{Cl}_2$ are added to $100 \mathrm{~cm}^3$ of water.
Calculate the time taken for the concentration of $\mathrm{Pt}\left(\mathrm{NH}_3\right)_2 \mathrm{Cl}_2$ to fall to $2.50 \times 10^{-6} \mathrm{moldm}^{-3}$.
time taken $=$ $[2]$ [Total: 10]
▶️Answer/Explanation
Ans:
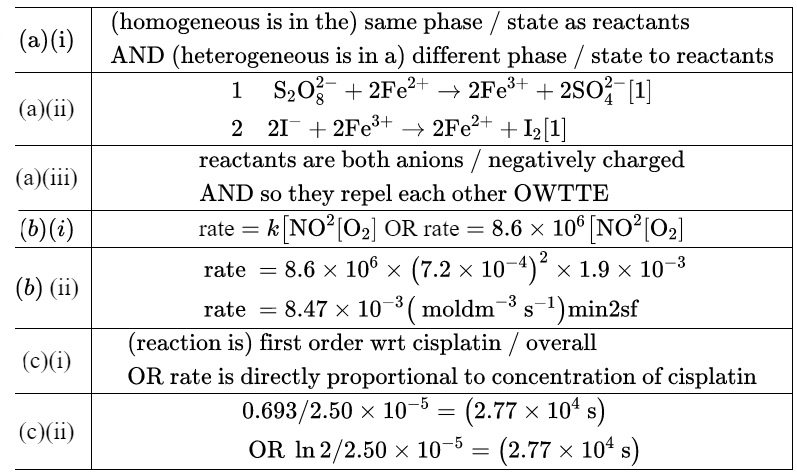
(c)(iii)
initial concentration is $8.0 \times 10^{-5} \mathrm{~mol} \mathrm{dm}^{-3}$ – five half-life periods have elapsed [1]
$$
\text { time }=5 \times 27720=1.39 \times 10^5 \mathrm{~s}[1] \mathrm{min} 2 \mathrm{sf}
$$
Question 10
Compounds $\mathbf{F}$ and $\mathbf{J}$ are shown in Fig. 4.1.
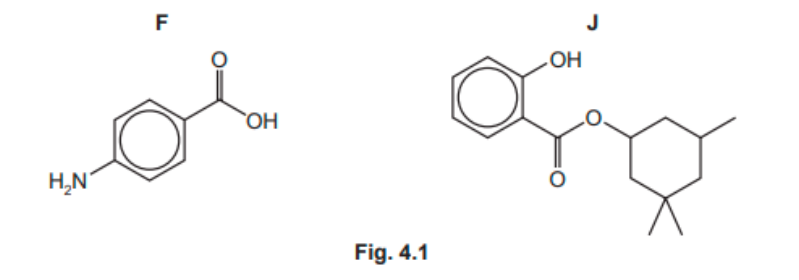
(a) $\mathbf{F}$ and $\mathrm{J}$ both contain the arene functional group.
(i) Identify the other functional groups in $\mathbf{F}$ and $\mathbf{J}$.
F:…………………………………………………………………………………………………
J:…………………………………………………………………………. [2]
(ii) State the number of chiral centres in a molecule of $\mathbf{F}$ and in a molecule of $\mathrm{J}$.
number of chiral centres in: $\mathbf{F}=$ $\mathbf{J}=$ [1]
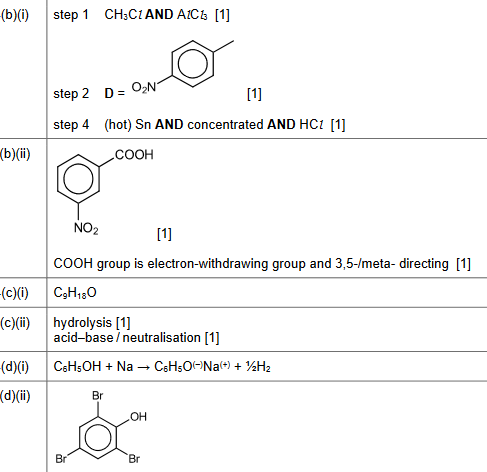
(b) A student proposes a multi-step synthesis of $\mathbf{F}$ from benzene, as shown in Table 4.1.
(i) Complete Table 4.1 by providing relevant details of the reagents and conditions for steps 1 and 4 , and the structure of product $\mathbf{D}$.
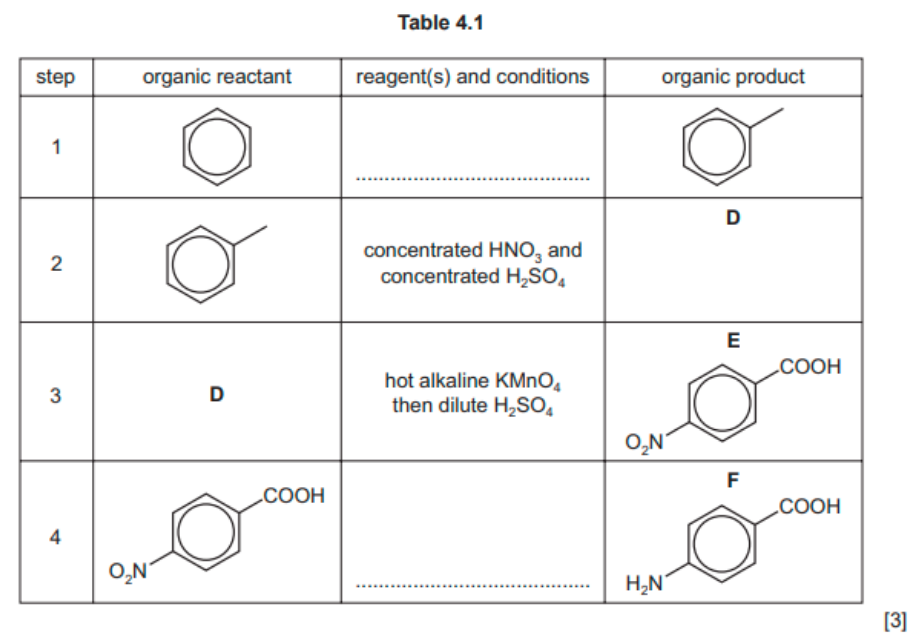
(ii) In a second multi-step synthesis, the student changes the order in which the reagents and conditions are used.
The reaction scheme is shown in Fig. 4.2.
$\mathbf{G}$ is the major product of this synthesis.
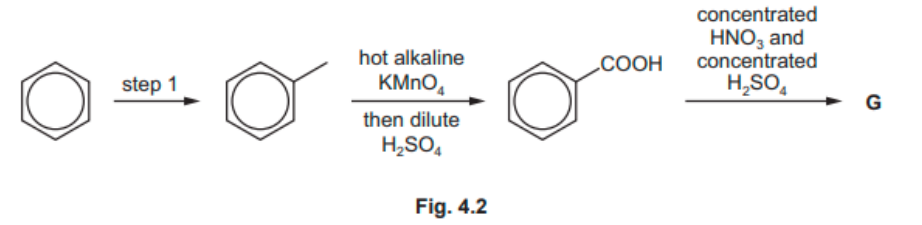
Draw the structure of $\mathbf{G}$.
Explain why $\mathbf{G}$ is the major product of the synthesis rather than $\mathbf{E}$.

…………………………………………………………………………………………………[2]
(c) $\mathrm{J}$ reacts under suitable conditions with $\mathrm{NaOH}(\mathrm{aq}$ ). After acidification of the reaction mixture, compounds $\mathbf{K}$ and $\mathbf{L}$ form.
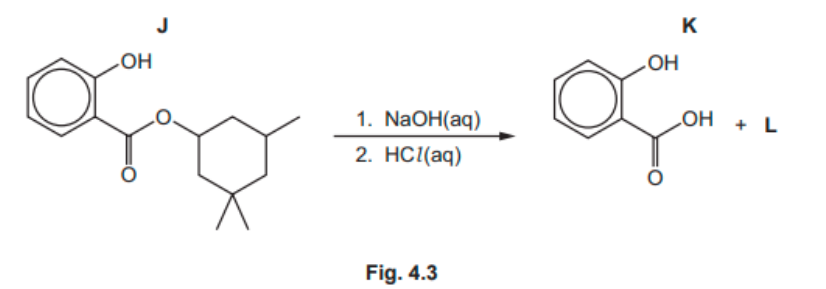
(i) Give the molecular formula of $\mathbf{L}$.
(ii) State the two types of reaction that occur when $\mathbf{J}$ reacts with $\mathrm{NaOH}(\mathrm{aq})$.
1…………………………………………..
2……………………………………………… $[2]$
(d) $\mathrm{K}$ can also be synthesised from phenol, $\mathrm{C}_6 \mathrm{H}_5 \mathrm{OH}$.
Fig. 4.4 shows several reactions of phenol.
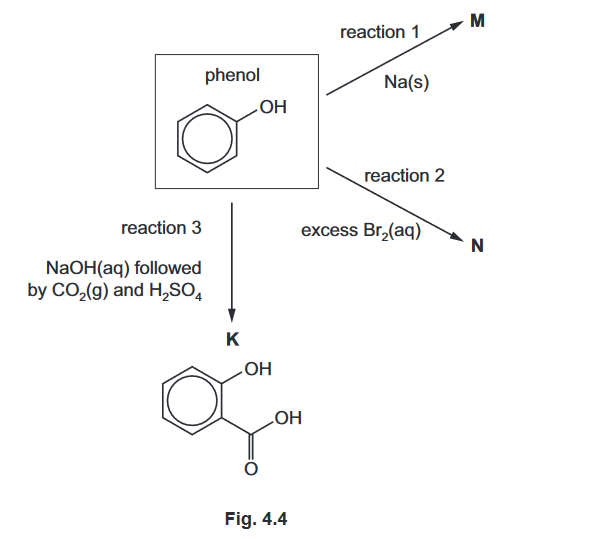
(i) Write an equation for the formation of $\mathbf{M}$ in reaction 1 .
(ii) Draw $\mathbf{N}$, the product of reaction 2 …………………………………..[1]
(iii) Explain why phenol is a weaker acid than $\mathbf{K}$………………………………………………[2]
(e) Phenol and benzene both react with nitric acid, as shown in Fig. 4.5.
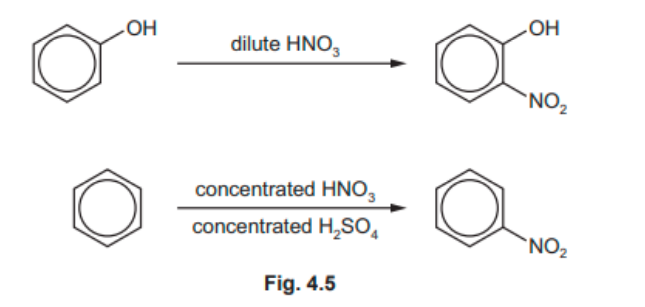
Explain why the reagents and conditions for these two reactions are different.
……………………………………………………………………………………………………..[3] [Total: 18]
▶️Answer/Explanation
Ans:
(a)(i)
In F: (phenyl)amine AND carboxylic acid
In J: phenol AND ester
Any two for one mark
All four for two marks
(a)(ii) 0 (zero) in F AND 2 (two) in J
(d)(iii)
• (CO)O—H bond weaker / more easy to donate H$^+$ in K
• owing to negative inductive / electron withdrawing effect of C=O / COOH group
• carboxylate anion stabilised / phenoxide anion is less stabilised
All three for two marks
(e)
p-orbital on oxygen overlaps with ring / π system OR lone pair of e – on oxygen is delocalised into the ring [1]
electron density in ring increases
attracts/polarises electrophile better
Question 11
(a) Aqueous solutions of copper(II) salts contain the blue-coloured $\left[\mathrm{Cu}\left(\mathrm{H}_2 \mathrm{O}\right)_6\right]^{2+}$ complex ion. Separate portions of this blue solution react with aqueous sodium hydroxide and with concentrated hydrochloric acid.
Give the following information for each of these reactions.
- reaction with aqueous sodium hydroxide ionic equation type of reaction colour and state of the copper-containing product
- reaction with concentrated hydrochloric acid ionic equation type of reaction colour and state of the copper-containing product [6]
(b) Chloride ions can be identified using aqueous silver nitrate, $\mathrm{AgNO}_3(\mathrm{aq})$.
$
\mathrm{Ag}^{+}(\mathrm{aq})+\mathrm{Cl}^{-}(\mathrm{aq}) \rightarrow \mathrm{AgCl}(\mathrm{s})
$
$0.303 \mathrm{~g}$ of a chloride of sulfur is completely hydrolysed with water. All the chlorine atoms present in the chloride of sulfur are converted into chloride ions. The solution is diluted to $100.0 \mathrm{~cm}^3$. A $25.00 \mathrm{~cm}^3$ sample of this solution is titrated with $0.0500 \mathrm{moldm}^{-3} \mathrm{AgNO}_3(\mathrm{aq})$. The titration requires $22.40 \mathrm{~cm}^3$ of $0.0500 \mathrm{~mol} \mathrm{dm}^{-3} \mathrm{AgNO}_3(\mathrm{aq})$.
Calculate the empirical formula of the chloride of sulfur. Show all your working.
▶️Answer/Explanation
Ans:
(a)
$\left[\mathrm{Cu}\left(\mathrm{H}_2 \mathrm{O}\right)_8\right]^{2+}+2 \mathrm{OH}^{-} \rightarrow \mathrm{Cu}(\mathrm{OH})_2+6 \mathrm{H}_2 \mathrm{O}$
precipitation
blue precipitate
$\left[\mathrm{Cu}\left(\mathrm{H}_2 \mathrm{O}\right)_8\right]^{2+}+4 \mathrm{Cl}^{-} \rightarrow \mathrm{CuCl}_4^{2-}+6 \mathrm{H}_2 \mathrm{O}$
ligand exchange / displacement/ substitution / replacement
yellow solution
(b)
amount of $\mathrm{Ag}^{+}=0.050 \times 0.0224=1.12 \times 10^{-3} \mathrm{~mol}\left(\right.$ in $\left.25 \mathrm{~cm}^3\right)$
amount of $\mathrm{Ag}^{+}=1.12 \times 10^{-3} \times 4=4.48 \times 10^{-3} \mathrm{~mol}\left(\right.$ in $100 \mathrm{~cm}^3$ )
amount of $\mathrm{Cl}^{-}=4.48 \times 10^{-3} \mathrm{~mol}\left(\right.$ in $100 \mathrm{~cm}^3$ )
mass of $\mathrm{Cl}^{-}=4.48 \times 10^{-3} \times 35.5=0.159 \mathrm{~g}\left(\right.$ in $\left.100 \mathrm{~cm}^3\right)$
mass of $S=0.303-0.159=0.144 \mathrm{~g}$ (in $100 \mathbf{c m}^3$ ) ecf
moles of $S=0.144 / 32.1=4.49 \times 10^{-3}$
molar ratio $\mathrm{S}: \mathrm{Cl} 1: 1 \rightarrow \mathbf{S C l}$ ecf