Question
Carbon exists in several different forms. Two of these forms are buckminsterfullerene and
graphene. Buckminsterfullerene is a fullerene allotrope of carbon.
Which statements about buckminsterfullerene and graphene are correct?
1 Both have delocalised electrons.
2 Buckminsterfullerene has a giant molecular structure.
3 The carbon atoms in graphene form a tetrahedral lattice
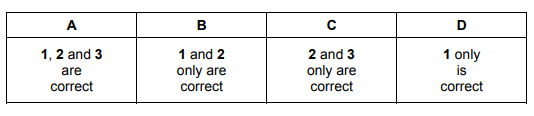
▶️Answer/Explanation
Ans:D
Question
Which ion has both more electrons than protons and more protons than neutrons? $\left[\mathrm{H}={ }_1^1 \mathrm{H} ; \mathrm{D}={ }_1^2 \mathrm{H} ; \mathrm{O}={ }_8^{16} \mathrm{O}\right]$
A $\mathrm{D}^{-}$
B $\mathrm{H}_3 \mathrm{O}^{+}$
C $\mathrm{OD}^{-}$
D $\mathrm{OH}^{-}$
▶️Answer/Explanation
Ans:D
Question
An article in a science magazine contains the following statement.
‘It is lighter than a feather, stronger than steel, yet incredibly flexible and more conductive than copper.’
Which form of carbon is being described?
A buckminsterfullerene
B diamond
C graphene
D graphite
▶️Answer/Explanation
Ans:C
Question
Graphene, graphite and the fullerene \(C_{60}\) are allotropes of carbon.
Which statements are correct for all three of these allotropes of carbon?
1 Delocalised electrons are present in the structure.
2 All bond angles are 120°.
3 It has a giant molecular crystalline lattice structure.
The responses A to D should be selected on the basis of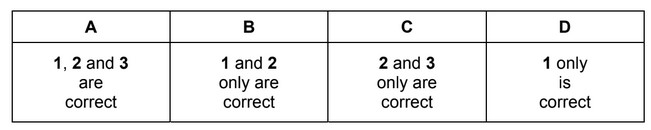
No other combination of statements is used as a correct response.
Answer/Explanation
Ans: D
Question
The 68Ge isotope is medically useful because it undergoes a natural radioactive process to give an isotope of a different element, 68X, which can be used to detect tumours. This transformation of 68Ge occurs when an electron enters the nucleus and changes a proton into a neutron. Which statement about the composition of an atom of 68X is correct?
A It has 4 electrons in its outer p orbitals.
B It has 13 electrons in its outer shell.
C It has 37 neutrons.
D Its proton number is 32.
Answer/Explanation
Answer: C
Question
When nuclear reactions take place, the elements produced are different from the elements that reacted. Nuclear equations, such as the one below, are used to represent the changes that occur.
\(_{235}^{92}\textrm{U}+_{1}^{0}\textrm{n}\rightarrow _{144}^{56}\textrm{Ba}+_{89}^{36}\textrm{Kr}+3_{0}^{1}n\)
The nucleon (mass) number total is constant at 236 and the proton number total is constant at 92. In another nuclear reaction, uranium-238 is reacted with deuterium atoms, \(_{1}^{2}\textrm{H}\). An isotope of a new element, J, is formed as well as two neutrons.
\(_{92}^{238}\textrm{U}+_{1}^{2}\textrm{H}\rightarrow J+2_{0}^{1}\textrm{n}\)
What is isotope J?
A 238Np B 238Pu C 240Np D 240Pu
Answer/Explanation
Answer: A
Question
Use of the Data Booklet is relevant to this question.
The isotope 99Tc is radioactive and has been found in lobsters and seaweed adjacent to nuclear fuel reprocessing plants.
Which statements are correct about an atom of 99Tc?
1 It has 13 more neutrons than protons.
2 It has 43 protons.
3 It has 99 nucleons.
The responses A to D should be selected on the basis of
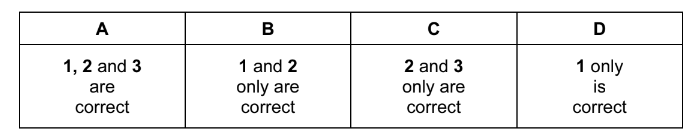
Answer/Explanation
Ans:A
Question
Use of the Data Booklet is relevant to this question.
The isotope 99Tc is radioactive and has been found in lobsters and seaweed adjacent to nuclear fuel reprocessing plants.
Which statements are correct about an atom of 99Tc?
- It has 13 more neutrons than protons.
- It has 43 protons.
- It has 99 nucleons.
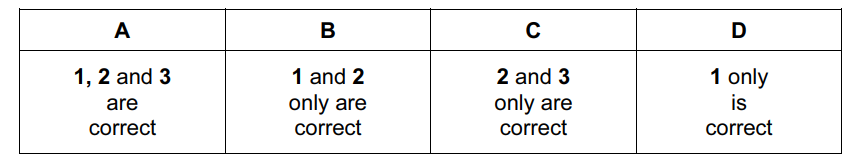
Answer/Explanation
Ans:
A
Question
Use of the Data Booklet is relevant to this question.
The 68Ge isotope is medically useful because it undergoes a natural radioactive process to give a gallium isotope, 68Ga, which can be used to detect tumours. This transformation of 68Ge occurs when an electron enters the nucleus, changing a proton into a neutron.
Which statement about the composition of an atom of the 68Ga isotope is correct?
- It has 4 electrons in its outer p subshell.
- It has 13 electrons in its outer shell.
- It has 37 neutrons.
- Its proton number is 32.
Answer/Explanation
Ans:
C