2.4 Proteins
Essential Idea:
Proteins have a very wide range of functions in living organisms
Understandings:
- Amino acids are linked together by condensation to form polypeptides
- There are 20 different amino acids in polypeptides synthesised on ribosomes
- Amino acids can be linked together in any sequence giving a huge range of possible polypeptides
- The amino acid sequence of polypeptides is coded for by genes
- A protein may consist of a single polypeptide or more than one polypeptide linked together
- The amino acid sequence determines the three-dimensional conformation of a protein
- Living organisms synthesise many different proteins with a wide range of functions
- Every individual has a unique proteome
Applications:
- Rubisco, insulin, immunoglobulins, rhodopsin, collagen and spider silk as examples of the range of protein functions
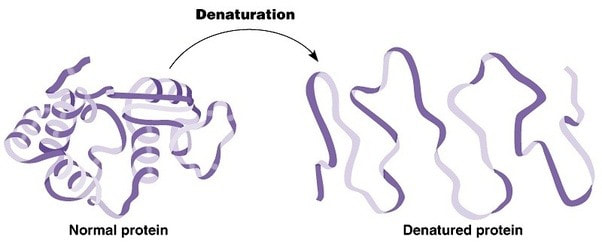
- Denaturation of proteins by heat or by deviation of pH from the optimum
Skills:
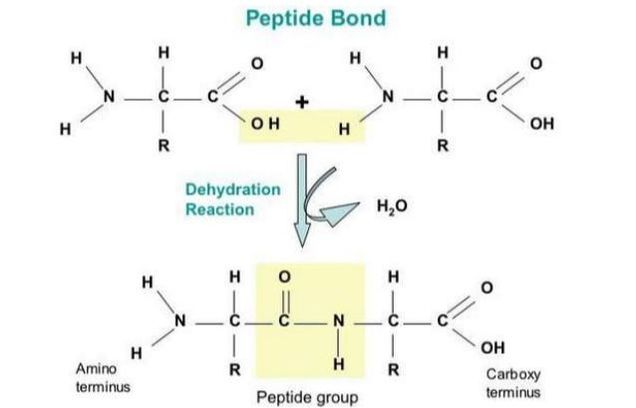
- Drawing molecular diagrams to show the formation of a peptide bond
2.4.U1 Amino Acids are linked together by condensation to form polypeptides.
- Describe polypeptide chain formation in terms of the formation of peptide bonds and condensation reactions.
- Determine the number of peptide bonds given the number of amino acids in a polypeptide.
- Define dipeptide, oligopeptides and polypeptide.
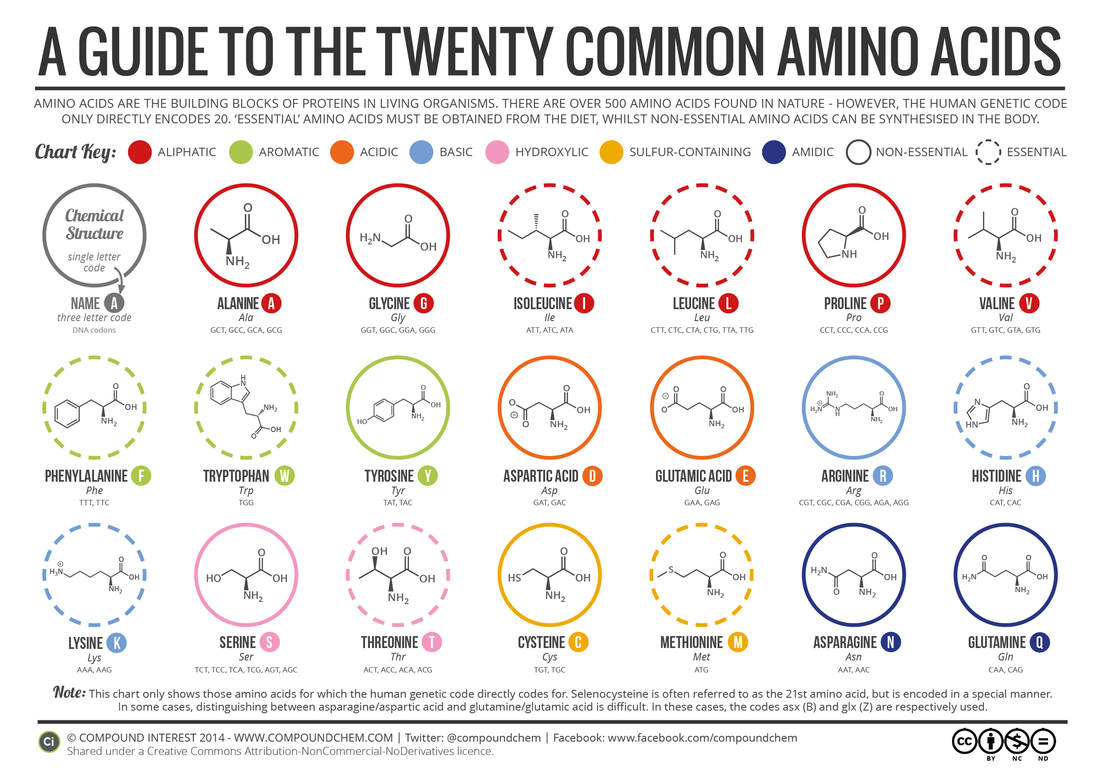
2.4.U2 There are 20 different amino acids in polypeptides synthesized on ribosomes.
- State the number of amino acids used by living organisms to make polypeptides.
- Given an image of an amino acid, classify the amino acid chemical properties based on R group properties.
- Outline the role vitamin C plays in the conversion of proline to hydroxyproline.
2.4.U3 Amino Acids can be linked together in any sequence giving a huge range of possible polypeptides.
- Calculate the possible number of amino acid sequences given n number of amino acids.
2.4.U4 The amino acid sequence of polypeptides is coded for by genes.
- Outline the relationship between genes and polypeptides.
2.4.U5 A protein may consist of a single polypeptide or more than one polypeptide linked together.
- Outline the structure and function of three example proteins composed of two or more polypeptides linked together.
2.4.U6 The amino acid sequence determines the three-dimensional conformation of a protein.
- Contrast the structure of globular proteins with the structure of fibrous proteins.
- Describe the structure of membrane bound globular proteins.
2.4.U7 Living organisms synthesize many different proteins with a wide range of functions.
- Contrast the generalized function of globular proteins with generalized function of fibrous proteins.
- List ten functions of proteins in a cell or organism.
- Describe the function of enzyme proteins.
- Describe the function of hormone proteins.
- Describe the function of immunoglobulin proteins.
- Describe the function of pigment proteins.
- Describe the function of structural proteins
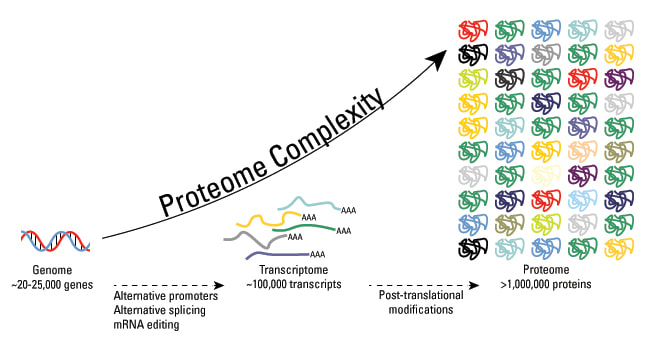
2.4.U8 Every individual has a unique proteome.
- Define proteome.
- Contrast proteome with genome.
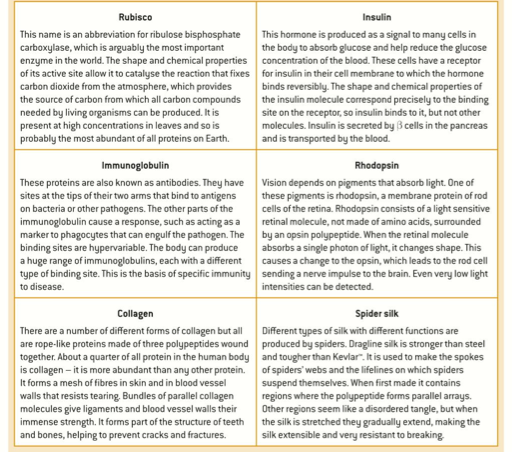
2.4.A1 Rubisco, insulin immunoglobulins, rhodopsin, collagen and spider silk as examples of the range of protein functions.
- State the function of each of the following proteins: rubisco, insulin, immunoglobulin, rhodopsin. collagen, spider silk, actin, myosin, casein, hemoglobin, acetylcholine receptor, oxytocin, prolactin, ferritin, billirubin, fibrinogen, transferrin and albumin.
2.4.A2 Denaturation of proteins by heat or by deviation of pH from the optimum.
- Define denaturation.
- Outline the effect of heat and pH on protein structure.
2.4.S1 Drawing molecular diagrams to show the formation of a peptide bond.
- Draw peptide bond formation in a condensation reactions.
2.4.NOS Looking for patterns, trends, and discrepancies- most but not all organisms assemble proteins from the same amino acids.
- Explain the trend of organisms assembly of polypeptides from the same amino acids.
- Describe a discrepancy of the trend of all organisms using the same amino acids to assemble polypeptides.
Proteins
Any of a class of nitrogenous organic compounds which have large molecules composed of one or more long chains of amino acids and are an essential part of all living organisms.
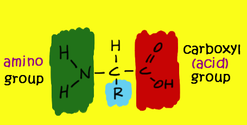
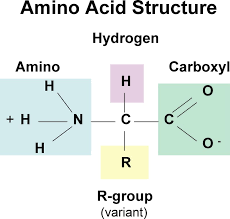
The building blocks of proteins are known as Amino Acids. There are 22 naturally occurring amino acids found in nature. Amino Acids are made up of Carbon, Hydrogen, Oxygen and Nitrogen. So, single amino acid is made up of Amino Group, Carboxyl Group, Hydrogen Atom and distinctive side chain, all bonded to alpha-carbon.

Fig.1. Structure of Amino Acid
When Amino Acids are dissolved in water, it exists in solution as the Dipolar Ion or Zwitterion. They can either acts as proton donor or proton acceptor.
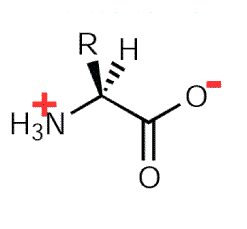
Fig. 2. Zwitterion
All Amino Acids are optically active, that is, they can rotate the plane of polarized light. Optically active molecules contain chiral carbon except glycine. A tetrahedral carbon atom with four different constituents are said to be chiral.
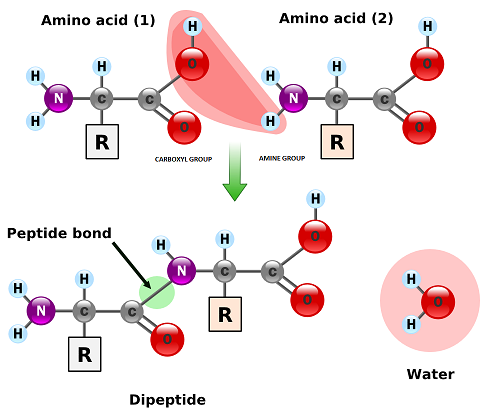
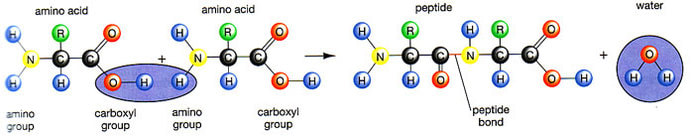
Peptide is a compound consisting of two or more amino acids. When two amino acids are linked together via peptide bond, they are said to form a dipeptide. Three Amino Acids joined to form tripeptide etc. Peptide chains of 12 to 20 amino acids form oligopeptide. When many amino acids are joined, they form polypeptide. The first amino acid is known as N Terminal or Amino Terminal and Last Amino Acid is said to be C terminal or carboxyl terminal.
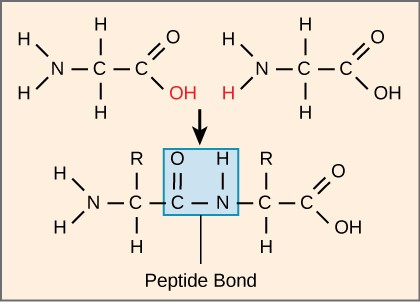
Fig. 3. Formation of peptide bond
Protein Structure
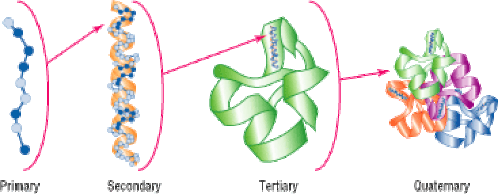
Proteins have four levels of protein organization. They are as follows:
Primary Structure of the protein is its sequence of amino acids. Amino Acids are joined by a peptide bond.
Secondary Structures are higher level of protein organization which includes- alpha helix and beta sheets. Both of them are stabilized by hydrogen bonds between carbonyl and N-H groups in a polypeptide backbone.
Alpha Helix is a rigid, rod like structure that forms when a polypeptide chain twists into a helical conformation.
Beta Pleated Sheets are formed when two or more polypeptide chain segment line up side by side. Each individual segment is known as Beta Strand.
Tertiary Structures refer to the three-dimensional conformations that a protein assumes as a consequence of the interactions between the side chains in their primary structure. They are stabilized by hydrophobic interactions, electrostatic interactions, hydrogen bonds, van der waal forces and covalent bonds.
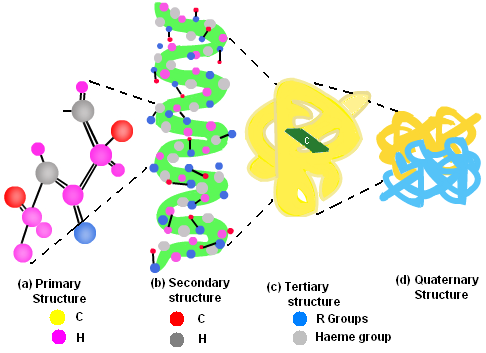
Fig. 4. Levels of protein organization
Quaternary Structures are composed of two or more polypeptide chains. Polypeptides are held together via hydrophobic interactions, electrostatic interactions, and hydrogen bonds etc. For Example: Hemoglobin.
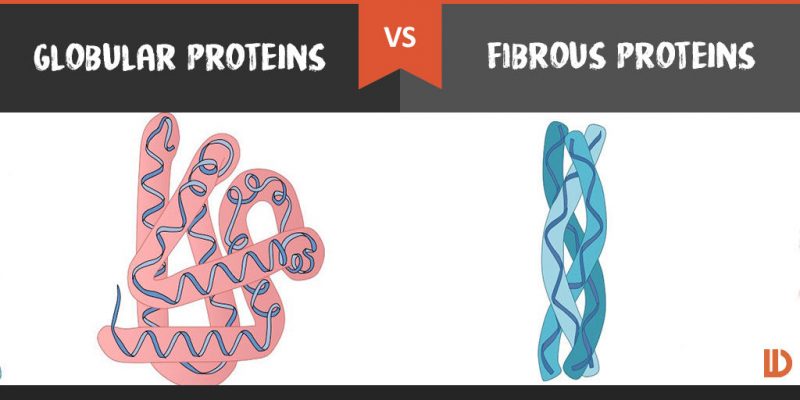
Fibrous and globular proteins
Fibrous are long, rod shaped molecules which are insoluble in water. They are generally protective and structural in nature. Globular proteins are compact spherical molecules that are usually water soluble.
Nucleic Acids
Nucleic Acid was first discovered by Friedrich Miescher from the nuclei of pus cells. There two types of Nucleic Acids- Deoxyribonucleic Acids and Ribonucleic Acids.
The monomeric unit of nucleic acid is known as Nucleotides. Nucleotide is made up of three components: A Nitrogenous Base, A Five-Carbon Sugar and Phosphoric Acid.
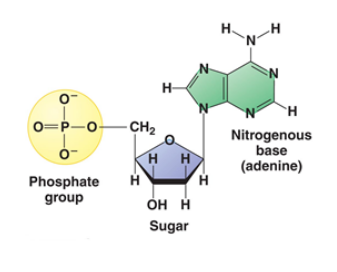
Fig. 5. Structure of Nucleotide
Nitrogenous bases are heterocyclic, aromatic molecules. There are basically two types of Nucleic Acids: Purines and Pyrimidines.
Purines are of two types – Adenine and Guanine whereas pyrimidines can be thymine, cytosine, or uracil.
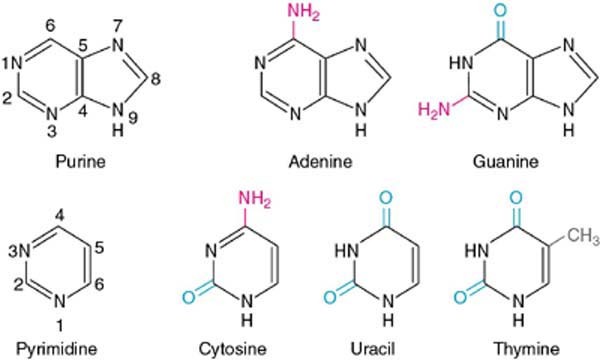
Fig. 6. Structure of purines and pyrimidines
The 5- carbon sugar found in DNA is deoxyribose and for RNA is ribose. Nucleotide without phosphate is known as nucleoside.
Structure of Double Stranded DNA
DNA are of different types such as:
B DNA
- Two long polynucleotide strands are coiled around the axis.
- Strands are antiparallel to each other.
- Guanine base pairs with cytosine and adenine base pairs with thymine.
- Guanine forms 3 hydrogen bonds with cytosine whereas adenine forms two hydrogen bonds with thymine.
Z DNA
- They are thinner than B DNA.
- They have alternating purine and pyrimidine bases.
- They are stabilized by high salt concentration.
Denaturation of DNA
When DNA is subjected to varying pH, temperature etc, two DNA strands separate out. That is the DNA is said to be denatured. If the denaturant such as temperature, pH is removed, the DNA regains its original or native structure. This is known as Renaturation.
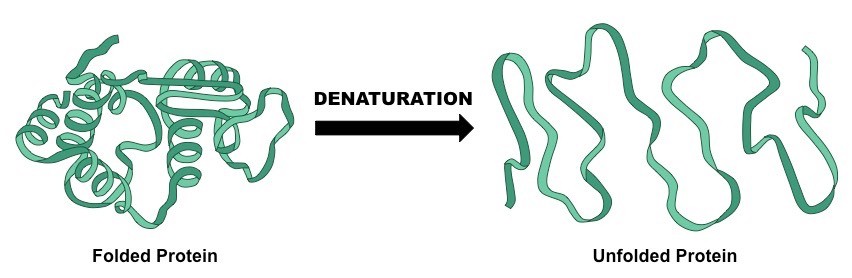
Fig. 7. Process of denaturation
RNA
RNA known as Ribonucleic Acid. It usually exists as single strand. RNA exists in cells in various forms as explained below-
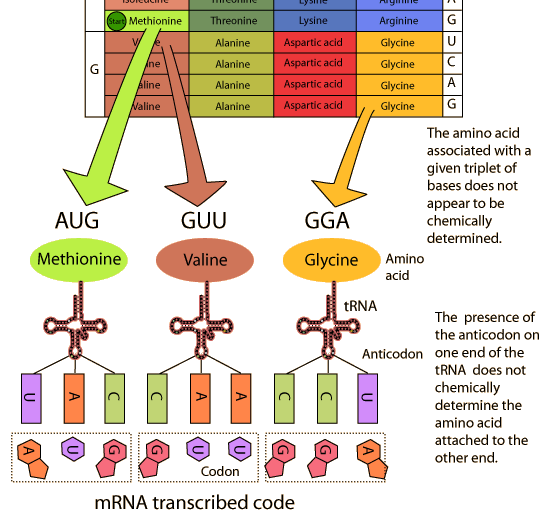
Messenger RNA carries genetic information from DNA in the form of codons (three nucleotides forms a codon), which codes for amino acids or protein.
Transfer RNA plays an important role during protein synthesis. They act as an interface between nucleic acid language and protein language. They are fund in the cytosol of the living cell.
Ribosomal RNA is a component of ribosomes. They have important roles during protein synthesis in eukaryotes and prokaryotes.
Topic 2.4: proteins
In the Basics of Biochemistry unit students are introduced to the major classes of biologically important molecules and the types of reactions used to build and break apart those molecules. The structure and function of water, as the medium of life, is also a focus.
The unit is planned to take 3 school days.
- Proteins have a very wide range of functions in living organisms.
- Looking for patterns, trends and discrepancies—most but not all organisms assemble proteins from the same amino acids. (3.1)
- Explain the trend of organisms assembly of polypeptides from the same amino acids.
- Describe a discrepancy of the trend of all organisms using the same amino acids to assemble polypeptides.
2.4.U.1 Amino acids are linked together by condensation to form polypeptides.
- Describe polypeptide chain formation in terms of the formation of peptide bonds and condensation reactions.
- Determine the number of peptide bonds given the number of amino acids in a polypeptide.
- Define dipeptide, oligopeptides and polypeptide.
Polypeptides are chains of amino acids that are made by linking together amino acids by condensation reactions- this happens on ribosomes by a process called translation. Amino acids are the main component of proteins and in many proteins they are the only component
Polypeptides can contain one protein some contain more
The condensation reaction involves the amine group (-NH2) of one amino acid and the carboxyl group (-COOH) of another. Water is removed as in all condensation reactions, and a new bond is formed between the two amino acids, called a peptide bond
2.4.U.2 There are 20 different amino acids in polypeptides synthesized on ribosomes.
- State the number of amino acids used by living organisms to make polypeptides.
- Given an image of an amino acid, classify the amino acid chemical properties based on R group properties.
- Outline the role vitamin C plays in the conversion of proline to hydroxyproline.
2.4.U.3 Amino acids can be linked together in any sequence giving a huge range of possible polypeptides.
- Calculate the possible number of amino acid sequences given n number of amino acids.
Polypeptides are molecule consisting of many amino acids linked by peptide bonds. The polypeptides can contain any number of amino acids through chains of fewer than 20 amino acids are usually referred to as oligopeptides rather than polypeptides.
- insulin – small protein that contains two polypeptides, one with 21 amino acids and the other with 30
Ribosomes link amino acids together on at a time until a polypeptide is fully formed ribosome can make peptide bonds between any pair of amino acids so any sequence of amino acids is possible amount of amino acid sequences can be calculated starting with dipeptides bother amino acids in a dipeptide can be any of the 20 so there are 20 times 20 possible sequences – there are 20 x 20 x 20 possible tripeptide sequences for a polypeptide of n amino acids there are 20 n possible sequences # of amino acids in a Polypeptide can be from 200 to 1000s example, 400 amino acids there are 20^400 possible amino acid sequences
2.4.U.4 The amino acid sequence of polypeptides is coded for by genes.
- Outline the relationship between genes and polypeptides.
The number of amino acids sequences that could be produced is immense but living organisms only actually produce a small fraction of these. A typical cell produces polypeptides with thousands of different sequences and must store the information needed to do this- the amino acid of sequence of each polypeptide is stored in a coded form in the base sequence of a gene.
Some genes have other roles but most genes in a cell store the amino acid sequences of a polypeptide- using a genetic code to do this. Three bases of the gene code are needed to code for each amino acid in the polypeptide. The base sequence that actually codes for a polypeptide is known to molecular biologists as the open reading frame- one puzzle is that open reading frames only occupy a small proportion of the total DNA of a species.
2.4.U.5 A protein may consist of a single polypeptide or more than one polypeptide linked together.
- Outline the structure and function of three example proteins composed of two or more polypeptides linked together.
Some proteins are single polypeptides but other are composed of two or more Polypeptides linked together
- a dipetide is a molecule consisting of two amino acids linked by a peptide bond
- amino acids that are linked together by ribosomes to make polypeptides all have some identical structural features – a carbon atom in the centre of the molecule is bonded to an amine group, a carboxyl group and a hydrogen atom
- carbon atom is also bonded to an R group = different in each amino acids
- amine groups and the carboxyl groups are used up in forming the petide bond – R group of the amino acids that give a polypeptide its character
- repertoire of R groups allows living organisms to make and use an amazingly wide range of proteins
- certain proteins possess a fourth level of structural organisation called a quaternary structure
- quaternary structures are found in proteins that consist of more than one polypeptide chain linked together
- alternatively, proteins may have a quaternary structure if they include inorganic prosthetic groups as part of their structure
- not all proteins will have a quaternary structure – many proteins consist of a single polypeptide chain
2.4.U.6 The amino acid sequence determines the three-dimensional conformation of a protein.
- Contrast the structure of globular proteins with the structure of fibrous proteins.
- Describe the structure of membrane bound globular proteins.
The order of the amino acid sequence is called the primary structure and determines the way the chain will fold. The different amino acid sequences will fold into different configurations due to the chemical properties of the variable side chains. The amino acid sequences will commonly fold into two stable configurations, called secondary structures.
Alpha helices occur when the amino acid sequence folds into a coil / spiral arrangement. Beta-pleated sheets occur when the amino acid sequence adopts a directionally-oriented staggered strand conformation. Both α-helices and β-pleated sheets result from hydrogen bonds forming between non-adjacent amine and carboxyl groups
Where no secondary structure exists, the polypeptide chain will form a random coil conformation of a protein is its three dimensional structure.
The overall three-dimensional configuration of the protein is referred to as the tertiary structure of the protein The tertiary structure of a polypeptide chain will be determined by the interactions between the variable side chains
These interactions may include hydrogen bonds, disulphide bridges, ionic interactions, polar associations, etc.
- determined by the amino acid sequence of a protein and its constituent polypeptides
- fibrous proteins such a collagen are elongated usually with a repeating structure
- many proteins are globular with an intericate shape that oftern includes parts that are helical or sheet like
- amino acids are added one by one to form a polypeptide
- they are always added in the same sequence to make a particular polypeptide
- in globular proteins the polypeptides gradually fold up as they are made to develop the final conformation= this is stabilized by bonds between the R groups of the amino acids that have been brought together by the folding
- globular proteins that are soluble in water- there are hydrophilic R groups on the outside of the molecules and there are usually hydrophobic groups on the inside
- in globular membrane proteins there are regions with hydrophobic R groups on the outside of the molecules which are attracted to the hydrophobic centre of the membrane
- in fibrous proteins the amino acid sequence prevents folding up and ensures that the chain of amino acids remains in an elongated form.
2.4.U.7 Living organisms synthesize many different proteins with a wide range of functions.
- Contrast the generalized function of globular proteins with generalized function of fibrous proteins.
- List ten functions of proteins in a cell or organism.
- Describe the function of enzyme proteins.
- Describe the function of hormone proteins.
- Describe the function of immunoglobulin proteins.
- Describe the function of pigment proteins.
- Describe the function of structural proteins
- Catalysis – there are thousdans of different enzymes to catalyse specific chemical reactions within the cell oroutside it
- Muscle contraction – actin and myosin together cause the muscle contractions used in locomotion and transport around the body
- Cytoskeletons – tubulin is the subunit of microtubules that give animal cells their shape and pull on chromosomes during mitosis
- Tensil strengthening – fibrous proteins give tensile strength needed in skin, tendons, ligaments and blood vessel walls
- Blood clotting – plasma proteins act as clotting factors that cause blood to turn from a liquid to a gel in wounds
- Transport of nutrients and gases – proteins in blood help transport oxygen, carbon dioxide, iron and lipids
- Cell adhesion – membrane proteins cause adjacent animal cells to stick to each other within tissue
- Membrane transport – membrane proteins are used for facilitated diffusion and active transport, and also for elecgtron transport during cell respiratoi and photosynthesis
- Hormones – some such as insulin FSH and LH are proteins, but hormones are chemicall yver diverse.
- Receptors – binding sites in membranes and cytoplasm for hormones, neurotransmitters, tastes and smells, and also receptors for light in the eye and in plants.
- Packing of DNA – histones are associated with DNA in eukaryotes and help chromosomes to condense during mitosis
- Immunity – this is the most diverse group of proteins, as cells cab make huge numbers of different antibodies.
2.4.U.8 Every individual has a unique proteome.
- Define proteome.
- Contrast proteome with genome.
The proteome is the totality of proteins expressed within a cell, tissue or organism at a certain time. The proteome of any given individual will be unique, as protein expression patterns are determined by an individual’s genes
- all of the proteins produced by a cell, a tissue or an organism .
- compared to the genome is all of the genes of a cell, a tissue or an organism
- to find out how many different proteins are being produced mixtures of proteins are extracted from a sample and are then separated by gel electrophoresis
- this is to indentify whether or not a particular protein is present, antibodies to the protein that have been linked to a fluorescent marker can be used – if the cell fluoresces the protein is present
- BUT the genome of an organism is fixed,the proteome is variable because different cells in an organism make different proteins
- even in a single cell the proteins that are made vary over time depending on the cells activities – the proteome therefore reveals what is actually happening in an organism, not what potentially could happen
- within a species there are strong similarities in the proteome of all individuals, but also differences
- proteome of each individual is unique partly because of differences of activity but also because of differences in the amino acid sequence of proteins- with the possible exception of identical twins, none of us have identical proteins, so each of us has a unique proteome, even the proteome of identical twins can become different with age.
2.4.A.1 Rubisco, insulin, immunoglobulins, rhodopsin, collagen and spider silk as examples of the range of protein functions.
- State the function of each of the following proteins: rubisco, insulin, immunoglobulin, rhodopsin. collagen, spider silk, actin, myosin, casein, hemoglobin, acetylcholine receptor, oxytocin, prolactin, ferritin, billirubin, fibrinogen, transferrin and albumin.
2.4.A.2 Denaturation of proteins by heat or by deviation of pH from the optimum.
- Define denaturation.
- Outline the effect of heat and pH on protein structure.
Denaturation of proteins involves the disruption and possible destruction of both the secondary and tertiary structures. Since denaturation reactions are not strong enough to break the peptide bonds, the primary structure (sequence of amino acids) remains the same after a denaturation process. Denaturation disrupts the normal alpha-helix and beta sheets in a protein and uncoils it into a random shape
Denaturation of proteins can usually be caused by two key conditions – temperature and pH
Temperature
- High levels of thermal energy may disrupt the hydrogen bonds that hold the protein together
- As these bonds are broken, the protein will begin to unfold and lose its capacity to function as intended
- Temperatures at which proteins denature may vary, but most human proteins function optimally at body temperature (~37ºC)
pH
- Amino acids are zwitterions, neutral molecules possessing both negatively (COO–) and positively (NH3+) charged regions
- Changing the pH will alter the charge of the protein, which in turn will alter protein solubility and overall shape
- All proteins have an optimal pH which is dependent on the environment in which it functions (e.g. stomach proteins require an acidic environment to operate, whereas blood proteins function best at a neutral pH)
2.4.S.1 Drawing molecular diagrams to show the formation of a peptide bond.
- Draw peptide bond formation in a condensation reactions.
The amine group loses a hydrogen atom (H) and the carboxylic acid loses a hydroxyl (OH) – this forms water (H2O)
Carbohydrates, lipids and proteins
3.2.1 Distinguish between organic and inorganic compounds.
Organic compounds are compounds that are found in living organisms and contain carbon. Inorganic compounds are the ones that don’t contain carbon. Although, there are a few compounds found in living organisms which also contain carbon but are considered as inorganic compounds. These include carbon dioxide, carbonates and hydrogen carbonates.
3.2.2 Identify amino acids, glucose, ribose and fatty acids from diagrams showing their structure.
3.2.3 List three examples each of monosaccharides, disaccharides and polysaccharides.
- Glucose, galactose and fructose are all monosaccharides.
- Maltose, lactose and sucrose are all disaccharides.
- Starch, glycogen and cellulose are all polysaccharides.
3.2.4 State one function of glucose, lactose and glycogen in animals, and of fructose, sucrose and cellulose in plants.
In animals, glucose is used as an energy source for the body and lactose is the sugar found in milk which provides energy to new borns until they are weaned. Finally, glycogen is used as an energy source (short term only) and is stored in muscles and the liver.
In plants, fructose is what makes fruits taste sweet which attracts animals and these then eat the fruits and disperse the seeds found in the fruits. Sucrose is used as an energy source for the plant whereas cellulose fibers is what makes the plant cell wall strong.
3.2.5 Outline the role of condensation and hydrolysis in the relationships between monosaccharides, disaccharides and polysaccharides; between fatty acids, glycerol and triglycerides; and between amino acids and polypeptides.
3.2.6 State three functions of lipids.
- Lipids can be used for energy storage in the form of fat in humans and oil in plants.
- Lipids can be used as heat insulation as fat under the skin reduces heat loss.
- Lipids allow buoyancy as they are less dense than water and so animals can float in water.
3.2.7 Compare the use of carbohydrates and lipids in energy storage.
Carbohydrates and lipids can both be used as energy storage however carbohydrates are usually used for short term storage whereas lipids are used for long term storage. Carbohydrates are soluble in water unlike lipids. This makes carbohydrates easy to transport around the body (from and to the store). Also, carbohydrates are a lot easier and more rapidly digested so their energy is useful if the body requires energy fast. As for lipids, they are insoluble which makes them more difficult to transport however because they are insoluble, lipids do not have an effect on osmosis which prevents problems within the cells in the body. They also contain more energy per gram than carbohydrates which makes lipids a lighter store compared to a store of carbohydrates equivalent in energy.