BASIC CONCEPTS OF ORGANIC CHEMISTRY
ELECTRONEGATIVITY
A covalent bond, where the electrons are shared equally is called a nonpolar bond (eg H–H) and an unequal sharing of the pair of bonding electrons results in a polar bond. The unequal sharing of electrons is due to the ability of an atom to attract electrons towards itself which is known as Electronegativity.
Elements with higher electronegativity values have greater attraction for bonding electrons.
Electronegativity increases from left to right and decreases from top to bottom.
INDUCTIVE EFFECT (I)
The displacement of shared pair of electrons towards the more electronegative atom in a molecule is called inductive effect. It is a permanent effect e.g.
It develops polarity in a bond or molecule.
It is transmitted along a chain of atoms but the intensity goes on decreasing with the increase in the size of chain. For example,
Hence transmission can be ignored after the second C-atom. An atom or group which attracts electrons more strongly than hydrogen is said to have a negative inductive effect (–I). An atom or group which attracts electrons less strongly than hydrogen is said to have a positive inductive effect (+I).
Inductive effect does not change the covalency. The more the inductive effect between a bond, the more is the ionic character of the bond.
APPLICATIONS
ACID CHARACTER OF ACIDS
Formic acid is stronger than acetic acid.
The oxygen atom in acetic acid holds the hydrogen atom more tightly after acquiring negative charge due to +I effect of methyl group. Hence it is less ionised
Acid character of halogens substituted acids. Chloro substituted acetic acids follow the following order for acid character.
The O – H bond is more ionic in nature in trichloroacetic acid and its ionic character decreases from right to left.
Dispersal of the negative charge after ionisation decreases from left to right which is the cause of decreasing the acid character.
Dissociation constants (×10–5) for acids
Acetic acid | Monochloro acetic acid | Dichloro acetic acid | Trichloro acetic acid |
1.8 | 155 | 500 | 13,000 |
Monofluoro acetic acid | Monochloro acetic acid | Monobromo acetic acid | Monoiodo acetic acid |
217 | 155 | 138 | 75 |
Inductive effect of halogens F > Cl > Br > I
Dissociation constant (×10–5) of a, b and g mono chlorobutyric acids
n-Butyric acid 1.5
ɑ-Monochloro butyric acid 139
β-Monochloro butyric acid 8.8
γ-Monochloro butyric acid 3.0
The transmission of the inductive effect along a chain of carbon atoms weakens as the chain gets longer.
REACTIVITY OF ALKYL HALIDES
It follows the following order
Due to +I effect the intermediate carbonium ions  are stabilised in the order t > s > p > methyl. Hence the order of reactivity of alkyl halides decreases from right to left.
are stabilised in the order t > s > p > methyl. Hence the order of reactivity of alkyl halides decreases from right to left.
BASIC CHARACTER OF AMINES
It follows the order
Although electron density on nitrogen is maximum in  but due to steric hindrance it is less basic.
but due to steric hindrance it is less basic.
INDUCTIVE EFFECT AND DIPOLE MOMENT
Inductive effect leads to a dipole moment. The measured dipole moments of some alkyl halides are given below
CH3Br C2H5Br (CH3)2CHBr (CH3)3CBr
1.79 1.88 2.04 2.21
CH3I C2H5I (CH3)2CHI (CH3)3CI
1.64 1.78 1.84 2.13
+I effect increases from –CH3 to –C(CH3)3 gp. –I effect of Br is more than I.
ESTIMATION OF PERCENTAGE IONIC CHARACTER OF BONDS
% Ionic character of covalent bond = . 
INDUCTOMETRIC EFFECT
Consider the inductive effect in a bond 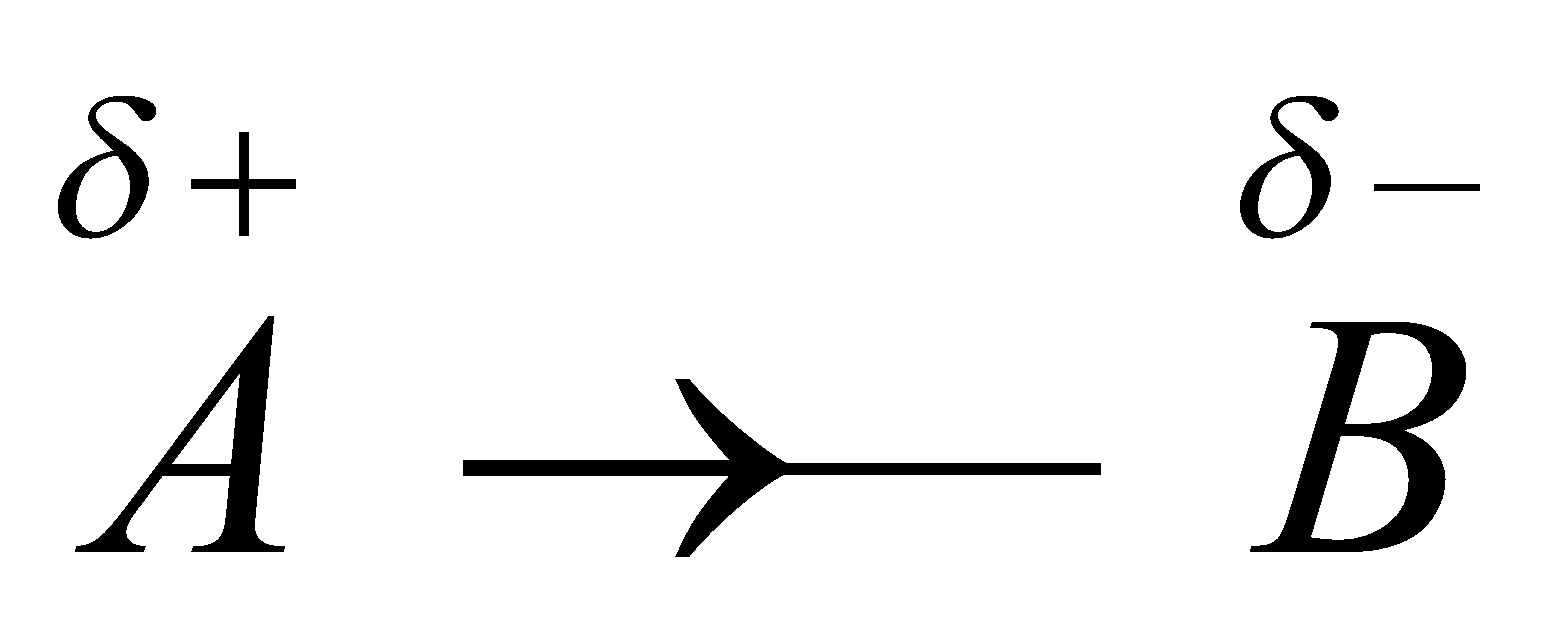 . When some negatively charged ion approaches A, the inductive effect between A – B is temporarily increased which is known as inductometric effect.
. When some negatively charged ion approaches A, the inductive effect between A – B is temporarily increased which is known as inductometric effect.
ELECTROMERIC EFFECT (E)
It involves the complete transference of p pair of electrons to one of the atoms joined a multiple bond. It is temporary effect and takes place at the requirement of attacking reagent. Consider the addition of HCl to propene.
Addition takes place according to Markownikoff’s rule which states that the negative portion of attacking reagent goes to carbon atom containing lesser number of hydrogen atoms. The reason for this is that it results in the formation of more stable intermediate secondary carbonium ion.
PEROXIDE EFFECT, KHARASCH EFFECT
In presence of oxygen or peroxide the addition of HBr to unsymmetrical alkene takes place anti to Markovnikov’s rule which is known as peroxide effect or Kharasch effect.
The attack of Br• on terminal carbon atom (see step III) results in the formation of more stable secondary free radical. This is the reason that addition takes place anti to markownikoff’s rule. HCl and HI do not show peroxide effect. HCl does not give  atoms and HI gives molecular I2.
atoms and HI gives molecular I2.
RESONANCE
Representation of certain molecules by various electronic configurations is known as Resonance. Electronic configurations differ only in location of electrons, the atoms must stay in the same conditions. e.g.
The real structure is a combination of the resonance forms and is called Resonance hybrid.
FEATURE OF RESONANCE
- Resonance is a permanent effect.
- It involves the delocalisation of electrons, lone pair of electrons and p pair of electrons.
- The number of unpaired (not lone pairs) electrons must stay the same.
- Resonating structure with lowest energy contributes more towards resonance.
- Negative charges are most stable on electronegative atoms.
- Resonating structures with maximum bonds and little charge contribute more.
- Real structure resemble the major contributor more than the minor contributor.
- All the atoms participating in resonance must lie in the same plane or must at least nearly do so.
APPLICATIONS OF RESONANCE
ACID CHARACTER OF PHENOLS
Phenols are acidic and Alcohols are neutral.
Resonance stabilisation of Phenoxide ion
Resonance stabilisation of Phenol molecule
Resonance stabilisation of phenoxide ion is more than the Resonance stabilisation of Phenol molecule itself. Hence Phenol will ionise to give phenoxide and H+ ions.
BASIC CHARACTER OF AMINES
Aromatic amines are less basic than aliphatic amines
Due to resonance the unshared pair of electrons present on nitrogen atom is delocalised within benzene nucleus and not available for protonation (to accept H+). Hence basic character is suppressed.
STABILITY OF CATIONS
Methoxy methyl cation is more stable by 76 kcal/mol than methyl Cation.
UNEXPECTED ADDITION PRODUCTS
A is more stable than B
RESONANCE AND BOND LENGTHS :
Benzene 
Normal C – C = 1.54 Å, C = C = 1.34 Å,
All C – C bonds in benzene 1.39Å.
Carboxylate ion 
Normal C = O = 1.22Å, C – O = 1.4Å,
All C – O bonds in carboxylate ion = 1.28Å
Nitro group 
Normal N – O = 1.36Å, N = O = 1.15Å
All N – O bonds in nitro group 1.21 – 1.23 Å
RESONANCE AND BOND ORDER
It is obtained by the following relation.
RESONANCE AND DIPOLE MOMENT
Resonance affects the Dipole moment. D.M. of ethyl chloride is 2.05 Debye.
In vinyl chloride 
Inductive and resonance induced D.M. operate in opposite direction, hence value is 1.44 Debye.
In chlorobenzene Inductive and Resonance induced D.M. operate in opposite direction. 
The value of DM is 1.55 Debye.
RESONANCE ENERGY (E)
It is given by the equation
ER = E0 – EC
ER = Resonance energy, E0 = Observed heat of formation and EC = Calculated heat of formation of the most stable of the resonating structures.
In case of unsaturated compounds, Resonance energy is the difference between a measured and calculated heat of hydrogenation e.g.,
Calculated value of heat of hydrogenation of benzene
= 28.6 × 3 = 85.8 kcal/mol, RE = 36.0 kcal/mol.
The greater the RE, the more is the stability.
MESOMERIC EFFECT (ME)
It is a permanent effect and similar to electromeric effect.
Like Inductive effect it may be +ME or –ME.
+ME atoms or groups donate electrons to the double bond or conjugated system e.g. –Cl, –Br, –I, NH2, –NHR, –NR2, –OH, –OR, –SH, –SR etc.
–ME atoms or groups withdraw electrons eg. –NO2, CN, COOH, CHO, HSO3.
CONJUGATION
The compounds containing alternate single and double bonds are known as conjugated compounds. Such compounds exhibit certain abnormal properties due to interaction between single and double bonds, known as conjugation
ABNORMAL ADDITION REACTIONS
Addition of HBr to 1,3-butadiene.
EXTRA STABILITY
Each C-atom in 1, 3-butadiene is sp2 hybridised and contains one pz atomic orbital parallel to each other and perpendicular to the plane of hybrid atomic orbitals. By sidewise overlapping these pz atomic orbitals form a delocalised p molecular orbital which provides the extra stability to the molecule.
BOND LENGTH
Conjugation affects the bond length. The C2 – C3 bond length in 1,3-butadiene is 1.47 Å and C1 – C2 bond length is 1.35Å due to conjugation.
HEAT OF HYDROGENATION
Calculated heat of hydrogenation of 1,3 butadiene = 28.6 × 2 = 57.2 kcal.
Observed heat of hydrogenation of 1,3-butadiene = 53.7 kcal.
R.E. = 57.2 – 53.7 = 3.5 kcal/mole
Thus, due to conjugation 1,3-butadiene is stabilised by 3.5 kcal/mol.
HYPERCONJUGATION
Introduced by Baker and Nathan (1935). The electron release by C–H bond by the effect similar to electromeric effect is known as hyperconjugation. It is a permanent effect.
Since there is no apparent bond between C and H+, the hyperconjugation is also known as No bond Resonance. The magnitude of inductive effect and hyperconjugation follows the order.
Increasing Inductive Effect
EFFECTS OF HYPERCONJUGATION
HEAT OF HYDROGENATION OF SUBSTITUTED OLEFINS :
The greater the number of H.C. forms the more is the stability.
Compound Heat of hydrogenation
CH2 = CH2 Ethylene 32.8 kcal/mol
CH3–CH = CH2 Propylene 30.1 kcal/mol
(Due to H.C. forms)
BOND LENGTHS
Dimethyl acetylene
Normal C–C = 1.54Å; C1 – C2 = 1.46Å
HYPERCONJUGATION AND DIPOLE MOMENT
Calculated dipole moment of nitro methane is 2.59 Debye and the observed value is 3.15 Debye.
CLEAVAGE OF COVALENT BOND
HETEROLYTIC CLEAVAGE / HETEROLYSIS
shared pair of electrons is retained by one atom
or 
Species carrying negative charge are known as anions; they are rich in electrons hence nucleophilic in nature. In a chemical reaction such species always attack at the point of low electron density. Species carrying positive charge are known as cations, they are electrons deficient hence electrophilic in nature. In a chemical reaction they attack at the point of high electron density.
HOMOLYTIC CLEAVAGE / HOMOLYSIS
The atoms retain one electron each.
The resulting species are neutral, contain at least one unpaired electron hence known as free radicals, electron deficient hence electrophilic in nature, very reactive, paramagnetic in nature, hydrogen abstractor. Homolytic cleavage usually occurs in non polar bonds at high temperature or in presence of UV radiations.
TYPES OF REAGENTS
ELECTROPHILIC REAGENTS
They have high affinity for electrons
They may be neutral in nature also AlCl3, BF3, ZnCl2, FeCl3, SnCl4 etc.
NUCLEOPHILIC REAGENTS
Electron rich species and have affinity towards nucleus (which is positively charged).
Neutral nucleophiles are capable of donating a pair of electrons e.g.
Nucleophilicity is defined by the rate of attack on an electrophilic carbon atom.
- Species with negative charge are stronger nucleophiles than analogous species without a negative charge
- Nucleophilicity decreases from left to right across the periodic table
- Nucleophilicity increases down the periodic table
REACTION INTERMEDIATES
CARBOCATIONS (CARBONIUM IONS)
These are the species carrying positive charge on the carbon atom, which is sp2 hybridised, with planar structure. The vacant p-orbital lies perpendicular to the plane of the other atoms. They are strong electrophiles. They are stabilised by alkyl substituents by
INDUCTIVE EFFECT
HYPERCONJUGATION
Partial overlapping of filled orbitals with empty ones
RESONANCE STABILISATION
Unsaturated carbocations are stabilised by Resonance. e.g.
Allyl Carbonium ion
Benzyl Carbonium ion
The order of stability of different carbonium ions
REACTIONS OF CARBOCATIONS
- Combination with a nucleophile
- Elimination of Proton
- Rearrangement
If 1,2-shift of hydrogen or alkyl can form a more stable carbocation, then such a rearrangement takes place
CARBANIONS (CARBO ANIONS)
The species carrying negative charge on the carbon atom which is sp3 hybridised and tetrahedral. They are nucleophilic in nature and their structure resembles an amine. The stability order is
INDUCTIVE EFFECT
+ I effect of R increases electron density on C making it less stable.
RESONANCE STABILISATION
Allyl Carbanion
Benzyl Carbanion
Resonance stabilisation is more effective than other factors.
FREE RADICALS
They are sp2 hybridised and planar. The perpendicular p-orbital contains an odd electron. They lack in octet hence electrophilic in nature. The order of stability is
INDUCTIVE EFFECT
NO BOND RESONANCE STABILISATION / HYPERCONJUGATION
RESONANCE STABILISATION
Allyl free radical
Benzyl free radical
Order of stability of various free radicals 

CARBENES R=C:
These are uncharged reactive intermediates that contain a divalent carbon atom which is sp2 hybridised. There is a perpendicular vacant p-orbital.
GENERATION
SINGLET CARBENES
Multiplicity is A = 2s + 1 = 2 × 0 + 1 = 1
TRIPLET CARBENES
Multiplicity is A = 2 × 1 + 1 = 3
Triplet is more stable than singlet.
NITRENES
Nitrenes are nitrogen analogs of carbenes, nitrogen is sp2 hybridised.
GENERATION
SINGLET
Multiplicity A = 2s + 1 = 2 × 0 + 1 = 1
TRIPLET
Multiplicity A = 2s + 1 = 2 × 1 + 1 = 3
ARYNES
The derivatives of benzyne are called arynes :
GENERATION
TYPES OF REACTIONS
SUBSTITUTION REACTIONS OR REPLACEMENT REACTIONS
Such reactions take place in two stages
Strength of nucleophile not important | Strong nucleophiles required |
Good ionising solvent required | May go faster in less polar solvent |
Rate = K [RX] | Rate = K [RX] [Nu–] |
Possible rearrangements | No rearrangements |
Lead to racemisation | Lead to inversion. |
ELIMINATION REACTIONS
E1 : ELIMINATION UNIMOLECULAR
E2 : BIMOLECULAR ELIMINATION
E1 | E2 |
Good ionising solvent required | Solvent polarity not so important |
Base strength not important | Strong bases are required |
Rate = K [RX] | Rate = K [RX] [B–] |
Saytzeff orientation | Saytzeff orientation |
Rearrangements are common | No rearrangements |
ADDITION REACTIONS
ELECTROPHILIC ADDITION REACTIONS, initiation by electrophile e.g.
NUCLEOPHILIC ADDITION REACTIONS, initiation by nucleophile e.g.
FREE RADICAL ADDITION REACTIONS, initiation by free radical
REARRANGEMENT REACTIONS
POLYMERISATION REACTIONS
DIRECTIVE INFLUENCE OF ATOMS AND GROUPS
(For electrophilic substitution reactions)
When monosubstitution product of benzene is converted into di-substitution product, the position of second incoming group is decided by the atom or group already present in the benzene nucleus. This is known as directive influence of atoms and groups.
Directive influence is governed by three effects :
- Inductive effect (I)
- Electromeric effect (E)
- Resonance (M)
Any effect that pushes the electrons towards the benzene nucleus is taken as positive and activates the benzene nucleus for further substitution. The effect that pushes the electrons away from benzene nucleus is taken as negative and deactivates the benzene nucleus for further substitution.
Here we will consider Inductive effect and Mesomeric effect (Resonance) to decide the directive influence of atoms and groups. The electromeric effect is similar to Mesomeric effect and always operate in the same direction, the only difference is the former is temporary and latter is permanent.
DIRECTIVE INFLUENCE OF OH GROUP
INDUCTIVE EFFECT (I)
MESOMERIC EFFECT (M)
Ortho and para positions become the points of high electron density as + M > > – I. The electrophilic reagent will attack at o- and p- positions. Hence OH gp. is o, p-directing in nature with activation of benzene nucleus.
Other examples are
DIRECTIVE INFLUENCE OF –CH3GROUP
INDUCTIVE EFFECT (I)
HYPERCONJUGATION (HC)
The o, p-positions become the points of high electron density. The electrophilic reagent will attack at o- and p- positions. Hence methyl group is o,p-directing in nature with activation of benzene nucleus.
Other examples are : etc.
DIRECTIVE INFLUENCE OF –CN GROUP :
INDUCTIVE EFFECT (I)
MESOMERIC EFFECT (M)
The o,p-positions become the points of low electron density, therefore the electrophilic reagent will attack at the m position. Hence CN is meta directing in nature with deactivation of benzene nucleus.
Other examples are : 
DIRECTIVE INFLUENCE OF –CL ATOM
INDUCTIVE EFFECT (I)
MESOMERIC EFFECT (M)
By mesomeric effect the o,p positions become the points of high electron density. Further –I > +M, hence Cl is o, p directing in nature with deactivation of benzene nucleus.
Other examples are : –F, Br, I
EASE OF ELECTROPHILIC SUBSTITUTION OF BENZENE AND ITS DERIVATIVES
- Strongly activating (o, p directing)
- Moderately activating (o, p directing)
- Weakly activating (o, p-directing)
- Benzene itself.
- Deactivating (o, p – directing) : F, Cl, Br, I
- Deactivating (m-directing) :
Again,
COMMON ELECTROPHILIC SUBSTITUTION REACTIONS
- Nitration
Electrophile 
- Sulphonation
Electrophile  or SO3
or SO3
- Halogenation
Electrophile 
- Friedel Craft’s alkylation
Electrophile R+
- Friedel crafts acylation
Electrophile 
NUCLEOPHILIC SUBSTITUTION OF BENZENE
It does not occur with benzene itself, but it does occur with some substituted benzenes.
H– much less stable hence some oxidising reagent with which H– can react facilitate the nucleophilic substitution.