Biomolecules Class 11 Notes Biology Chapter 9
Topics and Subtopics in for Class 11 Biology Chapter 9 Biomolecules:
| Section Name | Topic Name |
| 9 | Biomolecules |
| 9.1 | How to Analyse Chemical Composition? |
| 9.2 | Primary and Secondary Metabolites |
| 9.3 | Biomacromolecules |
| 9.4 | Proteins |
| 9.5 | Polysaccharides |
| 9.6 | Nucleic Acids |
| 9.7 | Structure of Proteins |
| 9.8 | Nature of Bond Linking Monomers in a Polymer |
| 9.9 | Dynamic State of Body Constituents – Concept of Metabolism |
| 9.10 | Metabolic Basis for Living |
| 9.11 | The Living State |
| 9.12 | Enzymes |
| 9.13 | Summary |
A cell is composed of variety of molecules (like carbon, hydrogen, oxygen) which perform various functions.
Other than these basic elements, some metals and non-metals are also present as cellular materials, thence, all these materials combines in different ways in order to form various biomolecules, which are found in cells of organisms.
These molecules are not living, but perform various living functions. Thus, biomolecules are the organic substances (e.g., Carbohydrates, proteins, lipids, etc.) that play a major role in the structure and function of the living organism.
Water is also an important and most abundant chemical compound present in the body of living organism.
Topic 1 Analysis of Basic
Units of Biomolecules
Chemical Analysis & Cyanic Compounds
To analyse the chemical composition of any organic compound in a living organism, the chemical analysis of living tissue is done. As organic compounds play a major role, it is essential to have knowledge of their molecular formula and structure.
This can be easily predicted out by performing following steps
(i) Weight of a living tissue (like a piece of a liver, fruit, vegetable or any other body tissue) is taken.
(ii) The tissue is then grinded in trichloroacetic acid (Cl3CCOOH) with the help of mortar and pestle.
(iii) The thick slurry obtained is then filtered through a cheese-cloth or cotton.
This will generate two fractions of solution
(a) Filtrate or the acid-soluble fraction.
(b) Retentate or the acid-insoluble fraction.
(iv) The extract can then be subjected to numerous separation techniques, in order to obtain the desirable compound from all other components.
By successfully performing all the above steps, we can get an idea of the molecular formula and the probable structure of the compound.
(v) All the carbon compounds that we get from living tissues can be called biomolecules.
Ash Analysis for Inorganic Compound and Elements
After the analysis of chemical composition of an organic compound in a tissue, it is necessary to do the analysis of inorganic elements and compounds. It can be done easily by performing a destructive experiment that separate inorganic compounds from organic compounds in the form of ash (contains inorganic compounds and elements).
Ash is the remaining part of apy living tissue which remains back after burning of all organic compounds.
Ash analysis is done in order to analyse the chemical composition of different inorganic compounds and elements. It is done in the following way
(i) For analysis, a small amount of a living tissue is taken, which is oven dried till all the water evaporates.
(ii) This gives the dry weight.
(iii) After that tissue is burnt completely which results in the formation of ash.
Thus, ash formed contains inorganic elements like potassium, sodium, calcium, magnesium, etc (inorganic compounds are also seen in the acid soluble fraction).
Cellular Pool
The sum total of different types of biomolecules, compounds and ions present in the cell is called cellular pool. It contains more than 5000 chemicals.
List of Representative Inorganic Constituents of Living Tissues
Components Formula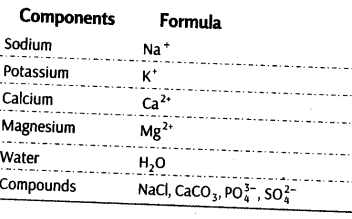
Biomicromolecules
Biomicromolecules are small in size, with low molecular weight (18-1800 Da) highly soluble (if polar) and have simple molecular conformation. Micromolecules can be inorganic such as water, minerals salts, gases or organic compounds such as sugars, amino acids, nucleotides, etc.
All these compounds mentioned above are soluble fraction of filtrate except the lipids which occur as insoluble fraction of filtrate as they are mosdy found in cell membrane and thereby forms vesicles, which are separated as an insoluble pool.
Various micromolecules in detail are as follows
1. Carbohydrates
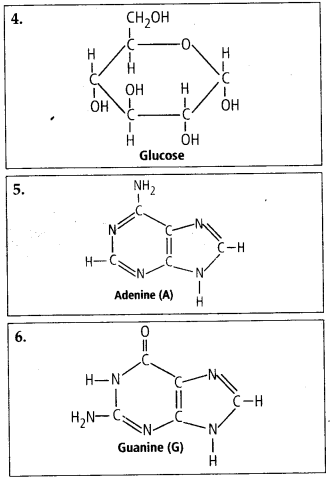
These are the organic compound mainly made up of C, H and O. They are defined as polyhydroxy aldehydes and ketones. These are produced directly by the plants during photosynthesis. Carbohydrates are also known as saccharides because their major constituents are sugars.
These are divided into following types
i- Monosaccharides
These are simplest carbohydrates which cannot be hydrolysed further into smaller components. These are generally composed of three to seven carbon atoms per molecule.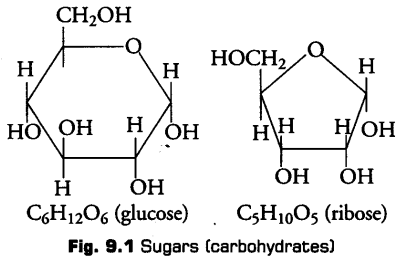
Monosaccharides are also known as reducing sugars, because they have a free aldehydic (—CHO) or ketonic (> C = O) group and can also reduce Cu2+ (cupric ions) of Benedict’s or Fehling’s solution to Cu+ (cuprous ions)., e.g., Ribose, glucose, erythrose, etc.
1. Oligosaccharides
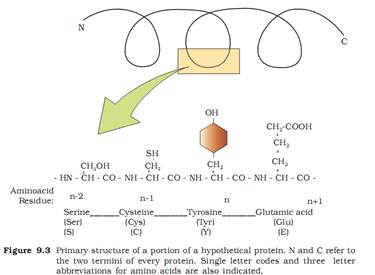
These are formed by condensation of 2-6 monosaccharide molecules. The bond between two monosaccharide units is called a glycosidic bond.
They are classified according to the number of their monosaccharide units or monomers as follows
(a) Disaccharides These are the sugars containing two monomeric units and can be further hydrolysed into smaller components. These are known as non-reducing sugars because the free aldehyde or ketone group is absent, e.g., Sucrose, maltose, lactose, etc.
(b) Trisaccharide It contain three monomers. e.g., Raffinose.
(c) Tetrasaccharides, e.g., Stachyose and so on.
2 Amino Acids
Amino acids are organic compounds containing an amino group and an acidic group as substituent on the same carbon, i.e., the a-carbon. Hence, they are called a-amino acids. These are substituted methanes.
a-carbon also bears a hydrogen and a variable group designated as R group. Thus, there are four substituent
groups present on a-carbon which occupy the four different valency position. These are hydrogen, carboxyl, amino and R group.
Based on the nature of R group, there are many amino acids. However, those which occur in proteins are only of twenty types.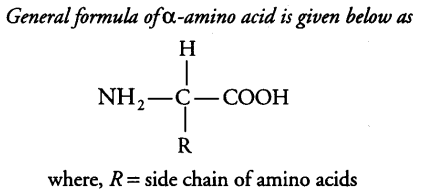
The amino group accepts a proton whereas, the carboxyl group donates a proton. So, an amino acid can act as both acid and base. Hence, it is amphoteric in nature.
The R group in these proteinaceous amino acids could be a hydrogen (glycine), a methyl group (alanine), hydroxyl methyl (serine), etc.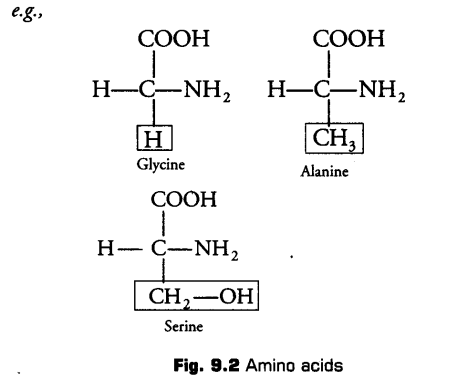
The chemical and physical properties of amino acids are essentially due to the amino, carboxyl and functional groups present.
Based on the number of amino and carboxyl group present, amino acids are categorised into following types
i. Acidic Amino Acids
These contain one amino group and two carboxyl group per molecule, e.g., glutamic acid and aspartic acid.
ii. Basic Amino Acids
These contain two amino groups and one carboxyl group per molecule, e.g., Arginine, lysine and histidine.
iii. Neutral Amino Acids
These contain one amino group and one carboxyl group per molecule, e.g., Methionine, isoleucine, serine, threonine, cysteine, glycine, alanine, valine, leucine, aspargine, glutamine and proline.
iv. Aromatic Amino Acids
These contain aromatic rings in their side chain, e.g., Phenylalanine, tyrosine and tryptophan.
Zwitter Ion
Zwitter ion formation is anoth’er particular property of amino acid. It is a neutral molecule (with positive and negative charge), having the ionizable nature of —NH2 and —COOH groups. Hence, in splutions of different pHs, the structure of amino acid changes variably.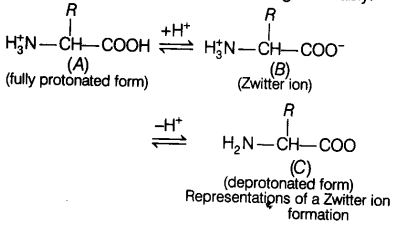
3. Lipids
Lipids are the esters of fatty acids and alcohol. These are generally insoluble in water. They could be simply fatty acids.
Fatty acids are the organic acids having hydrocarbon chains that end in a carboxylic group (—COOH). The carboxylic group is attached to an R group that could be a methyl (—CH3) or ethyl (—C2H5) or higher number of —CH2 groups (1 carbon to 19 carbons), e.g., Palmitic acid has 16 carbons including carboxyl carbon.
Arachidonic acid has 20 carbon atoms including the carboxyl carbon.
Depending upon the types of bonds present, fatty acids are of following two types
i.Saturated Fatty Acids
Fatty acids which do not have double bonds, (C—C). These are generally solid at room temperature.
ii. Unsaturated Fatty Acids
Fatty acids which contain one or more than one double bonds (C = C). These are generally liquid at room temperature.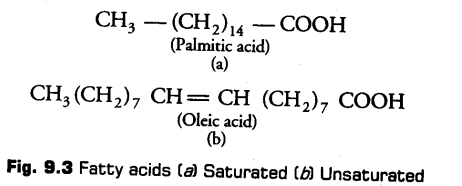
Difference between saturated and unsaturated fatty acids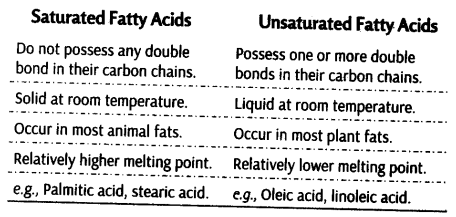
Simple Lipids
These are esters of fatty acids and various alcohol.
They are of further two types
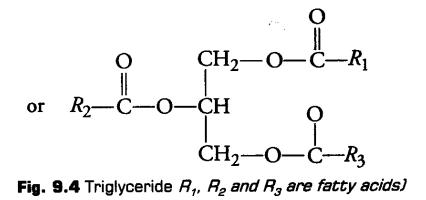
(a) Neutral or True Fats These are esters of fatty acids with glycerol (glycerine). They are also called glycerides.
Glycerol is a simple lipid which is known as trihydroxypropane as it is an alcohol with a backbone of three carbon atoms, each carrying an —OH group.
When glycerol is esterified with fatty acid it is known as triglyceride.
The ester is called monoglyceride, diglycerlde and triglyceride depending on the number of fatty acids attached to a glycerol.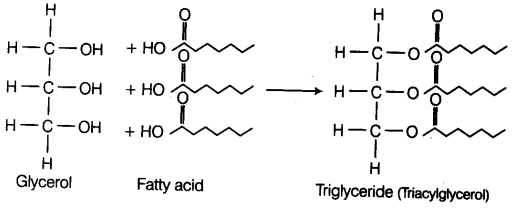
ii- Compound or Conjugated Lipids
These are the esters of fatty acids and alcohol but contain other substances also, e.g., Phospholipids, glycolipids, cutin, suberin etc.
Phospholipids are lipids which have phosphorus and phosphorylated organic compound in them. One of the common example of phospholipid is lecithin.
Some tissue have complex structure of lipids, e.g., Neural tissues.
(b) Oils are usually liquid at room temperature because they have low melting point, e.g., groundnut (peanut) oil, cotton seed oil, mustard oil, etc.
As oils have low melting points. They remain as oils in winters also, e.g., Gingely oil.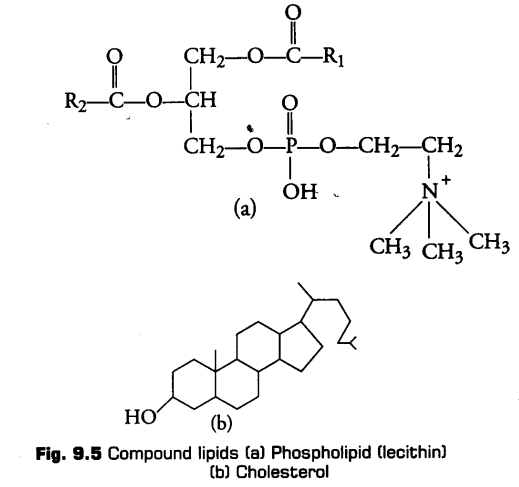
iii. Derived Lipids
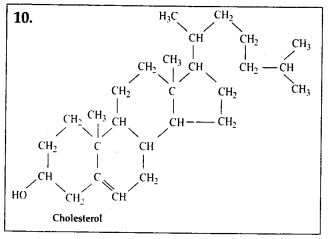
These are lipid-like substances such as sterol or derivatives of lipids, e.g., steroids, prostaglandins and terpenes.
Fats are also differentiated into two main types, on the basis of their melting points at room temperature as follows
(a) Hard Fats are solids at room temperature and contain long chains of fatty acids, e.g., Animal fat.
Softness of butter is due to the good quantity of short chain fatty acid it contains.
4. Nucleotides
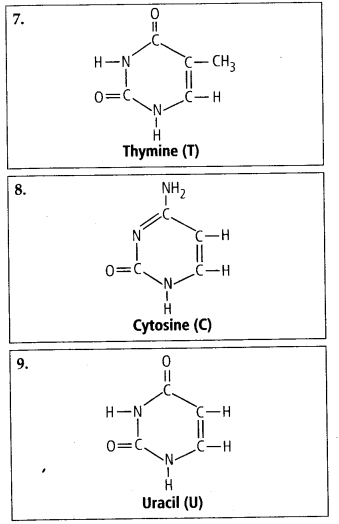
These are the monomers of nucleic acids.The nucleotides are made up of three molecules, i.e., a pentose sugar, a cyclic nitrogenous base and a phosphoric acid (phosphate group), e.g., Adenylic acid, thymidylic acid, guanylic acid, uridylic acid and cytidylic acid.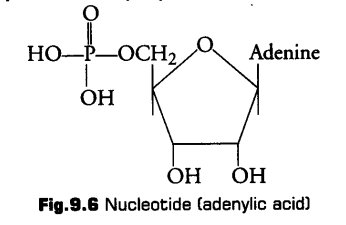
1. Pentose Sugar
It occurs in pentagon or fiiranose form with four carbon and one 02 forming a ring. It is present in the form of ribose or deoxyribose sugar in RNA and DNA respectively.
i. Nitrogenous Bases
These are the flat heterocyclic compounds having nitrogen and carbon in ring structure.
These are of basically two types
(a) Purines It is larger and composed of two rings. They are further of two types, i.e., Adenine (A) and Guanine (G).
(b) Pyrimidines It is smaller and composed of single ring. They are of further three types, i.e., Cytosine (C), Thymine (T) and Uracil (U).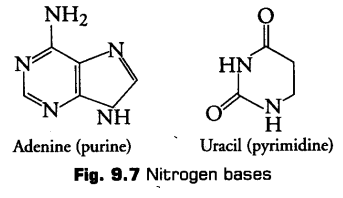
iii. Phosphoric Acid (Phosphate Group)
It is composed of phosphoric acid. A nucleotide may have 1, 2 or 3 phosphate groups. It gives acidic nature to the nucleotide.
Nucleoside
If a pentose sugar is attached to a nitrogen base by a glycosidic bond, it is called nucleoside.
e.g., adenine + ribose —> adenosine.
Likewise guanosine, thymidine, uridine and cytidine are the examples of nucleoside.
The nucleoside combines with a phosphate group at 5-position by an ester bond to form a nucleotide.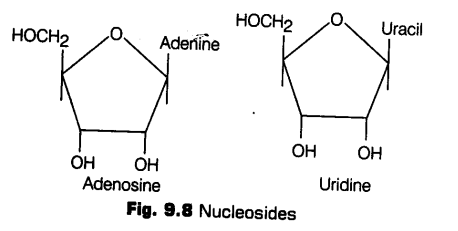
Differences between Nucleoside and Nucleotide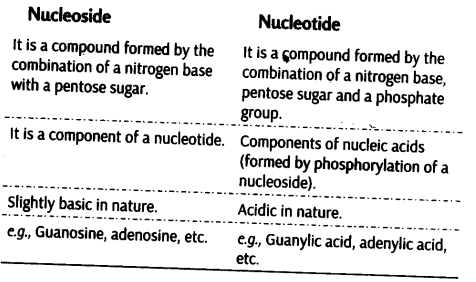
Primary and Secondary Metabolites
A large number of organic biomolecules are present in the cells which are used in various metabolic reactions of cell. Hence, these compounds are called metabolites. These are divided into two types
1. Primary Metabolites
These are metabolites which are found in animal tissues. Their functions are easily indentifiable. They play specific known roles in the normal physiological processes, e.g., Amino acids, carbohydrates, proteins, nitrogen bases, nucleic acids, etc.
2. Secondary Metabolites
These are metabolites which are generally found in plant, fungal and microbial cells. These are the products of certain metabolic pathways. Their functions are not identifiable in host organism and are not yet understood, e.g., Alkaloids, flavonoids, rubber, essential oils, antibiotics, coloured pigments, scents, gums, spices.
Some Secondary Metabolites
Note:
* Nucleic acids (DNA and RNA) are composed of only nucleotides, both DNA and RNA acts as a genetic material.
* Uracil is found only in RNA in place of thymine.
* Ribose molecule differs from deoxyribose molecule in having a -OH group instead of H at carbon 2.
* Heterocyclic compounds have more than one kind of atoms.
Functions
Both primary and secondary metabolities serves the followingjunctions
(i) Many of them are useful in human welfare, e.g., Rubber, drugs, spices, scents, pigments.
(ii) Some have ecological importance.
Topic 2 Biomacromolecules
Biomacromolecules are large in size, higher molecular weight molecules 10,000 daltons (Da) and above (except lipids).
These are generally formed by linking a number of micromolecules commonly known as monomers.
All these compounds are found in the acid insoluble pool. These are of four major types, i.e., proteins, polysaccharides, nucleic acids and lipids.
Except lipids all other macromolecules are formed by polymerisation (condensation) of monomeric subunits.
The molecules which are found in living organisms are divided into two main types
(i) Biomicromolecules These are molecules which have their molecular weight less than 10,000 Da.
Biomicromolecules has already been described in detail in topic 1 of the chapter. .
(ii) Biomacromolecules These are molecules which have their molecular weight, 10,000 Da and above.
All these macromolecules are actually polymers of their biomicromolecules.
For example, Polysaccharides are polymers of monosaccharides, proteins are polymers of amino acids and nucleic acids are polymers of nucleotides.
Hence, on the basis of the number and types of monomer present, polymers are of following two types
(i) Homopolymers, are those which have only one type of monomer present. These monomer can be repeated n number of times in a chain, e.g., Starch, insulin, etc.
(ii) Heteropolymers, are those which have two or more than two types of monomers, e.g., Proteins.Various macromolecules and their major roles are described under as
Lipids
The molecules in the insoluble fraction are polymeric substances except lipids.
Athough lipids have their molecular weight not exceeding above 800 Da, but still it comes under acid insoluble fraction, i.e., biomacromolecular category.
This happens because these are small moleculer weight compounds and are present not only as such, but also arranged in structures like cell membranes and other membranes.
Thus, when we grind a tissue, we disrupt the cell structure, cell membrane and other membranes are broken down into pieces and form vesicles that are not water soluble. Therefore, these are separated along with acid insoluble pool and are placed in macromolecules.
Lipids are not strictly biomacromolecule.
If representation of the chemical- composition of living tissue is done from abundance point of view and arranged class-wise, it is observed that water is the most abundant chemical in living organisms.
Average Composition of Cells
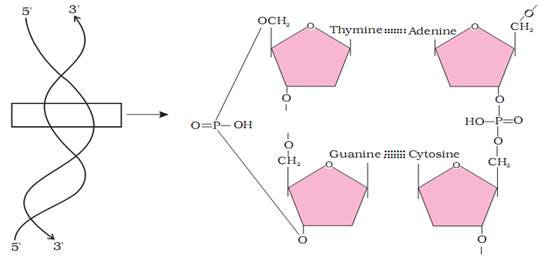
i. Primary Structure
It is the description of basic structure of a protein. This includes number and sequence of amino acids in each polypeptide. The distance between two adjacent peptide bonds is about 0.35 nm.
A protein is imagined as a line whose left end is represented by the first amino acid, also called as the N-terminal amino acid and the right end is represented by the last amino acid called the C-terminal amino add, e.g., Insulin, ribonudease.
Proteins
These are the most important and abundant intracellular organic biomoLecules. These are polypeptides having chains of amino acid arranged linearly that are linked by peptide bonds.
Thus, each protein is a polymer of amino acids (as studied earlier in the chapter), there are 20 types of amino acids, e.g., Alanine, valine arginine, leucine, histidine, etc.
So, proteins are considered as heteropolymer.
These amino acids are divided into two main types, on the basis of their utility
(i) Essential Amino Adds These are those amino acids that are essential for our health so, are need to be supplied through our diet. The dietary proteins are the source of essential amino acids, e.g., Leucine, isoleucine, etc.
(ii) Non-essential Amino Adds These are those amino acids, which our body can synthesise, e.g., Proline, serine.
Human adults require an additional essential amino acid named threonine while children need two more arginine and histidine. These are. called semi-essential amino acids.
Structure of Proteins
As mentioned earlier, proteins are heteropolymers containing strings of amino acids. Biologists describe the protein structure at four different levels, i.e., primary, secondary, tertiary and quaternary.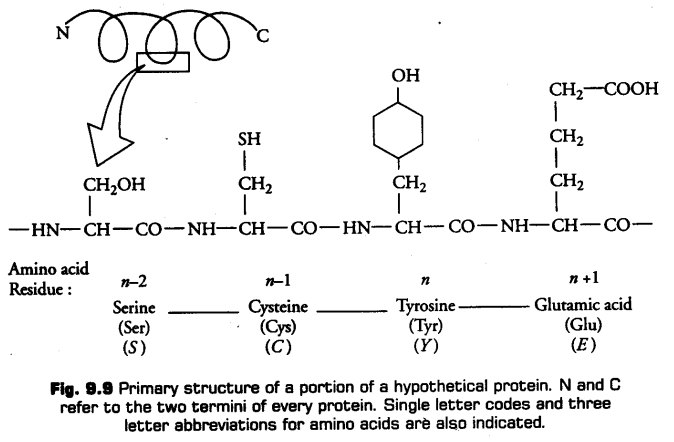
ii. Secondary Structure
The thread of the primary protein is folded in the form of a-helix. The a-helix is stabilised by hydrogen bonds between oxygen of the carboxylic group of one amino acid residue and —NH group of the next fourth amino acid residue, e.g., Keratin.
In β-pleated secondary structure, two or more polypeptide chains get interconnected by hydrogen bonds. Adjacent strands of polypeptide may run in the same direction or in opposite direction, e.g., Silk fibre.
In proteins, only right handed helices are observed.The polypeptide chain curls The protein is more distended and longitudinally by the action of the hydrogen bond forms a zig-zag hydrogen bonds forming a shaped protein structure called spiral or helix. (which combines and forms p-sheet).
Differences between α-helix and β–pleated Structure of Proteins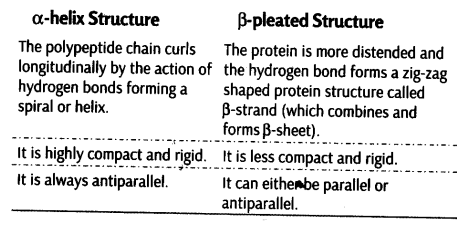
iii. Tertiary Structure
There is bending and folding of various types to form a hollow wollen ball-like spheres, rods or fibres. Tertiary structure is stabilised by several types of bonds-hydrogen bonds, ionic bonds, van der waals interactions, covalent bonds and hydrophobic bonds. It gives information about a 3-dimensional (3-D) conformation of the protein, e.g., Myoglobin.
Tertiary structure is helpful for many biological activities of proteins.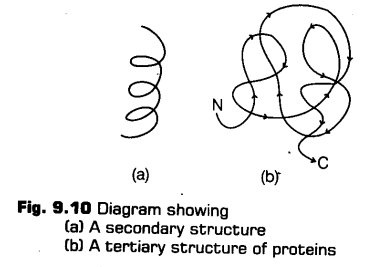
iv. Quaternary Structure
Certain proteins consist of an asembly of more than one polypeptide or subunits. Thus, the individual polypeptide or subunit are arranged with respect to one another (linear strings of spheres, spheres arranged one upon each other in the form of a cube or plate, etc.) e.g., Haemoglobin, lactic acid dehydrogenase enzyme.
This type of structure is found only in the oligomeric proteins (proteins having two or more polypeptide chains).
Structure of Haemoglobin (Hb)
An adult human haemoglobin is a iron containing pigment that acts as an oxygen carrier. It has a quaternary structure because it is made up of four monomeric sub-units each about the size of many normal individual proteins.
Every subunit has its own tertiary structure and is identical to each other. Hence, two subunits of α-type and two sub-units of β-type together constitute the human haemoglobin (Hb). Insulin is an another example of protein having quaternary structure.
Types of Proteins
Proteins are classified on the basis of shape, chemical composition and function.
Accordingly on the basis of shape these are of two main types
i. Fibrous Proteins
The proteins have spiral secondary polypeptide chains wound around each other in order to form fibres. These are insoluble in water generally, but soluble in concentrated acids, alkalis and salts, e.g., Collagen of connective tissue, keratin of hair, etc.
ii. Globular Proteins
They are rounded in shape and are generally soluble in water and in dilute acids, alkalis, salts, e.g., Egg albumin, serum globulins.
Note:
Collagen, the most abundant protein of animal world and Ribulose Bisphosphate Carboxylase Oxygenase (RuBisCO) is the most abundant protein in plants and the whole of the biosphere.
Functions of Proteins
Proteins have various basic functions in living organism given below . Helps in transportation of nutrients across the cell membrane by acting as protein transporter.
(ii) Helps in fighting with infectious organism.
(iii) These are helpful in movement of muscles, e.g., Myosin and actin.
(iv) Helps in maintenance of pH and regulation of the volume of body fluids.
(v) Helps at the time of injury in blood clotting and acts as antibodies and provide immunity.
(vi) Helps in growth and repair of body tissues.
(vii) Some proteins function as hormones and some functin as enzymes and catalyse the reactions.
Denaturation of Proteins
When proteins are exposed to extreme change in pH, acids or temperature (or bases or high salt concentrations) the weak bonds holding the tertiary and the quaternary structure gets disrupted so, that the protein unfold (into primary structure). This unfolding is known as denaturation of proteins or loss of its functioning.
Denaturation is not strong enough to break peptide bonds thus, primary structure remains unaffected.
A denatured protein may spontaneously refold into its original structure when suitable condition are re-provided. This is called renaturation.
Polysaccharides
These are another class of macromolecule that are present in the acid insoluble fraction. Polysaccharides are long chains of sugars. They are not sweet and are insoluble in water. Polysaccharide chain (like glycogen) is made up of two ends, whose right end is called reducing end and the other left end is called non-reducing end. They- ace threads containing different monosaccharides as building blocks.
Types of Polysaccharides
Polysaccharides are of two types as given below
i. Homopolysaccharides
These are those complex carbohydrates which are formed by polymerisation of only one type of monosaccharide monomers, e.g., Starch, glycogen and cellulose (these all are composed of single type of monosaccharide unit namely glucose).
Some of them are as fallows
a. Cellulose
It is a polymeric polysaccharide which consists of only one type of monosaccharide monomer, i.e., glucose. It is known to be a rigid and insoluble polysaccharide found in cell wall of most algae, certain protists, fungi and some higher plant.
Paper made from pulp of plant and cotton fibre are also made up of cellulose. As cellulose is not composed of complex helices so, it cannot hold iodine (I2) and cannot give colour with iodine.
b. Starch
It is a storage polysaccharide because it helps in storing energy in plant tissues. Chemically, the starch is formed of two glucose monomers, r.e.,α-amylose and amylopectin.
Starch forms helical secondary structures. Thus, it can hold iodine (I2) molecules in the helical portion. Therefore, gives blue colour with iodine solution.
c. Glycogen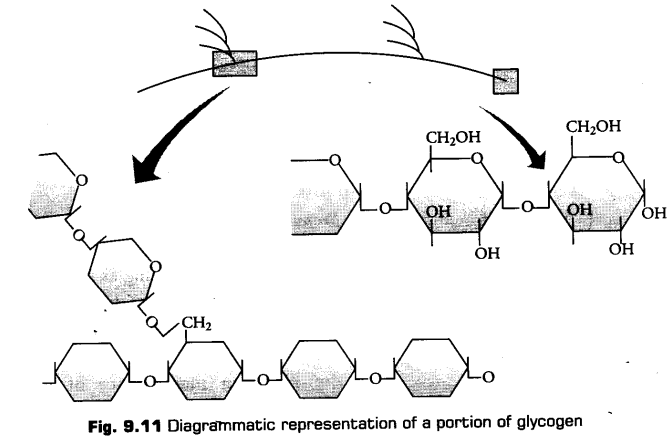
It is also storage polysacchiaride found in animals only (in liver cells and muscles). It is also known as animal starch. It gives red colour on reaction with iodine.
It is a polymer of fructose. It is a naturally occurring polysaccharide produced by many types of plants. It is used by some plants in storing energy.
Plants that synthesis and store inulin are unable to store other forms of carbohydrates like starch, etc.
Agar, xylan, araban, etc, are some other types of homopolysaccharides found.
ii. Heteropolysaccharides
These are complex carbohydrates formed by the polymerisation of two or more than two types of monosaccharide monomers, e.g., Chitin, pectin, peptidoglycans (murein), hyaluronic acid.
iii. One ofthem is explained below Chitin
It is the second most abundant natural polymer, found in exoskeleton of arthropods (e.g., prawns, crabs, etc.) and in cell wall of fungi. It has building blocks of amino sugars and chemically modified sugar.
iv. acetylglucosamine units interlinked by glycosidic bond
Glucosamine also acts as building block (like N-acetyl glucosamine) in other types of heteropolysaccharide.
Functions of Polysaccharide
Polysaccharide plays multiple function and can be used in the following ways
(i) Acts as structural compounds in cell wall of plants certain fungi and protists, e.g., Cellulose, chitin.
(ii) Helps in anticoagulation and prevents blood clotting inside the vessels, e.g., Heparin.
(iii) Helps in lubrication of joints between bones, e.g., Hyaluronic acid.
(iv) Also used in tissue culture, e.g. Agar.
(v) Acts as a reserve food, e.g., Starch.
Nucleic Acids
The other type of macromolecule found as a part of acid insoluble fraction of any living tissue is the nucleic acids. These are polymeric compounds of nucleotides, i.e., polynucleotides.
A nucleotide (as discussed previously in the chapter) is composed of three chemically distinct components
(i) Heterocyclic compound-nitrogen base (adenine, guanine, uracil, cytosine and thymine).
(ii) Monosaccharide (ribose or deoxyribose).
(iii) Phosphoric acid or phosphate.
A nucleic acid which contains deoxyribose sugar is called deoxyribonucleic acid (DNA), while that which contains ribose sugar is ribonucleic acid (RNA).
Deoxyribonucleic Acid (DNA)
DNA is genetic material found in the nucleus of all living cells except some viruses.
In eukaryotic organisms linear DNA is found in nucleus, in the mitochondria and chloroplasts, whereas in prokaryotes, DNA is circular in structure and is found in the cytoplasm.
Structure of DNA
The structure of DNA was elucidated by Watson and Crick based on X-ray diffraction studies. They proposed a double helix model of DNA. According to this model, DNA exists as a double helix and consists of two strands of polynucleotides that are antiparallel to each other, i.e., both run in opposite directions, one in 5′–> 3′ direction and other in 3’—> 5’direction. ,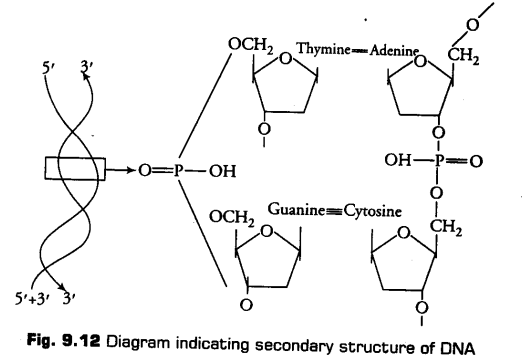
The backbone of DNA is formed by the sugar phosphate-sugar chain. The nitrogen bases are projected more or less perpendicular to the backbone of DNA and faces inside. A and G of one strand base pairs with T and C respectively on the other strand.
Between A and T (A== T), there are two hydrogen bonds while, there are three hydrogen bonds between G and C(G=C).
DNA has a uniform thickness of 20 A and pitch of is 34 nm. Thus, one turn of DNA measures 3.4 nm (rise per base pair) and consists of 10 nucleotides (or ten base pairs). This form of DNA is called B-DNA.
Functions of Nucleic Acids
Nucleic acidplays multiple role in living organism these are given as follows
(i) It enables cell to grow, maintain and divide by directing the synthesis of structural proteins.
(ii) Acts as a genetic material, i.e., transfer hereditary characters from one generation to the next.
Differences between DNA and RNA are given below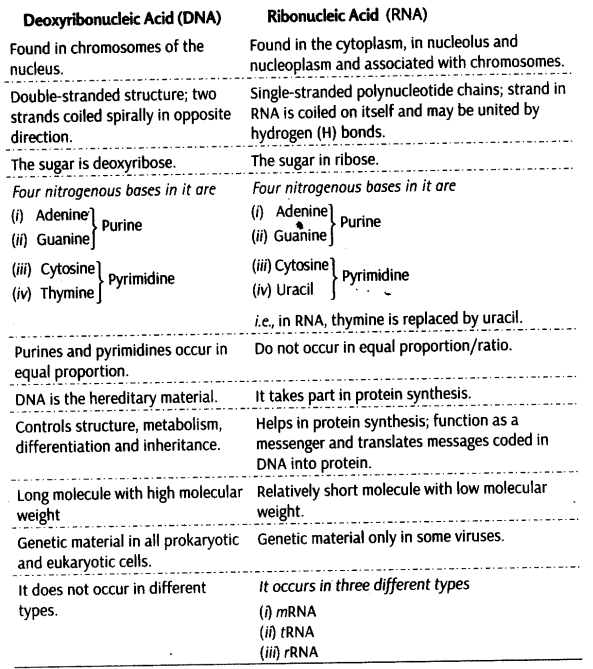
Some of them are as follows
1. Peptide Bond
In a polypeptide or a protein, amino acids are linked by a peptide bond. Formed when the carboxyl group (—COOH) of one amino acid reacts with the amino group (—NH2) of the next amino acid with elimination of water.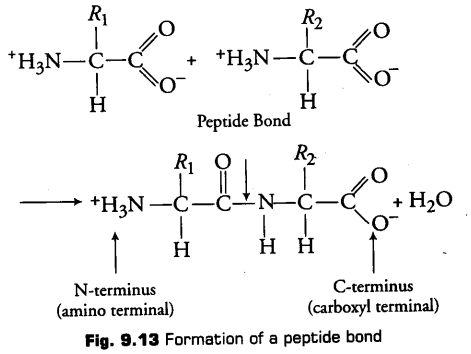
2. Glycosidic Bond
It is formed between two carbon atoms of two adjacent monosaccharides, thus it forms a polysaccharide by linking individual monosaccharides. This bond is also formed by dehydration (removal of water).
3. Phosphodiester Bond
In a nucleic acid a phosphate moiety links the 3,carbon of one sugar of one nucleotide to the 5’carbon of the sugar of the succeeding nucleotide.
The bond between the phosphate and hydroxyl group of sugar is an ester b id. As there is one such ester bond on either side, it is called phosphodiester bond.
Dynamic State of Body Constituents
Nature of Bond Linking Monomers in a Polymer
The polymers described above in the topic are formed by the combination or linking of one or more type of monomer units. So, in order to link these units together various types of bonds are required depending on the nature and the type of macromolecule.
Concept of Metabolism
Each cell contain thousands of organic compounds. These compounds or biomolecules are present in living organisms in various concentrations. Turn over of biomolecules is one of the greatest discoveries. It is the phenomenon in which biomolecules change constantly into some other biomolecules or made from some other biomolecules.
All these, transfer of one biomolecule into other occur due to chemical reaction which continuously take place in an organism. The chemical reactions together are called metabolism.
Each metabolic reaction results in the process of transformation, e.g., an amino acid when transforms intq an amine, C02 is removed, removal of amino group in a nucleotide base, etc.
Majority of these metabolic reactions do not occur in isolation, instead they take place in a series of linked reaction known as metabolic pathways. These pathways are either linear or circular and criss-cross each other, i.e., there are traffic functions.
Flow of metabolites through metabolic pathway has a definite rate and direction and this metabolic flow is called the dynamic state of body constituents. Also these metabolic reactions are always catalysed reaction, i.e., no uncatalysed metabolic conversion is present in living systems. The catalysts which hasten the rate of a given metabolic conversion are also proteins. These proteins with catalytic power are called enzymes.
Metabolic Basis for Living
Metabolic pathways in living organisms are divided into two main types
i. Anabolic Pathways
These include the formation of complex structure from simple ones, e.g., formation of cholesterol from acetic acid, protein synthesis, etc. These are energy consuming pathways.
ii. Catabolic Pathways
Glycolysis
Glucose is degraded to lactic acid in human skeletal muscle, liberating energy. This metabolic pathway from glucose to lactic acid which occurs in ten metabolic steps is called glycolysis.
This liberated energy is stored in the form of chemical bonds and this bond energy can be utilised in various biosynthetic, osmotic and mechanical work when needed.
Adenosine Triphosphate (ATP)
The most important form of energy currency present in living systems is the bond energy in a chemical compound of ATP.
The Living State
Various chemical compounds (metabolites or biomolecules) are present at a concentration characteristic of each of them, i.e., all living organisms exist in a steady state characterised by concentrations of each of these biomolecules. It is the most important fact of biological systems. These metabolites are in a state of metabolic flux. Hence, the living system is kept in a non-equilibrium state by metabolic flux, which enables it to perform work as living organism.
It has to work continuously and are unable to reach equilibrium.
Therefore, metabolism is helpful in providing a mechanism which enables energy production.
It can be stated that the living state and . metabolism are synonymous and are correlated. Thus, metabolism and living state are incomplete without each other.
These include the formation of simpler structures, i.e., the breakage of complex structures into simpler ones, e.g., Conversion of glucose into lactic acid in skeletal muscles. These are energy releasing
CBSE Class 11 Biology Chapter-9 Important Questions
1 Marks Questions
1. Which is the important energy carrier in the cell?
Ans. Adenosine tri phosphate (ATP)
2. Name the monomer subunits which form Nucleic acids?
Ans. Nucleotide.
3. What are macromolecules? Give example.
Ans. Macromolecules are large complex molecules formed by polymerization of micromolecules & have high molecular weight.
4.Identify the polymer which makes exoskeleton of insects.
Ans. Chitin a polymer of glucosamine that forms exoskeleton of insects,
5.Name the following:- i) sugar present is DNA ii) Base not found in DNA
Ans.(i) Deoxyribose sugar (ii) Uracil
6.Why proteins are called biological polymer?
Ans. As proteins are able to perform multiple functions eg. Protection mechanical support, transportation, movement etc, they are called as biological polymers.
7.Which molecule has the capacity to duplicate?
Ans. Deoxyribonucleic acid (DNA)
8.Name the abundant proteins in biosphere?
Ans. RUBISCO
9.Lipids are not biomacromolecules why?
Ans. Lipids are not biomacromolecule because their molecular weight does not exceed 800.
10.Which lipid can cause heart ailment?
Ans. Cholesterol.
11.What are micro- nutrients?
Ans. Minerals required by plants in trace quantity eg. Mn, Co, Zn, B, etc. are called micronutrients.
12.Why do oils generally remain in liquid state even in winters?
Ans. Oils are unsaturated lipids, hence have lower melting points.
13. Name an element found in proteins but not in lipids and carbohydrates.
Ans. Nitrogen.
14. What is the difference between RNA and DNA in terms of nitrogenous base?
Ans. RNA has uracil instead of thymine.
15. What does an enzyme do in terms of energy requirement of a reaction?
Ans. Lowers the activation energy of reaction.
16. What is the function of ATP in cell metabolism?
Ans. Are the energy currency of cell.
17. Name the protein which form the intercellular ground substance.
Ans. Collagen.
2 Marks Questions
1.Differentiate between nucleotide & nucleoside?
Ans.
| NUCLEOTIDE | NUCLEOSIDE |
| i) Nucleotide is made up of base, sugar & phosphoric acid. | i) Nitrogenous base & sugar form a nucleoside |
| ii) Nucleotide of RNA is called ribonucleotide & nucleotide of DNA is called deoxyribonucleotid | ii) Nucleoside of RNA is called ribonucleoside & nucleoside of DNA is called deoxyribonucleoside |
| iii) E.g adenylic acid, guanylic acid, thymidylic acid, uridylic acid | iii) Eg. Adenosine, fuanosine, cytidine, thymidine, uridine |
2.How are glycosidic bonds formed?
Ans. The glycosidic or ketone group of a monosaccharide can react & bind with an alcoholic group of another organic compound to join the two compounds together. This bond is known as glycosidic bond.
3.What do you mean by steady state?
Ans. An open system always remains in steady state i.e. the rate of in put of energy & matter is always equal to the output of energy & matter.
4.What is metabolism? Mention the role of enzymes is metabolism ?
Ans. Metabolism is defined as the sum total of the living processes in the body. Enzymes direct metabolic pathways. Enzymes act as catalysts. Enzymes are highly specialized organic catalysts produced by living cell. Biochemical pathways refer to the reactions occurring in the cells in sequences. Enzymes guide the biochemical pathways along desired directions. They have active site. The substrate binds at active site of enzyme & form enzyme substrate complex.
5.Why are enzymes called as biocatalyst?
Ans. The substances which changes the rate of chemical reaction without altering the equilibrium point of reaction is called catalyst. The catalysts of the organism are called enzymes & they are synthesized in the living cell. Hence called as Biocatalysts.
6.Give the functions of carbohydrates?
Ans.
(i)Carbohydrates play role in all metabolic reactions of body & formed as intermediate compounds in pathways of the processes.
(ii)Ribose & deoxyribose sugar are found in nucleic acids.
(iii)Glucose is oxidised in respiration to yield energy.
(iv)Glucose is used in synthesis of fats as well as proteins.
7.What do you meant by activation energy?
Ans. Activation energy is the energy required to initiate a chemical or biochemical reaction. Activation energy overcomes the energy barriers of the reactants which occurs amongst the reactants due to i) presence of electrons over their surface ii) Absence of precise & forceful collisions essential for bringing the reactive sites of the chemical together.
8.List the different types of lipids.
Ans. Lipids are of three types:-
(i) Simple lipids:- they are of alcohols or triglycerides containing fatty acid & glycerol.
(ii) Compound lipids:- They are simple lipids with a biologically active compound in them eg. glycolipids ( carbohydrate lipid) lipoprotein ( protein + lipids)
(iii) Derived lipids:- They are hydrolysed products of simple lipids such as fatty acids & alcohol.
9.Enlist three properties of enzymes?
Ans. (i) An enzyme is specific for a substrate & catalyses only a particular reaction. because of the specific shape of active site & substrate.
(ii) Every enzyme requires an optimum temperature for its functioning.
(iii) The enzymes are sensitive to PH & each enzyme shows its maximum activity at a specific PH called optimum PH.
10.Enumerate differences between DNA & RNA?
Ans.
| DNA | RNA |
| i) it consists of a double helical of two polynucleotide chains | i) It consists of only one helical of single polynucleotide chain. |
| ii) Deoxyribose sugar is present in the nucleotides. | ii) Ribose sugar is present in nucleotide |
| iii) Pyrimidine bases are thymidine & cytosine. | iii) Pyrmidine bases are uracil & cytosine |
| iv) DNA contains all the genetic information | iv) RNA helps in protein synthesis. |
11.Why are monosaccharide’s sugars are are known as reducing sugars?
Ans. Monosaccharides sugars are called reducing sugars because they have a free aldehyde or ketone group & can reduce Cu2+ to Cu+. Disaccharides like sucrose does not reduce Cu2+ to Cu+ so, it not a reducing sugar.
12.How does temperature affects enzyme catalysed reaction?
Ans. The temperature affects the velocity of enzyme action. When the temperature is high, there is a sudden decrease in enzyme action due to denaturation. Mostly enzymatic reactions occur below 450c
13.What is enzymatic competitive inhibition? Give one example?
Ans. Some chemicals prevent the enzyme to function, are known as inhibitors. Enzymatic competitive inhibition is done by the substrate which very closely resembles the substrate in its molecular structure.
Enzyme + Inhibitor Enzyme inhibitor complex.
Enzyme inhibitor complex.
Eg. malonate inhibits the action of succinate dehydrogenase because it shows close resemblance with succinate substrate.
14. Why are aminoacids also known as substituted methane?
Ans. The a-carbon has 4 substituted groups occupying the 4 valency position – H. –COOH, 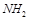 and -R group.
and -R group.
15. Amino acid exist as zwitter ions. Givc its structure. Why is it formed?
Ans.
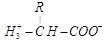
Duetoionizable nature of  groups.
groups.
16. Why do starch give blue black colour with iodine?
Ans. Starch forms helical secondary structures which can hold 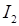
17. Why are starch and glycogen more suitable than glucose as a storage product?
Ans. Occupy lesser space as less bulky and can hydrolysed to glucose when required.
18. What would happed when salivary amylase which acts on starch in mouth and in stomach?
Ans. In mouth, salivary amylase changes starch into maltose. Action of amylase stops in stomach as it cannot act in an acidic medium.
19. Differentiate between homopolysaccharides and heterosaccharides.
Ans.
| Homopolysaccharides | Heteropolysaccharides |
a)Constituted of single type of monosaccharide units b) e. g., starch, glycogen, cellulose | Constituted by two or more type of monosaccharide unit and their derivatives e.g., Peptidoglyeans, chiten |
20.Why do physicians recommend vegetable oils rich in poly unsaturated fat for persons suffering from cardiovascular diseases?
Ans. Polyunsaturated oils contain fatty acids having one or more double bonds which does not clog arteries due to high proportion of polyunsaturated fatty acids
21. Why does the shelf life of fruits and vegetables increase in a refrigerator?
Ans. Low temperature prevents growth of food spoiling micro organisms and also inhibits the action of enzymes are in the food, because enzymesam inactivated at low temperature.
3 Marks Questions
1.Enumerate the functions of lipids?
Ans. i)Most of the plants & animals fats constitute storage compound. Fat is stored mainly in adipose cells in the animals.
ii)In oil seed plants, oil provides nourishment to developing embryo during seed germination. Oil extracted from these seeds is used in cooking.
iii)Fats provide energy to the body.
iv)Fats serve as insulators & protect body from cold. It gets deposited underneath skin.
v) Phospholipid form an structural component of all bio- membranes in cell.
vi)Cholesterol acts as precursor for synthesis of various hormones, vitamins & bile salts.
vii)The lipid form the white matter, grey matter of brain & myelin sheath of neurons.
2.Describe the lock & key hypothesis of enzyme action?
Ans. According to Fischer’s lock & key hypothesis of enzyme action:- if the right key fits in the right lock, the lock can be opened otherwise not. To explain the above in context with enzyme action it is bedewed that molecules have specific geometric shapes. Proteins are able to act as enzyme because their shape provides space configuration into which other molecules can fit. The molecules which are acted upon by the enzymes are called substrates of the enzymes.
Under the above assumption only those substrate molecule with proper geometric shape can fit into the active site of the enzymes. However, under special circumstances some other molecules which are similar to the substrate can also combine with active site of enzyme. In such cases molecules may compete with substrate & the reaction may either slow down or stop. This is called competitive inhibition.
3.Describe the structure & function of ATP?
Ans. ATP is primary & universal carrier of chemical energy in the cell living cell capture store & transport energy in a chemical form, largely ATP & it is the ATP which is the carrier & intermediate source of chemical energy to those reactions in the cell which do not occur simultaneously. These reactions can take place only if chemical energy is released.
The ATP molecule consists of a nitrogenous base adenine a pentose sugar of ribose type & three inorganic phosphate molecules two phosphate bonds are high energy bonds & one is relatively poor in energy.
Energy released in living cell is thus stored in the chemical bonds of the ATP molecule which then serve as major energy yielding & energy requiring substance in the cell. ATP is broken down into ADP whenever energy is needed.
ATP –> ADP + ip + energy.
4.Differentiate between cofactors, coenzymes & prosthetic group.
Ans.
| COFACTORS | COENZYMES | PROSTHETIC GROUP |
| i) It is a non protein substance or group that gets attached to an enzyme. | i) it is a non protein group which is loosely attached to the open enzyme in a functional enzyme | i) it is a non protein part or group which gets attached to open enzyme. |
| ii) It is essential for functioning it may be organic or inorganic or metallic factor | ii) NAD is coenzyme for dehydrogenase | ii) Some prosthetic group have porphyrin of the cytochrome. |
5.How does enzymes brings about high rate of chemical conversions?
Ans. A chemical that is converted into a product is known as the substrate. Therefore the enzymes with tertiary structures including an active site convert a substrate into a product. The substrate ‘S’ must bind enzymes at its active site within a given cleft. So an obligatory formation of an ES substrate complex occurs. At a state when the substrate is bound to an enzyme active site, a new structure of substrate is formed.
In the graph, if ‘P’ is at lower level than ‘S’ reaction is exothermic i-e energy is supplied to make product ‘P’. The ‘S’ has to go through much higher energy state known as “transition state. The enzymes brings down energy barrier making transition of ‘S’ to ‘P’ more easy. The difference in average energy content between that of ‘S’ & this transition state is termed as activation energy.
6.What are nucleic acids? Describe the structure of DNA.
Ans. Nucleic acids are found in acid soluble fraction of living tissue. They are linear polymers of deoxyribonucleotides or ribonucleotides A nucleotide has 3 distinct components.
DNA is a double stranded structure & each strand is a polymer of deoxyribonucleotide. The backbone of the nucleic acid is uniformly consisting of alternating pentose sugar & phosphate group
i)The steps composed of nitrogenous bases adenine guanine cytosine & thymine & hydrogen bonds hold two strands together.
ii)Two strands are complementary to each other.
iii)They run in an antiparallel manner.
iv)It is genetic material in all organisms.
v)It has the property to replicate
vi)At one end of strand, 5-c of pentose sugar is free on other end; third carbon of pentose is free.
7. (a) What is anzyme?
(b) Give an example of co-enzyme.
(c) Distinguish between apoenzyme and co-enzyme.
Ans. (a) Are biocatalysts.
(b) NADP, NAD
(c) The enzymes which work only in the presence of co-factors am known as apoenzymes.
An organic non-protein cofactor which is easily separable from the apoenzyme is called co- enzyme.
5 Marks Questions
1. Explain briefly four levels of protein structure?
Ans. FOUR LEVELS OF PROTEIN STRUCTURE:-
a) PRIMARY STRUCTURE:- The protein exists as a long chain of amino acids arranged in a particular sequence such a polypeptide is non- functional
b) SECONDARY STRUCTURE:-first amino acid is N-terminal amino acid & last is known as c-terminal amino acid. There is interaction between every fourth amino acid by formation of hydrogen bond the polypeptide is folded in a helical shape eg. keratin. When two or more polypeptide chains are held together by intermolecular hydrogen bonds the structure is known as pleated sheet.
c) TERTIARY STRUCTURE:- The polypeptide becomes stabilized by folding & coating by the formation of ionic bonds or hydrophobic bonds or disulfide bridges. It is called tertiary structure. It gives a three dimensional view of proteins. Biological activity of protein depends on its tertiary structure.
d) QUATERNARY STRUCTURE:- Such proteins are farmed of more than one polypeptide or subunits each one having primary secondary & tertiary structure. This is called quaternary structure. Each polypeptide chain functions as subunit of the proteins.

NCRT TEXTBOOK QUESTIONS SOLVED
1. What are macromolecules? Give examples.
Solution: Macromolecules are large high molecular weight substances with complex molecular structure and occur in colloidal state (being insoluble) in intracellular fluid. These are formed by polymerization of large number of micromolecules. Polysaccharides, proteins and nucleic acids are few examples.
2. Illustrate a glycosidic, peptide, and a phospho- diester bond.
Solution. (i) Glycosidic bond is the type of chemical linkage between the monosaccharide units of disaccharides, oligosaccharides and polysaccharides, which is formed by the removal of a molecule of water.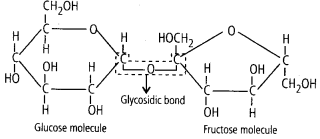
(ii)Peptide bonds are formed by the reaction between carboxyl (- COOH) of one amino acid and amino (- NH2) group of other amino acid with the elimination of water.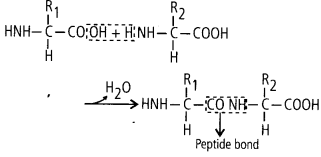
(iii) In a polynucleotide chain, adjacent nucleotides are joined together by a bond called phosphodiester bond. This bond links a phosphate group and sugar group of two adjacent nucleotides by means of an oxygen bridge.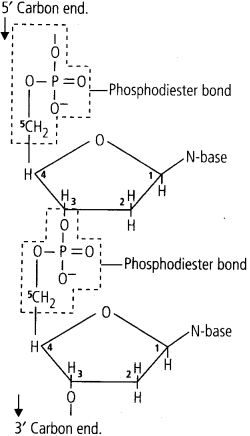
3. What is meant by tertiary structure of proteins?
Solution: The helical polypeptide molecule may fold on itself and assume a complex but specific form-spherical, rod-like or any form in between these. These geometrical shapes,are known as tertiary (3°) structure of protein molecules. The coils and folds of the polypeptide molecules are so arranged as to hide the non-polar amino acid chains inside and to expose the polar side chains. The tertiary structure of a protein brings distant amino acid side chains nearer to form active sites of enzymatic proteins. The tertiary structure is maintained by weak bonds such as hydrogen, ionic, disulphide and hydrophilic – hydrophobic bonds, formed between one part of a polypeptide and another. This structure is easily disrupted by pH, temperature and chemicals stopping the function of proteins.
4. Find and write down structures ©f 10 interesting small molecular weight biomolecules.
Solution: Interesfing small molecular weight biomolecules are minerals (like sodium, potassium, calcium, zinc, iodine etc), gases (like Oz, N2, C02, NH3) sugars – (ribose, deoxyribose, glucose, fructose), lipids, amino acids, nucleotides (pyrimidines & purine). Structures of 10 interesting small molecular weight biomolecules are as follows: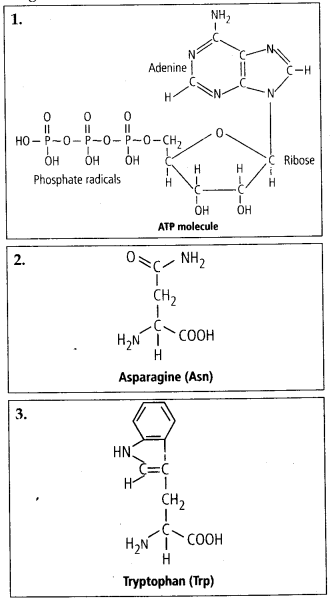
5. Proteins have primary structure. If you are given a method to know which amino acid is at either of two termini (ends) of a protein, can you connect this information to purity or homogeneity of a protein?
Solution: There are several methods provided by several scientists to find out the sequence of amino acids. Frederick Sanger proposed Sanger’s reagent to know the amino acid sequence in a polypeptide chain.
Sanger used 1-fluoro 2, 4 dinitrobenzene (FD NB) to determine insulin structure. FDNB specifically binds with N-terminal amino acid to form a dinitrophenyl (DNP) derivative of peptide. This DNP- derivative peptide can be identified by chromatography. The identified sequence of amino acids shows the homogeneity of a protein molecule.
6. Find out and make a list of proteins used as therapeutic agents. Find other applications of proteins.
Solution: Proteins used as therapeutic agents are: thrombin, fibrinogen, enkephalins, antigens, antibodies, streptokinase, protein tyrosine kinase, diastase, renin, insulin, oxytocin, vasopressin etc. Proteins are also used in cosmetics, dairy industries, textile industries, research techniques, biological buffers etc.
7. Explain the composition of triglycerides. jSfflTriacylglycerols (triglycerides) are the esters of glycerol with fatty acids.
Solution: They are insoluble in water and non-polar in character and commonly known as neutral fats. The neutral or depot fats are composed of carbon, hydrogen and oxygen like carbohydrates but have far fewer oxygen atoms than carbon atoms unlike the carbohydrates.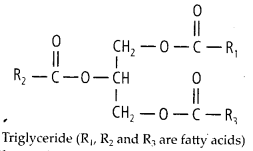
(i) Glycerol – A glycerol molecule has 3
carbons, each bearing a hydroxyl (-OH) group. .
(ii) Fatty acids – A fatty acid molecule is an unbranched chain of carbon atoms with each carbon atom (C) forming four bonds to other atoms. It has a carboxyl group- COOH at one end and hydrogen atom (H) bonded to all or most carbon atoms forming a hydrogen chain. The carbon- hydrogen bonds are non-polar. Therefore, the hydrocarbon chain does not dissolve in water. Because the carboxyl group contains the polar C = O and OFI groups. It tends to dissolve in water even though the rest of fatty acid molecule will not. Triacylglycerols of plants, in general, have higher content of unsaturated fatty acids as compared to that of animals.
8. Can you describe what happens when milk is converted into curd or yoghurt, from your understanding of proteins.
Solution: Milk is converted into curd or yoghurt due to denaturation of proteins. In denaturation, disruption of bonds that maintains secondary and tertiary structure leads to the conversion of globular proteins into fibrous proteins. This involves a change in physical, chemical and biological properties of protein molecules.
9. Can you attempt building models of biomolecules using commercially available atomic models (Ball and stick models).
Solution: Yes, models of biomolecules can be prepared using commercially available atomic models.
Ball and stick models and space filling models are 3D or spatial molecular models which serve to display the structure of chemical products and substances or biomolecules. With ball and stick models, the centers of the atoms are connected by straight lines which represent the covalent bonds. Double and triple bonds are often represented by springs which form curved connections between the balls. The bond angles and bond lengths reflect the actual relationships, while the space occupied by the atoms is either not represented at all or only denoted essentially by the relative sizes of the spheres.
10. Attempt titrating an amino acid against a weak base and discover the number of dissociating (ionizable) functional groups in the amino acid.
Solution: The existence of different ionic forms of amino acids can be easily understood by the titration curves. The number of dissociating functional group is one in case of neutral and basic amino acids and two in case of acidic amino acids.
11. Draw the structure of the amino acid, alanine.
Solution: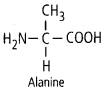
12. What are gums made of ? Is fevicol different ?
Solution: Gums are hetero-polysaccharides (poly-mers) of large number of different monosac-charide units. Yes, fevicol is a different kind of polymer. It is a synthetic sticky substance called resin which is manufactured by esteri-fication of organic compounds.
13. Find out a qualitative test for proteins, fats and oils, amino acids and test, any fruit juice, saliva, and urine for them.
Solution: Biuret test for protein : The biuret test is a chemical test used for determining the presence of peptide bonds. In a positive test, a copper II ion (Cu2+ ion) is reduced to copper I (Cu+) which forms a complex with the nitrogen and carbon of peptide bonds in an alkaline solution. A violet colour indicates the presence of proteins.
Ninhydrin test for amino acid: Ninhydrin (2,2 Dihydroxy indane-l,3-dione) is a chemical used to detect ammonia or primary and secondary amines. When reacting with these free amines, a deep blue or purple colour known as Ruhemann’s purple is evolved. Amino acid analysis of proteins is also done by ninhydrine. Most of the amino acids (including a-amino acids) are hydrolysed and reacted with ninhydrin except proline (a secondary amine). Amino acid containing a free amino group and a free carboxylic acid group reacts together with ninhydrin to produce coloured product. When the amino group is secondary, the condensation product is yellow.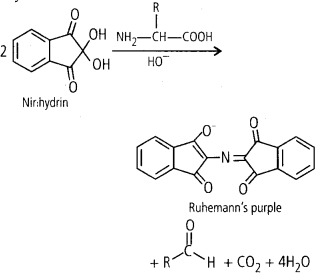
Solubility test for fats and oils : A positive solubility test for fats is that the fat dissolves in lighter fluid and not in water. In this test, 5 drops of fat or oil are added in two test tubes containing 10 drops of lighter fluid and 10 drops cold water respectively.
Fruit juice contains sugar so it cannot be tested by the above-mentioned tests. Saliva contains proteins, mineral salts, amylase etc., so it can be tested for protein and amino acids. Urine contains proteins, so it can be tested for it.
14. Find out how much cellulose is made by all the plants in the biosphere.
Solution: About 100 billion tonnes of cellulose is prepared per year by the plants of the world.
15. Describe the important properties of enzymes.
Solution: The important properties of enzymes are as follows:
(i) The enzymes are generally proteins which are high molecular weight complex globular proteins. They can associate with non-protein substance for their activity.
(ii) The enzymes do not start a chemical reaction but only accelerate it. They combine temporarily with the substrate molecules and are not consumed or changed permanently in the reaction which they catalyse.
(iii) The enzyme controlled reactions are reversible.
(iv) The enzymes are specific in action. An enzyme catalyses only a particular kind of reaction or acts on a particular substrate only.
(v) The enzymes are thermolabile i.e., heat sensitive and can function best at an optimum temperature. Similarly, enzymes show maximum activity at optimum pH.
(vi) The enzymes are inactivated by poisons and radiation.