Structure of Atom : Notes and Study Materials -pdf
- Concepts of Structure of Atom
- Structure of Atom Master File
- Structure of Atom Revision Notes
- Structure of Atom MindMap
- NCERT Solution Structure of Atom
- NCERT Exemplar Solution Structure of Atom
- Structure of Atom: Solved Example 1
- Structure of Atom: Solved Example 2
- Structure of Atom : Practice Paper 1
- Structure of Atom : Practice Paper 2
- Structure of Atom : Practice Paper 3
Subtopics Of Class 11 Chemistry Chapter 2 Structure Of The Atom
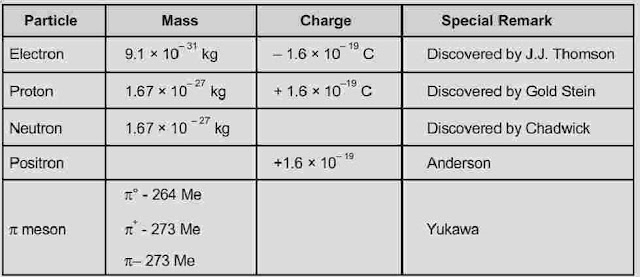
- Sub-atomic Particles
- Discovery Of Electron
- Charge To Mass Ratio Of Electron
- Charge On The Electron
- Discovery Of Protons And Neutrons
- Atomic Models
- Thomson Model Of Atom
- Rutherford’s Nuclear Model Of Atom
- Atomic Number And Mass Number
- Isobars And Isotopes
- Drawbacks Of Rutherford Model
- Developments Leading To The Bohr’s Model Of Atom
- Wave Nature Of Electromagnetic Radiation
- Particle Nature Of Electromagnetic Radiation: Planck’s Quantum Theory
- Evidence For The Quantized* Electronic Energy Levels: Atomic Spectra
- Bohr’s Model For Hydrogen Atom
- Explanation Of Line Spectrum Of Hydrogen
- Limitations Of Bohr’s Model
- Towards Quantum Mechanical Model Of The Atom
- Dual Behaviour Of Matter
- Heisenberg’s Uncertainty Principle
- Quantum Mechanical Model Of Atom
- Orbitals And Quantum Numbers
- Shapes Of Atomic Orbitals
- Energies Of Orbitals
- Filling Of Orbitals In Atom
- Electronic Configuration Of Atoms
- Stability Of Completely Filled And Half Filled Subshells.
Structure of Atom Class 11 Notes Chemistry Chapter 2
Introduction
In the previous chapter, we have discussed about Some Basic Concept of Chemistry but in this chapter, we shall study about Structure of Atom. Atom is made up of still smaller particles like electrons, protons and neutrons. The arrangement of these particles within the atom was put forward by Rutherford (in 1911) on the basis of his “Scattering experiments“.
The word “atom” has been derived from the Greek word ‘atoms’ which mans ‘indivisible’. These early ideas were mere speculation and there was no way to test them experimentally.
Atomic Structure
Atom is made up of smaller units like proton, neutron and electron. Some other particles like positron, neutrino, antineutrino, π-meson, μ-meson, k meson etc are also present which are very short lived.
Discovery of Electron
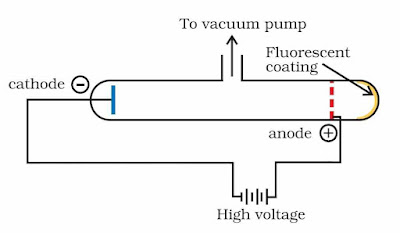
In 1879, William Crooks studied the conduction of electricity through gases at low pressure. He performed the experiment in a discharge tube which is a cylindrical hard glass tube about 60 cm in length. It is sealed at both the ends and fitted with two metal electrodes. The electrical discharge through the gases could be observed only at very low pressures and at very high voltages.
J.J. Thomson took a discharge tube and applied a voltage of a 10000 volt potential difference across it at a pressure of 10–2 mm of Hg. He found some glowing behind anode. It means some invisible rays produced at cathode strike behind anode and produce fluorescence. He named them cathode rays.
Properties of Cathode Rays
- These rays have mechanical energy and travel in straight line.
- These rays are deflected towards positive plate of electric field. It means these are made up of negatively charged particle called electron.
- Colour observed is independent from nature of gas.
- Mulliken determined the charge on electron which is 1.602 × 10–19 C.
- Specific charge on electron is calculated by J.J. Thomson.
Charge to mass ratio
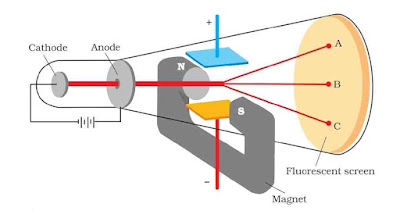
J.J. Thomson for the first time experimentally determined charge/mass ratio called e/m ratio for the electrons. For this, he subjected the beam of electrons released in the discharge tube as cathode rays to influence the electric and magnetic fields. These were acting perpendicular to one another as well as to the path followed by electrons.
According to Thomson, the amount of deviation of the particles from their path in presence of electrical and magnetic field depends on
- Magnitude of the negative charge on particle
- Mass of particle
- Strength of magnetic field
When electric field is applied, deviation from path takes place. If only electric field is applied, cathode rays strike at A. If only magnetic field is applied, cathode rays strike at C. In absence of any field, cathode rays strike at B.
By carrying out accurate measurements on the amount of deflections observed by the electrons on the electric field strength or magnetic field strength, Thomson was able to determine the value of
e/me = 1.758820 x 1011 C kg-1
where me = Mass of the electron in kg
e = magnitude of charge on the electron in coulomb (C).
Discovery of anode rays
In 1886, Goldstein modified the discharge tube by using a perforated cathode. On reducing the pressure, he observed a new type of luminous rays passing through the holes or perforations of the cathode and moving in a direction opposite to the cathode rays. These rays were named as positive rays or anode rays or as canal rays. Anode rays are not emitted from the anode but from a space between anode and cathode.
Properties of anode rays
- These rays deflect towards negative plate of applied electric field. It means these are made up of positively charged particle.
- Property of anode rays depends on nature of gas.
- These rays travel in straight line and have mechanical energy.
Discovery of Neutron
Chadwick in 1932 found the evidence for the production of neutron in given reaction.
4Be9 + 2He4 ⟶ 6C12 + 0n1
Neutron is chargeless particle and have mass equal to proton.
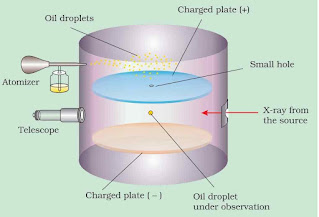
Millikan’s Oil Drop Experiment
In this experiment, some fine oil droplets were allowed to enter through a tiny hole into the upper plate of electrical condenser. These oil droplets were produced by atomiser. The air in the chamber was subjected to the ionization by X-rays. The electrons produced by the ionization of air attach themselves to the oil drops.
Thus oil droplets acquire negative charge. When sufficient amount of electric field is applied, the motion of the droplets can be accelerated, retarded or made stationary. Millikan observed that the smallest charge found on them was –1.6 × 10–19 coulomb and the magnitude of electrical charge, q on the droplets is always an integral multiple of the electrical charge ‘e’ i.e., q = ne
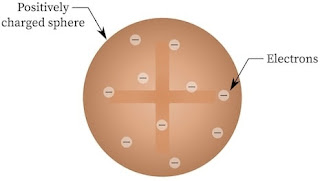
Thomson’s Model of Atom
J.J. Thomson in 1898, proposed a model of atom which looked more or less like plum pudding or raisin pudding. He assumed atom to be a spherical body in which electrons are unevenly distributed in a sphere having positive charge which balance the electron’s charge. It is called Plum pudding model.
Important Feature of This Model : The mass of the atom is assumed to be uniformly distributed over whole atom.
Failure : This model was able to explain the overall neutrality of the atom, it could not satisfactorily, explain the results of scattering experiments carried out by Rutherford in 1911.
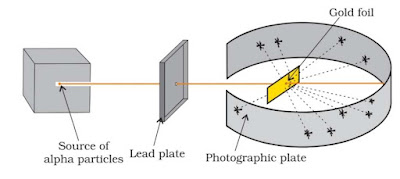
Rutherford’s Model
Rutherford in 1911, performed some scattering experiments in which he bombarded thin foils of metals like gold, silver, platinum or copper with a beam of fast moving a-particles. The thin gold foil had a circular fluorescent zinc sulphide screen around it. Whenever a-particles struck the screen, a tiny flash of light was produced at that point.
From these experiments, he made the following observations:
- Most of the α-particles pass without any deviation.
- Few particles deviate with small angle.
- Rare particles retrace its path or show deflection greater than 90°.
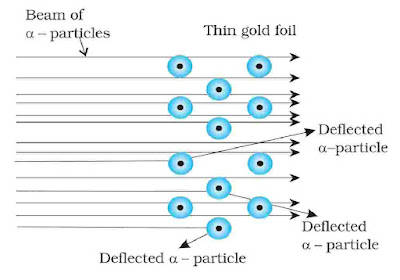
On the basis of these observation, he proposed a model.
- Atom is of spherical shape having size of order 10–10 meters.
- Whole mass is concentrated in centre called nucleus having size of order 10–15 meters.
- Electron revolves around the nucleus in circular path like planets revolve around sun.
Limitation : This model could not explain stability of atom. According to Maxwell’s classic theory, an accelerated charged particle liberates energy. So, during revolution, it must radiate energy and by following the spiral path it should comes on nucleus.
Atomic number
It is equal to the number of protons present in the nucleus of an atom. Atomic number is designated by the letter ‘Z’. In case of neutral atom atomic number is equal to the number of protons and even equal to the number of electrons in atom.
Z = Number of protons (p) = Number of electrons (e)
Mass number
It is equal to the sum of the positively charged protons (p) and electrically neutral neutrons (n). Mass number of an atom is designated by the letter ‘A’.
Mass number (A) = Number of protons (p or Z) + Number of neutrons (n)
Note : The atom of an element X having mass number (A) and atomic number (Z) may be represented by a symbol ZXA.
Isotopes
Atoms with identical atomic number but different atomic mass number are known as Isotopes. Isotopes of Hydrogen 1H1, 1H2 and 1H3
Isobars
Isobars are the atom with the same mass number but different atomic number, for example 6C14 and 7N14
Electromagnetic Waves Theory
This theory was put forward by James Clark Maxwell in 1864. Electromagnetic Waves are the waves which are produced by varying electric field and magnetic field which are perpendicular to each other in the direction perpendicular to both of them.
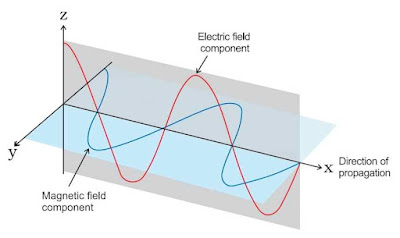
The main points of this theory are as follows:
- The energy is emitted from any source continuously in the form of radiations and is called the radiant energy.
- The radiations consist of electric and magnetic fields oscillating perpendicular to each other and both perpendicular to the direction of propagation of the radiation.
- The radiations possess wave character and travel with the velocity of light 3 x 108 m/sec.
- These waves do not require any material medium for propagation. For example, rays from the sun reach us through space which is a non-material medium.
Characteristics of a Wave
Wavelength (λ) : It is the distance between two consecutive crests or troughs and is denoted by λ.
Frequency (v) : It is the number of waves passing through a given point in one second. The unit frequency is hertz or cycle per second.
Wave number : It is the number of waves in a unit cycle. wave number `=\frac{1}{λ}`
Velocity : Velocity of a wave is defined as the linear distance travelled by the wave in one second. It is represented by c and is expressed in m/sec.
Amplitude : Amplitude of a wave is the height of the crest or the depth of the through. It is represented by V and is expressed in the units of length.
Black Body Radiations
Black-body is an ideal body which emits and absorbs radiations of all frequencies. The radiation emitted by these bodies is called black-body radiation.
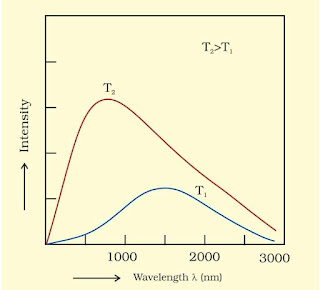
At a given temperature, the intensity and frequency of the emitted radiation depends is temperature. At a given temperature, the intensity of radiation emitted increases with decrease of wavelength.
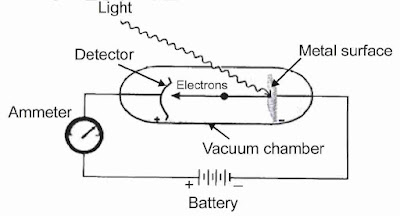
Photoelectric Effect
When light of a suitable frequency is allowed to incident on a metal, ejection of electrons take place. This phenomenon is known as photo electric effect.
Observations in Photoelectric Effect
- Only photons of light of certain minimum frequency called threshold frequency (v0) can cause the photoelectric effect. The value of v0 is different for different metals.
- The kinetic energy of the electrons which are emitted is directly proportional to the frequency of the striking photons and is quite independent of their intensity.
- The number of electrons that are ejected per second from the metal surface depends upon the intensity of the striking photons or radiations and not upon their frequency.
Explanation of Photoelectric Effect
Einstein in (1905) was able to give an explanation of the different points of the photoelectric effect using Planck’s quantum theory as under:
- Photoelectrons are ejected only when the incident light has a certain minimum frequency (threshold frequency v0).
- If the frequency of the incident light (v) is more than the threshold frequency (v0), the excess energy (hv–hv0) is imparted to the electron as kinetic energy.
- On increasing the intensity of light, more electrons are ejected but the energies of the electrons are not altered.
K.E. of the ejected electron.
`\frac{1}{2}mv^{2}=hv–hv_{0}`
Planck’s Theory
According to this theory, energy cannot be absorbed or released continuously but it is emitted or released in the form of small packets called quanta. In case of light this quanta is known as photon. This photon travels with speed of light. Energy of the photon is directly proportional to frequency.
`E\propto\nu`
`E=h\nu`
h is Planck’s constant, value is 6.62 × 10–34 Js
Bohr’s Model
Niels Bohr in 1913, proposed a new model of atom on the basis of Planck’s Quantum Theory. The main points of this model are as follows:
- Atom is of spherical shape having size (of order 10–10 metre).
- Whole mass is concentrated in centre called nucleus (having order of size 10–15 metre).
- Electron revolves around nucleus only in limited circular path and he assumed that electron does not radiate energy during its revolution in permitted paths.
- Only those orbits are allowed whose orbit angular momentum is integral multiple of `h/{2\pi}`.
`mvr=frac{nh}{2\pi}`, where n = 1, 2, 3, 4…
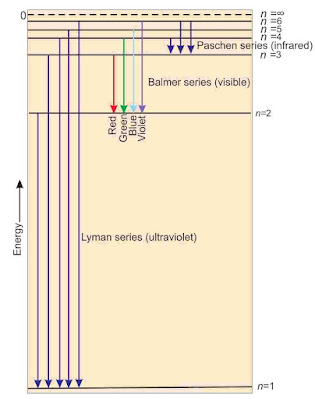
- When electron absorbs energy, it jumps to higher orbit and when it comes back, it radiates energy. This postulate explain spectra.
Achievements of Bohr’s Theory
- Bohr’s theory has explained the stability of an atom.
- Bohr’s theory has helped in calculating the energy of electron in hydrogen atom and one electron species.
- Bohr’s theory has explained the atomic spectrum of hydrogen atom.
Limitations of Bohr’s Model
- The theory could not explain the atomic spectra of the atoms containing more than one electron or multielectron atoms.
- Bohr’s theory failed to explain the fine structure of the spectral lines.
- Bohr’s theory could not offer any satisfactory explanation of Zeeman effect and Stark effect.
- Bohr’s theory failed to explain the ability of atoms to form molecule formed by chemical bonds.
- It was not in accordance with the Heisenberg’s uncertainty principle.
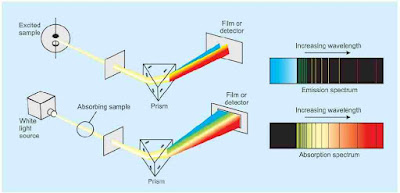
Spectra
The most compelling evidence for the quantization of energy comes from spectroscopy. Spectrum word is taken from Latin word which means appearance. The record of the intensity transmitted or scattered by a molecule as a function of frequency or wavelength is called its spectrum.
Cosmic rays < gamma rays < x rays < ultraviolet rays < visible rays < infra red < micro waves < radio waves
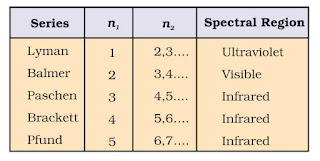
Line Spectrum of Hydrogen Atom
When electric discharge is passed through hydrogen gas enclosed in discharge tube under low pressure and the emitted light is analysed by a spectroscope, the spectrum consists of a large number of lines which are grouped into different series. The complete spectrum is known as hydrogen spectrum.
On the basis of experimental observations, Johannes Rydberg noted that all series of lines in the hydrogen spectrum could be described by the following expression:
wave number = `\frac{1}{\lambda}=R(\frac{1}{n_{1}^{2}}-frac{1}{n_{2}^{2}})`
R = Rydberg constant
R = 109678 cm–1
Zeeman Effect
When spectral line (source) is placed in magnetic field, spectral lines split up into sublines. This is known as zeeman effect.
Stark Effect
If splitting of spectral lines take place in electric field, then it is known as stark effect.
Dual Behaviour of Matter (de Broglie Equation)
de Broglie in 1924, proposed that matter, like radiation, should also exhibit dual behaviour i.e., both particle like and wave like properties. This means that like photons, electrons also have momentum as well as wavelength.
Assume light have wave nature, then its energy should be given by Planck’s theory
`E=h\nu` …(i)
If it have particle nature, then its energy should be given by Einstein relation
E = mc2 …(ii)
On comparing equation (i) and (ii),
`h\nu=mc^2`
`\lambda=h/{mc}` (for light) …(iii)
For other matter,
`\lambda=h/{mv}` …(iv)
`\lambda=h/p` …(v)
where p = momentum
This equation is called de Broglie equation.
Heisenberg’s Uncertainty Principle
It states that, “It is impossible to measure simultaneously the exact position and exact momentum of a microscopic particle”.
If uncertainty in position = Δx and
Uncertainty in momentum = ΔP
When both are measured simultaneously, According to this principle,
`Δx.ΔP\geq\frac{h}{4\pi}`
Quantum Numbers
There are a set of four quantum numbers which specify the energy, size, shape and orientation of an orbital. To specify an orbital only three quantum numbers are required while to specify an electron all four quantum numbers are required.
(i). Principal quantum number (n)
It identifies shell, determines sizes and energy of orbitals. It is indicated by ‘n’ and its values are 1, 2, 3, 4…
(ii). Azimuthal quantum number (l)
Azimuthal quantum number. ‘l’ is also known as orbital angular momentum or subsidiary quantum number. l. It identifies sub-shell, determines the shape of orbitals, energy of orbitals in multi-electron atoms along with principal quantum number and orbital angular momentum, i.e., The number of orbitals in a sub shell = 2l + 1. For a given value of n, it can have n values ranging from 0 to n-1.
(iii). Magnetic quantum number (ml)
It gives information about the spatial orientation of the orbital with respect to standard set of co-ordinate axis.For any sub-shell (defined by ‘l’ value) 2l+1 values of ml are possible. For each value of l, ml = – l, – (l–1), – (l–2)… 0,1…(l–2), (l–1), l
Electron spin quantum number (ms)
It refers to orientation of the spin of the electron. It can have two values +1/2 and -1/2. +1/2 identifies the clockwise spin and -1/2 identifies the anti-clockwise spin.
Shape of Atomic Orbitals
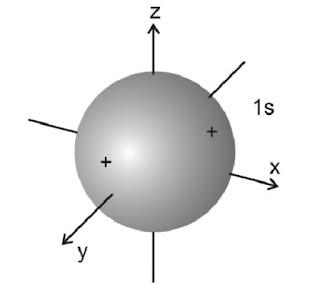
(i). Shapes of s-orbitals
s-orbital is present in the s-sub shell. For this sub shell, l = 0 and ml = 0. Thus, s-orbital with only one orientation has a spherical shape with uniform electron density along all the three axes. The probability of Is electron is found to be maximum near the nucleus and decreases with the increase in the distance from the nucleus.
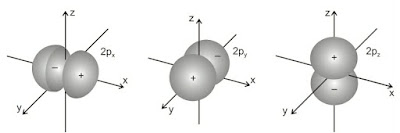
(ii). Shapes of p-orbitals
p-orbitals are present in the p-subshell for which l = 1 and ml can have three possible orientations –1, 0, +1. Thus, there are three orbitals in the p-subshell which are designated as px, py and pz orbitals depending upon the axis along which they are directed. The general shape of a p-orbital is dumb-bell consisting of two portions known as lobes.
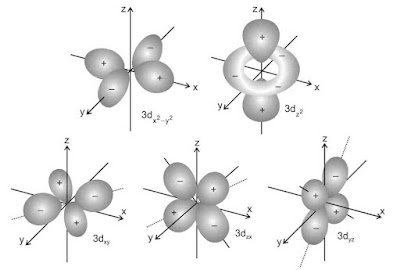
(iii). Shapes of d-orbitals
d-orbitals are present in d-subshell for which l = 2 and ml = -2, -1, 0, +1 and +2. This means that there are five orientations leading to five different orbitals. d orbitals are of five types :dxy, dyz, dzx, dx2-y2, dz2
Electronic Configuration
Distribution of electron in various orbitals is known as electronic configuration. The electrons filled in orbitals must obey the following rules –
- Aufbau’s principle
- Pauli’s exclusion principle
- Hund’s rule of maximum multiplicity
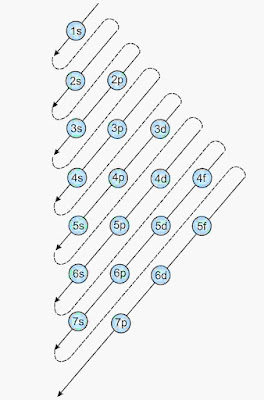
(i). Aufbau’s principle
According to this principle, orbitals with lowest energy are filled before the orbitals having higher energy.
1s < 2s < 2p < 3s < 3p < 4s < 3d < 4p < 5s < 4d < 5p < 6s < 4f < 5d < 6p < 7s < 5f < 6d < 7p
(n + l) rule (Bohr Bury’s Rule)
According to this, The orbital which has lower value of (n + l) is lower in energy.
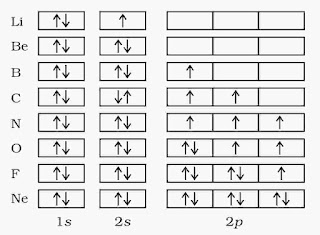
(ii). Pauli’s exclusion principle
According to this principle, in an atom, no two electrons have same value of all the four quantum numbers. In the same orbital, electron always accommodate in opposite spins. An orbital can have a maximum of two electrons, with opposite spin.
(iii). Hund’s rule of maximum multiplicity
According to this rule, electrons are distributed among the orbital of a subshell in such a way so as to give the maximum number of unpaired electrons with a parallel spin.
Summary
- Atomic number : It is equal to the number of protons in the nucleus of an atom.
- Mass number : It is equal to the sum of the positively charged protons (p) and electrically neutral neutrons (n).
- Isotopes : Isotopes are the atoms of the same element which have the same atomic number but different mass numbers.
- Isobars are the atoms of different elements having the same mass number but different atomic numbers.
- Isoelectronic species : These are those species which have same number of electrons.
- Radiations : These are defined as the emission or transmission of energy through space in the form of waves.
- Electromagnetic waves : The waves which consist of oscillating electric and magnetic fields are called electromagnetic waves.
- Electromagnetic radiations : Those radiations which are associated with electric and magnetic field are called electromagnetic radiations.
- Electromagnetic spectrum : The arrangement of the various types of electromagnetic radiations in the order of increasing or decreasing wavelengths or frequencies is known as electromagnetic spectrum.
- Wavelength (λ) : It is the distance between successive points of equal phase of a wave.
- Frequency (f) : The number of waves that pass a given point in one second is known as the frequency.
- Time period (T) : Time taken by the wave for one complete cycle or vibration is called time period.
- Velocity (v) : It is the distance travelled by a wave in one second.
- Wave number : It is defined as the number of wavelengths per unit length.
- Threshold frequency : It is the minimum frequency of light needed to cause the photoelectric effect.
- Continuous spectrum : The combination of light of different frequencies in continuous manner is called continuous spectrum.
- Line spectrum : The spectrum of atoms consist of sharp well-defined lines corresponding to definite frequencies is called line spectrum.
- Spectroscopy : The study of emission or absorption spectra is called spectroscopy.
- Quantization : The restriction of a property to discrete values and not continuous values is called quantization.
- Quantum mechanics : The branch of science that takes into account the dual behaviour of matter is called quantum mechanics.
- Atomic orbital : It is the region of space where the probability of finding the electron is maximum.
- Quantum numbers may be defined as a set of four numbers with the help of which we can get complete information about electron in an atom.