Solid State : Notes and Study Materials -pdf
Class 12th Chemistry notes for the chapter Solid State . Every concept is explained in a detailed way and after the concept student can find the verious solved questions asked from same.
The compete chapter is com[pleted as per the title and sub tiltle of Class 12th NCERT Chemistry book. Let’s start the chapter with a detail introduction
INTRODUCTION:
Three states of matter are solid, liquid and gas. Amongst them liquid and gases are called fluids because of their ability to flow. But solid cannot flow. The reason for that is particles in solid are not free to move in available space. There is strong intermolecular force of attraction in between the particles in solid. The constituents particles in solid have fixed position and can only oscillate about their mean position. This gives the rigidity to the solid and hence a fixed shape also. In brief we can say that:
- “Solid state of matter possesses fixed mass, volume, shape and rigidity “.
Solids are classified on the basis of arrangement of constituent particles. Due to their specific arrangements, it shows wide range of properties and hence varied applications like as superconductors, magnetic materials, polymers etc.
GENERAL CHARACTERISTICS OF SOLID STATE:
In nature the particular state of matter is governed by two opposing forces at given set of temperature and pressure. These forces are intermolecular force of attraction and thermal energy. If intermolecular force of attraction is high as compared to thermal energy, particles remains in closest position and hence very less movement in particles is observed. In this case solid state is the preferred state of matter.
Let us revise the general characteristics of solid:
i) Fixed mass, volume and shape
ii) Strong intermolecular force of attraction
iii) Least intermolecular space
iv) Fixed position of constituent particles
v) Incompressible and rigid
CLASSIFICATION OF SOLIDS:
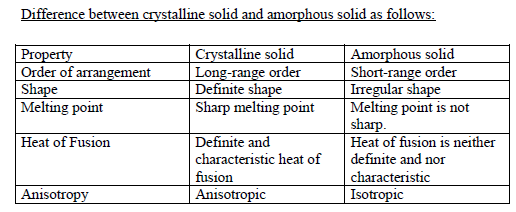
Solids are classified on the basis of arrangement of their constituent particles. If the arrangement of constituent particles is same throughout the solid (long range order) it is called crystalline. If the arrangement of particles does not follow any regular pattern throughout the solid (short range order) it is called amorphous solid.
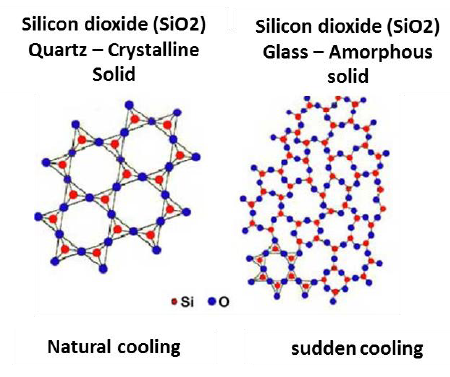
Characteristics of crystalline solid:
- It consists of large number of small crystals having a definite geometrical shape.
- The arrangement of constituent particles is regular throughout the solid (long range order). That is a fixed pattern of constituent particles repeat itself periodically over the entire range of solid.
- They have sharp melting points.
- They are anisotropic in nature.
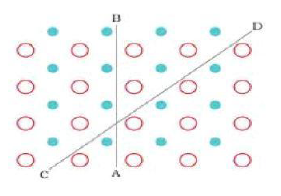
Anisotropy is defined as” Difference in properties when measured along different axes or different directions”.
Crystalline solid show different values of some of the physical properties like electrical resistance, refractive index etc.when measured along the different directions. The anisotropy arises due to the different arrangement of particles in different directions. Look the different arrangement of particles along the axis AB and CD in diagram given below.
Characteristics of amorphous solid:
- The arrangement of constituent particles is irregular throughout the solid. Regular pattern of constituent particle is visible in small areas only. That is it shows short-range order.
- Melting point is not sharp. Amorphous solid melts over a range of temperature.
- They have tendency to flow at slower rate.
- They are isotropic in nature.
“Isotropic means no difference in properties taken from any direction.”
Intext Questions:
Q.1 Classify the following solids as crystalline and amorphous.
Sodium chloride, quartz glass, quartz, rubber, polyvinyl chloride, Teflon
A.1 Crystalline solid: Sodium chloride, Quartz
Amorphous solid: Quartz glass, rubber, polyvinyl chloride, Teflon.
Q.2 why glass is considered as super cooled liquid?
A.2 Glass shows the tendency to flow at slower rate like liquid. Hence they considered as super cooled liquid.
Q.3 why the window glass of old buildings show milky appearance with time?
A.3 Glass is an amorphous solid. Amorphous solid has the tendency to develop some crystalline character on heating. Due to heating in day over the number of years, glass acquires some crystalline character and show milky appearance.
Q.4 why the glass panes fixed to window or doors of old building become slightly thicker at bottom?
A.4 Glass is super cooled liquid. It has the tendency to flow down very slowly. Due to this glass pane becomes thicker at the bottom over the time.
Q.5 Sodium chloride is a crystalline solid. It shows the same value of refractive index along all the direction. True/False. Give reason.
A.5 False
Crystalline solid shows anisotropy in properties. That is, it shows different values for the given physical property in different direction. All the crystalline solids show anisotropy in refractive index. Therefore sodium chloride will show different values of refractive index on different directions.
Q.6 Crystalline solid are anisotropic in nature. What does this statement means?[CBSE 2011]
A.6 Anisotropy is defined as” Difference in properties when measured along different axis or from different directions”. Crystalline solid show different values of some of the physical properties like electrical resistance, refractive index etc.when measured along the different directions. The anisotropy in crystalline solid arises due to the different arrangement of particles in different directions.
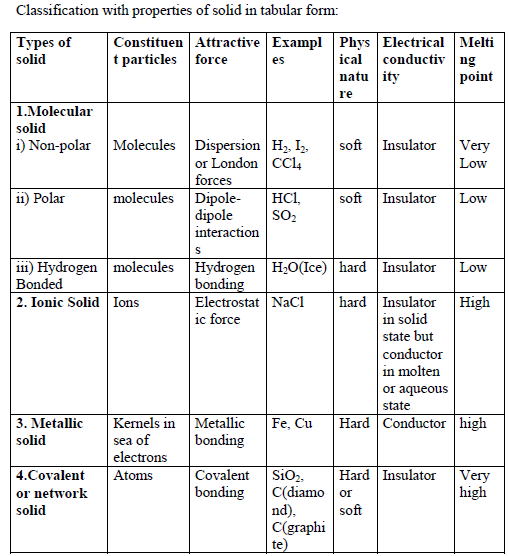
CLASSIFICATION OF CRYSTALLINE SOLIDS:
Crystalline solid can be classified on the basis constituent particles and intermolecular force of attraction in between them. Constituent particles are molecules, ions, metal kernel in sea of electrons and atoms. Force of attraction operate in between the particles are dispersion force, dipole-dipole interaction, hydrogen bonding, electrostatic attraction, metallic bonding and covalent bonding.
Classification with properties of solid in tabular form:
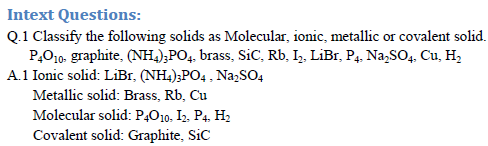
Q.2 what type of interactions hold the molecules together in a polar molecular solid.[CBSE 2010]
A.2 The molecules in a solid are held together by van der Waals forces. The term van der Waals forces include hydrogen bonding, dipole-dipole attraction and London dispersion forces. All molecules experience London dispersion forces. In addition, polar molecules can also experience dipole-dipole interactions. So, the interactions that holds the molecule together in polar molecular solid are London dispersion force and dipole-dipole interactions.
Q.3 Write a feature that will distinguish a metallic solid from an ionic solid. [CBSE 2010]
A.3 Metals are malleable and ductile whereas ionic solid are hard and brittle. Metallic solid has typical metallic lustre. But ionic solid looks dull.
Q.4 Write a point of distinction between a metallic solid and an ionic solid other than metallic lustre? [CBSE 2012]
A.4 Metals are malleable and ductile whereas ionic solid are hard and brittle.
Q.5 Write a distinguish feature of metallic solid. [CBSE 2010]
A.5 The force of attraction in between the constituent particles is special kind of electrostatic attraction. That is the attraction of positively charged kernel with sea of delocalized electrons.
Q.6 which group of solid is electrical conductor as well as malleable and ductile? [CBSE 2013]
A.6 Metallic solid
Q.7 why graphite is good conductor of electricity although it is a network (covalent solid)?
A.7 The exceptional property of graphite is due to its typical structure. In graphite, each carbon is covalently bonded with 3 atoms in same layer. The fourth valence electron of each atom is free to move in between different layers.
This free electron makes the graphite a good conductor of electricity.
Q.8 What is London Dispersion Force
A.8 The London dispersion force is the weakest intermolecular force. The London dispersion force is a temporary attractive force that results when the electrons in two adjacent atoms occupy positions that make the atoms form temporary dipoles. This force is sometimes called an induced dipole-induced dipole attraction.
CRYSTAL LATTICE AND UNIT CELL
The characteristic feature of crystalline solid is regular and repeating arrangement of constituent particles in space. The regular three dimensional arrangement of constituent particle in a crystal is known as crystal lattice.
The smallest part of the crystal lattice is known as unit cell. Look at the role of unit cell in crystal lattice.
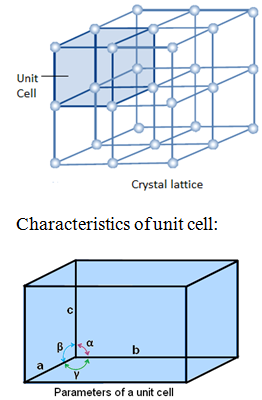
A unit cell is characterised by 6 parameters:
- 3- edges a, b and c (refer the diagram given above). The edges may or may not be perpendicular to each other.
- 3 angles α (between b and c) ,β (between a and c) and γ (between a and b).
Classification of unit cell:
i) Primitive unit cells: The constituent particles are only present at corners of unit cell.
ii) Centred unit cells: The constituent particles occupy other positions also beside the corners.
Centred unit cell can be further classified into 3-types:
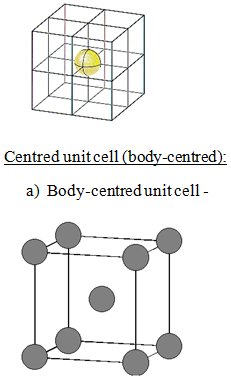
a) Body- centred unit cells: Other than corner it contains one constituent particle at the centre of the body of unit cell.
b) Face-centred unit cells: Other than corner it contains one constituent particle at the centre of each face of unit cell.
c) End- centred unit cells: Other than corner it contains one constituent particle at the centre of any two opposite faces of unit cell.
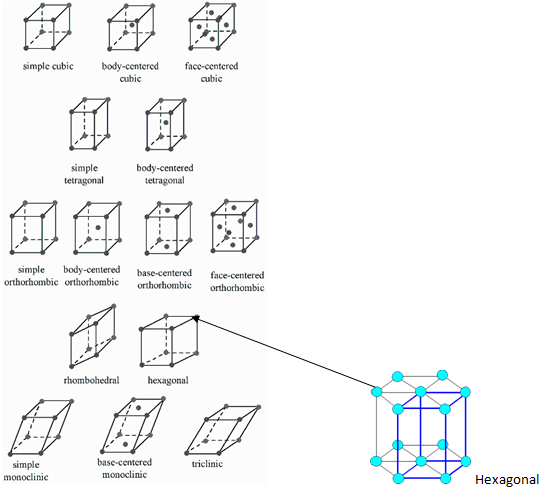
On the basis of 6 parameters (3 edges and 3 angles) of unit cell mentioned above, total seven types of primitive unit cells are possible. The primitive unit cells show variations in form of centred unit cells. The total number of possible unit cells (primitive and centred) in 3-dimensional lattice is 14. This is called Bravais Lattice. The detail of the lattice structure in tabular form is given below.
Point to be noted is primitive unit cell structure is 7 but total (primitive + centred) is 14.
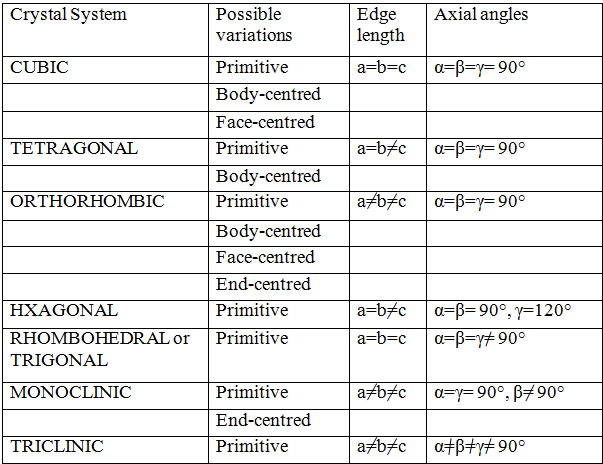
The structure of above mentioned 14 Bravias lattice is as follows:
NUMBER OF ATOMS IN UNIT CELL
Primitive unit cell: In Primitive unit cell, atoms are present at corner only. An atom is shared by 8 unit cell. Hence only 1/8 th of the atom belong to particular unit cell. There are total 8 corners in each unit cell therefore, 1/8 part of 8 atoms would give the value of number of atoms per unit cell. In brief
8 corners , 1 atom at each corner
1/8 of each atom in unit cell
Number of atoms present at different position is as follows:
- 8 corners, 1 atom at each corner (1/8 of each atom in unit cell)
- 1 atom at centre of body ( 1 atom completely present in the unit cell)
Total number of atom present in each unit cell:
= Contribution of atoms at corner + contribution of atom at the centre of the body
a) Face-centred unit cell –
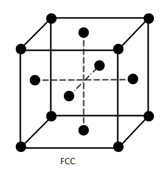
Number of atoms present at different position is as follows:
- 8 corners, 1 atom at each corner (1/8 of each atom in unit cell)
- 6 faces, 1 atom at each face of unit cell ( ½ of each atom in the unit cell)
Total number of atom present in each unit cell:
= Contribution of atoms at corner + contribution of atom at the face of unit cell
Intext Questions:
Q.1 How many atoms can be assigned to its unit cell if an element forms (i) body centred cubic cell, and (ii) a face centred cubic cell? [CBSE 2005, 2008, 2009]
A.1 (i) For body centred unit cell:2
(ii) For face centred unit cell:4
Q.2 How many lattice points are there in one unit cell of following lattices? (i) body centred cubic cell, and (ii) a face centred cubic cell?
A.2 (i) For body centred unit cell:
8 lattice points at corner + 1 lattice point at centre of the body = 9 lattice points
(ii) For face centred unit cell:
8 lattice points at corner + 6 lattice point at face-centre of each face = 14 lattice points
Q.3 A cubic solid is made of two elements X and Y. Atoms Y are at the corners of the cube and X at the body centre. What is the formula of the compound? [CBSE 2006]
A.3 Number of X atom per unit cell = 1
Number of Y atom per unit cell = 1/8 x 8 = 1
Formula of compound = XY
Q.4 What is the number of atom in a unit cell of simple cubic crystal?[CBSE (F) 2010]
A.4 8 Corners x 1/8 atom at each corner =1 atom per unit cell
Q.5 A cubic solid is made of two elements X and Y. Atoms Y(anions) are at the corners of the cube and X(cations) at present at face-centre of the cubic lattice. What is the formula of the compound?
A.5 Number of X atom per unit cell = 1/2 x 6 = 3
Number of Y atom per unit cell = 1/8 x 8 = 1
Formula of compound = X3Y
CLOSED PACKED STRUCTURE
As we know the constituent particles in solid are in form of sphere. The spheres in solid are arranged in different way to leave minimum vacant space. These arrangements of spheres in different layers form the closed packed structure of solid. The crystals are formed in closed packed structures. Similarly spheres in solid are also arranged in 3-dimension to form close-packed structure.
a) Close-packing in one-dimension :
- Spheres are arranged in a row with touching each other as shown below :
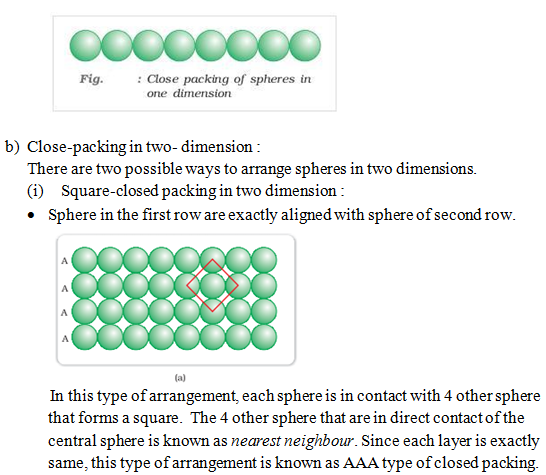
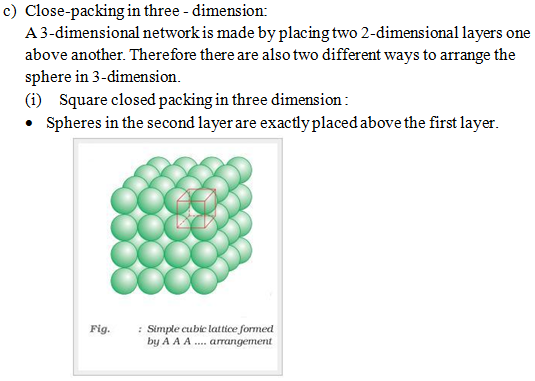
In this type of arrangement spheres of both the layers are perfectly aligned horizontally and vertically. This type of arrangement is known as AAA type closed packing of sphere in 3-dimension. The possible smallest geometrical 3-dimensional shape would be cube. Thus this type of arrangement generate simple cubic lattice with primitive type of unit cell.
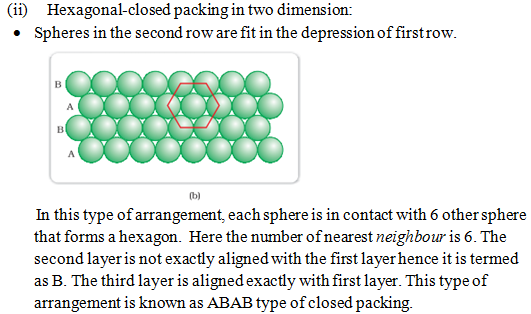
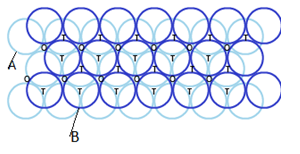
(i) Hexagonal closed packing in three dimension:
- Placing second layer over the first layer-
– Spheres in the second layer are fit in the depression of first layer.
Let the number of close packed spheres be N, then:
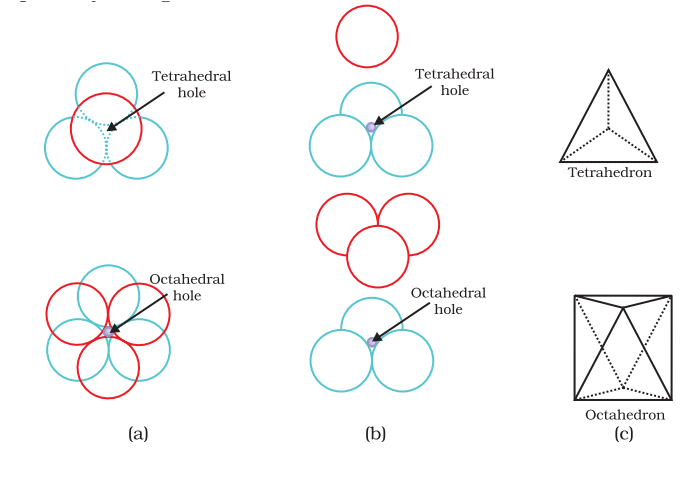
The number of octahedral voids generated = N
The number of tetrahedral voids generated = 2N
- Placing the third layer above second layer –
– Covering tetrahedral voids : If all the tetrahedral voids of second layer is covered by third layer, then third layer would be exactly aligned with the first layer as shown below :
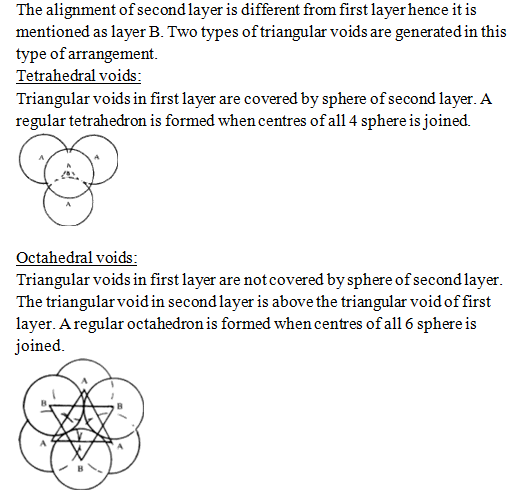
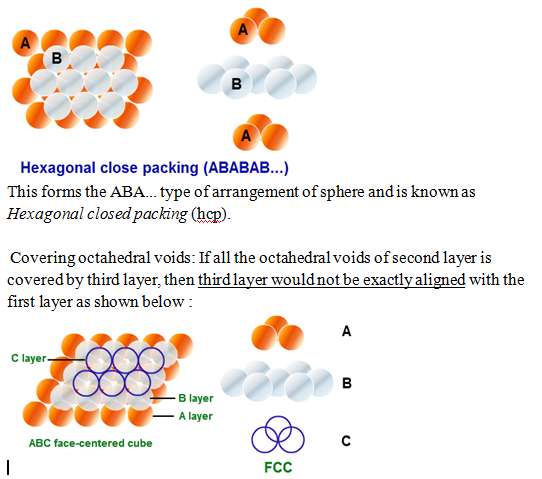
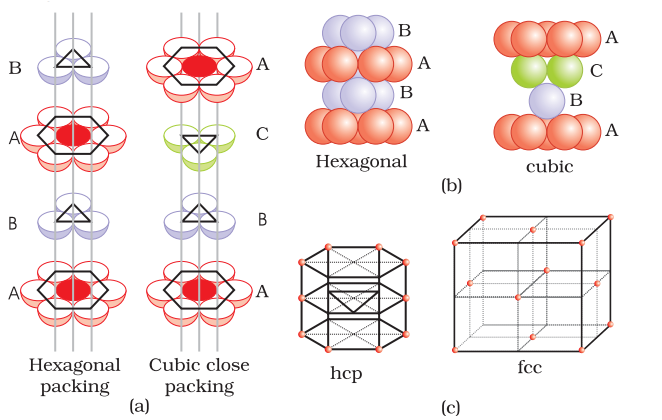
This forms the ABCABC… type of arrangement of sphere and is known as Cubic closed packing (ccp) or face centred cubic (fcc) structure.
Coordination number: The number of nearest neighbour touching a particle in closed packed structure is known as the coordination number of constituent particles.
In both type of crystal lattice (hcp, ccp or fcc) the coordination number for the constituent particle is 12.
Formula of compound:
Formula of the compound is deduced by calculating number of atoms present at lattice point and number of atoms present in voids. Generally anions are bigger and they occupy the lattice point while cations are occupied in voids.
In a given compound, it is not necessary that all the voids are occupied by constituent particles. Some time only fraction of voids are occupied depend on the formula of compound. Therefore it is necessary to know the position of voids in crystal lattice.
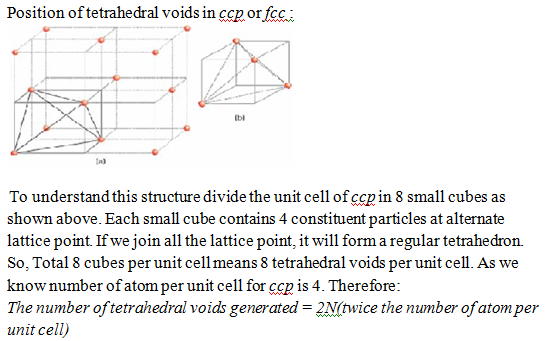
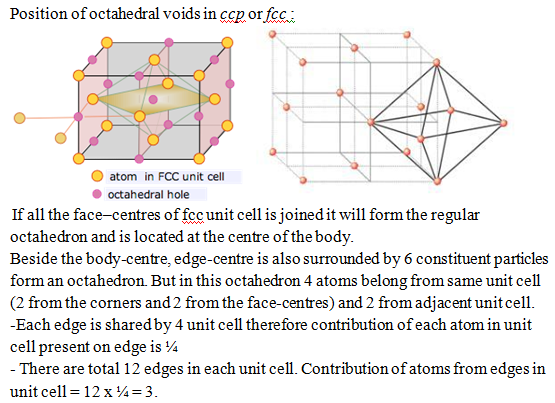
Total number of octahedral voids = 1(body-centre) + 3 (edge-centre) = 4
As we know number of atom per unit cell for ccp is 4. Therefore:
The number of octahedral voids generated = N(number of atoms per unit cell)
Intext Questions:
Q.1 A compound is formed by two element A and B. Element B occupy all the ccp position and element A occupy all the octahedral voids. Find the formula of compound.
A.1 In ccp number of atoms per unit cell = 4
Number of octahedral voids = same as number of atoms present at ccp = 4
Number of atoms of element B (at ccp) = 4
Number of atoms of element A (octahedral voids) = 4
Ratio of element A : B = 4 : 4 = 1 : 1
Formula of compound = AB
Q.2 A compound is formed by two elements A and B. The element B form ccp and element A occupy 2/3 of tetrahedral voids. What is the formula of compound?
A.2 Let the number of atoms of element B form ccp: N
The number of tetrahedral voids would be: 2N
Since 2/3 of tetrahedral voids are occupied therefore number of atom of element A would be: 2N x 2/3 = 4/3 N
Ratio of element A: B = 4/3: 1 or
Simple whole number ratio A: B = 4: 3
Formula of compound: A4B3.
Q.3 An oxide of aluminium is formed where oxide ions occupy all the hcp positions and aluminium ion occupy 2/3 of octahedral voids. What is the formula of compound?
A.3 Let the number of atoms of oxide form hcp: N
The number of aluminium ions in octahedral voids would be: N
Since 2/3 of octahedral voids are occupied therefore number of aluminium ion would be: N x 2/3 = 2/3 N
Ratio of element Aluminium ion: oxide ion = 2/3N: 1N or
Simple whole number ratio Aluminium ion: oxide ion = 2: 3
Formula of compound: Al2O3.
Q.4 What is the coordination number of each type of ion in rock-salt type crystal structure. [CBSE 2008]
A.4 Rock salt (NaCl) has fcc type of crystal structure.
In fcc the cations in voids touches 6 nearest neighbour as well as anion at lattice point also touches 6 nearest neighbour.
Coordination number of Na+ = 6
Coordination number of Cl– = 6
Q.5 what is the two-dimensional coordination number of a molecule in square-closed packed layer. [CBSE(F)2013]
A.54
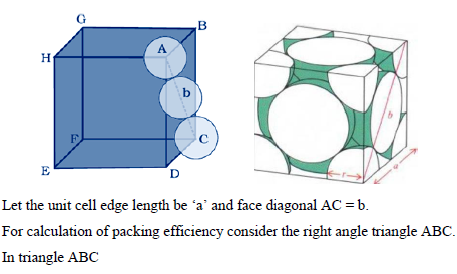
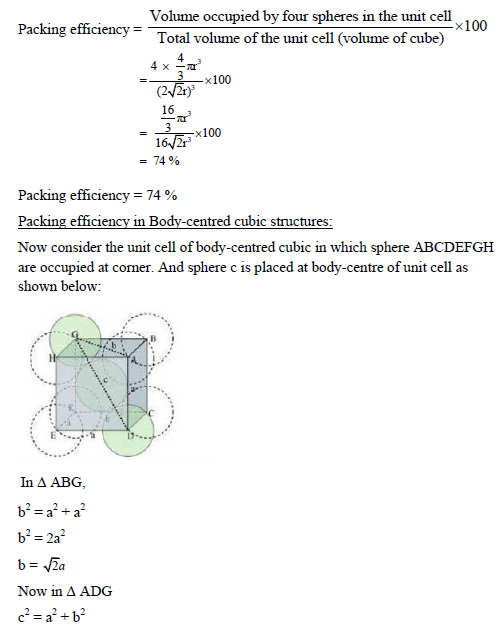
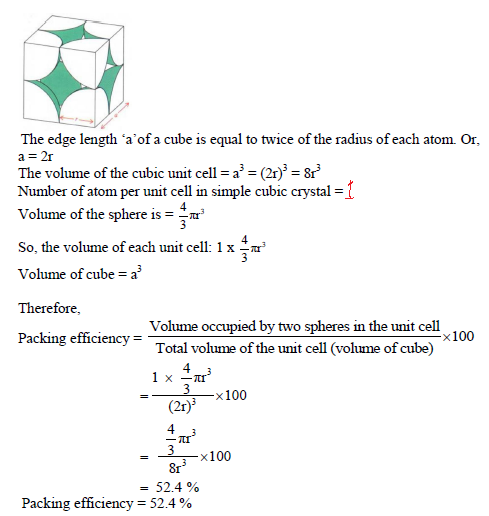
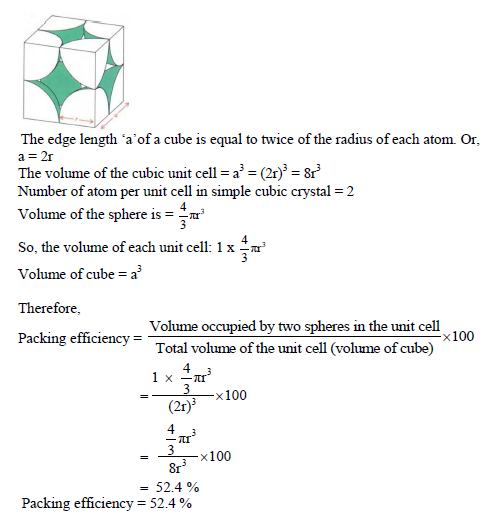
PACKING EFFICIENCY:
Packing efficiency is defined as percentage of total space filled by the constituent particles in crystal.
Packing efficiency in hcp and ccp structures:
Let us consider the unit cell of ccp in which sphere ABCDEFGH are occupied at corner. And sphere a,b,c,d,e,f are placed at centre of face.

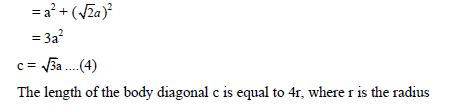












IMPERFECTION IN SOLIDS
Although in crystalline solid there is regular arrangement of constituent particles but yet the crystals are not perfect. There is always some king of irregularity in arrangement of constituent particles in small crystals. These
Irregularities are known as defects in crystal. There are 2 types of defects known in crystal lattice.
- Point defect
- Line defect
In our syllabus only point defects are included so we will focus our study on point defects only.
POINT DEFECTS:
- Impurity defect
- Stoichiometric defects
- Non- stoichiometric defects
Types of stoichiometric defects and Non- stoichiometric defects are mentioned in tabular form.
Stoichiometric defects | Non- stoichiometric defects |
| Vacancy defect | Metal excess defect |
| Interstitial defect | Metal deficiency defect |
| Frenkel defect | |
| Schottky defect |
Impurity defect:
Impurity defect is arises due to addition of small amount of impurity in ionic solid. It actually creates some kind of cationic vacancy in ionic solid. For example, if some SrCl2 is added in molten salt of NaCl it takes the position of Na+ in the crystal. But charge on Sr is 2+. After removing 2 ions of Na+, Sr2+ occupy one point only. In this way it causes vacancy of 1 point.
Vacancy defect:
It arises when some of the lattice point remains unoccupied during the crystal formation.
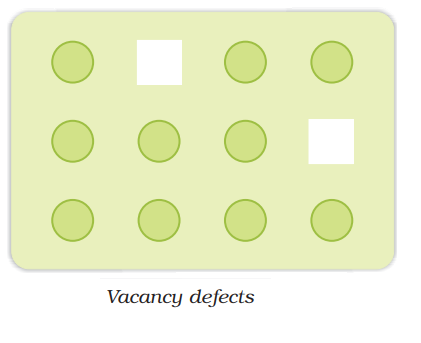
- It occurs in non-ionic compounds
- It decreases the density of solid
- It can be created by heating
Interstitial defect:
It arises when some of the constituent particles occupy the interstitial sites other than the lattice points.
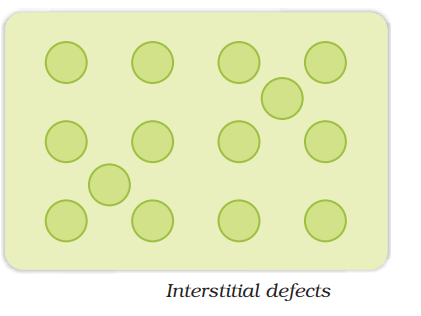
It occurs in non-ionic compounds
It increases the density of solid
Frenkel defect:
It is actually the combination of vacancy defect and interstitial defect in ionic compound. It arises when smaller ion is dislocated from its normal site and occupies an interstitial site. It is also known as dislocation defect.
- It occurs in ionic compounds
- It does not change the density of solid
- Shown by ionic compound having large difference in their constituent ions.
For example: ZnS, AgBr, AgI etc.
Schottky defect:
It is actually a vacancy defect. But it causes vacancy of cation and anion both.
- It occurs in ionic compounds
- It decrease the density of solid
- Shown by ionic compound having similar size of cation and anion.
For example: NaCl, KCl, AgBr.
Important point: AgBr shows both Frenkel and schottky defect.
Metal excess defect:
· Due to anionic vacancies:
anionic vacancies are created due to reaction of anion of the crystal with excess of metal present in the atmosphere. For example when crystal of NaCl is heated in presence of sodium vapour, the sodium atoms are deposited on the surface of crystal and later on forms the bond with Cl– ion by losing an electron.
In this way it creates a vacancy for Cl– ion. But the electron removed by sodium metal diffuse in the crystal and occupies the anionic site. This creates a F-centre.The presence of unpaired electron at vacant anionic site in metallic crystal is known as F-centre.
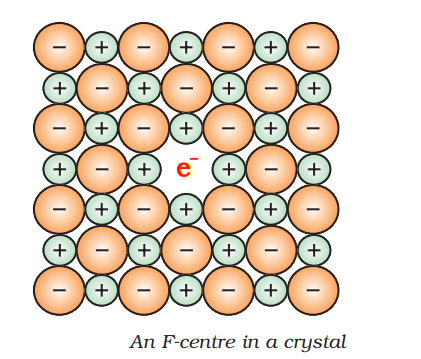
This F-centre is responsible for imparting colour to the crystal. Like as:
NaCl – yellow colour
LiCl – pink
KCl-violet.
- It occurs in ionic compounds
- It is responsible for imparting colour to the crystal through F-centres.
For example: NaCl, KCl, LiCl.
Due to presence of extra cations:
Excess cationic sites are generated due to the loss of anion of crystal after any reaction. The excess of cation occupies the interstitial site. ZnO on heating loss oxide ion from the crystal. The excess of Zn2+ ions occupy the interstitial site and electron lost by oxide ion occpy the neighbouring site, in this way maintain the electrical neutrality of crystal.
- It occurs in ionic compounds
- It is responsible for imparting colour to the crystal through free electrons.
For example: ZnO (white at room temperature, yellow on heating)
Metal deficiency defect:
Number of metal cations is less as required by metallic crystal. For example in FeO the actual composition ranges from Fe0.93O to Fe0.95O.
ELECRTICAL PROPERTIES:
Solid are classified as conductor, semi-conductor and insulator on the basis of the magnitude of electrical conductivity.
- Conductor: electrical conductivity range 104 to 107 ohm-1m-1
- Semi-Conductor: electrical conductivity range 10-6 to 107 ohm-1m-1.
- Insulator: electrical conductivity range 10-20 to 10-10 ohm-1m-1
CONDUCTION OF ELECTRICITY
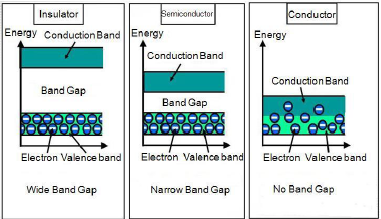
As we know that free electrons are responsible for the conduction of electricity in metals. Electrons are occupied in atomic orbital. The atomic orbital forms molecular orbital in metallic crystal. Molecular orbital are very closer in energy and known as bands. To be a conductor there must be some electrons in conduction band. But electrons are mainly occupied in valance band. On the basis of conductivity solids are classified in 3 types: Conductor, semi-conductor, insulator.
- In case of conductor there is an overlapping of valance band and conduction band.
- In semi-conductor there is small gap of energy between valance band and conduction band. Some of the electron may jump and show some activity. The metal showing this type of activity is known as intrinsic semi-conductors. For example : Silicon and Germanium
- In case of insulator there is a large energy difference between valance band and conduction band

Classification of semi-conductors:
The electrical conductivity of semi-conductors can be increased by adding some electron rich impurity or electron deficient impurity. This is called doping.
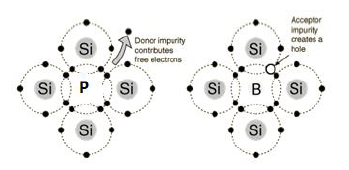
n-type semiconductor : When the conductivity of semi-conductor is increased by adding electron rich impurity. It generates n-type semi-conductor. For Example: when Silicon is doped with phosphorus, phosphorus also occupies some lattice site. The covalency of Si is 4 but that of P is 5. So, one electron per atom left unused and it delocalise from its location. This delocalise electron help in increasing conductivity of semi-conductor.
p-type semiconductor : When the conductivity of semi-conductor is increased by adding electron deficient impurity. It generates p-type semi-conductor. For Example: when Silicon is doped with boron, boron also occupies some lattice site. The covalency of Si is 4 but that of B is 3. So, one of the position of electron is left unused. This is called electron hole. The electron from neighbouring atom moves to fill this hole but creates a new hole. This looks like movement of electron hole throughout the system. Electrons moves through theses hole under the influence of electric fields. In this way electrical conductivity increases.

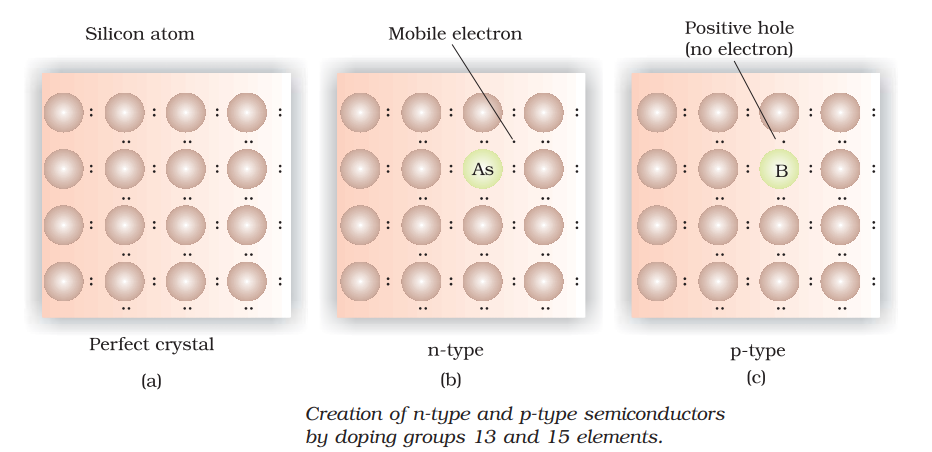
Application of n-type and p-type semiconductors:
By the combination of n-type and p-type semi-conductors many electronic devices are prepared.
- Diode (is a device that allows the current in one direction only) is used as amplifier
- pnp and npn sandwiching is done in making transistors
- As photo diode in solar cells
- In lasers
MAGNETIC PROPERTIES:
Electrons are charged particles and it generates a magnetic field around itself. The magnetic field arises due to the spinning of electron at its own axis and movement of atomic orbital around nucleus.
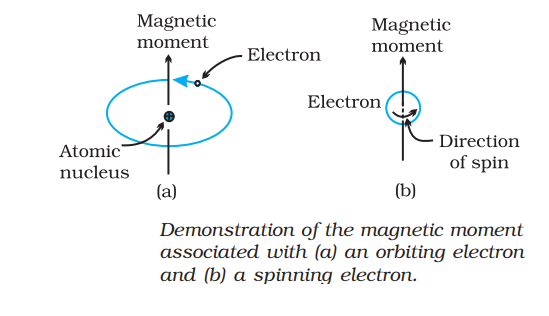
Classification of magnetic properties:
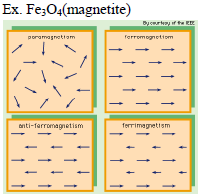
I. Paramagnetism :
- Weakly attracted by magnetic fields
- Alignment of magnetic dipole in the same direction of magnetic field
- Loose magnetism in absence of magnetic field
- They have unpaired electrons
Ex. Cu2+, Fe3+
II. Diamagnetism :
- Weakly repelled by magnetic fields
- Alignment of magnetic dipole in the opposite direction of magnetic field
- They have all the electrons paired
Ex. H2O, NaCl
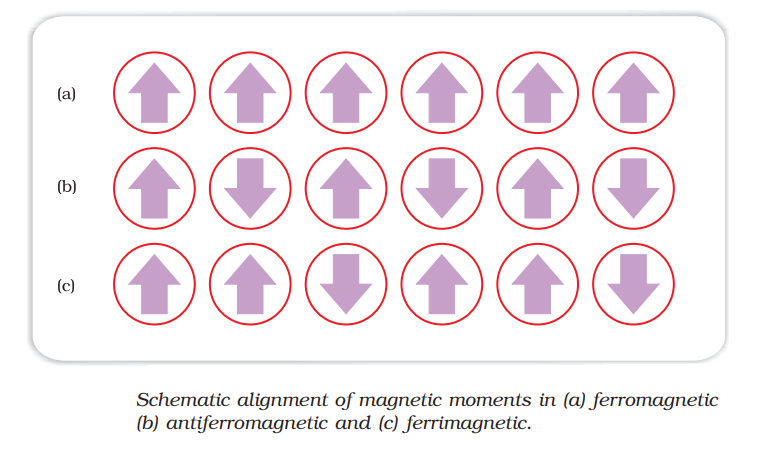
III. Ferromagnetism :
- strongly attracted by magnetic fields
- Permanently magnetised.
Ex. Fe, Co, Ni etc.
IV. Anti-Ferromagnetism :
- strongly repelled by magnetic fields
- Alignment of magnetic domains (group of metal ions)in the opposite direction of magnetic field..
Ex. MnO
V. Ferrimagnetisms :
- Weakly attracted by magnetic fields
- Alignment of magnetic domains (group of metal ions)in the parallel and anti parallel direction with direction of magnetic field and unequal in number.
- Loss magnetic moment on heating.
Ex. Fe3O4(magnetite)

Intext Questions:
Q.1 what is a semi-conductor? Describe the two main types of semi-conductors and contrast their conduction mechanism. [CBSE Delhi 2008]
A.1 Answer in text.
Q.2 What type of substances exhibit antiferromagnetism ? [CBSE Delhi 2008]
A.2 Substances in which the domains are aligned in opposite direction. So, the net magnetic moment is zero.
Q.3 Which crystal defect lowers the density of solid? [CBSE (AI)2008,2009]
A.3 Schottky defect.
Q.4 Name an element with which silicon may be doped to give p-type semi-conductor. [CBSE (AI) 2008]
A.4 Boron.
Q.5 which point defect in the crystal does not alter the density of solid? [CBSE Delhi 2009, 2010]
A.5 Frenkel defect
Q.6 Which point defect in its crystal unit increases the density of a solid? [CBSE Delhi 2009, 2011: (AI) 2012]
A.6 Interstitial defect
Q.7 How do metallic and ionic substances differ in conducting electricity? [CBSE (AI)2009]
A.7 In metallic substance the electrical conduction takes place by the movement of electrons. But in ionic solid it takes place by the movement of free ions.
Q.8 what are F-centres? [CBSE (AI) 2008]
A.8 The free electrons at anionic vacancies are known as F-centres.
Q.9 What is meant by doping in a semi-conductor? [CBSE Delhi 2012]
A.9 The electrical conductivity of semi-conductors can be increased by adding some electron rich impurity or electron deficient impurity. This is called
doping.
Q.10 what type of substance would make better permanent magnet, ferromagnetic or ferromagnetic? [CBSE Delhi 2013]
A.10 Ferromagnetic
Q.11 What type of stoichiometric defect is shown by AgCl? [CBSE Delhi 2013]
A.11 Frenkel defect.
Q.12 what type of semi-conductor is obtained when silicon is doped with arsenic? [CBSE (AI) 2010]
A.12 n-type semiconductor.
Q.13 How may the conductivity of an intrinsic semiconductor be increased? [ CBSE (AI) 2012]
A.13 The conductivity of semiconductor can be increased by doping it with electron rich impurity or electron deficient impurity.
Q.14 What is mean by intrinsic semiconductor? [CBSE (F) 2011]
A.14 The pure semiconductor also shows some amount of electrical conductivity. But that is very low and has no practical application. These type of semiconductors are known as intrinsic semiconductor. For example : pure silicon and germanium.
Q.15 what are n-type semiconductor? [CBSE(AI) 2012]
A.15 when the semiconductor is doped with electron-rich impurity, it increases the electrical conductivity. These are known as n-type semiconductor. For example: Silicon is doped with Germanium.
Q.16 What is meant by ‘forbidden zone’ in reference to the band theory of solid?[CBSE (AI) 2008; (F) 2012]
A.16 The energy gap between the valance band and conduction band is known as forbidden zone. As shown below:
Summary:
- Solids have definite mass, volume and shape. This is due to the fixed position of their constituent particles and strong intermolecular force of attraction.
- Solids are classified as amorphous and crystalline.
- In amorphous solids, the arrangement of constituent particles has only short
- Range order. The melting point is not sharp. They are isotropic in nature. In crystalline solids there is long range order in the arrangement of their constituent particles. The melting point is sharp. They are anisotropic in nature.
- Properties of crystalline solids depend upon the nature of interactions between their constituent particles. On this basis, they can be divided into four categories, namely: molecular, ionic, metallic and covalent solids.
- The constituent particles in crystalline solids are arranged in a regular pattern which extends throughout the crystal. This arrangement is often depicted in the form of a three dimensional array of points which is called crystal lattice. Each lattice point gives the location of one particle in space. In all, fourteen different types of lattices are possible which are called Bravais lattices. Each lattice can be generated by repeating its small characteristic portion called unit cell
- A unit cell is the smallest portion of space lattice which when repeated in different directions , generate the entire lattice. A unit cell is characterised by its edge lengths and three angles between these edges. Unit cells can be either primitive which have particles only at their corner positions or centred. The centred unit cells have additional particles at their body centre (body-centred),at the centre of each face (face-centred) or at the centre of two opposite faces (end-centred)
- There are seven types of primitive unit cells. Taking centred unit cells also into account, there are fourteen types of unit cells in all, which result in fourteen Bravais lattices
- Close-packing of particles is obtained in two highly efficient lattices, hexagonal close-packed (hcp) and cubic close-packed (ccp). The latter is also called facecentredcubic (fcc) lattice. In both of these packings 74% space is filled. Other types of packing are not close-packings and have less efficient packing of particles. While in body-centred cubic lattice (bcc) 68% space is filled, in simple cubic lattice only 52.4 % space is filled
- The remaining space is present in the form of two types of voids-octahedral voids and tetrahedral voids
- Solids are not perfect in structure. There are different types of imperfections or defects in them. Point defects and line defects are common types of defects
- Point defects are of three types – stoichiometric defects, impurity defects and non-stoichiometric defects
- Vacancy defects and interstitial defects are the two basic types of stoichiometric point defects. In ionic solids, these defects are present as Frenkel and Schottky defects
- Impurity defects are caused by the presence of an impurity in the crystal. In ionic solids, when the ionic impurity has a different valence than the main compound, some vacancies are created
- Nonstoichiometric defects are of metal excess defect and metal deficient defect
- On the basis of electrical conductivity solids are classified as conductor, semiconductor and insulator
- Sometimes calculated amounts of impurities are introduced by doping in semiconductors that change their electrical properties. Such materials are widely used in electronics industry. They are of two types namely, n-type semiconductor and p-type semiconductor
- Solids show many types of magnetic properties like paramagnetism, diamagnetism, ferromagnetism, antiferromagnetism and ferrimagnetism
Solid State MCQ Chapter 1
A Solid is one of the three basic states of matter in this universe, along with the gas and liquid. A solid forms mostly when a gas or liquid is compressed because the energy of the particles decreases, and end up forming a 3-D structure.
Solids show a certain type of character that differentiates them from the liquids and gases. Some of them being like, they have a definite and rigid shape and their particles vibrate in their fixed positions.
Below are some of the very important NCERT Solid State MCQ of Class 12 Chemistry Chapter 1 with Answers.
The Solid State Class 12 MCQs Questions with Answers
Question 1.
Which one of the following is non-crystalline or amorphous?
(a) Diamond
(b) Graphite
(c) Glass
(d) Common Salt
Answer
Answer: (c) Glass
Question 2.
NaCl typecrystal (with coordination no. 6 : 6) can be converted into CsCl type crystal (with coordination no. 8 : 8) by applying
(a) high temperature
(b) high pressure
(c) high temperature and high pressure
(d) low temperature and low pressure
Answer
Answer: (b) high pressure
Question 3.
How many chloride ions are surrounding sodium ion in sodium chloride crystal ?
(a) 4
(b) 8
(c) 6
(d) 12
Answer
Answer: (c) 6
Question 4.
In NaCl structure
(a) all octahedral and tetrahedral sites are occupied
(b) only octahedral sites are occupied
(c) only tetrahedral sites are occupied
(d) neither octahedral nor tetrahedral sites are occupied
Answer
Answer: (b) only octahedral sites are occupied
Question 5.
In Zinc blende structure
(a) zinc ions occupy half of the tetrahedral sites
(b) each Zn2- ion is surrounded by six sulphide ions
(c) each S2- ion is surrounded by six Zn2+ ions
(d) it has fee structure
Answer
Answer: (c) each S2- ion is surrounded by six Zn2+ ions
Question 6.
A unit cell of BaCl2 (fluorite structure) is made up of
(a) four Ba2+ ions and four Cl– ions
(b) four Ba2- ions and eight Cl– ions
(c) eight Ba² ions and four Cl– ions
(d) four Ba² ions and six Cl– ions
Answer
Answer: (b) four Ba2- ions and eight Cl– ions
Question 7.
Alkali halids do not show Frenkel defect because
(a) cations and anions have almost equal size
(b) there is a large difference in size of cations and anions
(c) cations and anions have low coordination number
(d) anions cannot be accommodated in voids
Answer
Answer: (a) cations and anions have almost equal size
Question 8.
The coordination number of Y will be in the XY types of crystal:
(a) 6
(b) 8
(c) 12
(d) 4
Answer
Answer: (a) 6
Question 9.
The fraction of the total volume occupied by the atoms present in a simple cube is
(a) \(\frac{π}{4}\)
(b) \(\frac{π}{6}\)
(c) \(\frac{π}{3√2}\)
(d) \(\frac{π}{4√2}\)
Answer
Answer: (b) \(\frac{π}{6}\)
Question 10.
Edge length of unit cell of chromium metal is 287 pm with bcc arrangement. The atomic radius is of the order
(a) 287 pm
(b) 574 pm
(c) 124.27 pm
(d) 143.5 pm
Answer
Answer: (c) 124.27 pm
Question 11.
The edge length of fee cell is 508 pm. If radius of cation is 110 pm, the radius of anion is
(a) 110 pm
(b) 220 pm
(c) 285 pm
(d) 144 pm
Answer
Answer: (d) 144 pm
Question 12.
The density of a metal which crystallises in bcc lattice with unit cell edge length 300 pm and molar mass 50 g mol-1 will be
(a) 10 g cm-3
(b) 14.2 g cm-3
(c) 6.15 g cm-3
(d) 9.3 2 g cm-3
Answer
Answer: (c) 6.15 g cm-3
Question 13.
How many lithium atoms are present in a unit cell with edge length 3.5 Å and density 0.53 g cm-3? (Atomic mass of Li = 6.94):
(a) 2
(b) 1
(c) 4
(d) 6
Answer
Answer: (a) 2
Question 14.
The distance between Na– and CL– ions in NaCl with a density 2.165 g cm-3 is
(a) 564 pm
(b) 282 pm
(c) 234 pm
(d) 538 pm
Answer
Answer: (b) 282 pm
Question 15.
An element with atomic mass 100 has a bcc structure and edge length 400 pm. The density of element is
(a) 10.37 g cm-3
(b) 5.19 g cm-3
(c) 7.29 g cm-3
(d) 2.14 g cm-3
Answer
Answer: (b) 5.19 g cm-3
Question 16.
Fe3O4 (magnetite) is an example of
(a) normal spinel structure
(b) inverse spinel structure
(c) fluoride structure
(d) anti fluorite structure
Answer
Answer: (b) inverse spinel structure
Question 17.
Which of the following crystals does not exhibit Frenkel defect?
(a) AgBr
(b) AgCl
(c) KBr
(d) ZnS
Answer
Answer: (c) KBr
Question 18.
What type of stoichiometric defect is shown by ZnS?
(a) Schottky defect
(b) Frenkel defect
(c) Both Frenkel and Schottky defects
(d) Non-stoichiometric defect
Answer
Answer: (b) Frenkel defect
Question 19.
Silver halides generally show
(a) Schottky defect
(b) Frenkel defect
(c) Both Frenkel and Schottky defects
(d) cation excess defect
Answer
Answer: (c) Both Frenkel and Schottky defects
Question 20.
Which of the following will have metal deficiency defect?
(a) NaCl
(b) FeO
(c) KCl
(d) ZnO
Answer
Answer: (b) FeO
Question 21.
In which pair most efficient packing is present?
(a) hep and bcc
(b) hep and ccp
(c) bcc and ccp
(d) bcc and simple cubic cell
Answer
Answer: (b) hep and ccp
Question 22.
What is the effect of Frenkel defect on the density of ionic solids?
(a) The density of the crystal increases
(b) The density of the crystal decreases
(c) The density of the crystal remains unchanged
(d) There is no relationship between density of a crystal and defect present in it
Answer
Answer: (c) The density of the crystal remains unchanged
Question 23.
In a Schottky defect
(a) an ion moves to interstitial position between the lattice points
(b) electrons are trapped in a lattice site
(c) some lattice sites are vacant
(d) some extra cations are present in interstitial space
Answer
Answer: (c) some lattice sites are vacant
Question 24.
To get n-type of semiconductor, germanium should be doped with
(a) gallium
(b) arsenic
(c) aluminium
(d) boron
Answer
Answer: (b) arsenic
Question 25.
p-type semiconductors are formed When Si or Ge are doped with
(a) group 14 elements
(b) group 15 elements
(c) group 13 elements
(d)group 18 elements
Answer
Answer: (c) group 13 elements
Question 26.
Which of the following conditions favours the existence of a substance in the solid state?
(a) High temperature
(b) Low temperature
(c) High thermal energy
(d) Weak cohesive forces
Answer
Answer: (b) Low temperature
Solid State MCQ
- Isomorphous salts are
- Epsom salt, Green vitriol
- Blue vitriol, Gypsum salt
- Epsom salt, Green vitriol, White vitriol
- White vitriol, Blue vitriol, Green vitriol
Answer/Explanation
Answer:
1. (1)
Isomorphous substances are those which have the same crystal structure.
Blue vitriol = CuSO4.5H2O
Epsom salt = MgSO4.7H2O
Green vitriol = FeSO4.7H2O
White vitriol = ZnSO4.7H2O
As epsom salt, green vitriol and white vitriol have the same water of crystallization, they are isomorphous salts.
- Glass is a
- Liquid crystal
- Crystalline solid
- Ionic solid
- Super-cooled liquid
Answer/Explanation
2. (2)
Glass is an amorphous supercooled liquid. As it has a tendency to flow, though slowly, it is called a supercooled liquid.
- The smallest repetitive unit of the crystal structure is known as
- Atoms
- Compounds
- Unit Cell
- Lattice
Answer/Explanation
3. (3)
Crystal is built up of a regular arrangement of atoms in 3D, this arrangement can be represented by a repeated unit called unit cell, thus unit cell is defined as the smallest repeating unit which shows the full symmetry of the crystal structure.
- What makes molecular crystals solid at room temperature?
- Van der waal force
- Sharing of electron
- Transfer of electron
- All of the above
Answer/Explanation
4. (1)
Van der Waal forces make the molecular crystals, solids at low temperature. Its properties are dictated by the weak nature of these inter-molecular forces.
- Solid CO2 is an amorphous solid of which category?
- Ionic crystal
- Metallic crystal
- Molecular crystal
- Covalent crystal
Answer/Explanation
5. (3)
CO2 is an example of molecular solid
- Crystalline solids do not have
- Definite latent heat of fusion
- Regular atomic arrangement
- Incompressibility
- Free movement of atoms
Answer/Explanation
6. (4)
Crystalline solids have definite heat of fusion, regular atomic arrangement, incompressibility and the atoms are closely packed.
- Number of Bravais lattices present are?
- 10
- 7
- 14
- 21
Answer/Explanation
7. (3)
There are total 14 Bravais lattices present
- AB has NaCl type structure. If atoms from the edge centers along an axis connecting the opposite edge center on a face are removed, the empirical formula of the remaining solid would be?
- A8B5
- A7B8
- A3B5
- A3B8
Answer/Explanation
8. (2)
In NaCl structure, Cl– ions form FCC and Na+ ions are present at edge centres and body centres.
Let B form the FCC and A is present at body centre and edge centre.
Number of atoms of B = 4 (in unit cell)
Number of atoms of A = (14 x 12) + 1 = 3 + 1 = 4 (in unit cell)
If atoms from edge centers along an axis joining opposite edges on a face are removed then,
Effective number of atoms of A removed = 14 x 2 = 12
Effective number of atoms of A present = 4 – 12 = 72
So, A : B ↠ 72 : 4 = 7 : 8
Therefore the formula becomes A7B8
- In rock salt structure, the number of formula units per unit cell
- 2
- 4
- 6
- 8
Answer/Explanation
9. (2)
Na+ atoms are at the edge centres and at the body centre = (12 x 14) + 1 = 4
- The ratio of number of atoms per unit cell in simple cubic, body centered cubic and face centered cubic is?
- 1:2:3
- 1:2:4
- 4:2:1
- 1:3:2
Answer/Explanation
10. (2)
ZSC = 8 x 18 = 1
ZBCC = (8 x 18) + 1 = 2
ZFCC = (8 x 18) + (6 x 12) = 4
Ratio = 1 : 2 : 4
Solid State MCQ (11-20)
- A compound having BCC structure has atomic mass 100 and edge length is 290 pm. Calculate the density.
- 13.6 g/cm3
- 136 g/cm3
- 1.36 g/cm3
- 0.136 g/cm3
Answer/Explanation
11. (1)
Let,
Edge length ‘a’ = 290 pm = 2.9 x 10-6 cm
Atomic mass ‘M’ = 100 g/mol-1
ZBCC = 2
Density = (Z x M)/(a3x NA) = (2 x 100)/[(2.9 x 10-6)3 x 6.023 x 1023] = 13.6 g cm-3
- Which of the following has the largest void percentage?
- Body Centred Cubic
- Simple Cubic
- Face Centred Cubic
- End Centred Cubic
Answer/Explanation
12. (2)
Packing fraction in :-
Simple Cubic = 58%
Body Centred Cubic = 68%
Face Centred Cubic = 74%
Therefore, void fraction in :-
Simple cubic = 48%
Body Centred Cubic = 32%
Face Centred Cubic = 26%
Hence, Simple cubic has the highest percentage of voids
- In a cube, A atoms are present at corners and B atoms are present in the face centres of a cube. Number of A and B are?
- 8,8
- 6,8
- 8,6
- 6,6
Answer/Explanation
13. (3)
Number of corners present in a cube = 8
Number of face-centres present in a cube = 6
Therefore,
A = 8, B = 6
- Packing fraction in CCP and BCC?
- 54% and 79%
- 74% and 68%
- 32% and 26%
- 48% and 48%
Answer/Explanation
14. (2)
Packing fraction in :-
Simple Cubic = 58%
Body Centred Cubic = 68%
Face Centred Cubic = 74%
- What is a F-centre?
- Anion present in interstitial site
- Anion vacancy occupied by electron
- Cationic vacancy occupied by electron
- Anionic vacancy occupied by unpaired electrons
Answer/Explanation
15. (4)
When anions leave their site and form anionic vacancy, the electrons reach and occupy the anionic vacancy. When the crystal is heated, the electrons get excited and impart colour to the crystal.
- What type of stoichiometric defect is shown by NaCl?
- Frenkel
- Schottky
- Both Frenkel and Schottky
- Non-Stoichiometric Defect
Answer/Explanation
16. (2)
NaCl shows a schottky defect.
- Total number of octahedral and tetrahedral voids present in the bcc lattice respectively are?
- 2,6
- 6,4
- 2,4
- 4,6
Answer/Explanation
17. (3)
BCC has two atoms per unit cell.
Thus, it has 2 octahedral voids and 2 x 2 = 4 tetrahedral voids.
- If R forms cubic close packing S occupy 2/3 of octahedral voids in it, then the general formula of the compound is?
- R2S3
- R3S2
- RS
- R3S
Answer/Explanation
18. (2)
In cubic close packing, there are six atoms per unit cell. Hence, R = 6 (no. of octahedral void = number of atoms per unit cell )
S = 2/3 of 6, S = 4R : S = 6 : 4 = 3 : 2
Formula becomes, R3S2
- Number of tetrahedral voids present in each body diagonal in a CCP unit cell is?
- 1
- 2
- 3
- 4
Answer/Explanation
19. (2)
Number of tetrahedral voids present in each body diagonal in a CCP unit cell is 2.
- Which of the following crystal systems is most unsymmetrical?
- Triclinic
- Orthorhombic
- Rhombohedral
- Monoclinic
Answer/Explanation
20. (1)
In triclinic, a ≠ b ≠ c and ɑ ≠ ꞵ ≠ γ
Solid State MCQ (21-30)
- Graphite is an example of which crystal lattice?
- Orthorhombic
- Trigonal
- Tetragonal
- Hexagonal
Answer/Explanation
21. (4)
Graphite has a hexagonal crystal lattice.
- Calculate number of atoms in a cubic unit cell having one atom on each corner and two on each body diagonal.
- 8
- 6
- 9
- 4
Answer/Explanation
22. (3)
Atoms at corner = 8 x (1/8) = 1
Atoms at the body diagonal = 2 x 4 = 8
Total atoms = 1 + 8 = 9
- Ortho-rhombic crystal can show how many possible variations?
- 1
- 2
- 3
- 4
Answer/Explanation
23. (4)
Orthorhombic crystals have all four kinds of lattices which are primitive, body centre, face centre and end centre.
- A tetrahedral void in FCC is formed by atoms at
- 3 face centres + 1 corner
- 2 face-centre + 2 corners
- 3 corners + 1 face centre
- 2 face-centre + 2 corners + 1 body centre
Answer/Explanation
24. (1)
A tetrahedral void is formed by atoms at 3 face centres and at 1 corner.
- What is the coordination number of a crystal having ABC ABC type layer?
- 8
- 12
- 6
- 4
Answer/Explanation
25. (2)
Cubic close packing shows the ABCABC type layer and it has a coordination number of 12.
- Element Y forms CCP and atoms of X occupy 1/3rd of tetrahedral voids. What’s the formula of the compound?
- X2Y3
- X3Y2
- XY
- X2Y
Answer/Explanation
26. (1)
Effective number (Z) of Y (it forms CCP)= 4
X occupies 1/3 of tetrahedral voids
No. of tetrahedral voids in a unit cell = 2Z = 2 x 4 = 4
Effective number of X = (1/3) x 8 = 83
X = 8/3 and Y = 4
Ratio = X : Y = 8 : 12 = 2 : 3
Formula becomes X2Y3
- Which of the following is an example of crystalline solid?
- Fused Silica
- Snowflakes
- Bakelite
- Pitch Tar
Answer/Explanation
27. (2)
Snowflakes have crystal-like structures.
- Which of the following statements is not correct about amorphous solid?
- They are anisotropic
- They have a long range order
- They melt over a range of temperature
- They have an irregular pattern of atoms arranged
Answer/Explanation
28. (2)
Amorphous solids have short-range order.
- In the structure of sphalerite
- Each S2- ion is surrounded by 6 Zn2+
- It has an FCC structure
- Zn ions occupy half of the tetrahedral site
- Each Zn2+ ion is surrounded by 6 S2- ion
Answer/Explanation
29. (3)
In ZnS structure,
- there are equal number of Zn2+ and S2-
- each Zn2+ is surrounded tetrahedrally by four S2- and each S2- is surrounded tetrahedrally by four Zn2+ ions
- Zn2+ ions are arranged in CCP arrangement and S2- are arranged in the corners and face centres
- only half of the tetrahedral voids are occupied by Zn2+
- What is the volume occupied by atoms per unit cell in BCC?
- (1/6)πr3
- (4/3)πr3
- (8/3)πr3
- None of the above
Answer/Explanation
30. (1)
In SC, a = 2r
Volume = (4/3)πr3 = (4/)3π (a2)3 = (4/3)π(a3/8)= (πa3)/6
Solid State MCQ (31-40)
- Which of the following solids have the highest melting point?
- Metallic Solid
- Ionic Solid
- Molecular Solid
- Covalent Solid
Answer/Explanation
31. (1)
Metallic solids show the highest melting point because the bonds between the molecules are the strongest compared to other bonds.
- Copper crystallizes in a FCC lattice with a unit cell length of 361pm. What is the radius of a copper atom?
- 108 pm
- 157 pm
- 181 pm
- 128 pm
Answer/Explanation
32. (4)
In FCC, √2a = 4r
r = (√2a)/4 = (√2 x 361)/4 = 128 pm
- Which element is used to make P-type semiconductor?
- Boron
- Phosphorus
- Carbon
- Tin
Answer/Explanation
33. (1)
P-type semiconductors are formed by the 13 group elements which include elements Boron, Aluminum, Gallium,Indium and Thallium.
- What is the radius ratio of octahedral void?
- 0.155-0.225
- 0.225-0.414
- 0.414-0.732
- 0.732-0.999
Answer/Explanation
34. (3)
Radius ratio of :-
Triangular planar void= 0.155 – 0.225
Tetrahedral void = 0.225-0.414
Octahedral void = 0.414-0.732
Cubic void = 0.732-0.999
- What is the coordination number of the ZnS structure?
- 8:8
- 4:4
- 6:6
- 2:2
Answer/Explanation
35. (2)
ZnS has 4:4 coordination.
- M crystallizes into FCC and BCC structure depending on the surrounding conditions. The ratio of densities in FCC and BCC structures is?
- 8/(3√6)
- 1/2
- 8/√6
- 16/(3√6)
Answer/Explanation
36. (1)
In FCC, a = (4r)/√2
In BCC, a = (4r)/√3
Ratio of density = d1/d2= (Z1/Z2) x (a2/a1)3 = (4/2) x (4r/√3)3 x (√2/4r)3 = (4√2)/(3√3) = 8/(3√6) (after rationalizing)
- Number of unit cells in 117g of NaCl is approximately?
- 1.5 x 1023
- 3.011 x 1023
- 6.022 x 1023
- 0.24 x 1024
Answer/Explanation
37. (2)
Let,
Edge length = a
Radius of each atom = r
As the atoms touch along the face diagonals, i.e,
Face diagonal = √2a = 4r
a = 4r/(√2)= 2√2 x 124 pm = 350.7 pm
Hence, edge length of the unit cell is 350.7 pm
- A truncated tetrahedron has ________
- 12 vertices
- 8 faces
- 18 edges
- 3 hexagonal faces
Answer/Explanation
38. (1)
A truncated octahedron has 12 vertices.
- The packing with more efficiency in a layer is?
- Trigonal Close Packing
- Square Close Packing
- Hexagonal Close Packing
- None of the above
Answer/Explanation
39. (3)
Hexagonal Close Packing has the most efficient packing.
- Which of the following defects is seen in FeO?
- Metal excess defect
- Displacement defect
- Impurity defect
- Metal deficient defect
Answer/Explanation
40. (2)
FeO shows metal deficiency defect.
Solid State MCQ (41-50)
- Lattice energy of an ionic compound depends on?
- Packing of ions only
- Size of the ion only
- Charge on the ion only
- Charge on the ion and size of the ion
Answer/Explanation
41. (4)
Lattice energy of an ionic compound depends on both charge on the ion and size of the ion.
- AgBr shows both Schottky and Frenkel defect because
- Size of Ag >>> Br
- Size of Ag <<< Br
- AgBr is highly ionic
- Both B and C
Answer/Explanation
42. (1)
AgBr shows both Schottky and Frenkel defect because the size of Ag ion is very big in size compared to Br ion.
- Atomic radius of nickel is 124 pm. Nickel crystallizes in a face-centred cubic lattice. What is the length of the edge of the unit cell expressed in pm?
- 350.7 pm
- 420 pm
- 268.3 pm
- 359.2 pm
Answer/Explanation
43. (1)
Let,
Edge length = a
Radius of each atom = r
As the atoms touch along the face diagonals, i.e,
Face diagonal = √2a = 4r
a = (4r)/√2= 2√2 x 124 pm = 350.7 pm
Hence, edge length of the unit cell is 350.7 pm
- Which arrangement of the electrons describes ferrimagnetism?
- ↑↑↑↑↑↑
- ↑↓↑↓↑↓
- ↑↑↓↓↓↑
- None of the above
Answer/Explanation
44. (3)
The substance in which the magnetic moments of domains are aligned parallel and antiparallel directions in unequal numbers, are ferrimagnetic substances.
Their domains are arranged as:- ↑↑↓↑↓↓↑↑↑↓
- An example of a body-centred cube is
- Magnesium
- Sodium
- Zinc
- Copper
Answer/Explanation
45. (2)
Metals like sodium, potassium, chromium, barium, vanadium etc. have body centred-cubic structure.
- The interionic distance for caesium chloride crystals will be?
- a
- a/2
- (a√3)/2
- (2a)/√3
Answer/Explanation
46. (3)
CsCl has a body-centred structure, i.e. its diagonal length is (a√3)/2.
- Which of the following is not true about hexagonal close packing?
- They have 26% empty space
- They have ABABAB arrangement
- Coordination number is 8
- None of the above
Answer/Explanation
47. (3)
HCP has coordination number 12
- What is the approximate number of unit cells present in 1 g of gold. Given that gold crystallizes in FCC lattice.
- 5.68 x 109
- 8.94 x 1010
- 3.57 x 109
- 7.64 x 1010
Answer/Explanation
48. (4)
1 mole of gold = 197 g = 6.022 x 1023 atoms
Therefore, the number of atoms present in 1 g of gold = (6.022 x 1023)/197 x (1/4) = 7.64 x 1020
Hence, 7.64 x 1020 unit cells are present in 1 g of gold.
- Which of the following metal oxide is antiferromagnetic in nature?
- MnO2
- TiO2
- VO2
- CrO2
Answer/Explanation
49. (4)
CrO2 is antiferromagnetic in nature.
- A semiconductor of Ge can be made p-type by adding
- trivalent impurity
- tetravalent impurity
- pentavalent impurity
- divalent impurity
Answer/Explanation
50. (1)
P-type semiconductors are prepared by adding group 13 elements also called trivalent impurity.
Assertion/Reasoning MCQs
Codes
(a) Both A and R are true and R is the correct explanation of A
(b) Both A and R are true and but R is not a correct explanation of A
(c) A is true but R is false
(d) A is false, but R is true
1 . Assertion (A) Quartz glass is crystal is solid and Quartz is an amorphous solid
Reason (R) Quartz glass has no long range order
Answer/Explanation
1. (d)
The structure of quartz is crystalline and that of quartz glass is amorphous. The two structures are almost identical yet in case of amorphous quartz glass, there is no long range order.
2. Assertion (A) Graphite is a good conductor of electricity however Diamond belongs to the category of insulators
Reason (R) Graphite is soft in nature on the other hand diamond is very hard and brittle
Answer/Explanation
2. (b)
Diamond is a bad conductor of electricity because cell valence electrons of carbon are involved in bonding. In graphite however three out of four valence electrons are involved in bonding and the fourth electron remain free between adjacent layers which makes it a good conductor. Graphite is soft because parallel layers are held together by weak Vander waal’s force. However, diamond is hard due to compact 3D network of bonding.
3. Assertion (A) In crystalline solids the value of resistance is different in different directions
Reason (R) Crystalline solids are isotropic in nature
Answer/Explanation
3. (c)
Crystalline solids are anisotropic in nature that is, some of their physical properties like electrical resistance show different values along different directions due to different arrangement of particles in different directions.
4. Assertion (A) Glass panes fixed to windows or panes of old building are found to be slightly thicker at the bottom
Reason (R) Amorphous solids have a tendency to flow
Answer/Explanation
4. (a)
Solids have a tendency to flow, though very slowly. Glass is sometimes called a supercooled liquid because it does not form a crytalline structure, but instead forms an amorphous solid that allows molecules in the material to continue to move.
5. Assertion (A) Face centred cubic cell has 4 atoms per unit cell
Reason (R) In FCC unit there are 8 atoms at the corner and six atoms at face centres
Answer/Explanation
5. (a)
The face centered cubic structure has atoms located at each of the corners and the centres of all the cubic faces. Each of the corner atoms is the corner of another cube so the corner atom is shared among eight unit cell.
6. Assertion (A) CsCl has body-centered cubic arrangement
Reason (R) CsCl has one Cs ion and 8 Cs in its unit cell
Answer/Explanation
6. (c)
CsCl has one Cs+ and one Cl– in its unit cell.
7. Assertion (A) In crystal lattice the size of the tetrahedral hole is large then an octahedral hole
Reason (R) The cation occupy less space than anions in crystal packing
Answer/Explanation
7. (d)
Tetrahedral holes are smaller in size than octahedral voids. Cations usually occupy less space than ions.
8. Assertion (A) On heating ferromagnetic or ferrimagnetic substances, they become paramagnetic
Reason (R) The electrons change their spin on heating
Answer/Explanation
8. (a)
All magnetically ordered solids transform to the paramagnetic state at high temperature due to the randomisation of the spins.
9. Assertion (A) The total number of atoms present in a simple cubic unit cell is one
Reason (R) Simple cubic unit cell has atoms at its corner, each of which is shared between 8 adjacent unit cell
Answer/Explanation
9. (a)
In the simple cubic unit cell, the total number of atoms is 8 x (1/8) = 1
10. Assertion (A) The packing efficiency is maximum for the FCC structure
Reason (R) the coordination number is 12 in FCC structure
Answer/Explanation
10. (b)
In FCC unit cell, CCP arrangement is present with packing efficiency of 74.01% (max).
Case Study Based MCQ
1. Read the passage given below and answer the following questions
Point defects play an important role in determining the physical properties of most crystalline substances, most notably those controlling the transport of matter and the properties that stem from it.
Even a crystal of high purity under conditions of no irradiation contains point defects in thermal equilibrium some.
Some lattice sites are vacant and some atoms are displaced from their normal lattice sights into interstitial positions or onto ‘wrong’ lattice sites.
For stoichiometric compounds of high purity the concentrations of these point defects are very low, even at temperatures up to melting point.
A meaningful model, then, is to consider the crystal as a solvent containing a very dilute solution of simple, individual vacancies and interstitials.
Long range in fractions among the defect and with impurity atoms and short range interactions that produce pair or other clusters can be produced in a first-order approximation.
The following questions are multiple choice questions. Choose the most appropriate answer
i) Which one of the given below statement is wrong about frenkel defect
- It is a combination of vacancy and interstitial defect
- Cation leave their actual lattice site and occupy the interstitial space in the solid
- Density remains the same
- Density of the Crystal increases
Answer/Explanation
Answer: 4
ii) Which one of the following is an interstitial void?
- Octahedral void
- Tetrahedral void
- None of the above
- Both a and b
Answer/Explanation
Answer: 4
iii) This type of defect arises due to absence of equal number of cations and anions from lattice sites in the crystalline solid of the type A B and it lowers the density of the Crystal.
- Vacancy defect
- Schottky defect
- Interstitial defect
- Frenkel defect
Answer/Explanation
Answer: 2
iv) Which one of the following cannot be called as a non-stoichiometric defect
- Metal excess defect due to anion vacancies
- Metal excess defect due to presence of extrication
- Metal deficiency due to absence of cations
- Combination of vacancies and interstitial defects
Answer/Explanation
Answer: (a) 4
2. Read the passage given below and answer the following questions
Unit calls are comprised of empty spaces existing between spheres. This is called void space or hole. Two types of interstitial voids are present in a three dimensional close packing system.
Tetrahedral voids and octahedral voids. All the atoms in a crystal lattice passes vibrational energy and at temperature above absolute zero, a finite number of atoms acquire sufficient energy to break their bonds and get free from their positions.
Thus, point defects are created. There are mainly two types of stoichiometric point defects present – Schottky defects and frenkel defects.
In three questions, a statement of assertion followed by a statement of reason is given. Choose the correct answer out of the following choices.
Codes
(a) Both A and R are true and R is the correct explanation of A
(b) Both A and R are true and but R is not a correct explanation of A
(c) A is true but R is false
(d) A is false, but R is true
i) Assertion (A) The number of tetrahedral void is double the number of octahedral void
Reason (R) The size of the tetrahedral void is half of that of octahedral void
Answer/Explanation
Answer: d
ii) Assertion (A) In BCC arrangement coordination number is 8
Reason (R) In BCC arrangement atoms occupy cubic void
Answer/Explanation
Answer: c
iii) Assertion (A) Due to Frenkel defect there is no effect on density of a solid
Reason (R) Ions shift from lattice sites to interstitial sites in frenkel effect
Answer/Explanation
Answer: c
iv) Assertion (A) Schottky defect is generally shown by the compound with high coordination number
Reason (R) In schottky defect is equal number of cations and anions are missing from their lattice sites
Answer/Explanation
Answer: c
v) Assertion (A) In NaCl crystal, the Na+ ions occupies the octahedral void while Cl ions occupy the vertices of octahedron
Reason (R) The radius ratio of an Na+:Cl minus ions lies between 0.4 to 0.7
Answer/Explanation
Answer: d