Solid State : Notes and Study Materials -pdf
Notes and Study Materials
- Concepts of Biomolecules
- Master File Biomolecules
- NCERT Solutions for – Biomolecules
- NCERT Exemplar Solutions for – Biomolecules
- Mind Map of Biomolecules
- Concept of Biomolecules
- Past Many 12th Board Years of Biomolecules
Examples and Exercise
CBSE Class 12th Chemistry Notes: Biomolecules
This article provides you the revision notes on CBSE Class 12 Chemistry: Chapter – Biomolecules, to give you a quick glance of the chapter. These quick notes are prepared strictly according to the latest CBSE syllabus for Class 12th Chemistry
This article provides you the revision notes on Class 12 Chemistry: Chapter – Biomolecules, to give you a quick glance of the chapter. In this particular part, you will learn about carbohydrates and some of its important forms like glucose, sucrose, starch, etc. These quick notes are prepared strictly according to the latest CBSE syllabus for Class 12th Chemistry.
The main topics covered in this part are:
• Biomolecules
• Carbohydrates
o Classification
• Glucose
o Introduction
o Structure
o Preparation
o Chemical reactions
• Sucrose
• Glycosidic linkage
• Starch
• Cellulose
Biomolecules
Biomolecules are the organic molecules that are involved in the maintenance and metabolic processes of living organisms. Thus, they build up the living system and are responsible for their growth and maintenance.
Some of the common biomolecules are:
• Carbohydrates
• Proteins
• Lipids
• Nucleic acids
• ATP molecules
Carbohydrates
• Carbohydrates are polyhydroxy aldehydes or ketones or the compounds which upon hydrolysis produce polyhydroxy aldehydes or ketones.
• They are optically active due to the presence of chiral ‘C’.
• They are also called saccharides (From Latin word Saccharum = sugar) due to sweet taste.
Classification of Carbohydrates
Depending upon the reaction of carbohydrates with water, they can be classified into the following three types:
1. Monosaccharides: These are the carbohydrates which cannot get hydrolyzed into simpler molecules.
For example glucose,fructose, galactose etc.
2. Disaccharides: These are the carbohydrates that yield two to ten monosaccharide units, on hydrolysis.
For example sucrose, lactose, maltose.
3. Polysaccharides: These are the high molecular mass carbohydrates which on hydrolysis yield a large number of monosaccharides.
For example starch, cellulose, glycogen, gums, etc.
Note: Monosaccharides and oligosaccharides are crystalline solids, soluble in water and sweet in taste. These are known as Sugars. Polysaccharides are amorphous, insoluble in water and tasteless. There are called Non-sugars.
Based upon reducing and non-reducing properties, carbohydrates can be divided into the following two categories:
1. Reducing sugars: These are the carbohydrates which contain free aldehydic or ketonic group and reduces Fehling’s solution or Tollen’s reagent.
For example: Maltose, lactose.
2. Non Reducing Sugars: These are the carbohydrates which do not contain free functional group and do not reduce Fehling’s or Tollen’s reagent.
For example: Sucrose.
Some important carbohydrates are described below:
Glucose
It is a monosaccharide’s with molecular formula C6H12O6. It is present in sweet fruits and in honey.
Preparation of Glucose:
(i) From sucrose, C12H22O11:
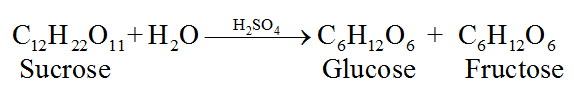
(ii) From starch, C6H10O5:
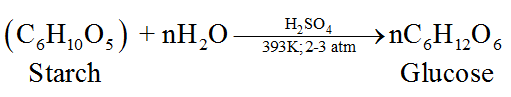
Structure of Glucose:
• Open chain structure (Fisher model):
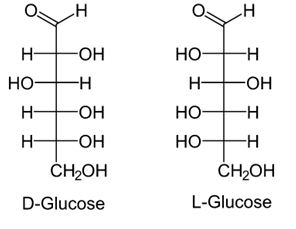
These two optical isomers differ in configuration around any other C atom other than C1 atom.
In D-Glucose, −OH group on first chiral `C’ from the bottom is on right hand.
In L-Glucose, −OH group points to the left of chiral carbon.
• Cyclic Structure:
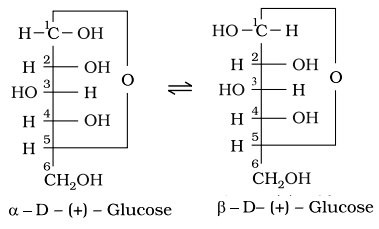
CBSE Class 12th Chemistry Notes: Aldehydes, Ketones and Carboxylic Acids
Chemical Reactions of Glucose
• Reaction with HI:
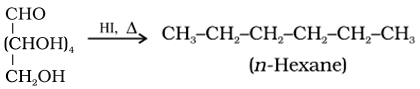
• Reaction with hydroxylamine, NH4OH: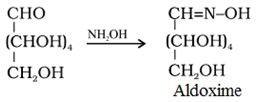
• Reaction with bromine water:
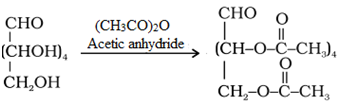
• Acetylation reaction: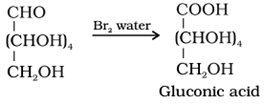
• Oxidation:
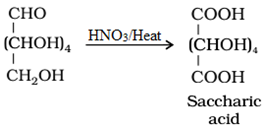
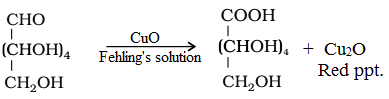
• Reaction with Fehling’s solution:
• Reaction with Tollen’s reagent:
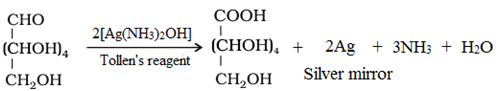
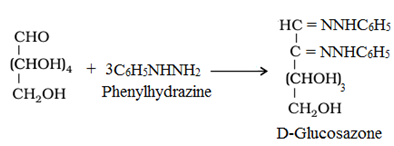
• Reaction with Phenylhydrazine, C6H5NHNH2:
Sucrose:
It is one of the common disaccharides, which on hydrolysis gives equimolar mixture of D (+) -glucose and D (−) fructose.
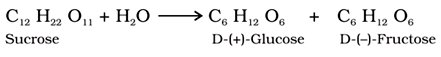
Glycosidic Linkage
The ether linkage combining two monosaccharides is known as glycosidic linkage.
For example: In sucrose, the glycosidic linkage is present between glucose and fructose.
Starch
• Main sources of starch are wheat, maize, rice, potatoes and barley.
• It is a white amorphous powder, in-soluble in cold water.
• It is a polymer of α-glucose and consists of two components- Amylose and Amylopectin.
o Amylose: It is a linear polymer of α‒ glucose. It is water soluble and gives blue colour with iodine solution.
o Amylopectin: It is a branched chain polymer of α‒ glucose. It is water in-soluble and does not give blue colour with iodine solution.
Cellulose
• Cellulose is a linear polymer of β-glucose. It consists of bundles of polymeric chains which are held together by hydrogen-bonding.
• Main sources are wood (45 –50%), cotton (90 – 95%). It is the main constituent of cell walls.
• It is colourless amorphous solid that decomposes on heating.
• It does not reduce Tollen’s reagent or Fehling’s solution.
• It cannot be digested by human stomach.
• Cell wall of bacteria and plants is made up of cellulose
• Cellulose forms a number of important products, such as:
o Cellotape used for packaging.
o Viscose rayon used in textile industry.
o Gun cotton used as an explosive.
In Part-II, you will get to know about the Proteins, Amino acids and Peptides along with their various types. These quick notes are prepared strictly according to the latest CBSE syllabus for Class 12th Chemistry.
The main topics covered in this part are:
• Proteins
• Amino Acids
o Classification
• Zwitter Ion
• Isoelectric point
• Peptides
o Peptide linkage
o Classification
• Classification of Proteins
• Structure of Proteins
• Denaturation of Proteins
The key notes of the chapter are as follows:
Proteins
Proteins are high molecular mass complex biopolymers made up of smaller units called amino acids. All proteins contains C, H, O, N and S. Some of these also contain phosphorus, iodine and traces of metals like Fe, Cu, Zn, Mn. All proteins on hydrolysis first give polypeptides and finally give amino acids.
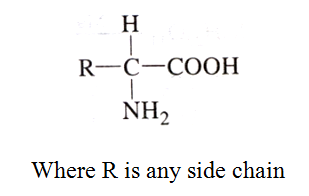
Amino Acids
The compounds containing amino group (-NH2) and carboxylic group (-COOH) are called amino acids. The general structure is:
Classification of Amino Acids
Depending upon the relative position of amino group with respect to carboxyl group, the amino acids can be classified as α, β, γ, δ and so on.
For example:

All naturally occuring amino acids are laevoro-tatory (except glycine).
• Depending upon the relative number of amino and carboxyl groups in their molecule, Amino acid may be categorized as:
o Neutral: These are the amino acids containing equal number of NH2 and COOH groups.
o Acidic: These are the amino acids containing more COOH groups than NH2 groups
o Basic: These are the amino acids containing more NH2 groups than COOH groups
• Depending upon their synthesis, amino acids can be categorizd as:
o Essential Amino Acids: The amino acids which cannot be synthesised in the body and must be obtained through diet, are known as essential amino acids. Examples: Valine, Leucine
o Non-essential Amino Acids: The amino acids, which can be synthesised in the body, are known as non-essential amino acids. Examples: Glycine, Alanine
Zwitter ion
• Amino acid contains both basic acidic (carboxyl group) and basic (amino group) groups in the same molecule. In aque-ous solution, the carboxyl group loses a proton which is accepted by the NH2 group which results in the formation of a dipolar ion known as Zwitter ion. This is neutral but contains both positive and negative charges.
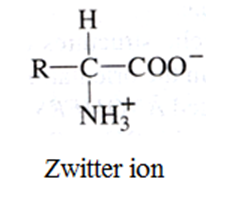
• In zwitter ionic form, amino acids show arnphoteric behaviour as they react both with acids and bases.
Isoelectric point
The pH at which this dipolar ion stops moving towards the respective electrode is known as isoelectric point. At isoelectric point, the amino acids have the least solubility in water.
Peptides
Peptides are condensation products of two or more amino acids.
Peptide Linkage
Peptide linkage is an amide linkage formed by condensation of two amino acids involving −NH2 group of one amino acid and the −COOH group of the other amino acid with the loss of water.
For example:
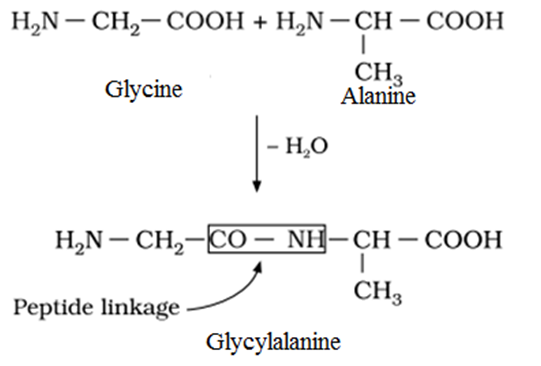
Classification of Peptides:
• On the basis of number of amino acids undergoing the condensation, the peptides can be classified as:
o Dipeptide: It is a peptide composed of two amino-acid residues.
o Tripeptide: It is a peptide composed of three amino-acid residues.
o Polypeptide: It is a peptide composed of more than ten amino-acid residues.
Protein
Peptide having molecular mass higher than 10,000 u is called a protein.
Classification of Proteins
• On the basis their molecular shape, proteins can be classified as:
o Fibrous proteins: The polypeptide chains run parallel and are held together by hydrogen and disulphide bond forming a fibre like structure.
They are generally insoluble in water.
For example: Keratin (present in hair, wool, silk) and myosin (present in muscles), etc.
o Globular proteins: The polypeptide chains coil around to give a spherical shape. These are soluble in water.
For example: Insulin and albumins.
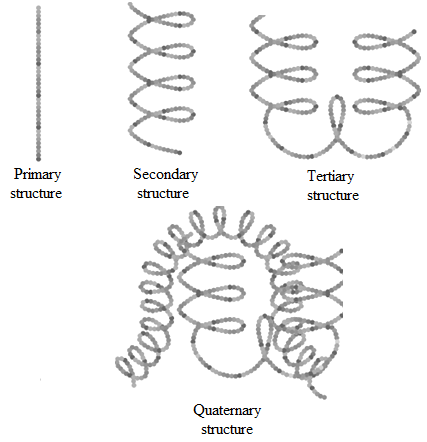
Structure of Proteins
• Primary structure: The sequence of amino acids is said to be the primary structure of a protein.
• Secondary structure: This structure of a protein refers to the shape in which a long polypeptide chain can exist.
There are two secondary structures of proteins:
o α-Helix: In α-Helix, the polypeptide chain coil itself into a right handed screw with the −NH group attached to the −C=O of an adjacent turn of the helix by the hydrogen bonding.
It generally exists when R- group is large
o β-Helix: In this structures, the peptide chains are stretched out to nearly maximum extension and then laid side by side, held together by intermolecular hydrogen bonds.
It generally exists when R- group is small.
• Tertiary structure: The tertiary structure of proteins represents overall folding and superimposition of the polypeptide chains, i.e., further folding of the secondary structure giving rise to a ball shape or spheroidal shape structure.
It is stabilised by covalent, ionic, hydrogen and disulphide bonds.
• Quaternary structure: Some of the proteins are composed of two or more polypeptide chains referred to as sub-units. The spatial arrangement of these subunits with respect to each other is known as quaternary structure.
Denaturation of Proteins
• The most stable conformation of a protein at a given temperature and the pH is known as its native state.
• The loss of biological activity of proteins when a protein in its native form, is subjected to physical change like change in temperature or chemical change like change in pH, is called denaturation of protein.
• For example: (i) Coagulation of egg white on boiling, (ii) curdling of milk which is caused due to the formation of lactic acid by the lactobacillus bacteria present in milk.
In Part-I & II you got acquainted to the Carbohydrates, Proteins, Amino acids and Peptides along with their various types. This Class 12 Chemistry: Chapter– Biomolecules, Part-III, explains you about the Enzymes, Nucleic Acids and Vitamins, their various types and structures. These quick notes are prepared strictly according to the latest CBSE syllabus for Class 12th Chemistry.
The main topics covered in this part are:
• Enzymes
o Nomenclature of Enzymes
o Mechanism of Enzyme Action
• Nucleic Acids
o Structure of Nucleic Acid
o Types of Nucleic Acid
o Structure of DNA
o Structure of RNA
o Importance of DNA
• Vitamins
o Introduction
o Classification
• Some important Vitamins
o Sources
o Deficiency Diseases
The key notes of the chapter are as follows:
Enzymes
• Enzymes are proteinaceous substances which are used as biological catalysts.
• Almost all the enzymes are globular proteins.
• Being proteins, they have colloidal nature and get inactivated during reactions and have to be constantly replaced by synthesis in the body.
• Enzymes are needed only in small quantities for the progress of a reaction.
• Enzymes are very selective and specific for a particular reaction.
• Enzymes lower the energy barrier that reactants must pass over to form the products.
• An enzyme molecule may contain a nonprotein part which is known as prosthetic group.
It is of two types:
o Cofactor: The prosthetic group which is covalently attached with the enzyme molecule is known as cofactor.
o Coenzyme: The prosthetic group which get attached to the enzyme at the time of a reaction is known as coenzyme.
Nomenclature of Enzymes
Enzymes are usually named by adding the suffix ‘ase’ to the root name of the substrate, e.g., urease, maltase, invertase, etc.
Mechanism of Enzyme Action
• There is a lock and key arrangement between the an enzyme and a substrate.
• Substrates bind at active site, temporarily forming an enzyme-substrate (E-S) complex.
• The E-S complex undergoes internal rearrangements that form the product.
• The enzyme gets regenerated for the next molecule of the substrate.
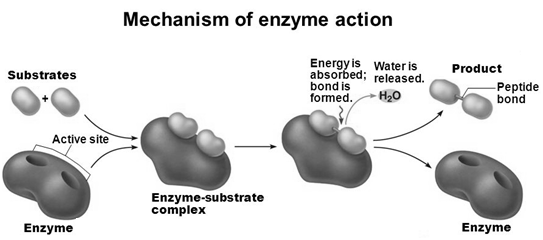
Nucleic Acids
Nucleic acids are chain like polymers of thousands of nucleotide units, hence they are also called polynucleotides.
Nucleoside
A unit formed by the attachment of a base to 1′ position of sugar is known as nucleoside.
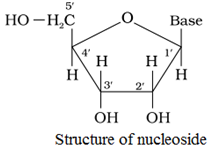
Nucleotide
A nucleotide consists of three subunits: a nitrogen containing heterocyclic aromatic compound which is called base, a pentose sugar and a molecule of phosphoric acid.
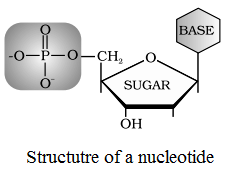
Structure of Nucleic Acid
Thus a nucleic acid chain is represented as shown below.
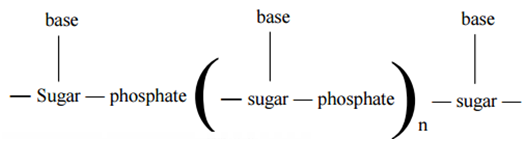
Types of Nucleic Acids
The nucleic acids are of two types:
(i) Deoxyribonucleic acid (DNA)
(ii) Ribonucleic acid (RNA)
• DNA is mainly localised in the nucleus, within the chromosome. While small amount is present in cytoplasm. RNA is also present in the nucleus as well as cytoplasm.
• DNA is mainly used in protein synthesis involving RNA and also a major source of genetic information.
• DNA contains doxyribosee while RNA contains ribose.
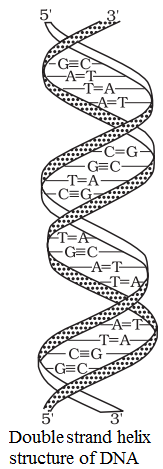
Structure of DNA
• DNA has a double helix structure in which the two strands are antiparellel and are held together by hydrogen bonds.
• In DNA molecule, adenine (A) pairs up with thymine (T) via two hydrogen bonds and guanine (G) pairs up with cytosine (C) via three hydrogen bonds. Therefore CG base pair has more stability than AT base pair.
Structure of RNA
It is usually a single strand of ribonucleotides and take up right handed helical conformation.
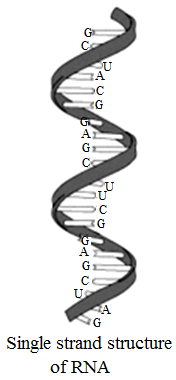
Structural difference between DNA and RNA
There are mainly two structural differences Between DNA and RNA
• Sugar part: DNA contains deoxyribose while RNA contain riboses.
• Base part: In DNA, four bases have been found. They are adenine (A), guanine (G), cytosine (C) and thymine (T). The first three of these bases are found in RNA but thymine (T) is replaced by uracil (U) in place of Thymine.
Importance of DNA
• DNA is the store house of the hereditary information of the organism.
• DNA is involed in protein synthesis.
Vitamins
• Vitamins are a group of organic compounds which are essential for normal growth and nutrition and are required in very small amounts for maintaining optimum growth and a good health.
• Their absence causes specific deficiency diseases.
• Most of the vitamins cannot be synthesised in our body but plants can synthesise almost all of them.
• Vitamin D is an exception because it can be made in the skin from exposure to sunlight.
Classification of Vitamins
On the basis of solubility in water, vitamins are classified into the following two types:
• Fat soluble vitamins: Vitamins A, D, E and K are oil soluble.
• Water soluble vitamins: The group includes Vitamins B and C. These are stored in much lesser amounts in the cells.
Note: Vitamin H (Biotin) is an exception, since it is neither soluble in water nor in fat.
Some important Vitamins, their Sources and their Deficiency Diseases are dictated in the table given below
Name of Vitamin | Important Sources | Deficiency Diseases |
Vitamin A | Fish liver oil, Milk, butter, egg yolk, green and yellow vegetables. | Night blindness, Xerophthalmia (hardening of cornea of eye). |
Vitamin B1 | Yeast, milk, green vegetables, cereals, fruits, egg yolk. | Beriberi (loss of appetite, retarded growth) |
Vitamin B2 | Egg yolk, liver, milk, green leafy vegetables. | Cracked lips, sore tongue, digestive disorders and burning sensation of the skin. |
Vitamin B6 | Milk, egg yolk, cereals, yeast, legumes. | Nervous disturbances and convulsions. |
Vitamin B12 | Meat, fish, kidney, eggs. | Pernicious anaemia (RBC deficient in haemoglobin) |
Vitamin C | Citrus fruits, amla and green leafy vegetables. | Scurvy (bleeding gums) |
Vitamin D | Exposure to sunlight, fish and egg yolk | Rickets (bone deformities in children) and osteomalacia (soft bones and joint pain in adults) |
Vitamin E | Milk, ghee, vegetable oils like wheat germ oil, sunflower oil, cotton seed oil. | Increased fragility of RBCs and muscular weakness |
Vitamin H | Milk, yeast, liver, kidney. | Loss of hair, dermatitis. |
Vitamin K | Green leafy vegetables, fish, meat, cereals. | Increased blood clotting time |
Biomolecules Class 12 Chemistry MCQs
1. RNA and DNA are chiral molecules, their chirality is due to
(a) chiral bases
(b) chiral phosphate units
(c) D-sugar component
(d) L-sugar component
Answer/Explanation
Answer: c
Explaination:
(c) D-sugar component.
2. Glucose does not react with
(a) NH2OH
(b) Cone. HNO3
(c) (CH3CO)2O
(d) NaHSO3
Answer/Explanation
Answer: d
Explaination:
(d) NaHSO3 does not react with glucose.
3. The glycosidic linkage involved in linking the glucose units in amylose part of starch is
(a) C1 -C4 β-linkage
(b) C1 -C6 α-linkage
(c) C1 -C4 α-linkage
(d) C1 -C6 β-linkage
Answer/Explanation
Answer: c
Explaination:
(c) C1-C4 α-linkage are involved in α-glucose, in amylose, linear polymer or a-glucose in starch.
4. A basic amino acid among the following is
(a) glycine
(b) valine
(c) histidine
(d) leucine
Answer/Explanation
Answer: c
Explaination:
(c) It has two amino groups and one —COOH group.
5. Glucose on oxidation with Br2(aq) gives
(a) Gluconic acid
(b) Tartaric acid
(c) Sachharic acid
(d) Meso-oxalic acid
Answer/Explanation
Answer: a
Explaination:
(a) Br2/H2O oxidises —CHO to —COOH.
6. Which of the following is non-reducing sugar?
(a) Glucose
(b) Sucrose
(c) Maltose
(d) Lactose
Answer/Explanation
Answer: b
Explaination:
(b) Sucrose.
7. Which of the following statements is not correct?
(a) Ovalbumin is a simple food reserve in egg white.
(b) Blood proteins thrombin and fibrinogen are involved in blood clotting.
(c) Denaturation makes the proteins more active.
(d) Insulin maintains sugar level in the blood of a human body.
Answer/Explanation
Answer: c
Explaination:
(c) Denaturation leads to biological activity.
8. Deficiency of vitamin B, causes the disease
(a) convulsions
(b) beri beri
(c) cheilosis
(d) sterility
Answer/Explanation
Answer: b
Explaination:
(b) It is caused by deficiency of water soluble B1.
9. Which one of the following metals is required as cofactor by all enzymes utilizing ATP in phosphate transfer?
(a) K
(b) Ca
(c) He
(d) Mg
Answer/Explanation
Answer: d
Explaination:
(d) Mg acts as cofactor.
10. In aqueous solution, an amino acid exist as
(a) cation
(b) anion
(c) zwitter ion
(d) neutral molecule
Answer/Explanation
Answer: c
Explaination: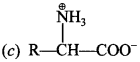
11. Glycogen is a branched chain polymer of α-D-glucose units in which chain is formed by C1—C4 glycosidic linkage whereas branching occurs by the formation of C1– C6 glycosidic linkage. Structure of glycogen is similar to __________ . [NCERT Exemplar]
(a) Amylose
(b) Amylopectin
(c) Cellulose
(d) Glucose
Answer/Explanation
Answer: b
Explaination:
(b) Glycogen is branched chain polymer of α-glucose like amylopectin.
12. Which of the following polymer is stored in the liver of animals? [NCERT Exemplar]
(a) Amylose
(b) Cellulose
(c) Amylopectin
(d) Glycogen
Answer/Explanation
Answer: d
Explaination:
(d) Glycogen is stored in liver of animals.
13. Which of the following pairs represents anomers? [NCERT Exemplar]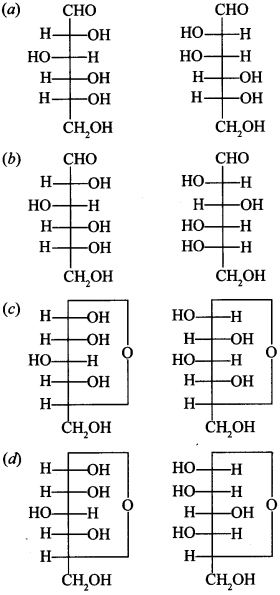
Answer/Explanation
Answer: c
Explaination:
(c) They differ in position of —OH group on C—1 carbon.
14. Proteins are found to have two different types of secondary structures viz. α-helix and β-pIeated sheet structure, α-helix structure of protein is stabilised by : [NCERT Exemplar]
(a) Peptide bonds
(b) van der Waals forces
(c) Hydrogen bonds
(d) Dipole-dipole interactions
Answer/Explanation
Answer: c
Explaination:
(c) H-bonds are present in α-helix.
15. Which of the following acids is a vitamin? [NCERT Exemplar]
(a) Aspartic acid
(b) Ascorbic acid
(c) Adipic acid
(d) Saccharic acid
Answer/Explanation
Answer: b
Explaination:
(b) Ascorbic acid is vitamin C, antioxidant, water soluble and its deficiency causes scurvy.
16. Dinucleotide is obtained by joining two nucleotides together by phosphodiester linkage. Between which carbon atoms of pentose sugars of nucleotides are these linkages present? [NCERT Exemplar]
(a) 5’and 3′
(b)1’and 5′
(c) 5′ and 5′
(d) 3′ and 3′
Answer/Explanation
Answer: a
Explaination:
(a) 5′ and 3′ carbon atoms.
17. Nucleic acids are the polymers of __________ . [NCERT Exemplar]
(a) Nucleosides
(6) Nucleotides
(c) Bases
(d) Sugars
Answer/Explanation
Answer: b
Explaination:
(b) Nucleic acids are polymers of nucleotides.
18. Each polypeptide in a protein has aminoacids linked with each other in a specific sequence. This sequence of amino acids is said to be __________ . [NCERT Exemplar]
(а) primary structure of proteins.
(b) secondary structure of proteins.
(c) tertiary structure of proteins.
(d) quaternary structure of proteins.
Answer/Explanation
Answer: a
Explaination:
(a) It is called primary structure having![]()
peptide bonds.
19. Which of the following B group vitamins can be stored in our body? [NCERT Exemplar]
(a) Vitamin B1
(b) Vitamin B2
(c) Vitamin B6
(d) Vitamin B12
Answer/Explanation
Answer: d
Explaination:
(d) Vitamin B12 is stored in our body as it is neither water soluble nor fat soluble.
20. Which of the following reactions of glucose can be explained only by its cyclic structure? [NCERT Exemplar]
(a) Glucose forms pentaacetate.
(b) Glucose reacts with hydroxylamine to form an oxime.
(c) Pentaacetate of glucose does not react with hydroxylamine.
(d) Glucose is oxidised by nitric acid to gluconic acid.
Answer/Explanation
Answer: c
Explaination:
(c) It means glucose pentaacetate has cyclic structure which does not have free aldehyde group.
21. The sugar present in milk is
(a) Sucrose
(b) Maltose
(c) Glucose
(d) lactose
Answer
Answer: d
22. α-D (+) glucose and β-D (+) – glucose are
(a) Enantiomers
(b) Geometrical isomers
(c) Anomers
(d) Epimers
Answer
Answer: c
23. Distinction between glucose and fructose can be done by
(a) Benedict’s solution
(b) Tollen’s reagent
(c) Selivanoff’s reagent
(d) Fehling solution
Answer
Answer: c
24. Which does not show mutarotation?
(a) Glucose
(b) Maltose
(c) Fructose
(d) Sucrose
Answer
Answer: d
25. The reagent used for obtaining osazone derivative of fructose is
(a) NH2OH
(b) NH2 – NH2
(c) NH2 – NHC6H5
(d) 2, 4-DNP
Answer
Answer: c
26. Amylopectin is a polymer of
(a) β-D-glucose
(b) α-D-glucose
(c) β-D-frutose
(d) α-D-fructose
Answer
Answer: b
27. Hydrolysis of sucrose gives
(a) Glucose only
(b) Glucose + fructo
(c) Glucose and galactose
(d) Maltose
Answer
Answer: b
28. The disease resulting from the intake of amino acid deficient diet is
(a) Kwasiorkar
(b) Pernicicres anaemia
(c) PEM
(d) Haemophilio
Answer
Answer: a
29. Kerating present in hair is an example of
(a) Fibrous protein
(b) Globular protein
(c) Conjugated protein
(d) Derived protein
Answer
Answer: a
30. DNA and RNA differ in
(a) Sugar
(b) Purines
(c) Pyrimidines
(d) Both (a) and (c)
Answer
Answer: d
31. The vitamin present in oils and fats are
(a) A and D
(b) B and C
(c) A and B
(d) A and C
Answer
Answer: a
Note: In the following questions two or more options may be correct. (Q.21 to Q.23)
32. Carbohydrates are classified on the basis of their behaviour on hydrolysis and also as reducing or non-reducing sugar. Sucrose is a __________ . [NCERT Exemplar]
(a) monosaccharide
(b) disaccharide
(c) reducing sugar
(d) non-reducing sugar
Answer/Explanation
Answer:
Explaination:
(b) and (d). It is disachharide made up of glucose and fructose and does not have free aldehyde group, therefore, non-reducing sugar.
33. Amino acids are classified as acidic, basic or neutral depending upon the relative number of amino and carboxyl groups in their molecule. Which of the following are acidic? [NCERT Exemplar]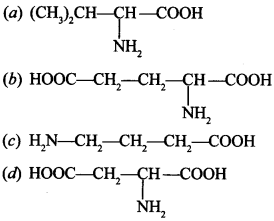
Answer/Explanation
Answer:
Explaination:
(b) and (d) are acidic amino acids.
∵ they have 2—COOH groups and one —NH2 group.
34.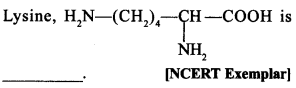
(a) α-Amino acid
(b) Basic amino acid
(c) Amino acid synthesised in body
(d) β-Amino acid
Answer/Explanation
Answer:
Explaination:
(a) and (b). It is a-amino acid.
∵ —NH2 and—COOH groups are attached to same carbon. It is basic because it has 2 amino groups and one —COOH group.
35. Match the vitamins given in Column I with the deficiency disease they cause given in Column II. [NCERT Exemplar]
| Column I (Vitamins) | Column II (Diseases) |
| (a) Vitamin A | (i) Pernicious anaemia |
| (b) Vitamin B1 | (ii) Increased blood clotting time |
| (c) Vitamin B12 | (iii) Xerophthalmia |
| (d) Vitamin C | (iv) Rickets |
| (e) Vitamin D | (v) Muscular weakness |
| (i) Vitamin E | (vi) Night blindness |
| (g) Vitamin K | (vii) Beri Beri |
| (viii) Bleeding gums | |
| (ix) Osteomalacia |
Answer/Explanation
Answer:
Explaination:
(a) (iii) and (vi)
(b) (vii)
(c) (i)
(d) (viii)
(e) (iv) and (i)
(f) (v)
(g) (ii)
Note: In the following questions a statement of assertion followed by a statement of reason is given. Choose the correct answer out of the following choices. (Q.25 to Q.26)
(a) Assertion and reason both are correct and reason is correct explanation of assertion.
(b) Assertion and reason both are wrong statements.
(c) Assertion is correct but reason is wrong statement.
(d) Assertion is wrong but reason is correct statement.
(e) Assertion and reason both are correct statements but reason is not correct explanation of assertion.
36. Assertion: D (+) – Glucose is dextrorotatory in nature.
Reason: ‘D’ represents its dextrorotatory nature. [NCERT Exemplar]
Answer/Explanation
Answer: c
Explaination:
(c) Assertion is correct but reason is wrong statement. ‘D’ represents configuration, i.e., – OH group on right side on first chiral carbon from the bottom (+) dextrorotatory, it is also denoted by d(+).
37. Assertion: Vitamin D can be stored in our body.
Reason: Vitamin D is fat soluble vitamin. [NCERT Exemplar]
Answer/Explanation
Answer: a
Explaination:
(a) Assertion and reason both are correct and reason is correct explanation of assertion.
38. Guanine and adenine belong to __________ and Thymine and Uracil are __________ bases.
Answer/Explanation
Answer:
Explaination: purines, pyrimidines
39. Enzymes are __________ proteins.
Answer/Explanation
Answer:
Explaination: globular
40. Cellulose is linear polymer of __________.
Answer/Explanation
Answer:
Explaination: β-glucose.
41. Invert sugar is mixture of glucose and fructose and is leavorotatory. [True/False]
Answer/Explanation
Answer:
Explaination: True
42. Amylose is linear water soluble, amylopectin is water insoluble, branch chain polymer of a-glucose are two components of starch. [True/False]
Answer/Explanation
Answer:
Explaination: True
43. During denaturation of proteins, tertiary and secondary structure are ruptured but primary structure remains the same. [True/False]
Answer/Explanation
Answer:
Explaination: True
44. What are monosaccharides?
Answer/Explanation
Answer:
Explaination:
Monosaccharides are simple sugars which cannot be hydrolysed, e.g. glucose, fructose, galactose, ribose, deoxyribose, etc. are the examples of monosaccharides.
45. Name the linkage connecting monosaccha-ride units in polysaccharides.
Answer/Explanation
Answer:
Explaination: Glycosidic linkage.
46. What is the structural feature characterising reducing sugar?
Answer/Explanation
Answer:
Explaination: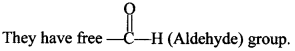
47. Give an example each of the following: [DoE]
(i) Reducing sugar
(ii) Non-reducing sugar
Answer/Explanation
Answer:
Explaination:
(i) Glucose
(ii) Sucrose
48. Under what conditions glucose is converted to gluconic acid and saccharic acid?
Answer/Explanation
Answer:
Explaination:
Bromine water converts glucose to gluconic acid, whereas cone. HNO3 converts to saccharic acid.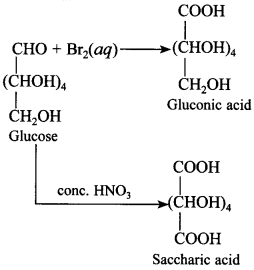
49. The letters ‘D’ or ‘L’ before the name of a stereoisomer of a compound indicate the correlation of configuration of that particular stereoisomer. This refers to their relation with one of the isomers of glyceraldehyde. Predict whether the following compound has ‘D’ or ‘L’ configuration.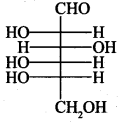
Answer/Explanation
Answer:
Explaination:
L-configuration because —OH group is on left side on first chiral ‘C’ from bottom.
50. Why does compound (A) given below not form an oxime?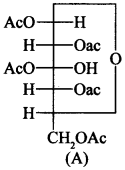
Answer/Explanation
Answer:
Explaination:
It is because it does not have free aldehyde group, therefore, it does not form oxime with hydroxyl amine (NH2OH).
51. Monosaccharides contain carbonyl group hence are classified as aldose or ketose. The number of carbon atoms present in the monosaccharide molecule are also considered for classification. In which class of monosaccharide will you place fructose ?
Answer/Explanation
Answer:
Explaination:
Fructose is keto hexose because it has 6 carbon atoms and ketone group.
52. Sucrose is dextrorotatory but the mixture obtained after hydrolysis is laevorotatory. Explain.
Answer/Explanation
Answer:
Explaination:
On hydrolysis, sucrose (dextrorotatory) gives » glucose (dextrorotatory +52.5) and fructose (laevorotatory, -92.4°). Since laevorotation of fructose is more than dextro-rotation of glucose, therefore, mixture is laevorotatory.
53. What are the products of hydrolysis of maltose? [AI 2014]
Answer/Explanation
Answer:
Explaination: 2 moles of Glucose.
54. Which disaccharide is found in milk?
Answer/Explanation
Answer:
Explaination: Lactose. It is more commonly known as milk sugar.
55. Which monosaccharide units are present in starch, cellulose and glycogen and which linkages link these units?
Answer/Explanation
Answer:
Explaination:
Starch is branched chain polymer of α-glucose, whereas cellulose is linear polymer of β-glucose. Glycogen is branched chain polymer of α-glucose.
In starch and glycogen, aα-glycosidic-linkage is present, whereas in cellulose β-glycosidic- linkage is present.
56. Which of the two components of starch is water soluble? [Delhi 2014; DoE]
Answer/Explanation
Answer:
Explaination:
Amylose is water soluble component in starch.
57. Which component of starch is a branched polymer of D-glucose and insoluble in water? [CBSE Sample Paper 2018; Delhi 2014]
Answer/Explanation
Answer:
Explaination:
Amylopectin.
58. Name the two components of D-glucose which constitute starch. [Foreign 2014]
Answer/Explanation
Answer:
Explaination:
Amylose and amylopectin are two components of starch.
59. Why is cellulose not digested in human body?
Answer/Explanation
Answer:
Explaination:
Cellulose is not digested in human body because we do not have enzymes which can metabolise cellulose.
60. Name animal starch. Why is it called so?
Answer/Explanation
Answer:
Explaination:
Glycogen. Since its structure is similar to amylopectin and is rather more highly branched so it is also known as animal starch. It is present in liver, muscles and brain.
61. Why do amino acids show amphoteric behaviour? [Uttarakhand 2019]
Answer/Explanation
Answer:
Explaination:
They are basic (-NH2) as well as acidic (-COOH), therefore, amphoteric in nature.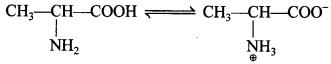
62. Out of a, p and y amino acids, which ‘ amino acids are obtained by hydrolysis of proteins? Give its general structure.
Answer/Explanation
Answer:
Explaination:
Only a-amino acids are obtained by hydrolysis of proteins. Its general structure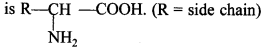
63. Give any two examples of essential amino acids.
Answer/Explanation
Answer:
Explaination: Valine and Leucine.
64. Write the name of linkage joining two amino acids. [AI 2013]
Answer/Explanation
Answer:
Explaination: Peptide linkage
65. Give an example for each of the fibrous protein and globular protein. [AI 2016]
Answer/Explanation
Answer:
Explaination:
Keratin is fibrous protein and albumin is globular protein.
66. What type of bonding occurs in P-pleated structure of proteins?
Answer/Explanation
Answer:
Explaination:
In p-pleated structure, the peptide chains are arranged side by side and these are held by a large number of intermolecular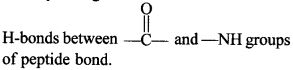
67. What is meant by tertiary structure of proteins?
Answer/Explanation
Answer:
Explaination:
Tertiary structure of proteins involves further folding and twisting of secondary structure of proteins. It has compact and folded structure. It involves H-bonding, disulphide linkage, ionic or salt bridges and hydrophobic interactions. It has van der Waals’ and electrostatic forces of attraction.
68. What are the structural changes found during denaturation of proteins?
Answer/Explanation
Answer:
Explaination:
During denaturation of proteins, 2° and 3° structures are distroyed but 1° structure remains intact.
69. What are biocatalysts? Give an example. [Foreign 2014]
Answer/Explanation
Answer:
Explaination:
Those catalysts which catalyse biochemical reactions are called biocatalysts, e.g. invertase catalyses hydrolysis of cane-sugar to form glucose and fructose.
70. Some enzymes are named after the reaction, where they are used. What name is given to the class of enzymes which catalyse the oxidation of one substrate with simultaneous reduction of another substrate.
Answer/Explanation
Answer:
Explaination:
Oxidoreductase.
71. How do enzymes help a substrate to be attacked by the reagent effectively?
Answer/Explanation
Answer:
Explaination:
Active site of enzyme holds the substrate molecule in suitable position so that it can be attacked by reagent effectively.
72. Out of the following groups, which group has all fat soluble vitamins:
(a) A, B-Complex, C, D
(b) A, D, E, K
(c) K, B-Complex, A, E
(d) C, A, E, D.
Answer/Explanation
Answer:
Explaination:
(b) A, D, E and K are the group of fat-soluble vitamin.
73. Name the deficiency diseases resulting from lack of vitamins A and E in the diet. [Delhi 2013(C); 2011(C); DoE]
Answer/Explanation
Answer:
Explaination:
(i) Night blindness is caused by lack of Vitamin A.
(ii) Loss of reproduction power is caused by deficiency of Vitamin E.
74. Which vitamin is linked with anti-sterility? [DoE]
Answer/Explanation
Answer:
Explaination: Vitamin E.
75. Aldopentoses named as ribose and 2-deoxyribose are found in nucleic acids. What is their relative configuration?
Answer/Explanation
Answer:
Explaination: D-configuration.
76. What are hormones? Give examples.
Answer/Explanation
Answer:
Explaination:
Hormones are chemical substances which are secreated by ductless endocrine glands and perform specific function e.g. Insulin metabolises glucose, testosterone in male, estradiol in female sex hormone.
77. What are non-steroid hormones? Name any one non-steroid hormone.
Answer/Explanation
Answer:
Explaination:
Non-steroid hormones do not contain steroid ring. An example of this type of hormone is insulin.
78. What is Thyroxine?
Answer/Explanation
Answer:
Explaination:
It is produced in thyroid gland, it is an iodine derivative of amino acid tyrosine.
79. What is cause of hypothyroidism? What are symptoms?
Answer/Explanation
Answer:
Explaination:
Low level of thyroxine is cause of hypothy-roidism, It is characterised by lethargyness and obesity. Nal should be added to common salt to prevent it.
80. What is hyperthyroidism?
Answer/Explanation
Answer:
Explaination:
Increased level of thyroxine leads to hyperthyroidism.
80. What is Addison’s disease?
Answer/Explanation
Answer:
Explaination:
If adrenal cortex does not function properly, it causes Addison’s disease, causing hypoglycemia (low level of glucose) weakness.
Biomolecules MCQ Chapter 14
Biomolecules are compounds which are produced by living cells in organisms
Below are some of the very important NCERT Biomolecules MCQ Class 12 Chemistry Chapter 14 with Answers. These Biomolecules MCQs have been prepared by expert teachers and subject experts based on the latest syllabus and pattern of CBSE Term 1 examination.
We have given these Biomolecules Class 12 Chemistry MCQs Questions with Answers to help students understand the concept.
MCQ Questions for Class 12 Chemistry are very important for the latest CBSE Term 1 and Term 2 pattern. These MCQs are very important for students who want to score high in CBSE Board, NEET and JEE exam.
We have put together these NCERT MCQ Questions of Biomolecules for Class 12 Chemistry Chapter 14 with Answers for the practice on a regular basis to score high in exams. Refer to these MCQs Questions with Answers here along with a detailed explanations.
MCQ (1-10)
- Which one is a fibrous protein?
- Globulin
- Collagen
- Hordein
- Gluten
Answer/Explanation
1 . (2)
Collagen is a fibrous protein which are thread like and occur either singly or in groups. It is insoluble in water.
- The relation between glucose and galactose is that they are
- metamers
- epimers
- anomers
- homomers
Answer/Explanation
2. (2)
Glusoce and galactose are epimers. Epimers are diasteriomers that contain more than one chiral center but differ from each other in the absolute configuration.
- Which of the following has maximum laevorotation?
- D-Fructose
- D-Glucose
- Sucrose
- Invert sugar
Answer/Explanation
3. (1)
D-(+)-Glucose has a specific rotation of +52.5°
D-(-)-Fructose has a specific rotation of -92.4°
Invert sugar has a specific rotation of -39.9°
- The human body does not produce
- enzymes
- DNA
- vitamin
- hormones
Answer/Explanation
4. (3)
Vitamins are not produced by the body.
- The deficiency of Vitamin C causes
- scurvy
- rickets
- pyorrhea
- anaemia
Answer/Explanation
5. (1)
The deficiency of vitamin C causes scurvy.
- The energy currency of the cell is
- ATP
- ADP
- AMP
- None of these
Answer/Explanation
6. (1)
The major energy currency molecule of the cell is ATP.
- The helical structure of protein is stabilized by
- Dipeptide bonds
- Hydrogen bonds
- Ether bonds
- Peptide bonds
Answer/Explanation
7. (2)
Hydrogen bonds
- Which of the following is not a pyrimidine?
- Uracil
- Cytosine
- Adenine
- Guanine
Answer/Explanation
8. (4)
Guanine is a purine
- The vitamin which is neither soluble in fat nor in water is
- biotin
- thiamine
- phylloquinone
- pyridoxine
Answer/Explanation
9. (1)
Biotin, also called (vitamin H) is neither soluble in water nor in fat.
- Invert sugar is
- cellobiose
- maltose
- sucrose
- lactose
Answer/Explanation
10. (3)
Biomolecules MCQ (11-20)
- Cellobiose is a
- monosaccharide
- polymer
- disaccharide
- trisaccharide
Answer/Explanation
11. (3)
Cellobiose is a disaccharide.
- Zwitter ion is a
- neutral species
- singly charged species
- doubly charged species
- multi functional species
Answer/Explanation
12. (3)
Zwitter ion is a doubly charged species.
- Which of the following sugars is least sweet?
- Glucose
- Fructose
- Sucrose
- Maltose
Answer/Explanation
13. (4)
Maltose is the least sweet.
- The pyrimidine bases present in DNA are
- cytosine and thymine
- cytosine and adenine
- cytosine and guanine
- cytosine and uracil
Answer/Explanation
14. (1)
The pyrimidine bases present in DNA are cytosine and thymine.
- Simplest amino acid is
- valine
- glycine
- alanine
- leucine
Answer/Explanation
15. (2)
Glycine is the simplest amino acid and is found in animal protein.
- The number of tripeptides formed by three different amino acids is
- 3
- 4
- 5
- 6
Answer/Explanation
16. (4)
The number of tripeptide bonds by three different amino acids as there are three different ways of arrangement (3!).
- The hormone that helps in the conversion of glucose into glycogen is
- cortisone
- bile acid
- adrenaline
- insulin
Answer/Explanation
17. (4)
Insulin helps in conversion of glucose to glycogen. This process is called glycogenolysis.
- The best source of vitamin A is
- oranges
- beans
- carrot
- wheat
Answer/Explanation
18. (3)
Best source of vitamin A is carrot.
- The sugar present in DNA is
- glucose
- deoxyribose
- ribose
- fructose
Answer/Explanation
19. (2)
Deoxyribose is the sugar present in DNA and ribose is present in RNA.
- The hormone which controls the process of burning of fats, proteins and carbohydrates and liberate energy in the body is
- thyroxine
- adrenaline
- insulin
- cortisone
Answer/Explanation
20. (1)
Thyroxine is the hormone which helps in the regulation of metabolism of the body.
Biomolecules MCQ (21-30)
- The number of amino acids commonly found in protein is
- 10
- 15
- 20
- 25
Answer/Explanation
21. (3)
There are 20 amino acids present protein.
- Which of the following is used in a chemical test to test the presence of sugar?
- Iodine solution
- Benedict’s solution
- Biuret reagent
- Bromothymol blue
Answer/Explanation
22. (2)
Benedict’s solutions is used for the chemical test for sugars.
- Which of the following is used in a chemical test to test for the presence of protein?
- Iodine solution
- Benedict’s solution
- Biuret reagent
- Bromothymol blue
Answer/Explanation
23. (3)
Biuret test is used is used for the detection of peptide bonds.
- The disease Beri Beri is caused by deficiency of vitamin
- B1
- A
- C
- D
Answer/Explanation
24. (1)
Beri-Beri is caused by the deficiency of B1 (B1 vitamin is also called thiamine).
- D galactose contains how many carbon atoms?
- 4
- 5
- 8
- 6
Answer/Explanation
25. (4)
D-galactose contains 6 carbon atoms.
- Starch is a polymer of
- glucose
- fructose
- galactose
- lactose
Answer/Explanation
26. (1)
Starch is the polymer of glucose.
- Which of the following is a linear polymer?
- amylopectin
- glycogen
- starch
- amylose
Answer/Explanation
27. (4)
Amylose is a linear polymer.
- Which of the following hormones contains iodine?
- Insulin
- Thyroxine
- Adrenaline
- Testosterone
Answer/Explanation
28. (2)
Thyroxine contains contains iodine.
- Sucrose is a
- reducing sugar
- non reducing sugar
- mixture of glucose
- monosaccharide and fructose
Answer/Explanation
29. (2)
Sucrose is a non-reducing sugar.
- Rice is deficient in
- lysine
- alanine
- glycine
- isoleucine
Answer/Explanation
30. (1)
Rice is deficient in the amino acid lysine.
Biomolecules MCQ (31-40)
- Which of the following statements about RNA is not correct?
- It has a single strand
- It does not undergo replication
- It does not contain any pyrimidine base
- It controls the synthesis of proteins
Answer/Explanation
31. (3)
Cytosine is a pyrimidine base which is present in RNA.
- Which of the following statements is true for protein synthesis (translation)?
- Amino acids are directly recognised by mRNA
- The third base of codon is less specific
- Only one codon codes for amino acid
- Every tRNA has more than one amino acid attachment
Answer/Explanation
32. (2)
The third base of codon is less specific.
- The enzymes which have control site in addition to active site are called
- holoenzymes
- coenzymes
- apoenzymes
- allosteric enzymes
Answer/Explanation
33. (4)
The enzymes which have control site in addition to active site are called allosteric enzymes.
- Which element is absent in protein
- carbon
- nitrogen
- sulphur
- phosphorus
Answer/Explanation
34. (4)
Phosphorus is absent in proteins.
- Which of the following does not react with glucose?
- NH3
- NaHSO3
- Schiff’s reagent
- All of these
Answer/Explanation
35. (3)
Glucose does not react with 2,4-DNP also called Schiff’s reagent.
- Successive nucleotides are covalently linked through
- phosphodiester bond
- hydrogen bond
- sulfide bonds
- any type of bonds
Answer/Explanation
36. (1)
Successive nucleotides are covalently linked through phosphodiester linkage.
- Burette test is given by the following amino acids
- arginine
- tryptophan
- histidine
- proline
Answer/Explanation
37. (3)
Histdine is the only amino acid which gives biuret test.
- The common feature amongst nucleus, chloroplast and mitochondria is
- DNA
- cell wall
- lamellae
- cristae
Answer/Explanation
38. (1)
The common feature amongst nucleus, chloroplast and mitochondria is DNA.
- Maximum amount of RNA is found in
- nucleolus
- ribosomes
- chloroplast
- cytoplasm
Answer/Explanation
39. (2)
Maximum amount of RNA is found in ribosomes.
- When adenine is attached to ribose sugar it is called adenosine. To make a nucleotide from it would require
- oxygenation
- addition of a base
- addition of phosphate
- hydrogenation
Answer/Explanation
40. (3)
When adenine is attached to ribose sugar it is called adenosine. To make a nucleotide from it would require addition of phosphate.
Biomolecules MCQ (41-50)
- Select the incorrect statement about amylose.
1 . It gives blue colour with iodine solution
2. It is unbranched
3. It is soluble in water
4. It has ɑ-C1-C6 linkages
Answer/Explanation
41. (4)
Amylose has ɑ-C1-C4 linkages.
- Vitamin B6 is known as
1 . pyridoxine
2. thiamine
3. tocopherol
4. riboflavin
Answer/Explanation
42. (1)
Vitamin B6 is also known pyridoxine.
- Which of the following disaccharide gives the ketose and an aldose only on hydrolysis?
1 . sucrose
2 . maltose
3. lactose
4. all of these
Answer/Explanation
43. (1)
Sucrose
- Sucrose on hydrolysis gives
1 . glucose and maltose
2. glucose and lactose
3. glucose and fructose
4. only glucose
Answer/Explanation
44. (3)
Sucrose on hydrolysis gives glucose and fructose.
- Glycogen is a
1 . linear polymer with no branching
2. contains more branching than amylopectin
3. contains less branching than amylopectin
4. cannot be digested by human beings
Answer/Explanation
45. (2)
Amylose is structurally similar to amylopectin but is extensively branched.
- Starch contains
1 . 20% of amylose and 80% of amylopectin
2. 30% of amylose and 80% of amylopectin
3. 80% of amylose and 20% of amylopectin
4. 70% of amylose and 30% of amylopectin
Answer/Explanation
46. (1)
20% of amylose and 80% of amylopectin
- Antibodies are
1 . Carbohydrates
2. Proteins
3. Lipids
4. Enzymes
Answer/Explanation
47. (2)
Antibodies are proteins.
- Which of the following is false for ꞵ-glucose?
1 . Melting point = 423 K
2. Specific rotation = +19.0°
3. Sweet taste
4. Dissolves in ether
Answer/Explanation
48. (4)
Glucose doesn’t dissolves in ether.
- The vitamins absorbed from intestine along with fats are
1 . A,D
2. A,B
3. A,C
4. D,B
Answer/Explanation
49. (1)
VItamin A and D are absorbed by the intestine.
- Lysine is least soluble in water in the pH range
1 . 3-4
2. 5-6
3. 6-7
4. 8-9
Answer/Explanation
50. (4)
Lysine is least soluble in water in the pH range 8-9.
Assertion And Reasoning MCQs
Codes
(a) Both A and R are true and R is the correct explanation of A
(b) Both A and R are true and but R is not a correct explanation of A
(c) A is true but R is false
(d) A is false, but R is true
1 . Assertion : When the native protein is subjected to physical changes such a change in temparature or chemical change such as pH, its H-bonds are disturbed. This disturbance unfolds the globules and uncoils the helix. As a result, the protein loses its biological activity. This loss of biological activity is called denaturation.
Reason : One of the examples of denaturation of proteins is the coagulation of egg white when an egg is boiled.
Answer/Explanation
1 . (a)
In a biological system, a protein is found to have a unique 3D structure and a unique biological activity. In such a situation, the protein is called native protein. However, when the native protein is subjected to physical changes, the H-bonds are disturbed. This disturbance uncoils the helix.
As a result, protein loses its biological activity and this is called denaturation. During this process, the secondary and tertiary structures of the protein gets destroyed but the primary structure remains intact. One of the examples of denaturation of proteins in boiling of egg.
2. Assertion : D (+)-glucose is dextrorotatory in nature.
Reason : ‘D’ represents the dextrorotatory nature.
Answer/Explanation
2. (c)
D corresponds to the position of -OH group on the right side on the fathest asymmetric carbon.
3. Assertion : The polypeptide chain in globular protein is folded around itself, giving rise to a spherical structure.
Reason : Some enzymes are globular proteins.
Answer/Explanation
3. (c)
All enzymes are globular proteins.
4. Assertion : DNA is responsible for the transmission of inherent characters from one generation to the next. This process of transmission is called heredity.
Reason : Nucleic acids are responsible for protein synthesis in a cell.
Answer/Explanation
4. (b)
Nucleic acids are responsible for protein synthesis in a cell. Even though the proteins are synthesized by the various RNA molecules in a cell, the message for synthesis of a particular protein is present in DNA.
5. Assertion : Glycine must be taken through diet.
Reason : It is an essential amino acid.
Answer/Explanation
5. (d)
Non-essential amino acids are those that can be produced in the body. Non-essential amino acids, such as glycine, are an example.
6. Assertion : Fibrous protein is a fibre-liked structure formed by the polypeptide chain. These proteins are held together by strong hydrogen and disulphide bonds.
Reason : It is usually soluble in water.
Answer/Explanation
6. (c)
Proteins that are fibrous A fibre-like structure is generated when polypeptide chains run parallel and are kept together by hydrigen and disulphide bonds. In most cases, these proteins are water insoluble.
7. Assertion : In presence of enzyme, substrate molecule can be attacked by the reagent effectively.
Reason : Active sites of enzyme hold the substrate molecule in a suitable position.
Answer/Explanation
7. (a)
Because active sites of enzymes retain the substrate molecule in a favourable place, it can be attacked by a reagent effectively when there is an enzyme present. As a result, enzyme-catalysed reactions are stereospecific.
Frequently Asked Questions (FAQs)
Q1. What are the 4 biomolecules?
Carbohydrates, Proteins, Nucleic Acid and Lipids
Q2. Which biomolecule is the most abundant in the human body?
Proteins are the most abundant biomolecule in the human body.
Q3. What is the largest protein in the body?
Titin is the largest protein in the body which is made of 27,000 amino acids.