Polymers : Notes and Study Materials -pdf
Notes and Study Materials
- Concepts of Polymers
- Master File Polymers
- NCERT Solutions for – Polymers
- NCERT Exemplar Solutions for – Polymers
- Mind Map of Polymers
- Concept of Polymers
- Past Many 12th Board Years of Polymers
Examples and Exercise
CBSE Class 12th Chemistry Notes: Polymers
This article provides you the revision notes on Class 12 Chemistry: Chapter– Polymers, to give you a quick glance of the chapter. These quick notes are prepared strictly according to the latest CBSE syllabus for Class 12th Chemistry.
This article provides you the revision notes on Class 12 Chemistry: Chapter– Polymers, to give you a quick glance of the chapter. In this particular part you will learn about polymers, their classification, methods of polymerization and preparation of some important polymers. These quick notes are prepared strictly according to the latest CBSE syllabus for Class 12th Chemistry.
• Important definitions
o Polymers
o Monomers
o Polymerization
• Classification of Polymers
• Types of Polymerisation Reactions
• Preparation of some important addition polymers
- The key notes of the chapter are as follows:
Polymer
Polymers are defined as high molecular mass macromolecules which consist of repeating structural units derived from the appropriate monomers.
Monomer
The unit molecules that combine with each other to to form a polymer is called a monomer.
Polymerisation
The process of formation of polymers from respective monomers is called polymerisation.
For example:
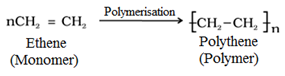
CBSE Class 12 Solved and Unsolved Papers
Classification of Polymers
On the basis of source of availability, polymers can be divided into the following types:
o Natural polymers: These are the polymers that exist in nature,i.e., are found in plants and animals.
For example: Starch, cellulose, rubber, silk.
o Semi-synthetic polymers: These are polymers that are prepared by making some modification in natural polymers by artificial means, in laboratory.
For example: Rayon, vulcanised rubber, gun cotton.
o Synthetic polymers: These are the man-made polymers,i.e., the polymers that are prepared in laboratory.
For example: Bakelite, Teflon, PVC, polystyrene, nylon.
On the basis of structure, polymers can be categorized into the following types:
o Linear polymers: These are the polymers in which the monomer units are linked to one another to form long and straight chains.
These chains are closely packed in space which causes the linear polymers to have high densities, tensile strength and high melting and boiling points.
For example: High density polyethene (HDPE), PVC, nylon, polyester.
o Branched chain polymers: These are the linear chain polymers having some branches.
Branching causes these polymers to be loosely packed in space due to which thay have low densities, low tensile strength as well as low melting and boiling points. For example: Low density polyethene (LDPE), amylopectin, glycogen.
o Cross-linked or network polymers: These are the polymers formed of various linear polymers connected to each other by strong covalent bonds.
These are polymers hard, rigid and brittle.
For example: Bakelite, formaldehyde polymer, glyptal, melamine-formaldehyde polymer.
On the basis of mode of polymerization, polymers are of following two types:
o Addition polymers: These are the polymers formed by the repeated addition of monomer molecules containing multiple bonds.
(i) Homopolymers: These are the polymers derived from the polymerisation of only one kind of monomers.
For example:
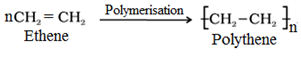
(ii) Copolymers: These are the polymers obtained by the polymerisation of two or more kind of monomers.
For example:
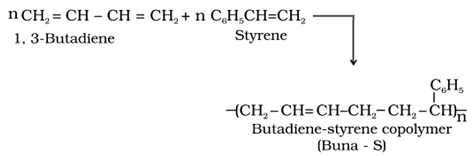
• Condensation polymers: These polymers are formed by the repeated condensation reaction of different bifunctional or trifunctional monomers, with the elimination of small molecules like H2O,HCl, CH3OH.
For example:
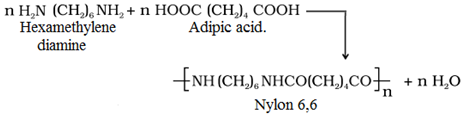
On the basis of the molecular forces, the polymers can be classified as:
o Elastomers: These polymers are held together by weak van der Waals, forces and have low elasticity. For example: Buna-S, buna-N, neoprene.
o Fibres: These are the polymer held together by strong hydrogen bonds. They have high tensile strength and sharp melting point. For example: Nylon, polyster, silk, wool, orlon, rayon.
o Thermoplastics polymers: They are the polymers having intermolecular forces of attraction intermediate between elastomers and fibers. They can be made soft and remoulded by heating. For example: Polythene, PVC, polystrene, polypropene.
o Thermosetting polymers: These are the hard, rigid, cross-linked polymers. They cannot be remoulded once set into a desired shape. For example: Melamine, bakelite.
Types of Polymerisation Reactions
There are two general methods of polymerisation:
(i) Addition polymerisations or chain growth polymerisation
(ii) Condensation polymerization or step growth polymerization
(iii) Copolymerisation
Addition polymerization
Molecules of the same monomer or different monomers simply add together to form a polymer.
The monomers used are mainly the unsaturated compounds.
Addition polymerization generally follows the free radical mechanism.
• Free radical polymerization: It involves formation of reactive intermediate such as free radical, a carbocation or a carbanion.
This is a three step process:
(i) Chain initiating step
(ii) Chain propagating step
(iii) Chain terminating step.
For example, the ethene is converted to polythene by free radical polymerization as follows:
o Chain initiating step:
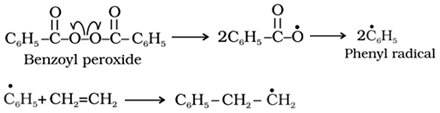
o Chain propagating step
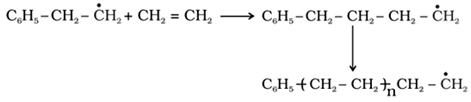
o Chain terminating step
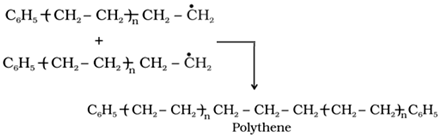
Preparation of some important addition polymers
Polyethene
The polythene is of two types:
(i) Low density polyethene (LDPE): It is obtained by polymerization of ethene at 350 to 750 K and 1000 to 2000 atm pressure.
It is chemically inert and tough but flexible and a poor conductor of electricity.
It is used in the insulation of electricity carrying wires and manufacture of squeeze bottles, toys and flexible pipes.
(ii) High density polyethene (HDPE): It is obtained by the addition polymerisation of ethene at 330 to 350 K at atmospheric pressure.
It is tough and hard with high tensile strength.
It is used in the manufacture of plastic containers, house wares, pipes.
Polyacrylonitrile
• It is obtained by the addition polymerisation of acrylonitrile in presence of a peroxide catalyst.

• It is used in the formation of substitute for wool as orlon or acrilan.
Polytetrafluoroethene (Teflon)
• It is obtained by the free radical polymerisation of tetrafluoroethene at high pressures.

• It is chemically inert and resistant to attack by corrosive reagents.
• It is used in the manufacture of oil seals and gasket and non-stick kitchen wares.
Condensation Polymerisation
Such polymerisation involves a repetitive condensation reaction between two bi-functional monomers.
It occurs in a stepwise manner with elimination of some smaller molecules like H2O, NH3, HCI, ROH, etc., therefore it is also named as step Growth Polymerisation.
For example: Dacron is obtained by the condensation polymerization of ethylene glycol and terephthalic acid.

Some important condensation polymerisation reactions are:
Polyamides
These are the polymers possessing amide linkages and are named as nylons.
Preparation and uses of some important polyamides are given below:
• Nylon 6: It is obtained by heating caprolactum with water at a high temperature.
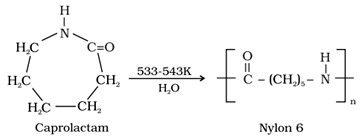
It is used for the manufacture of tyre cords, fabrics and ropes.
• Nylon 6,6: It is prepared by the condensation polymerisation of hexamethylenediamine with adipic acid under high pressure and at high temperature.

• It is used in making stocking, socks, ropes, Parachutes, fabrics, bristles of tooth brush.
Melamine – formaldehyde polymer
• It is obtained by the condensation polymerisation of melamine and formaldehyde.
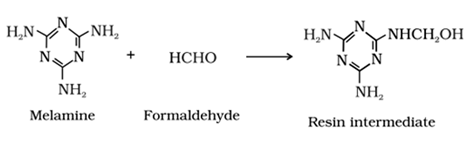
• It is used in the manufacture of unbreakable crockery.
In Part-I you got to learn about polymers, their classification, methods of polymerization and preparation of some important polymers. In Part-II, you will get to know about copolymerisation, biodegradable polymers and some commercially important polymers. These quick notes are prepared strictly according to the latest CBSE syllabus for Class 12th Chemistry.
The main topics covered in this part are:
• Copolymerisation
• Rubber
o Natural
o Synthetic
• Biodegradable Polymers
• Polymers of Commercial Importance
The key notes of the chapter are as follows:
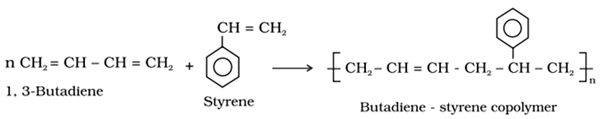
Copolymerisation
It is a type of polymerisation reaction in which a mixture of more than one monomeric species is allowed to polymerise and form a polymer called copolymer.
Copolymer contains multiple units of each monomer.
For example: 1, 3-Butadiene and styrene can undergo copolymerization to form butadiene –styrene copolymer.
Rubber
Rubber is of two types: Natural and synthetic
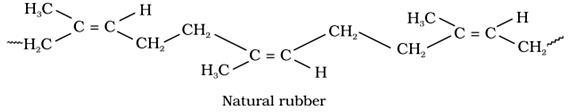
1. Natural Rubber
It is a natural polymer possessesing elastic properties.
It is a linear 1, 4-polymer of isoprene (2-methyl-1, 3-butadiene).
Drawbacks of Natural Rubber:
• It becomes soft at high temperature and brittle at low temperatures.
• It is non-resistant to the attack of oxidizing agents.
Vulcanisation of Rubber: The process of adding sulphur to rubber to improve its physical properties is called vulcanisation of rubber.
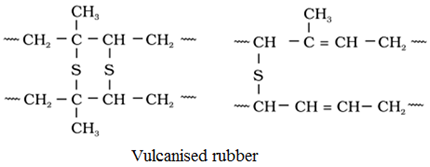
Vulcanisatlon is carried out by adding sulphur (3-5%) and zinc oxide to the rubber, and then heating the object at about 110°Cfor about 20-30 minutes.
Zinc oxide accelerates the rate of vulcanisation
Sulphur forms cross links at the reactive sites of double bonds and thus the rubber gets stiffened.
Thus about 5% sulphur is used for making tyre rubber and 30% of it for making battery case rubber.
The improved properties of vulcanised rubber are:
(i) High elasticity.
(ii) Low water-absorption tendency
(iii) Resistance to oxidation.
2. Synthetic Rubber
Synthetic rubbers are polymers derived from 1, 3 – butadiene or 1, 3 – butadiene derivatives.
The synthetic rubber is of two types:
• Neoprene
o It is a homopolymers of chloroprene (2-Chloro-1,3-butadiene).
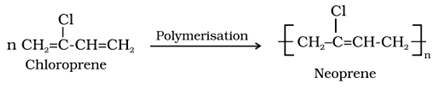
o Uses: It is used for manufacturing conveyor belts, gaskets and hoses.
• Buna-N
o It is a copolymer of 1,3-Butadiene with acrylonitrile (Buns-N) carried out in the presence of a peroxide catalyst.
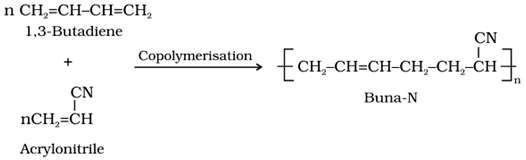
o Uses: It is used in making conveyor belts and printing rollers.
Biodegradable Polymers
These are the synthetic polymers that are hydrolysed by enzymes and to some extent degrade by oxidation.
These polymers contain functional groups similar to the functional groups present in biopolymers.
Some important examples of biodegradable polymers are given below:
1. Poly-hydroxybutyrate- co-β-hydroxy valerate (PHBV):
• Preparation: It is a copolymer of 3-hydroxy butanoic acid and 3-hydroxy pentanoic acid in which the monomeric units are connected by ester linkages.
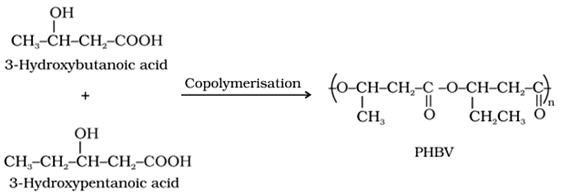
• Uses: It is used in packaging, orthopaedic devices and even in controlled drug release.
2. Suture Polymer:
• It is a copolymer of glycolic acid and lactic acid.
• Sutures help the healing process by closing and sealing a wound.
• Dextron was the first biodegradable suture made from biodegradable polyesters for post-operation sticher.
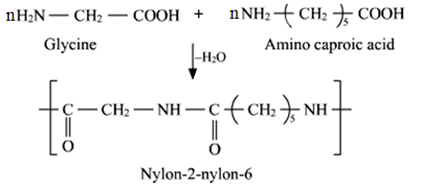
3. Nylon-2-Nylon-6:
It is a copolymer of (H2N–CH2–COOH) and amino caproic acid [H2N (CH2)5 COOH] and is biodegradable.
Polymers of Commercial Importance
Some Commercially Important Polymers, their monomers and uses are given below:
Polymer | Monomer | Uses |
Polyvinyl chloride (PVC) | CH2=CHCl Vinyl Chloride | Manufacture of rain coats, hand bags, vinyl flooring, and water pipes. |
Polyethylene | CH2=CH2 Ethylene | Manufacture of coats, wire insulators, bags |
Polystyrene | CH2=CH‒C6H5 Vinylbenzene (Styrene) | As insulator, wrapping material, manufacture of toys, radio and television cabinets |
Polyvinyl acetate (PVA) | CH2=CH‒OCOCH3 Vinylacetate | Making latex, paint |
BUNA rubber | CH2=CH‒CH=CH2 1,3-Butadiene | Manufacture of tyres and hoses |
Polymers Class 12 Chemistry MCQs
1. The monomer of polystyrene is
(a) C2H —CH=CH2
(b) CH2=CHCl
(c) C6H5CH=CH2
(d) CH2=CH—CHO
Answer/Explanation
Answer: c
Explaination:
(c) C6H5—CH=CH2 is monomer of polystyrene.
2.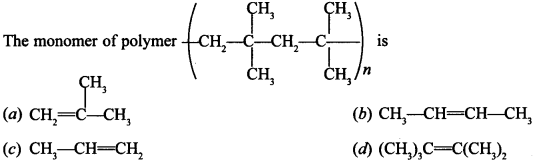
Answer/Explanation
Answer: a
Explaination: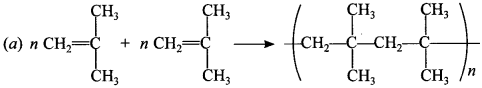
3.![]()
(a) addition polymer
(b) thermosetting polymer
(c) homopolymer
(d) condensation copolymer (Nylon-66)
Answer/Explanation
Answer: d
Explaination:
(d) It is condensation copolymer of Hexamethylene diamine (H2N(CH2)6NH2) and adipic acid,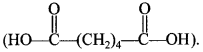
4. Which of the following statements is not true?
(a) Natural rubber is frans-polyisoprene
(b) Buna-S is addition copolymer of butadiene and styrene
(c) Natural rubber is cis 1, 4-polymer of isoprene
(d) Vulcanisation makes rubber hard due to cross linkages with sulphur.
Answer/Explanation
Answer: a
Explaination:
(a) Natural rubber is cis- polyisoprene
5. Which of the following is not correct?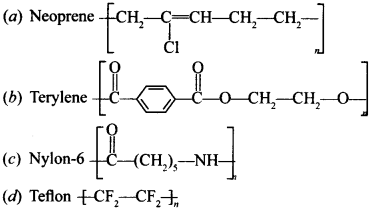
Answer/Explanation
Answer: a
Explaination: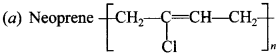
is polymer of 2-chloro-1,3-butadiene (Chloroprene)
6. Which of the following has ester linkages?
(a) Nylon
(b) Bakelite
(c) Terylene
(d) PVC
Answer/Explanation
Answer: c
Explaination:
(c) Terylene is polyester, it has many ester linkages.
7. Which of the following is thermosetting polymer
(a) Neoprene
(b) PVC
(c) Nylon-6,6
(d) Bakelite
Answer/Explanation
Answer: d
Explaination:
(d) Bakelite is cross linked thermosetting polymer of phenol and formaldehyde.
8. Caprolactum is used for preparation of
(a) Nylon-6
(b) Nylon-6,6
(c) Nylon 6, 10
(d) Nylon-2 – Nylon-6
Answer/Explanation
Answer: a
Explaination:
(a) Nylon-6 is polymer of caprolactum.
9. The polymer used in orthopaedic devices and in controlled drug release is
(a) Orion
(b) PTFE
(c) SBR
(d) PHBV
Answer/Explanation
Answer: a
Explaination:
(a) PHBV is biocompatible, used in orthopaedic devices and controlled drug release. Orion is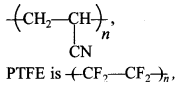
SBR is styrene butadiene rubber.
10. Which of the following is not correct regarding terylene?
(a) Step growth polymer
(b) Synthetic fibre
(c) also called dacron
(d) Thermosetting plastic
Answer/Explanation
Answer: d
Explaination:
(d) It is synthetic fibre condensation, step growth polymer also called dacron.
11. The polymer which is used in manufacture of squeeze bottles is
(a) Polystyrene
(b) Teflon
(c) Polypropene
(d) Low density polythene
Answer/Explanation
Answer: d
Explaination:
(d) LDP is used for making squeeze bottles, flexible hose pipe.
12. The polymer containing strong intermolecular H-bonding and crystalline nature is
(a) Polystyrene
(b) Natural rubber
(c) Teflon
(d) Nylon 6, 6
Answer/Explanation
Answer: d
Explaination:
(d) Nylon 6, 6 is fibre with strong intermolecular H-bonding and is crystalline in nature.
13. Which of the following is initiator in free radical polymerisation?
(a) Benzoyl peroxide
(b) AlCl3
(c) HNO3
(d) Bu-Li
Answer/Explanation
Answer: a
Explaination:
(a) Benzoyl peroxide undergoes homolytic fission to form free radicals and acts as initiator.
14. Novolac, the linear polymer used in paints is
(a) copolymer of buta 1, 3-diene and styrene
(b) polymer of methyl methacrylate
(c) condensation polymer of phenol and HCHO
(d) copolymer of melamine and HCHO
Answer/Explanation
Answer: c
Explaination:
(c) It is copolymer of phenol and HCHO, linear.
15. Which of the following is not a semisynthetic polymer? [NCERT Exemplar]
(a) cw-polyisoprene .
(b) Cellulose nitrate
(c) Cellulose acetate
(d) Vulcanised rubber
Answer/Explanation
Answer: a
Explaination:
(a) cis-polyisoprene is natural rubber, others are semisynthetic because these are obtained from natural polymers by suitable process.
16. The commercial name of polyacrylonitrile is __________ . [NCERT Exemplar]
(a) Dacron
(b) Orion (acrilan)
(c) PVC
(d) Bakelite
Answer/Explanation
Answer: b
Explaination: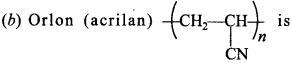
artificial wool, used for making carpets etc.
17. Zeigler – Natta catalyst is used in making
(a) LDPE
(b) HDPE
(c) Polystyrene
(d) PMMA
Answer
Answer: b
18. Natural rubber in a polymer of
(a) Butadiene
(b) Ethyne
(c) Styrene
(d) Isoprene
Answer
Answer: d
19. Which of the following is a biodegrable polymer?
(a) Cellulose
(b) Polyethene
(c) PVC
(d) Nylon-6
Answer
Answer: a
20. A polymer of butadiene and acrylonitrile is called
(a) Buna-2
(b) Buna-N
(c) Buna-S
(d) Buna-A
Answer
Answer: b
21. The manufacture of nylon-6 involves condensation of
(a) Phenol and formaldehyde
(b) Urea and formaldehyde
(c) Adipic acid and hexamethylene diamine
(d) Ethylene glycol and pthalic acid
Answer
Answer: c
22. Match List-I and List-II and select the correct answer using the codes below the lists: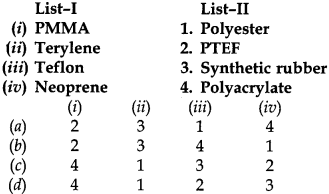
Answer
Answer: d
23. F2C = CF2 is a monomer of
(a) Teflon
(b) Glyptal
(c) Nylon-6
(d) Buna-S
Answer
Answer: a
24. Which of the following is used in tyre cords?
(a) Terylene
(b) Polyethylene
(c) Polypropylene
(d) Nylon-6
Answer
Answer: d
25. PHBV has _______ type of linkages
(a) Amide
(b) Ester
(c) Diene
(d) Nitrile
Answer
Answer: b
26. The vulcanised rubber has
(a) High water absorption, resistant to oxidation and good elasticity
(b) High water absorption, susceptible to oxidation and no elasticity
(c) High water absorption, susceptible to oxidation and good elasticity
(d) Low water absorption, resistance to oxidation and good elasticity.
Answer
Answer: d
27. Which of the following polymer is biodegradable? [NCERT Exemplar]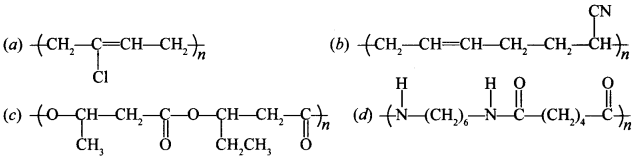
Answer/Explanation
Answer:
Explaination:
(c) is PHBV, biodegradable,
(a) is Neoprene
(b) Buna-N
(d) Nylon-6, 6.
28. In which of the following polymers ethylene glycol is one of the monomer units? [NCERT Exemplar]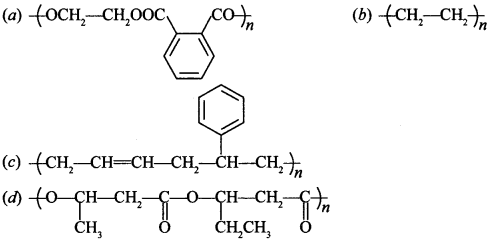
Answer/Explanation
Answer:
Explaination:
(a) has HOCH2—CH2—OH as one of the monomers of terylene
(b) Polythene
(c) Buna-S
(d) PHBV
29. Which of the following statements is not true about low density polythene? [NCERT Exemplar]
(a) Tough
(b) Hard
(c) Poor conductor of electricity
(d) Highly branched structure
Answer/Explanation
Answer: b
Explaination:
(b) It is not hard but flexible, branched, tough, poor conductor of electricity.
Note: In the following questions two or more options may be correct. (Q.20 to Q.22)
30. Which of the following polymers, need atleast one diene monomer for their preparation? [NCERT Exemplar]
(a) Dacron
(b) Buna-S
(c) Neoprene
(d) Novolac
Answer/Explanation
Answer:
Explaination:
(b) and (c). Buna-S needs buta 1, 3-diene and Neoprene needs 2-chloro 1,3-butadiene. Dacron and Novolac are condensation polymers.
31. Which of the folloiwng are characteristics of thermosetting polymers? [NCERT Exemplar]
(a) Heavily branched cross linked polymers,
(b) Linear slightly branched long chain molecules.
(c) Become infusible on moulding so cannot be reused.
(d) Soften on heating and harden on cooling, can be reused.
Answer/Explanation
Answer:
Explaination:
(a) and (c) e.g. Bakelite is used for making electrical switches and switch boards.
32. Which of the following polymers are thermoplastic? [NCERT Exemplar]
(a) Teflon
(b) Natural rubber
(c) Neoprene
(d) Polystyrene
Answer/Explanation
Answer:
Explaination:
(a) and (d), Teflon is used in non-stick kichen ware, polystyrene is used to make toys and radio and T.V. cabinets.
33. Match the polymer of column I with correct monomer of column II. [NCERT Exemplar]
| Column I | Column II |
| (a) High density polythene | (i) Isoprene |
| (b) Neoprene | (ii) Tetrafluoro- ethene |
| (c) Natural rubber | (iii) Chloroprene |
| (d) Teflon | (iv) Acrylonitrile |
| (e) Acrilan | (v) Ethene |
Answer/Explanation
Answer:
Explaination:
(a) (v)
(b) (in)
(c) (i)
(d) (ii)
(e) (iv)
34. Match the polymers given in Column I with their chemical names given in Column II. [NCERT Exemplar]
| Column I | Column II |
| (a) Nylon 6 | (i) Polyvinyl chloride |
| (b) PVC | (ii) Polyacrylonitrile |
| (c) Acrilan | (iii) Polycaprolactum |
| (d) Natural rubber | (iv) Low density polythene |
| (e) LDP | (v) cis-polyisoprene |
Answer/Explanation
Answer:
Explaination:
(a) (iii)
(b) (i)
(c) (ii)
(d) (v)
(e) (iv)
35. Match the polymers given in Column I with their commercial names given in Column II. [NCERT Exemplar]
| Column I | Column II |
| (a) Polyester of glycol and phthalic acid | (i) Novolac |
| (b) Copolymer of 1, 3-butadiene and styrene | (ii) Glyptal |
| (c) Phenol and formaldehyde resin | (iii) Buna-S |
| (d) Polyester of glycol and terephthalic acid | (iv) Buna-N |
| (e) Copolymer of 1, 3-butadiene and acrylonitrile | (v) Dacron |
Answer/Explanation
Answer:
Explaination:
(a) (ii)
(b) (iii)
(c) (i)
(d) (v)
(e) (iv)
36. Match the polymers given in Column I with their main applications given in Column II. [NCERT Exemplar]
| Column I | Column II |
| (a) Bakelite | (i) Unbreakable crockery |
| (b) Low density polythene | (ii) Non-stick cook-wares |
| (c) Melamine- formaldehyde resin | (iii) Packaging material for shock absorbance |
| (d) Nylon 6 | (iv) Electrical switches |
| (e) Polytetrafluo- roethane | (v) Squeeze bottles |
| (f) Polystyrene | (v) Tyre, cords |
Answer/Explanation
Answer:
Explaination:
(a) (iv)
(b) (v)
(c) (i)
(d)(vi)
(e) (ii)
(f) (iii)
37. Match the polymers given in Column I with the preferred mode of polymerisation followed by their monomers. [NCERT Exemplar]
| Column I | Column II |
| (a) Nylon-6,6 | (i) Free radical Polymeriaation |
| (b) PVC | (ii) Ziegler-Natta polymerisation or coordination polymerisation |
| (c) HDP | (iii) Anionic polymerisation |
| (iv) Condensation polymerisation |
Answer/Explanation
Answer:
Explaination:
(a) (iv)
(b) (i)
(c) (ii)
Note: In the following questions a statement of assertion followed by a statement of reason is given. Choose the correct answer out of the following choices. (Q.28 to Q.30)
(a) Assertion and reason both are correct and reason is correct explanation of assertion.
(b) Assertion and reason both are wrong statements.
(c) Assertion is correct but reason is wrong statement.
(d) Assertion is wrong but reason is correct statement.
(e) Assertion and reason both are correct statements but reason is not correct explanation of assertion.
38. Assertion: Rayon is a semi synthetic polymer and is taken as a better choice than cotton fabric. [NCERT Exemplar]
Reason: Mechanical and aesthetic properties of cellulose can be improved by acetylation.
Answer/Explanation
Answer: a
Explaination:
(a) Assertion and reason both are correct and reason is correct explanation of assertion.
39. Assertion: Most of the Synthetic polymers are not biodegradable. [NCERT Exemplar]
Reason: Polymerisation process induces toxic character in organic molecules.
Answer/Explanation
Answer: c
Explaination:
(c) Assertion is correct but reason is wrong statement.
40. Assertion: Olefinic monomers undergo addition polymerisation. [NCERT Exemplar]
Reason: Polymerisation of vinyl chloride is initiated by peroxides/ persulphates.
Answer/Explanation
Answer: e
Explaination:
(e) Assertion and reason both are correct statements but reason is not correct explanation of assertion.
41. Trans-polyisoprene, a rubber like material is called __________ .
Answer/Explanation
Answer:
Explaination: Gutta percha
42. High density polythene __________ polymer of ethene.
Answer/Explanation
Answer:
Explaination: linear
43. A copolymer can be addition or condensation. [True/False]
Answer/Explanation
Answer:
Explaination:
True, e.g. Nylon-66 (condensation copolymer), Buna-S (addition copolymer).
44.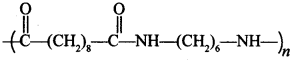
is Nylon 6, 10, synthetic fibre. [True/False]
Answer/Explanation
Answer:
Explaination: True
45.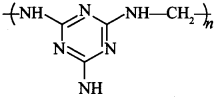
is Melamine-formaldehyde resin, a thermosetting polymer. [True/False]
Answer/Explanation
Answer:
Explaination: True
46. Which of the following is natural polymer? Buna-S, Protein, PVC [Chennai 2019; AI2014]
Answer/Explanation
Answer:
Explaination:
Protein is a natural polymer.
47. Give an example of semi-synthetic polymer.
Answer/Explanation
Answer:
Explaination:
Cellulose nitrate.
48. What are synthetic polymers?
Answer/Explanation
Answer:
Explaination:
These are man-made polymers extensively used in daily life as well as in industry, e.g. plastic (polythene)
49. Give an example of linear and branched chain polymer each.
Answer/Explanation
Answer:
Explaination:
Linear polymer – Polyvinyl chloride
Branched chain polymer – Low density polythene.
50. Identify the type of polymer given in the following figure. [HOTS]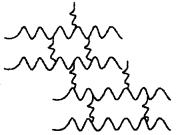
Answer/Explanation
Answer:
Explaination: Cross-linked polymer
51. Identify the type of polymer:
—A—A—A—A—A—A—
Answer/Explanation
Answer:
Explaination: Homopolymer
52.![]()
a homopolymer or a copolymer? [AI 2013]
Answer/Explanation
Answer:
Explaination: It is homopolymer.
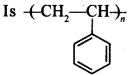
53.
a homopolymer or a copolymer? [AI 2013; NCERT]
Answer/Explanation
Answer:
Explaination: It is homopolymer.
54. Define the term, ‘homopolymerisation’ giving an example. [Delhi 2013(C); 2012]
Answer/Explanation
Answer:
Explaination:
The process in which only one type of monomer undergoes polymerisation is called homopolymerisation, e.g. formation of polythene from ethene is homopolymerisation.
55. How does a homopolymer differ from a copolymer? [AI 2014(C)]
Answer/Explanation
Answer:
Explaination:
Homopolymer is formed by the same monomer unit, whereas copolymer is formed by repeating two different monomers.
56. Identify the type of polymer:
—A—B—B—A—A—A—B—A—
Answer/Explanation
Answer:
Explaination: Copolymer
57. What is the repeating structural unit in polythene polymer? [AI 2011]
Answer/Explanation
Answer:
Explaination: (— CH2—CH2—)n
58. Draw the structure of the monomer for the following polymer: Polypropene [Delhi 2012]
Answer/Explanation
Answer:
Explaination:![]()
59. What is the primary structure feature necessary for a molecule to make it useful in a condensation polymerisation reactioris?
Answer/Explanation
Answer:
Explaination:
Monomers should have more than one functional group (bifunctional).
60. Give one example of a condensation polymer? [AI 2013]
Answer/Explanation
Answer:
Explaination: Nylon 6, 6.
61. Give an example of elastomers.
Answer/Explanation
Answer:
Explaination:
Natural rubber, Buna-S, Buna-N, Neoprene is an elastomer.
62. Based on molecular forces, what type of polymer is neoprene? [AI 2014]
Answer/Explanation
Answer:
Explaination: Elastomer.
63. Which of the following is a fibre?
Nylon, Neoprene, PVC [AI 2014]
Answer/Explanation
Answer:
Explaination: Nylon is fibre.
64. Which factor imparts crystalline nature to a polymer like nylon?
Answer/Explanation
Answer:
Explaination:
It is strong intermolecular forces of attraction like hydrogen bonding which leads to close packing of chains that impart crystalline nature.
65. Which of the following is a thermoplastic polymer?
Bakelite, Polystyrene, Urea-formaldehyde resin.
Answer/Explanation
Answer:
Explaination: Polystyrene.
66. Give an example of thermosetting polymer.
Answer/Explanation
Answer:
Explaination: Bakelite.
67. Out of chain growth polymerisation and step growth polymerisation, in which type will you place the following.
(—A—)m + (—A—)n → (A—)m (—A—)n or —(A—A—)m+n
Answer/Explanation
Answer:
Explaination: Chain growth polymer
68. Write the names of monomers of the following polymer: [AI 2016]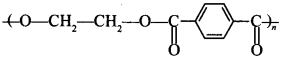
Answer/Explanation
Answer:
Explaination: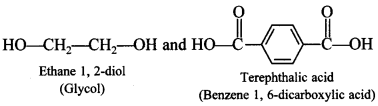
69. What does the part ‘6, 6’ mean in the name nylon-6, 6? [Chennai 2019]
Answer/Explanation
Answer:
Explaination:
It means each monomer of nylon has six carbon atoms. Adipic acid (HOOC—(CH2)4—COOH) and hexamethylenediamine (H2N—(CH2)6—NH2).
70. What is natural rubber?
Answer/Explanation
Answer:
Explaination:
It may be considered as a linear polymer of cis-isoprene (2-methyl-1,3-butadiene) and is also known as cis-1,4-polyisoprene.
71. Give the chemical reaction involved in the formation of Neoprene from chloroprene.
Answer/Explanation
Answer:
Explaination: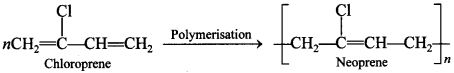
72. Why is the molecular mass of polymer is always expressed as an average?
Answer/Explanation
Answer:
Explaination:
The polymer sample contains chains of varying lengths, therefore, its molecular mass is always expressed as an average.
73. Give an example of biodegradable polymer.
Answer/Explanation
Answer:
Explaination:
Poly P-hydroxybutyrate-co-P-hydroxy valerate (PHBV)
74. Give two uses of polystyrene.
Answer/Explanation
Answer:
Explaination:
It is used as insulator and in manufacture of toys and television cabinets.
75. Can enzyme be called a polymer?
Answer/Explanation
Answer:
Explaination:
Yes. Enzymes are biocatalysts which are proteins, the polymers of amino acids.