Solutions : Notes and Study Materials -pdf
Notes and Study Materials
- Concepts of Solutions
- Master File Solutions
- NCERT Solutions for – Solutions
- NCERT Exemplar Solutions for – Solutions
- Brain Map of Solutions
- Past Many 12th Board Years of Solutions
- NCERT Book
Examples and Exercise
CBSE Class 12th Chemistry Notes: Solutions
Solution is an important chapter of CBSE Class 12 Physical Chemistry. Concepts used in this chapters are further used in other chapters of CBSE Class 12 Chemistry. The topics covered in these notes are: Solutions, types of solutions, Different Methods of Expressing Concentration of Solutions, Solubility and factors affecting solubility, Henry’s law.
Chapters in CBSE Class 12 Physical Chemistry are: Solid State, Solutions, Electrochemistry, Chemical Kinetics and Surface Chemistry.
The weightage of these chapters in CBSE Class 12 Chemistry board exam is 23 Marks (out of 70). Solution is an important chapter of CBSE Class 12 Physical Chemistry.
Introduction
A Solution is a homogenous mixture of two or more components. It is defined by using the terms solute and solvent.
Solvent: The component that is present in largest quantity is called solvent. It determines the physical state of solution.
- Solute: One or more components present in solution other than solvent is called solute.
Binary solutions: Solution consisting of two components only.
Types of Solutions
According to the phase of solvent, a binary solution can be classified in following types:
| Types of Solutions | Solute | Solvent | Examples |
| Gaseous solutions | Gas | Gas | Mixture of O2 and N2 |
| Liquid | Gas | Chloroform mixed with N2 Gas | |
| Solid | Gas | Camphor in nitrogen gas | |
| Liquid Solutions | Gas | Liquid | Oxygen dissolved in water |
| Liquid | Liquid | Ethanol dissolved in water | |
| solid | Liquid | Glucose dissolved in water | |
| Solid Solutions | Gas | solid | Solution of H2 and Pd |
| Liquid | solid | Amalgam of Hg with Na | |
| Solid | solid | Alloy |
In this chapter we are mainly focusing on binary solutions (solution made up of two components) of liquid known as liquid solutions.
CBSE Class 12 Chemistry Syllabus 2016 – 2017
Different Methods of Expressing Concentration of Solutions
The composition of solution is defined in terms of concentration. There are several ways to define concentration of solution as follows:

Intext Questions



Solubility
Solubility of a substance is the maximum amount that can be dissolved in given amount of solvent at specific temperature. Factors affecting the solubility:
– Nature of solute
– Nature of solvent
– Temperature
– Pressure
Solubility of Solid in a Liquid
Nature of solute and solvent:
According to the nature of solute and solvent the solubility of solid in a liquid follow the principle “ Like dissolves like”
If the nature of solute and solvent is same, the intermolecular force of interaction would be same. That helps in solubility of solute in solvent.
Polar solute dissolves in polar solvent. For example: NaCl and sugar dissolves in water. Non-polar solute dissolves in non-polar solvent. For example: Naphthalene and anthracene dissolves in benzene not in water.
Saturated solution: If the concentration of solute in solution remain constant at given set of temperature and pressure is called saturated solution. If we add more solute in it, it would precipitate out.
Un-Saturated solution: If the concentration of solute in solution can increase at given set of temperature and pressure is called un-saturated solution. If we add more solute in it, it would get dissolve and increase the concentration of solution.
Effect of temperature:
The solubility of solute in solvent always follows the dynamic equilibrium.
Solute + Solvent Solution.
It follows the Le Chateliers principle for the change in temperature at dynamic equilibrium.
If the solution is formed by giving heat means dissolution is endothermic. By increasing the temperature, the reaction will proceed in forward direction and solubility of solute increases.
If the heat is released in formation of solute means dissolution is exothermic. By increasing the temperature, the reaction will proceed in backward direction and solubility of solute decreases.
Effect of pressure:
Pressure has no significant effect on solubility of solid in liquid.
Solubility of Gas in a Liquid
Nature of solute and solvent:
Solubility of gas in liquid is also somewhat affected by nature of solute and solvent. Oxygen dissolves only a small extent in water but HCl is highly soluble in water because of polar nature of solute and solvent.
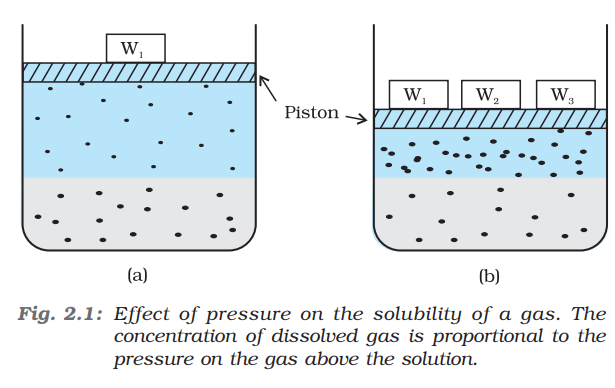
Effect of pressure:
Solubility of gas in liquid is highly affected by temperature and pressure. As the pressure of the gas above the surface of the liquid increases, it increases the solubility of gas in liquid. The quantitative relation of this equation is given by Henry’s Law.
Henry’s Law:
It states that, “At constant temperature, the solubility of gas in a liquid is directly proportional to the partial pressure of the gas present above the surface of the liquid or solution.”
If we consider mole fraction of gas in a solution to measure its solubility then it can be said that, “Mole fraction of gas in a liquid is proportional to the partial pressure of gas above the liquid or solution.”
Now the Henry’s Law can be stated as , “ The partial pressure of a gas in vapour phase (p) is proportional to the mole fraction of gas(x) in solution.”
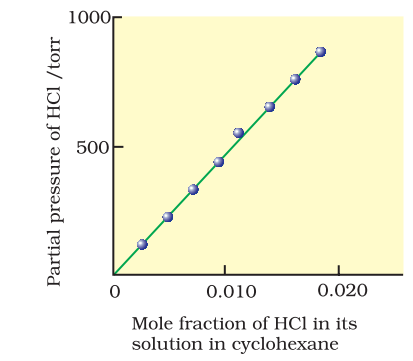
Experimental results for the solubility of HCl gas in cyclohexane at 293 K. The slope of the line is the Henry’s Law constant, KH.
Expression for Henry’s Law:
p ∝ x
p= KH. x
KH is Henry’s Law constant
Important point regarding Henry’s Law:
- Different gases have different KH values at the same temperature. That is KH depends on the nature of gas.
- Higher the value of KH at given pressure, lower is the solubility of gas in given liquid.
- KH value of particular gas increases with increasing temperature. It indicates that solubility of gas decreases with increasing temperature.
Effect of temperature:
Dissolution of gas in a liquid is an exothermic process. As dissolution process involves dynamic equilibrium, it follows Le Chaterlier’s principle. Hence the solubility of gas in liquid, decreases with increase in temperature.
Intext Questions
Question: State the formula relating pressure of a gas with its mole fraction in a liquid solution in contact with it. [CBSE 2005]
Sol: Henry’s Law give the relation between pressure of gas with its mole fraction in a liquid solution.
The required formula is
p= KH. x
Where,
p → Partial pressure of the gas in vapour phase
x → Mole fraction of the gas in solution
KH → Henry’s constant
Question: State Henry’s Law. What is the effect of temperature on the solubility of a gas in a liquid? [CBSE 2014,2012]
Sol: It states that, “At constant temperature, the solubility of gas in a liquid is directly proportional to the partial pressure of the gas present above the surface of the liquid or solution.”
If we consider mole fraction of gas in a solution to measure its solubility then it can be said that, “Mole fraction of gas in a liquid is proportional to the partial pressure of gas above the liquid or solution.”
Now the Henry’s Law can be stated as , “ The partial pressure of a gas in vapour phase (p) is proportional to the mole fraction of gas(x) in solution.”
Expression for Henry’s Law:
p ∝ x
p= KH. x
KH is Henry’s Law constant
The solubility of gases in liquid is dependent on temperature. An increase in temperature results in a decrease the solubility of gas in liquid, while a decrease in temperature results in an increase of solubility of gas in liquid.
Dissolution of gas in a liquid is an exothermic process. As dissolution process involves dynamic equilibrium, it follows Le Chaterlier’s principle. Hence the solubility of gas in liquid, decreases with increase in temperature. Or in other words, when the temperature increases the kinetic energy of the molecules also increase. This will result in more rapid motion of molecules, breaking intermolecular bonds and enable the molecules to escape from the solution. So, the dissolved gas evaporates more readily. That is rise of temperature will cause decrease in the solubility of gases in liquids.
Question: State Henry’s law correlating the pressure of a gas and its solubility in a solvent and mention two applications for the law
Sol: For Henry’s Law follow the text:
Applications of Henry’s Law:
Some of the applications of Henry’s law are given.
(i) To increase the solubility of CO2 in soft drinks and soda water, the bottles are sealed under high pressure.
(ii) The air used for scuba diving is diluted with He to prevent the medical condition known as bends.
In part I of CBSE Class 12 Chemistry: Chapter Notes on Solutions, we have covered basic concepts like definition of solution, types of solutions, various ways to express concentration of solution, solubility, solubility of solid in a liquid and some conceptual questions.
Now we will study the following topics and questions based on them:
- Vapour Pressure of a Liquid Solution
- Vapour Pressure of a Liquid-Liquid Solution
- Vapour Pressure of a Solid-Liquid Solution
- Ideal and Non Ideal Solutions
- Positive & Negative Deviation from Raoult’s law
- Azeotropes
• Minimum boiling azeotropes
• Maximum boiling azeotropes
The notes given below on above mentioned topics are important for CBSE board exams as well as competitive exams.
Vapour Pressure of a Liquid Solution
Liquid solution are formed when solvent is in liquid phase. Solute may be solid, liquid or gas. On the basis of solute, the liquid solution is classified in 3 types as:
(ii) Liquid in liquid
(iii) Gas in liquid
We will discuss the various properties of liquid in liquid solution and solid in liquid solutions.
Vapour Pressure of a Liquid-Liquid Solution
Let us take a binary solution made up of two volatile liquids 1 and 2. When taken in a closed vessel, both the components would evaporate and eventually an equilibrium would be established between vapour phase and the liquid phase . As we know that vapour pressure of liquid is proportional to its mole fraction. The quantitative relationship between the vapour pressure and mole fraction in binary solution is given by Roult’s Law.
Roult’s Law:
It states that for the solutions of volatile liquid, the partial vapour pressure of each component of the solution is directly proportional to its mole fraction present in solution.
For component 1,
p1 ∝ x1
p1 = p1° x1…(1)
p1 = Vapour pressure of component 1 in solution.
p1° = Vapour pressure of pure liquid component 1at the same temperature
x1 = Mole fraction of component 1 in solution.
Similarly for component 2 :
P2 = p2° x2 …(2)
According to Dalton’s Law of partial pressure, The total pressure (ptotal ) over the solution phase in the container will be the sum of partial pressure of the components in solution. If the solution is made up of two volatile liquids then total pressure above the solution is:
ptotal = p1 + p2 …(3)
ptotal = Total pressure over the solution phase
p1 = Vapour pressure of component 1 in solution
p2 = Vapour pressure of component 1 in solution
Substituting the values of p1 and p2 in eq…(3) we get:
ptotal = p1° x1 + p2° x2 …(4)
As, total mole fraction of components in any solution is 1. Therefore,
x1 + x2 = 1
x1 =(1 – x2)…(5)
Putting the value of x1 in eq (4) we get:
ptotal = p1°(1- x2) + p2° x2
ptotal = p1° – p1° x2 + p2° x2
ptotal = p1° +( p2°– p1°) x2 …(6)
The equation …(6) is known as the mathematical expression for Roult’s Law for the solution made up of two volatile liquids.
From this equation …(6) we can conclude the following points :
- ptotal can be related to mole fraction of any of the one component.
- ptotal varies linearly with x2.
- ptotal increases or decreases with increase of x1 .Depending on the vapour pressures of the pure components 1 and 2, total vapour pressure over the solution decreases or increases with the increase of the mole fraction of component 1
This can be represents on graph as follows assuming p1° < p2°:
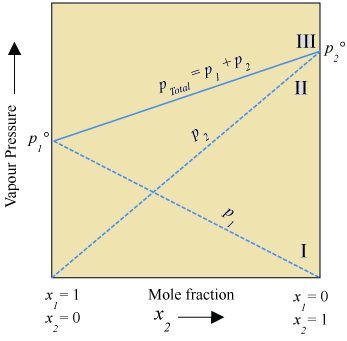
Conclusion from graph:
- Plot of p1° vs x1 is linear
- Plot of p2° vs x2 is linear
- Maximum value of ptotal = p2°
- Minimum value of ptotal = p1°
Composition of vapour phase at equilibrium is determined by using Dalton’s Law. Let y1 and y2 be the mole fraction of liquid 1 and 2 in solution then partial vapour pressure of each component is written as:
p1 = y1 ptotal… (7)
Equation …(7) gives you the value of partial vapour pressure of each component in vapour phase.
Vapour Pressure of a Solid-Liquid Solution
When a non-volatile solid is added to the solvent to form a solution, then the vapour pressure of solution is found lower than vapour pressure of the pure solvent at same temperature. The decrease in the vapour pressure of the solution is solely depends on the quantity of non-volatile solute present in solution.
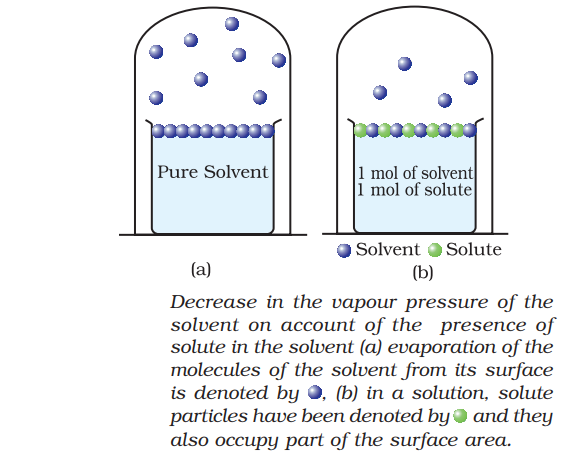
The vapour pressure of such solution is given by using general equation of Roult’s Law.
Assume that water is component 1 and non-volatile component is component 2, then vapour pressure of the solution will be equal to the vapour pressure of solvent in solution.
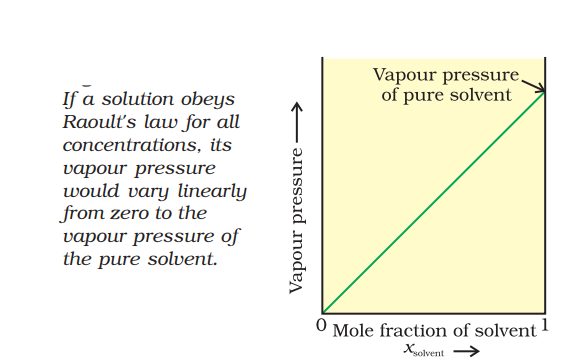
Vapour pressure of solvent p1 is proportional to its mole fraction in solution and given as :
p1 ∝ x1
p1 = p1° x1…(1)
p1 = Vapour pressure of component 1 in solution.
p1° = Vapour pressure of pure liquid component 1at the same temperature
x1 = Mole fraction of component 1 in solution.
Ideal and Non Ideal Solutions
Liquid-liquid solutions can be classified as ideal and non-ideal on the basis of certain properties.
Ideal Solution
- Obeys the Roult’s Law over the entire range of concentration.
- ΔHmixing = 0
- ΔVmixing = 0
- Example : solution of n-hexane and n-heptane, solution of bromoethane or chloroethane.
It can be summarised as: If a Solution formed by mixing the two components A and B, in which intermolecular force of attraction between A and B (A‒B) is nearly equal to intermolecular force of attraction between pure components (A‒A and B‒B) then no heat would be evolved or absorbed in forming the solution. Also volume of the solution will be equal to the total volume of the individual component taken to form the solution.
Non-Ideal Solution
- Does not obey the Roult’s Law over the entire range of concentration.
- ΔHmixing ≠ 0
- ΔVmixing ≠ 0
- Example: Solution of chloroform and acetone
It can be summarised as: If a Solution formed by mixing the two components A and B , in which intermolecular force of attraction between A and B (A‒B) is not equal to intermolecular force of attraction between pure components (A‒A and B‒B). This new interaction (A‒B) is either less than or more than the interaction of the pure components (A‒A and B‒B). This leads to the positive or negative deviations from Roult’s Law.
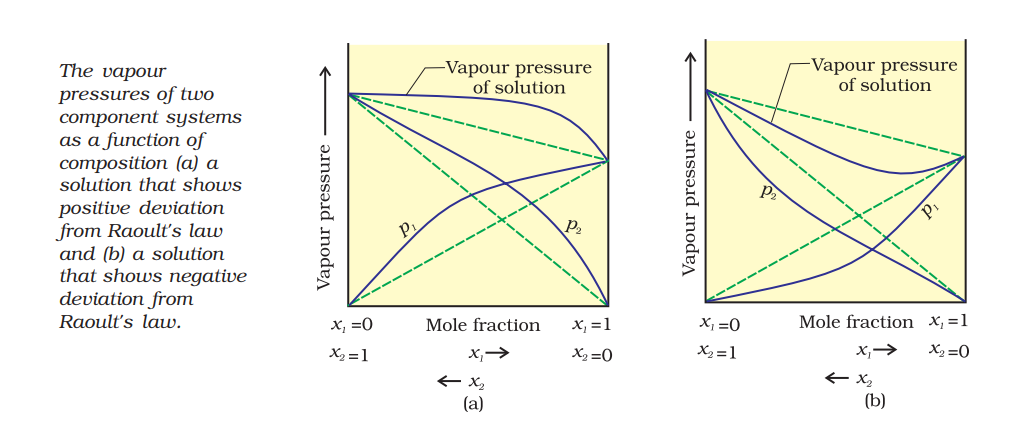
Positive Deviation from Roult’s Law
- The vapour pressure of solution formed by mixing two components is higher than predicted from Roult’s Law
- The new intermolecular interactions formed by mixing the component A and B (A‒B) are weaker than the intermolecular interactions of pure component (A‒A and A‒B).
- The cause for these deviations lie in the nature of interactions at the molecular level. In case of positive deviation from Raoult’s law, A-B
interactions are weaker than those between A-A or B-B, i.e., in this case the intermolecular attractive forces between the solute-solvent molecules
are weaker than those between the solute-solute and solvent-solvent molecules. This means that in such solutions, molecules of A (or B) will
find it easier to escape than in pure state. This will increase the vapour pressure and result in positive deviation - Example: mixture of ethanol and acetone, solution of carbon disulphide and acetone.
Negative Deviation from Roult’s Law
- The vapour pressure of solution formed by mixing two components is lower than predicted from Roult’s Law
- The new intermolecular interactions formed by mixing the component A and B (A-B) are stronger than the intermolecular interactions of pure component (A-A and A-B)
- Example: solution of phenol and aniline, chloroform and acetone.
Graph representing the positive and negative deviation:
Azeotropes
These are mixture of two liquids having same composition in liquid as well as vapour phase and boil at the constant temperature. This liquid mixture cannot be separated into pure component even on fractional distillation.
Types of Azeotropes
Minimum boiling azeotrope:
The solution which show large positive deviation from Raoult’s Law. Example: solution of 95% ethanol in water. Once this composition, known as azeotrope
composition, has been achieved, the liquid and vapour have the same composition, and no further separation occurs
Maximum boiling azeotropes:
The solution which show large negative deviation from Raoult’s Law. Example: solution of 68% nitric acid and 32% water by mass.
Intext Questions:
Question 1: What type of deviation is shown by a mixture of ethanol and acetone? Give reason. [CBSE 2014]
Solution 1: A mixture of ethanol and acetone shows positive deviation from Raoult’s Law. The new intermolecular interaction formed by mixing the component ethanol and acetone is weaker than the intermolecular interactions of pure component pure ethanol. Pure ethanol possesses hydrogen bonding. When acetone is mixed, the molecule of acetone takes the space in between the molecules of ethanol which results in breaking of some of the hydrogen bonds. Due to weakening of interactions, the vapour pressure of solution formed by mixing ethanol and acetone is higher than predicted from Roult’s Law. Hence the solution shows positive deviation from Raoult’s law.
Question 2: State Raoult’s law for the solution containing volatile components. What is the similarity between Raoult’s law and Henry’s law? [CBSE 2014]
Solution 2: Raoult’s law : It states that for the solutions of volatile liquids, the partial vapour pressure of each component of the solution is directly proportional to its mole fraction present in solution.
For component 1,
p1 ∝ x1
p1 = p1° x1…(1)
p1 = Vapour pressure of component 1 in solution.
p1° = Vapour pressure of pure liquid component 1at the same temperature
x1 = Mole fraction of component 1 in solution.
Similarly for component 2,
P2 = p2° x2 …(2)
Similarity between Raoult’s law and Henry’s law:
Both laws state that the partial pressure of the volatile component is directly proportional to its mole fraction in the solution. In case of Roult’s law it is liquid and in case of Henry’s law it is gas.
Equation for Roult’s law:
p = p° x
Equation for Henry’s law :
p= KH. x
Question 3: Some liquids on mixing form ‘azeotropes’. What are ‘azeotropes’? [CBSE 2014]
Solution 3: Azeotropes: These are mixture of two liquids having same composition in liquid as well as vapour phase and boil at the constant temperature. This liquid mixture cannot be separated into pure component even on fractional distillation. Azeotropic solution boils at constant temperature, regardless of difference in boiling points of respective components.
Question 4: Define an ideal solution and write one of its characteristics. [CBSE 2014, 2012]
Solution 4: The solutions that obey Raoult’s law over the entire range of concentration are called ideal solutions. Examples: n-hexane and n-heptane
Characteristics of ideal solutions:
Enthalpy of mixing (ΔmixH) of the pure components to form the solution is zero. Volume of mixing (ΔmixV) is also equal to zero.
Question 5: State Raoult’s law for a solution containing volatile components. How does Raoult’s law become a special case of Henry’s law? [CBSE 2013]
Solution 5: Similar to answer 2
Raoult’s Law as a Special Case of Henry’s Law
According to Raoult’s law, the vapour pressure of a volatile component in a given solution is p = p° x
According to Henry’s law, the partial vapour pressure of a gas(volatile component) in a liquid is p = KH. x
It can be observed that in both the equations, the partial vapour pressure of the volatile component varies directly with its mole fraction. The only difference is the proportionality constants which is KH in Henry’s law and p° in Roult’s Law. Thus Raoult’s law becomes a special case of Henry’s law in which KH is equal to p°.
Question 6: Define the following terms: (i) Ideal solution (ii) Azeotrope [CBSE 2013]
Solution 6: (i) Ideal solution ; same as answer 4
(ii) Azeotrope; same as answer 3
Question 7: Non-ideal solutions exhibit either positive or negative deviations from Raoult’s law. What are these deviations and why are they caused? Explain with one example for each type. [CBSE 2010]
Solution 7: Follow the above theory.
CBSE Class 12 Chemistry Important Questions Chapter 2 – Solutions
1 Mark Questions
1. Define the term –solubility?
Ans. The maximum amount of a substance that can be dissolved in a specified amount of solvent is called its solubility.
2. What is the effect of pressure on solubility of a gas?
Ans. The solubility of a gas increases with increases of pressure.
3. State Henry’s Law.
Ans. Henry’s Law states that at a constant temperature the solubility of a gas in a liquid is directly proportional to the pressure of the gas.
4. State Raoult’s Law.
Ans. Raoult’s Law states that for a solution of volatile liquids, the partial vapour pressure of each component in the solution is directly proportional to its mole fraction.
5. What are the factors on which vapour pressure depends?
Ans. The factors on which vapour pressure depends are –
1) Temperature of the liquid. 2) Nature of the liquid.
6. The vapour pressure of solvent gets lowered, when a non- volatile solute is
added to it. Why?
Ans. When a non-volatile solute is added to a solvent, the surface area for escape of solvent molecules decreases and vapour pressure gets lowered.
7. Name two ways by which vapour pressure of a liquid can be lowered.
Ans. The two ways by which vapour pressure can be lowered are –
1) By decreasing the temperature.
2) By adding a non- volatile solute.
8. Define‘solution’?
Ans. Solutions are homogeneous mixtures of two or more than two components.
9. Define the following terms : (a) Molality (b) Molarity
Ans. (a) Molality is defined as the number of moles of the solute per kilogram of solvent.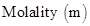

(b) Molarity (M) = Number of moles of solute dissolved in one litre of solution.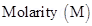
 Molarity (M)
Molarity (M)
10. How does change in temperature changes the molarity and molality values?
Ans. As the temperature increases, volume increases and molarity decreases whereas molality does not change with any change in temperature.
11. Define the term colligative properties?
Ans. The properties which depends upon amount of solute and not upon the nature of solute are called colligative properties.
12. What are the possible deviations from ideal behaviors?
Ans. There are two types of deviation from ideal behaviour – positive and negative deviations.
13. Give one example of each deviation?
Ans. Positive deviation – ethanol and acetone.
Negative deviation – chloroform and acetone.
14. At 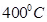 , the vapour pressure of water is 55.3 mm Hg .Calculate the vapour pressure at the same temperature over 10% aqueous solution of urea
, the vapour pressure of water is 55.3 mm Hg .Calculate the vapour pressure at the same temperature over 10% aqueous solution of urea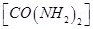 ?
?
Ans. 53.48 mm Hg.
15. How much urea (molar mass 60 g/mol) should be dissolved in 50g of water so that its vapour pressure at room temperature is reduced by 25%?
Ans. 41.7 g .
16. Why is the boiling point elevated when a non – volatile solute is dissolved in a liquid?
Ans. When a non – volatile solute is added the vapour pressure decreases and the solution is heated to a higher temperature, increasing the boiling point.
17. How is boiling point changed when mass of solvent is doubled?
Ans. 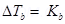
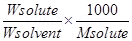
= when the amount of solvent is doubled, ∆Tb is halved.
18. What happens when red blood cells are placed in 0.1% NaCl solution?
Ans. Water from NaCl solution passes into cells &they swell. Finally they will burst.
19. How is osmotic pressure of a solution related to its concentration?
Ans. Osmotic pressure, π = CRT
C = concentration ,R = gas constant.
T= temperature
20. Calculate the osmotic pressure of 0.25 M solution of urea at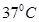 . R = 0.083 L bar/mol/k.
. R = 0.083 L bar/mol/k.
Ans. T = 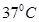 = 310k
= 310k =
=  RT
RT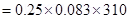 = 6.43 bar.
= 6.43 bar.
21. An aqueous solution of glucose, 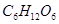 has osmotic pressure of 2.72 atm at 298k. How many moles of glucose were dissolved per litre of solution?
has osmotic pressure of 2.72 atm at 298k. How many moles of glucose were dissolved per litre of solution?
Ans. 0.111 mol.
22. When does the measurement of colligative property leads to abnormal molecular mass?
Ans. When the solute undergoes either association or disassociation abnormal molar mass is obtained.
23. When is the value of i less then unity?
Ans. When the solute under goes association in solution, I is less then unity.
24. The molecular mass of a solute is 120 g/mol and van’t Hoff factor is 4. What is its abnormal molecular mass?
Ans. Abnormal molecular mass = 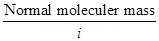
= 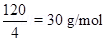 .
.
Solutions MCQ Chapter 2
Solution is a homogeneous mixture of two or more substances whose compositions can be varied continuously upto the limit of solubility.
Below are some of the very important NCERT Solutions MCQ Class 12 Chemistry Chapter 2 with Answers. These Solutions MCQs have been prepared by expert teachers and subject experts based on the latest syllabus and pattern of CBSE examination. We have given these Solutions Class 12 Chemistry MCQs Questions with Answers to help students understand the concept.
Solutions Class 12 Chemistry MCQs
1. The molality of pure water is
(a) 55.5
(b) 50.5
(c) 18
(d) 60.5
Answer/Explanation
Answer: a
Explaination:
(a) Molality = Number of moles/kg of solvent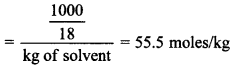
2. The number of moles of NaCl in 3 litres of 3M solution is
(a) 1
(b) 3
(c) 9
(d) 27
Answer/Explanation
Answer: c
Explaination:
(c) 3M solution means 3 moles in 1 litre.
∴ 9 moles in 3 litre.
3. 4L of 0.02 M aqueous solution of NaCl was diluted by adding one litre of water. The molality of the resultant solution is ________ .[NCERT Exemplar]
(a) 0.004
(b) 0.008
(c) 0.012
(d) 0.016
Answer/Explanation
Answer: d
Explaination:
(d) M1V1 = M2V2
0.02 × 4 = M2 × (4 + 1)
⇒ M2 = \(\frac{0.08}{5}\) = 0.016
4. Low concentration of oxygen in the blood and tissues of people living at high altitude is due to _________ .[NCERT Exemplar]
(a) low temperature
(b) low atmospheric pressure
(c) high atmospheric pressure
(d) both low temperature and high atmospheric pressure
Answer/Explanation
Answer: b
Explaination:
(b) Low atmospheric pressure will lead to low concentration of oxygen blood.
5. Considering the formation, breaking and strength of hydrogen bond, predict which of the following mixtures will show a positive deviation from Raoult’s law? [NCERT Exemplar]
(a) Methanol and acetone.
(b) Chloroform and acetone.
(c) Nitric acid and water.
(d) Phenol and aniline.
Answer/Explanation
Answer: a
Explaination:
(a) CH3OH and acetone, on mixing force of attraction will decrease.
6. Which of the following aqueous solutions should have the highest boiling point? [NCERT Exemplar]
(a) 1.0 M NaOH
(b) 1.0 M Na2SO4
(c) 1.0 M NH4NO3
(d) 1.0 M KNO3
Answer/Explanation
Answer: b
Explaination:
(b) Because i = 3, ∆Tb ∝ i, Boiling point ∝ ∆Tb.
7. In comparison to a 0.01 M solution of glucose, the depression in freezing point of a 0.01 M MgCl2 solution is _________ . [NCERT Exemplar]
(a) the same
(b) about twice
(c) about three times
(d) about six times
Answer/Explanation
Answer: c
Explaination:
(c) It will be nearly 3 times because number of particles in MgCl2 → Mg2+ + 2Cl– are thrice than glucose.
8. An unripe mango placed in a concentrated salt solution to prepare pickle, shrivels because _________ . [NCERT Exemplar]
(a) it gains water due to osmosis.
(b) it loses water due to reverse osmosis.
(c) it gains water due to reverse osmosis.
(d) it loses water due to osmosis.
Answer/Explanation
Answer: b
Explaination:
(d) Concentrated salt solution is hypertonic solution, therefore, fluids inside mango will come out and it shrivels.
9. Which of the following statements is false? [NCERT Exemplar]
(а) Two different solutions of sucrose of same molality prepared in different solvents will have the same depression in freezing point.
(b) The osmotic pressure of a solution is given by the equation π = CRT (where C is the molarity of the solution).
(c) Decreasing order of osmotic pressure for 0.01 M aqueous solutions of barium chloride, potassium chloride, acetic acid and sucrose is
BaCl2 > KCl > CH3COOH > sucrose.
(d) According to Raoult’s law, the vapour pressure exerted by a volatile component of a solution is directly proportional to its mole fraction in the solution.
Answer/Explanation
Answer:
Explaination:
(a) is false because ∆Tf will depend upon nature of solvent and their Kf.
10. The value of Henry’s constant KH is _________ .[NCERT Exemplar]
(а) greater for gases with higher solubility.
(b) greater for gases with lower solubility.
(c) constant for all gases.
(d) not related to the solubility of gases.
Answer/Explanation
Answer:
Explaination:
(b) Higher the value of KH, lower will be solubility.
11. Consider the figure and mark the correct option. [NCERT Exemplar]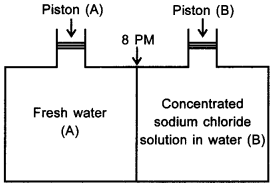
(a) water will move from side (A) to side (B) if a pressure lower than osmotic pressure is applied on piston (B).
(b) water will move from side (B) to side (A) if a pressure greater than osmotic , pressure is applied on piston (B).
(c) water will move from side (B) to side (A) if a pressure equal to osmotic pressure is applied on piston (B).
(d) water will move from side (A) to side (B) if pressure equal to osmotic pressure is applied on piston (A).
Answer/Explanation
Answer: b
Explaination:
(b) Reverse osmosis will take place.
12. We have three aqueous solutions of NaCl labelled as ‘A’, ‘B’ and ‘C’ with concentrations 0.1M, 0.01M and 0.001M, respectively. The value of van’t Hoff factor for these solutions will be in the order __________ . [NCERT Exemplar]
(a) iA < iB < iC
(b) iA > iB > iC
(c) iA = iB = iC
(d) iA < iB > iC
Answer/Explanation
Answer: c
Explaination:
(c) van’t Hoff factor (i) does not depend upon concentration.
13. A solution containing 10 g per dm3 of urea (molar mass 60 g mol-1) is isotonic with 5% solution of non-volatile solute, MB of solute is
(a) 300 g mol-1
(b) 350 g mol-1
(c) 200 g mol-1
(d) 250 g mol-1
Answer/Explanation
Answer: a
Explaination:
(a) \(\frac{1}{60}=\frac{5}{x}\)
⇒ x = 300 g mol-1
1000 cm3 contains 10 g
100 cm3 contains 1 g, i.e., 1%.
14. Cone. H2SO4 is 98 % H2SO4 by mass has d = 1.84 g cm-3. Volume of acid required to make one litre of 0.1 M H2SO4 is
(a) 5.55 mL
(b) 10 mL
(c) 20 mL
(d) 30 mL
Answer/Explanation
Answer: a
Explaination: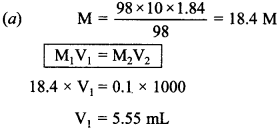
15. What is mole fraction of solute in 1.00 m aqueous solution?
(a) 0.0354
(b) 0.0177
(c) 0.177
(d) 1.770
Answer/Explanation
Answer: b
Explaination: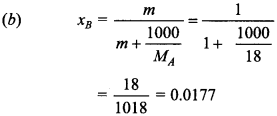
16. When 1 mole of benzene is mixed with 1 mole of toluene (vapour pressure of benzene – 12.8 kPa, Toluene = 3.85 kPa)
(a) The vapour will contain equal amount of benzene and toluene.
(b) Not enough information is given for prediction.
(c) The vapour will contain a higher percentage of benzene.
(d) The vapour will contain higher percentage of toluene.
Answer/Explanation
Answer: c
Explaination:
(c) It is because benzene has high vapour pressure, it will form more vapours as compared to toluene.
17. At 100°C, the vapour pressure of a solution of 6.5 g of solute in 100 g of water is 732 mm. If Kb is 0.52 K/m, the boiling point of solution will be [HOTS]
(a) 102°C
(b) 103°C
(c) 101 °C
(d) 100°C
Answer/Explanation
Answer: c
Explaination: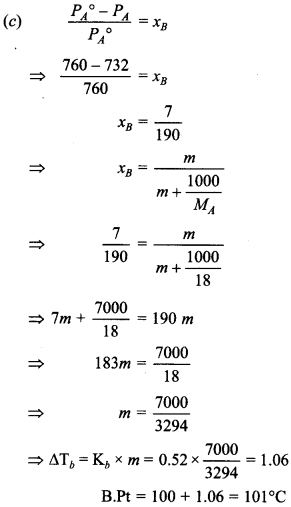
18. Which of the following is incorrect for an ideal solution?
(a) ∆Hmix =0
(b) ∆Vmix = 0
(c) ∆P = Pobs – Pcalculated = 0
(d) ∆Gmix = 0
Answer/Explanation
Answer: d
Explaination:
(d) ∆G cannot be equal to zero because mixing does not lead to equilibrium.
19. If molality of dilute solution is doubled, the value of molal depression constant (Kf) will be
(a) halved
(b) tripled
(c) unchanged
(d) doubled
Answer/Explanation
Answer: c
Explaination:
(c) Kf does not depend upon ‘mMt depends upon nature of solvent.
20. The temperature at which 10% aqueous solution of (W/V) of glucose will show the osmotic pressure of 16.4 atom is (R = 0.082 L atom K-1 mol-1)
(a) 360°C
(b) 180 K
(c) 300 K
(d) 360 K
Answer/Explanation
Answer: d
Explaination:
21. Which has the highest freezing point?
(a) 1 M glucose
(b) 1 M NaCl
(c) 1 M CaCl2
(d) 1 M AlF3
Answer/Explanation
Answer: a
Explaination:
(a) 1 M glucose solution has highest freezing point because it has lowest ∆Tf because i = l.
Note: In the following questions two or more options may be correct. (Q.22 to Q.24)
22. Which of the following is correct. [NCERT Exemplar]
(a) KJJ increases with increase in temperature (KH is Henry’s law constant).
(b) Solubility of gas in liquid decreases with increases in temperature.
(c) KJJ decreases with increase in temperature.
(d) Solubility of gas in liquid increases with increase in temperature.
Answer/Explanation
Answer: a
Explaination:
(a) and (b) are correct pgas = KH × xgas
KH increases with increase in temperature, decreases, i.e., solubility of gas in liquid decreases with increase in temperature.
23. Benzoic acid, when dissolved in benzene, which of the following is correct. [NCERT Exemplar]
(a) The benzoic acid will undergo dissociation.
(b) The benzoic acid will undergo association.
(c) Observed molar mass of benzoic acid in benzene will less than normal molar mass.
(d) Observed molar mass of benzoic acid in benzene is more than normal molar mass.
Answer/Explanation
Answer: b
Explaination: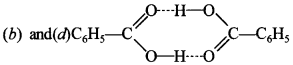
Molar mass will be nearly double due to dimerisation.
24. Relative lowering of vapour pressure is a colligative property because ________ . [NCERT Exemplar]
(а) It depends on the concentration of a non electrolyte solute in solution and does not depend on the nature of the solute molecules.
(b) It depends on number of particles of electrolyte solute in solution and does not depend on the nature of the solute particles.
(c) It depends on the concentration of a non electrolyte solute in solution as well as on the nature of the solute molecules.
(d) It depends on the concentration of an electrolyte or nonelectrolyte solute in solution as well as on the nature of solute molecules.
Answer/Explanation
Answer: a
Explaination:
(a) and (b) colligative property depends upon number of particles of solute in both electrolyte and non-electrolyte.
25. Match the items given in Column I with the type of solutions given in Column II. [NCERT Exemplar]
Answer/Explanation
Answer:
Explaination:
(a) – (v)
(b) – (iii)
(c) – (iv)
(d) – (ii)
(e) – (i)
26. In the following question a statement of assertion followed by a statement of reason is given. Choose the correct answer out of the following choices.
(a) Assertion and reason both are correct statements and reason is correct explanation for assertion.
(b) Assertion and reason both are correct statements but reason is not correct explanation for assertion.
(c) Assertion is correct statement but reason is wrong statement.
(d) Assertion and reason both are incorrect statements.
(e) Assertion is wrong statement but reason is correct statement.
Assertion: When methyl alcohol is added to water, boiling point of water increases. Reason: When a volatile solute is added to a volatile solvent elevation in boiling point is observed. [NCERT Exemplar]
Answer/Explanation
Answer: d
Explaination:
(d) Assertion and reason both are incorrect statements.
27. Mole fraction of glycerine C3H5(OH)3 in solution containing 36 g of water and 46 g of glycerine is
(a) 0.46
(b) 0.40
(c) 0.20
(d) 0.36
Answer
Answer: c
28. Out of molality (m), molarity (M), formality (F) and mole fraction (x), those which are independent of temperature are
(a) M, m
(b) F, x
(c) m, x
(d) M, x
Answer
Answer: c
29. Which of the following condition is not satisfied by an ideal solution?
(a) ΔHmixing = 0
(b) ΔVmixing = 0
(c) Raoult’s Law is obeyed
(d) Formation of an azeotropic mixture
Answer
Answer: d
30. The boiling point of an azeotropic mixture of water and ethanol is less than that of water and ethanol. The mixture shows
(a) no deviation from Raoult’s Law.
(b) positive deviation from Raoult’s Law.
(c) negative deviation from Raoult’s Law.
(d) that the solution is unsaturated.
Answer
Answer: b
31. Which has the lowest boiling point at 1 atm pressure?
(a) 0.1 M KCl
(b) 0.1 M Urea
(c) 0.1 M CaCl2
(d) 0.1 M A1Cl3
Answer
Answer: b
32. Osmotic pressure of a solution is 0.0821 atm at a temperature of 300 K. The concentration in moles/litre will be
(a) 0.33
(b) 0.666
(c) 0.3 × 10-2
(d) 3
Answer
Answer: c
33. People add sodium chloride to water while boiling eggs. This is to
(a) decrease the boiling point.
(b) increase the boiling point.
(c) prevent the breaking of eggs.
(d) make eggs tasty.
Answer
Answer: b
34. The van’t Hoff factor (i) accounts for
(a) degree of solubilisation of solute.
(b) the extent of dissociation of solute.
(c) the extent of dissolution of solute.
(d) the degree of decomposition of solution.
Answer
Answer: b
35. Which relationship is not correct?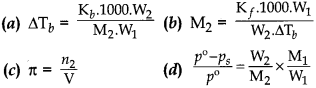
Answer
Answer: b
36. The molal elevation constant depends upon
(a) nature of solute.
(b) nature of the solvent.
(c) vapour pressure of the solution.
(d) enthalpy change.
Answer
Answer: b
37. The process used for desalination of water is _________ .
Answer/Explanation
Answer:
Explaination: reverse osmosis
38. Vapour pressure is _________ proportional to temperature.
Answer/Explanation
Answer:
Explaination: directly
39. Ethylene glycol is used as _________ .
Answer/Explanation
Answer:
Explaination: antifreeze
40. All intravenous injections must be isotonic with body fluids. [True/False]
Answer/Explanation
Answer:
Explaination: True.
41. Diabetic patients are likely to have high blood pressure. [True/False]
Answer/Explanation
Answer:
Explaination: True.
42. Common salt is non-electrolyte. [True/False]
Answer/Explanation
Answer:
Explaination: False, common salt is electrolyte.
43. State the main advantage of molality over molarity as the unit of concentration. [DoE]
Answer/Explanation
Answer:
Explaination:
Molality is more accurate than molarity because molality does not depend on temperature as mass does not change with temperature.
44. Define molality in terms of elevation in boiling point.
Answer/Explanation
Answer:
Explaination:
Molality is defined as the ratio of elevation in boiling point and KA (molal elevation constant).
45. State Raoulf s law for a solution containing volatile components. [Foreign 2012,11]
Answer/Explanation
Answer:
Explaination:
The vapour pressure of each component is directly proportional to the mole fraction of each component.
\(P_{A}=P_{A}^{\circ} x_{A}, P_{B}=P_{B}^{\circ} x_{B}\)
where PA and PB = Vapour pressure of components ‘A’ and ‘B’.
\(p_{A}^{\circ} \text { and } p_{B}^{\circ}\) = Vapour pressure of pure components ‘A’ and ‘B.
xA and xB = Mole fractions of ‘A’ and ‘B’.
46. Two liquids A and 5 boil at 145 °C and 190 °C respectively. Which of them has a higher vapour pressure at 80 °C?
Answer/Explanation
Answer:
Explaination:
‘A’ because lower the boiling point, higher will be vapour pressure.
47. What are the values of AH and AV for an ideal solution of two liquids?
Answer/Explanation
Answer:
Explaination:
∆H = 0, ∆V = 0 for an ideal solution of two liquids.
48. Give reason when 30 mL of ethyl alcohol and 30 mL of water are mixed, the volume of resulting solution is more than 60 mL.
Answer/Explanation
Answer:
Explaination:
It is because forces of attraction between ethyl alcohol and water are less than ethanol-ethanol and water-water. It shows positive deviation.
49. 10 mL of liquid A was mixed with 10 mL of liquid B. The volume of the resulting solution was found to be 19.9 mL. What do you conclude? [Delhi 2018(C) Blind]
Answer/Explanation
Answer:
Explaination:
It means solution shows -ve deviation from Raoult’s law due to increase in force of attraction, volume decreases, e.g. chloroform and acetone.
50. What are azeotropes? Give an example. [Delhi 2014]
Answer/Explanation
Answer:
Explaination:
Azeotropes are constant boiling mixtures which distill out unchanged in their composition, e.g. ethanol and water.
51. Define Ebullioscopic constant or molal elevation constant. [Foreign 2012; DoE]
Answer/Explanation
Answer:
Explaination:
Molal Elevation Constant (Ebullioscopic Constant): It is equal to elevation in boiling point of 1 molal solution, i.e. 1 mole of solute is dissolved in 1 kg of solvent. It is also called ebullioscopic constant. The units of Kb is K/m or °C/m or K kg mol-1, where ‘m’ is molality.
52. Calculate the freezing point of a solution containing 60 g of glucose (molar mass 180 g mol-1) in 250 g of water. [Kf for water = 1.86 K kg mol-1] [CBSE 2018]
Answer/Explanation
Answer:
Explaination: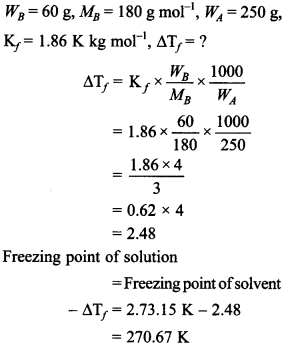
53. What is anti-freeze?
Answer/Explanation
Answer:
Explaination:
Anti-freeze is a substance which is added to solvent to lower its freezing point. It is used in car radiators to lower the freezing point of water, e.g. ethylene glycol.
54. What is ‘semipermeable’ membrane?
Answer/Explanation
Answer:
Explaination:
It is the membrane which has sub- microscopic pores through which small solvent molecules like water can pass but bigger solute particles cannot pass.
55. Define osmotic pressure. [AI 2013; DoE]
Answer/Explanation
Answer:
Explaination:
It is the extra pressure which must be applied on solution side so as to prevent the flow of solvent molecules from solution through semipermeable membrane.
56. Measurement of which colligative property is preferred for determination of molar mass 112 la^etAen- Objective Type Questions of macromolecules such as proteins and polymers. [Foreign 2013,17; DoE; CBSE 2016,18]
Answer/Explanation
Answer:
Explaination: Osmotic pressure.
57. A person suffering from high blood pressure should take less common salt, why? [DoE]
Answer/Explanation
Answer:
Explaination:
Common salt contains Na+ and Cl– which increase osmotic pressure of blood, therefore, increase blood pressure.
58. What is meant by ‘reverse osmosis’? [AI 2011; DoE]
Answer/Explanation
Answer:
Explaination:
Reverse Osmosis: If extra pressure is applied on the solution side and exceeds the osmotic pressure, the osmosis can be reversed. That is, pure water can be forced out of the solution to pass through the pores of the membrane in the opposite direction. This is called reverse osmosis.
59. Give an example of a material used for making semipermeable membrane for carrying out reverse osmosis.
Answer/Explanation
Answer:
Explaination: Cellulose acetate.
60. A 10% solution of urea is isotonic with 20% solution of V at same temperature. Calculate molecular weight of x.
Answer/Explanation
Answer:
Explaination:
61. Why do doctors advise gargles by saline water in case of sore throat? [HOTS]
Answer/Explanation
Answer:
Explaination:
Saline water is hypertonic solution, therefore, fluids causing irritation in throat will come out.
62. When outer shell of two eggs are removed, one of the eggs is placed in pure water and other is placed in saturated solution of NaCl, what will be observed and why? [HOTS]
Answer/Explanation
Answer:
Explaination:
The egg placed in pure water will swell, whereas the egg placed in saturated solution of NaCl will shrink.
63. Of 0.1 molal solutions of glucose and potassium chloride respectively, which one will have a higher boiling point? [CBSE 2018]
Answer/Explanation
Answer:
Explaination:
0.1 molal KC1 solution will have higher boiling point because KC1 dissociates into K+ and CF ions, therefore, number of particles will be doubled.
64. What is expected value of van’t Hoff factor for K3[Fe(CN)6].
Answer/Explanation
Answer:
Explaination:![]()
65. What would be the value of van’t Hoff factor for a dilute solution of K2SO4 in water?
Answer/Explanation
Answer:
Explaination:![]()
66. In the determination of molar mass of A+ B– using a colligative property, what may be the value of van’t Hoff factor if the solute is 50% dissociated?
Answer/Explanation
Answer:
Explaination: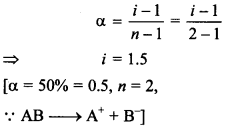
67. What possible value of ‘i’ will it have if solute molecules undergo association in solution? [AI 2014(C)]
Answer/Explanation
Answer:
Explaination:
i < 1, if solute molecules undergo association.
68. Predict whether van’t Hoff factor, (i) is less than one or greater than one in the following:
(i) CH3COOH dissolved in water.
(ii) CH3COOH dissolved in benzene. [Chennai 2019]
Answer/Explanation
Answer:
Explaination:
(i) It will be more than 1 because
CH3COOH will dissociate into CH3COO– and H+.
(ii) CH3COOH dissolved in benzene will form dimer i.e. undergo association, therefore, i < 1.
69. Why is osmotic pressure of l M KCl is higher than that of l M urea solution?
Answer/Explanation
Answer:
Explaination:
l M KCl solution dissociates into K+ and Cl–, therefore, its osmotic pressure is higher than that of l M solution of urea which does not dissociate.
70. State how does osmotic pressure vary with temperature.
Answer/Explanation
Answer:
Explaination:
Osmotic pressure increases with increase in temperature.
Solutions MCQ (1-10)
- Number of components in a binary solution is?
- 2
- 4
- 3
- 1
Answer/Explanation
1 . (1)
A binary solution is made by mixing two different species.
- Which among the following does not have any unit?
- Molality
- Molarity
- Mole Fraction
- None of the above
Answer/Explanation
2. (3)
Mole fraction is the ratio of the number of moles of a particular component to the total number of moles of the solution. Therefore, it is unitless.
- Vapour pressure of dilute aqueous solution of glucose is 730 mm of mercury at 373 K. The mole fraction of solute is?
- 1/10
- 3/76
- 1/76
- 3/28
Answer/Explanation
3. (2)
Vapour pressure of water = 760 mm
Vapour pressure of urea = 730 mm
We know,
(P°-P)/P° = Mole fraction = (760-730)/760 = 3/76
- With increase in pressure,
- Solubility of solids decreases
- Solubility of solids is not affected
- Solubility of gases increases
- Solubility of gases decreases
Answer/Explanation
4. (3)
According to Henry’s law, solubility of gas is directly proportional to the pressure of the gas present above the solution’s surface.
- Which of the following substances dissolves in water?
- C6H6
- C4H10
- CH4
- C6H12O6
Answer/Explanation
5. (4)
Glucose (C6H12O6) dissolves in water as both are polar in nature (like dissolves like).
- Which of the following is a method of expressing the concentration of a solution?
- Osmotic Pressure
- Lowering in freezing point
- Increase in boiling point
- Parts per million
Answer/Explanation
6. (4)
Parts per million is a method of expressing the concentration of a solution
- A 0.0020 molal aqueous solution of an ionic compound CO(NH3)5(NO2)Cl freezes at -0.00732°C. Number of moles of ions which 1 mol of ionic compound produces on being dissolved in water will be (kf = -1.86°C molal-1)
- 1
- 2
- 3
- 4
Answer/Explanation
7. (2)
ΔTf = i x kf x m
0.00732 = i x 1.86 x 0.002
i = 1.967 ≅ 2
- Which of the following shows negative deviation from Raoult’s law?
- Water + Nitric Acid
- Benzene + Methanol
- Acetone + Benzene
- Methyl alcohol + Water
Answer/Explanation
8. (1)
Water + Nitric Acid shows negative deviation from Raoult’s law
- 30 mL of liquid R and 20 mL of liquid S are mixed to form a solution of 50 mL. Then the solution is __________?
- Non-ideal with positive deviation
- Non-ideal with negative deviation
- Ideal Solution (.)
- Cannot be predicted
Answer/Explanation
9. (3)
30 mL + 20 mL = 50 mL
Final solution’s volume is also 50 mL.
As the change in volume is zero, therefore it forms an ideal solution.
- PCl5 (g) ⇌ PCl3 (g) + Cl2 (g). In the reaction, at equilibrium condition mole fraction of PCl5 is 0.2 and mole fraction of Cl2 is 0.4. What is the mole fraction of PCl3?
- 0.5
- 0.6
- 0.4
- 0.3
Answer/Explanation
10. (3)
Mole fraction’s sum is always equal to 1.
Mole fraction of PCl5 + Mole fraction of Cl2 + Mole fraction of PCl3 = 1
0.4 + 0.2 + Mole fraction of PCl3 = 1
Mole fraction of PCl3 = 1 – 0.6 = 0.4
Solutions MCQ (11-20)
- In case an electrolyte dissociates in the solution, the van’t hoff factor i is?
- >1
- <1
- 1
- >1 or <1
Answer/Explanation
11. (1)
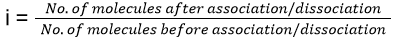
After dissociation, the number of molecules increases. Therefore i > 1
- Dissolving 120 g of urea (molecular weight 60 g mol-1) in 1000 g of water gave a solution of density 1.15 g mL-1. The molarity of the solution is
- 1.78 M
- 2.00 M
- 2.05 M
- 2.22 M
Answer/Explanation
12. (3)
Total mass of the solution = Mass of urea given + Mass of water given = 1000 + 120 = 1120 g
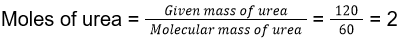
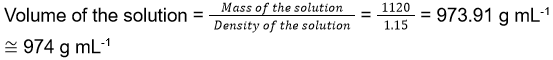
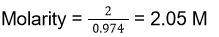
- Which has the highest freezing point at 1 atm?
- 0.1 molal NaCl solution
- 0.1 molal BaCl2 solution
- 0.1 molal sugar solution
- 0.1 molal FeCl3 solution
Answer/Explanation
12. (3)
Total mass of the solution = Mass of urea given + Mass of water given = 1000 + 120 = 1120 g
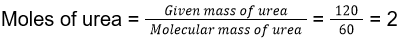
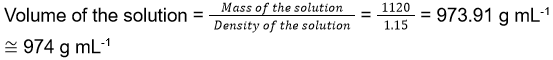
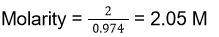
- Which of the following conditions is not satisfied by an ideal solution?
- ΔHmix = 0
- ΔSmix = 0
- ΔVmix = 0
- Raoult’s law is obeyed
Answer/Explanation
14. (2)
An ideal solution obeys Raoult’s law and has ΔHmix = 0 and ΔVmix = 0.
- A substance trimerizes when dissolved in a solvent. What is the Van’t Hoff factor (assume 100% association)?
- 1
- 2
- 3
- 1/3
Answer/Explanation
15. (4)
Van’t Hoff factor (i) = 1 – a + (a/n)
As there is 100% dissociation, a = 1
n = 3, i = 1/3
- An azeotrope solution of two liquids has boiling point lower than that of either of them if it
- Shows a negative deviation from Raoult’s law
- Shows a positive deviation from Raoutl’s law
- Shows no deviation from Raoult’s law
- Is saturated
Answer/Explanation
16. (2)
In case of positive deviation from Raoult’s law, the observed vapour pressure is higher than the ideal vapour pressure and boiling point of azeotrope becomes lower than either of pure liquid.
- Molarity of 900 g of water is?
- 50 M
- 55.5 M
- 5 M
- 5.55 M
Answer/Explanation
17. (2)
Volume of 900 g of water = 900 mL
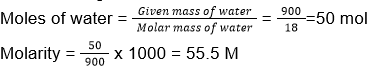
- Increase in the temperature of an aqueous solution will cause
- Decrease in molality
- Decrease in molarity
- Decrease in mole fraction
- Decrease in %w/w
Answer/Explanation
18. (2)
Increase in temperature increases the volume. As molarity is inversely proportional to the volume, so there will be a decrease in the molarity.
- Water boils at 96°C at a certain place. What is the amount of NaCl to be added to 1 L of water so that it boils at 100°C (Kb for water is 0.52°C molal-1
- 450 g
- 125 g
- 225 g
- 250 g
Answer/Explanation
19. (3)
ΔTb = i x kb x m
ΔTb = 100 – 96 = 4
NaCl has i = 2
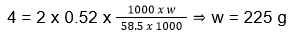
- An aqueous solution of methanol in water has vapour pressure
- Equal to that of water
- Equal to that of methanol
- More than that of water
- Less than that of water
Answer/Explanation
20. (3)
The vapour pressure of aq. methanol solution is more than H2O and less than methanol. This is due to the solute-solvent interaction more due to the formation of H-bonds than methanol but less in water.
- In case an electrolyte dissociates in the solution, the van’t hoff factor i is?
Solutions MCQ (21-30)
- If an aqueous solution of glucose is allowed to freeze, then crystals of which will separate out first?
- Water
- Glucose
- Both 1 and 2
- None of these
Answer/Explanation
21. (1)
Freezing point is the temperature at which the liquid and the solid form of the substance are in equilibrium and have the same vapour pressure. Due to lower vapour pressure of the solution, the solid form of the solution separates out at a lower temperature. When solid is the solute, it’s the solvent that freezes. Therefore, the water will separate first.
- Henry’s law constant for the solubility of N2 gas in water at 298 K is 1.0 x 105 atm. The mole fraction of N2 in air is 0.8. The number of moles of N2 from air dissolved in 10 mol of water at 298 K and 5 atm is
- 4.0 x 10-4
- 4.0 x 10-5
- 5.0 x 10-4
- 4.0 x 10-6
Answer/Explanation
22. (1)
According to Henry’s law,
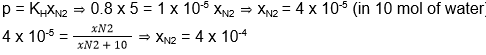
- At 80°C, the vapour pressure of pure liquid A is 520 mm Hg and that of pure liquid B is 1000 mm Hg. If a mixture of solutions A and B boils at 80°C and 1 atm pressure, the amount of A in the mixture is (1 atm = 760 mm Hg)
- 52 mol%
- 34 mol%
- 48 mol%
- 50 mol%
Answer/Explanation
23. (4)
Ptotal = p°AxA + p°BxB⇒ 760 = 520xA + p°B(1-xA) ⇒ xA = 0.5
- A solution showing a large positive deviation from ideal behavior has
- Higher boiling point that both the components
- ΔHmix is positive
- ΔHmix is negative
- None of the above
Answer/Explanation
24. (3)
A solution showing a large positive deviation from ideal behavior has ΔHmix negative.
- Which of the following colligative properties can help to determine the molar mass of a protein with the greatest precision?
- Osmotic pressure
- Depression in freezing point
- Elevation in boiling point
- Relative lowering of vapour pressure
Answer/Explanation
25. (1)
Osmotic pressure is used for the determination of molar mass of proteins as they are not stable at high temperature and polymers have poor stability.
- At a temperature, total vapour pressure in Torr of a mixture of volatile components A and B is given by p = 120 – 75 xB hence vapour pressure of pure A and B, respectively (in Torr) are
- 120 and 75
- 120 and 195
- 75 and 45
- 120 and 45
Answer/Explanation
26. (4)
Ptotal = 120 – 75xB = p°AxA + p°BxB
But, xA = 1 – xB
Ptotal = 120 – 75xB = p°A(1-xB) + p°BxB = p°A – (p°A – p°B)xB
p°A = 120, p°B = 120 – 45 = 75
- The osmotic pressure of a decinormal solution of BaCl2 at 27°C showing 80% degree of ionisation is
- 3.20 atm
- 4.20 atm
- 5.20 atm
- 6.40 atm
Answer/Explanation
27. (4)
i = 1 + ɑ(n-1)
ɑ = 0.8, n=3, i=2.6
Decinormal solution ⇒ C = 0.1M
π = i x C x R x T
π = 2.6 x 0.1 x 0.082 x 300 = 6.4 atm
- Which of the following statements is incorrect?
- Gibbs energy change of dissolution of a gas may be +ve or -ve
- Mole fraction of gas in the solution is directly proportional to the partial pressure of the gas above the solution
- Volume of gas dissolved measured at the pressure used is independent of the pressure of gas
- Solubility of gas is always an exothermic process
Answer/Explanation
28. (1)
Dissolution of a gas is an exothermic process. Gibbs free energy of an exothermic process is always negative.
- The values of observed and calculated molecular masses of calcium nitrate are , respectively, 65.6 g mol-1 and 164 g mol-1. The degree of dissociation of calcium nitrate will be
- 25%
- 50%
- 75%
- 60%
Answer/Explanation
29. (3)
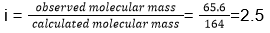
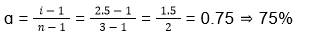
- Equal volumes of ethylene glycol (molar mass = 62 g mol-1) and water (molar mass = 18 g mol-1) are mixed. The depression in freezing point of water is (given kf of water = 1.86 K molal-1 and specific gravity of ethylene glycol is 1.11)
- 0.0033 K
- 0.333 K
- 3.33 K
- 33.3 K
Answer/Explanation
30. (4)
Mass = Density x Volume
Mass(ethylene glycol) = 1.11 x V
Mass(water) = 1.00 x V
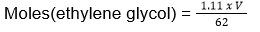
ΔTf = i x kf x m
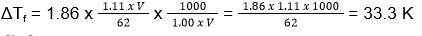
- If an aqueous solution of glucose is allowed to freeze, then crystals of which will separate out first?
Solutions MCQ (31-40)
- Vapour pressure of a dilute aqueous solution of glucose is 750 mm Hg at 373 K. The mole fraction of the solute is
- 1/10
- 1/(7.6)
- 1/35
- 1/76
Answer/Explanation
31. (4)
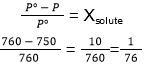
- Solutions which distill without change in composition liquid and vapour phase are called
- Amorphous
- Azeotropic mixture
- Supersaturated
- Ideal
Answer/Explanation
32. (2)
Solutions which distil without change in composition of liquid and vapour phase are called azeotropic mixtures.
- Depression in freezing point of 0.01 molal aqueous acetic acid acid solution is found to be 0.02046°C. One molal urea solution freezes at -1.86°C. Assuming molarity equal to molality, pH of the acidic solution is
- 2
- 3
- 3.2
- 4.2
Answer/Explanation
33. (2)
ΔTf = i x kf x m
Molarity = molality
Kf = 1.86
0.0204 = 1.86 x i x 0.01
i = 1.1
CH3COOH → CH3COO- + H+
C(1-ɑ) Cɑ Cɑ
i = 1 +ɑ
ɑ = 0.1
[H+] = Cɑ = 0.01 x 0.1 = 0.001
pH = -log[H+] = 3
- A 5% solution of cane sugar (molar mass = 342 g mol-1) is isotonic with a 1% solution of a substance X. The molecular weight of X is
- 34.2
- 171.2
- 68.4
- 136.8
Answer/Explanation
34. (3)
5% solution means 5g of solute is present in 100g of water
Density of water is 1g/cm3 so 100 g of water is equal to 100mL of water
Mass of solute = 5g
Volume of solution = 100 mL
Molar mass of solute = 342g
For substance X, molar mass = M
Given mass of solute
As mentioned in the question, solution of X and solution of cane sugar are isotonic with each other, i.e,
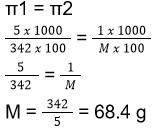
- The molal lowering of vapour pressure for water at 100°C will be
- 13.43 mm Hg
- 1.343 mm Hg
- 23.43 mm Hg
- 234.3 mm Hg
Answer/Explanation
35. (1)
Lowering of vapour pressure = XA
1 molal aqueous solution means 1 mole of solute in 1000 mL of water
Density of water = 1g/mL
1 mL of water = 1 g
1000 mL of water = 1000 g
No. of moles of water = 55.56XA = nA/(nA + nB) = 0.01768 atm or 13.43 mm Hg
- Which will form maximum boiling azeotrope?
- HNO3 + H2O
- C2H5OH + H2O
- C6H6 + C6H5CH3
- None of the above
Answer/Explanation
36. (1)
HNO3 + H2O is a maximum boiling azeotrope and shows negative deviation from Roult’s law.
- Which of the following statements is not correct for an ideal binary liquid solution AB (XA = mole fraction of A in vapour phase; XB = mole fraction of B in vapour phase)
- A plot between ptotal and XB is linear
- A plot between ptotal and XA is linear
- A plot between 1/ptotal and XA is linear
- A plot between ptotal and 1/XA is linear
Answer/Explanation
37. (c)
Plot between 1/ptotal and XA is linear is a wrong statement
- If two substances A and B have p°A : p°B = 1 : 2 and have mole fraction in solution 1 : 2 and have mole fraction of A in vapours is
- 0.33
- 0.25
- 0.52
- 0.20
Answer/Explanation
38. (4)
p°A/p°B = 1/2
p°A = 1, p°B = 2
nA = 1, nB = 2
PA = xA x p°A = (⅓) x 1 = ⅓
PA = xA x p°A = (⅔) x 2 = 4/3
PA = xA x PT
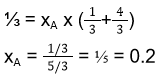
- Van’t Hoff factors x, y and z for association, dissociation and no change of solute in the solution, respectively, are in the order
- x < y < z
- x > z > y
- x < z < y
- x > y > z
Answer/Explanation
39. (3)
Van’t Hoff factor is the greatest for dissociation and lowest for association
- There is 100 mL of 0.1 M KCl solution. To make it 0.2 M, we have to
- Evaporate 50 mL of the solution
- Add 0.01 mol of KCl
- Use both 1 and 2
- Use neither 1 nor 2
Answer/Explanation
40. (1)
M1V1 = M2V2
100 x 0.1 = X x 0.2
X = 50 mL
We have to reduce the volume of the solution by 50 mL. Therefore, it can be done by evaporating the solution.
- Vapour pressure of a dilute aqueous solution of glucose is 750 mm Hg at 373 K. The mole fraction of the solute is
Solutions MCQ (41-50)
- A substance is deliquescent if its vapour pressure
- Is less than that of water vapour in the air
- Is more than that of water vapour in the air
- Is equal to that of water vapour in the air
- Is equal to atmospheric pressure
Answer/Explanation
41. (1)
A process by which a substance absorbs moisture from the atmosphere till it gets dissolved in the absorbed water and forms a solution is called deliquescence. It occurs only when vapour pressure is less than that of water vapour in the air.
- A pressure cooker reduces cooking time because
- The heat is more evenly distributed
- The higher pressure tenderizes the food
- The boiling point of water inside the cooker is elevated
- The boiling point of water inside the cooker is depressed
Answer/Explanation
42. (3)
The boiling point of water inside the cooker gets elevated due to which the cooking time reduces.
- The mass of glucose that should be dissolved in 50g of water in order to produce the same lowering of vapour pressure as produced by dissolving 1 g of urea in the same quantity of water is
- 1 g
- 3 g
- 6 g
- 8 g
Answer/Explanation
43. (3)
(Δp)glucose = (Δp)urea
(xB)glucose = (xB)urea
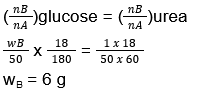
- Molarity of liquid HCl, if density of the solution is 1.17 g cm-3, is
- 36.5
- 18.25
- 42.10
- 32.05
Answer/Explanation
44. (4)
Density = 1.17 g cm-3 = 1170 g/L
Molar mass of HCl = 36.5
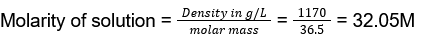
- Van’t Hoff factor for 0.1 M ideal solution is
- 1
- 0.1
- -0.01
- None of the above
Answer/Explanation
45. (1)
Non-electrolytes form ideal solution having van’t hoff factor equal to 1
- Which of the following remains independent of temperature?
- Molality
- Molarity
- Formality
- Normality
Answer/Explanation
46. (1)
Molality depends inversely on mass which remains unchanged with the change in temperature.
- The depression in freezing point for 1 M urea, 1 M glucose and 1 M NaCl are in the ratio
- 1 : 2 : 3
- 3 : 2 : 2
- 1 : 1 : 2
- None of the above
Answer/Explanation
47. (3)
iurea = 1
iglucose = 1
iNaCl = 2
Ratio = 1 : 1 : 2
- A liquid is in equilibrium with its vapour at its boiling point. On an average, the molecules in the two phases have equal
- Intermolecular force
- Potential energy
- Total energy
- Kinetic energy
Answer/Explanation
48. (4)
As temperature will be the same at the boiling points, kinetic energies will be the same as it is directly proportional to the temperature
- When mercuric iodide is added to the aqueous solution of potassium iodide, the
- Freezing point rises
- Freezing point decreases
- Freezing point remains the same
- Boiling point doesn’t change
Answer/Explanation
49. (1)
As HgI reacts with KI to form K2[HgI4], the number of ions decreases which decreases the van’t hoff factor. This also results in the decrease of freezing point as it is directly proportional to the van’t hoff factor. Thus, the freezing point is raised.
- Which of the following are homogeneous in nature? a) Ice b) Wood c) Soil d) Air
- a and b
- b and d
- a and d
- c and d
Answer/Explanation
50. (3)
Air and ice are homogeneous in nature as they have the same amount of their components throughout.
- A substance is deliquescent if its vapour pressure
Assertion And Reasoning MCQ
Codes
(a) Both A and R are true and R is the correct explanation of A
(b) Both A and R are true and but R is not a correct explanation of A
(c) A is true but R is false
(d) A is false, but R is true
1. Assertion (A) The concentration of pollutants in water or atmosphere is often expressed in terms of PPM.
Reason (R) Concentration in parts per million can be expressed as mass to mass volume to volume and mass to volume.
Answer/Explanation
1 . (d)
When a solute is present in trace quantities, it is convenient to express concentration in PPM.
2. Assertion (A) 0.1 M solution of KCl has greater osmotic pressure than 0.1 M solution of glucose at same temperature.
Reason (A) In solution KCl dissociates to produce more number of particles.
Answer/Explanation
2. (a)
KCl can dissociate in water but glucose does not. Due to dissociation of ions, solution exhibit higher colligative property.
3. Assertion (A) When a solution is separated from the pure solvent by a semipermeable membrane, the solvent molecules pass through the it from pure solvent side to the solution side.
Reason (R) Diffusion of solvent occurs from a region of high concentration solution to a region of low concentration solution.
Answer/Explanation
3. (c)
With the semipermeable membrane in place, and if one compartment contains the pure solvent, this can never happen; no matter how much liquid flows through the membrane, the solvent in the right side will always be more concentrated that that in the left side.
4. Assertion (A) In solution, amalgam of Mercury with sodium is an example of solid solutions.
Reason (A) Mercury is solvent and sodium is solute in the solution.
Answer/Explanation
4. (c)
Amalgam of mercury with sodium is an example of liquid in solid type solid solution. Here, mercury (liquid metal) acts as solute and sodium as solvent.
5. Assertion (A) Molarity of a solution in liquid state changes with temperature.
Reason (R) The volume of a solution changes with changes in temperature.
Answer/Explanation
5. (a)
Molarity is the number of moles of solute dissolved per litre of solution. Molarity changes as temperature changes as the volume of the solution changes.
6. Assertion (A) Pressure have any effect on solubility of solids in liquid.
Reason (A) Solids and liquids are not incompressible.
Answer/Explanation
6. (a)
Liquid and solids exhibit practically no change of solubility with changes in pressure. Gases as might be expected , increase in solubility with an increase in pressure.
7. Assertion (A) Elevation in boiling point is a colligative property.
Reason (R) Elevation in boiling point is directly proportional to molarity.
Answer/Explanation
7. (c)
Elevation in boiling point is directly proportional to molality.
8. Assertion (A) Azeotropic mixtures are not formed only by non-ideal solutions and they may have boiling points either greater than both the components or less than both the components.
Reason (R) The composition of the vapour phase is not same as that of liquid phase of azeotropic mixture.
Answer/Explanation
8. (b)
Non-ideal solutions with positive deviation, boils at a lower temperature than the components, while those with negative deviation, boil at a higher temperature.
9. Assertion (A) At equilibrium, vapor phase will not be always reach in component which is more volatile.
Reason (R) The composition of vapour phase in equilibrium with the solution is not determined by the partial pressure of the components.
Answer/Explanation
9. (d)
At equilibrium, the vapor phase will always be rich in volatile components. The higher liquids vapor pressure is at a given temperature the higher its volatility and the lower the liquid’s typical boiling point. The partial pressure of components determines the composition of the vapor phase in equilibrium with the solution.
10. Assertion (A) An ideal solution obeys Henry’s law.
Reason (R) In an ideal solution, solute-solute as well as solvents-solvent interaction are similar to solute-solvent interaction.
Answer/Explanation
10. (d)
An ideal solution obeys Raoult’s law.
Case Study Based MCQs
1. Read the passage given below and answer the following questions
The vapour pressure of a solvent decreases when a non volatile component is dissolved in the liquid phase. The depression of the vapour pressure of the solution (at constant temperature) results in rise of the the boiling point of the solution (at constant pressure).
The elevation in boiling point being a colligative property, depends on the concentration of the dissolved particles and on the nature of solvent. In dilute solution it is observed to be relatively independent of the nature of the solute
Codes
(a) Both A and R are true and R is the correct explanation of A
(b) Both A and R are true and but R is not a correct explanation of A
(c) A is true but R is false
(d) A is false, but R is true
i) Assertion (A) At boiling point, vapour pressure of a liquid is equal to the atmospheric pressure
Reason (R) Vapour pressure of a liquid decreases with a liquid
Answer/Explanation
Answer: b
ii) Assertion (A) Blood cells collapse when suspended in saline water, having more concentration compared to fluid inside the blood cell
Reason (R) Solvent molecules always flow from higher concentration to lower concentration
Answer/Explanation
Answer: c
iii) Assertion (A) When NaCl is added to water a depression in freezing point is observed
Reason (R) The lowering of the vapor pressure of a solution causes depression in the freezing point compared to the pure solvent
Answer/Explanation
Answer: a
iv) Assertion (A) Colligative property is used to determine the molecular mass of particles
Reason (R) Colligative properties depend upon number of solute particles in solution irrespective of the nature
Answer/Explanation
Answer: b
v) Assertion (A) Colloidal solution show colligative properties
Reason (R) Colloidal particles are large in size
Answer/Explanation
Answer: b
2. Read the passage given below and answer the following question
At 298K, the vapour pressure of pure benzene. C6H6 is 0.256 bar and the vapour pressure of pure toluene C6H5CH3 is 0.095 bar. Two mixtures are prepared as follows:
(I) 7.8g of C6H6 + 9.2g of toluene
(II) 3.9g of C6H6 + 13.8g of toluene
The following questions are multiple choice questions. Choose the most appropriate answer
i) The total vapour pressure (bar) of solution 1 is
- 0.128
- 0.174
- 0.198
- 0.258
Answer/Explanation
Answer: 2
ii) Which of the given solutions have higher vapour pressure
- I
- II
- Both have equal vapour pressure
- Cannot be predicted
Answer/Explanation
Answer: 1
iii) Mole fraction of benzene is vapour phase in solution I is
- 0.128
- 0.174
- 0.734
- 0.266
Answer/Explanation
Answer: 3
iv) Which of the following statement is/are correct
(a) Mole fraction of toluene in vapour phase is more in solution (I)
(b) More fraction of toluene in vapour phase is less than in solution (I)
(c) More fraction of benzene in vapour phase is less in solution (I)
- Only b
- Only a
- a and c
- b and c
Answer/Explanation
Answer: 1
v) Solution (I) is an example of a/an
- Ideal solution
- Non ideal solution with positive deviation
- Non ideal solution with negative deviation
- Can’t be predicted
Answer/Explanation
Answer: 1
Frequently Asked Questions FAQs
Q1. What are the two limitations of Henry’s law?
1. The pressure should be low and the temperature should be high, i.e, the gas should behave like an ideal gas.
2. The gas should not undergo compound formation with the solvent or associate or dissociate in the solvent.
Q2. What is an antifreeze?
An antifreeze is a substance which is added to lower the freezing point of a water based liquid and increase its boiling point. Ethylene glycol is used as an antifreeze.
Q3. Which is better, Molarity or Molality?
Molality is preferred over molarity because molality doesn’t changes with change in temperature as it is inversely proportional to the mass of solvent which doesn’t changes with temperature. Molarity, on the other hand changes with temperature as volume changes with the temperature.