Surface Chemistry : Notes and Study Materials -pdf
Notes and Study Materials
- Concepts of Surface Chemistry
- Master File Surface Chemistry
- NCERT Solutions for – Surface Chemistry
- NCERT Exemplar Solutions for – Surface Chemistry
- Mind Map of Surface Chemistry
- Concept Map of Surface Chemistry
- Past Many 12th Board Years of Surface Chemistry
Examples and Exercise
CBSE Class 12th Chemistry Notes: Surface Chemistry
Surface Chemistry is the most important chapter of CBSE Class 12th Chemistry. Every year 2 to 3 conceptual questions are frequently asked in CBSE Class 12th Board exams.
Surface Chemistry is the most important chapter of CBSE Class 12th Chemistry. Every year 2 to 3 conceptual questions are frequently asked in CBSE Class 12th Board exams. So, students must have deep understanding of the chapter in order to score maximum marks in CBSE Class 12th Chemistry Board Examination.
Here, you will get revision notes on CBSE Class 12 Chemistry: Chapter 5 – Surface Chemistry. These notes will give you a quick glance of the chapter.
The main topics covered in these quick notes are:
o Definition of
• Surface chemistry
• Adsorption
• Desorption
• Sorption
o Difference between adsorption and absorption
o Physisorption and chemisorption
o Adsorption of gases on solids
o Effect of temperature on adsorption
o Adsorption from solutions
o Catalysis
o Types of catalysis
• Homogeneous
• Heterogeneous
• Shape-selective catalysis
• Enzyme catalysis
The notes on the chpater are as follows:
Surface Chemistry:
Surface chemistry is a branch in chemistry which deals with the study of the phenomenon occurring at the interface of two phases. Surface represents the physical boundary of any condensed phase like liquid or solid. It separates one phase from the other.For example: Interfaces existing between two immiscible liquids like oil and water; between a metal and a gas like platinum and hydrogen; a liquid and a gas etc. The surface or interface between two phases is represented by a hyphen or slash. For example: Between the solid and the liquid, the interface is represented by solid/liquid. There is no interface between the gases as they are completely miscible with one another.
Adsorption:
The phenomenon of attracting and retaining the molecules of a substance on the surface of a liquid or a solid resulting into a higher concentration of the molecules on the surface is called adsorption. It is also called the positive adsorption. The surface on which adsorption takes place is called the adsorbent and the substance which is being adsorbed is called the adsorbate.
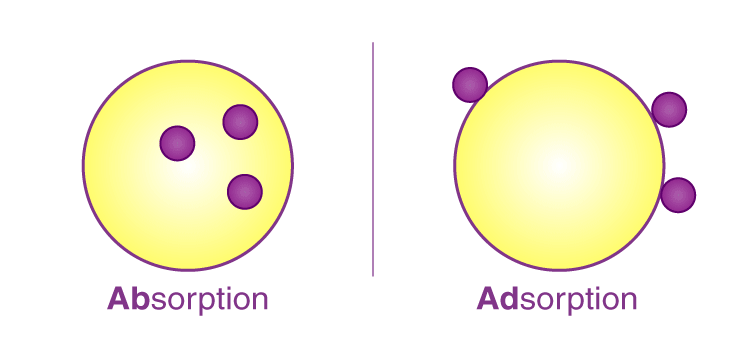
Inside surface On the surface
Types of adsorption:
Adsorption is of two types:
(i) Physical adsorption (physisorption): When a gas is held (adsorbed) on the surface of a soild by Vander Waals forces without forming any chemical bond between adsorbate and adsorbent it is called physical adsorption. E.g., Adsorption of CO2 gas on the surface of charcoal.
(ii) Chemical adsorption (Chemisorption): When a gas is held (adsorbed) on the surface of a solid by forces similar to those of a chemical bond the type of adsorption is called chemical adsorption. This type of adsorption leads to the formation of a surface compound.
Differences between Physisorption and Chemisorptions
Physisorption | Chemisorption |
Only van der Waals forces forces are present between adsorbate and surface of adsorbent. It usually takes place at a low temperature and decreases with increasing temperature. It is reversible. It is related to the ease of liquefaction of the gas. It forms multi-molecular layers. It does not require any activation energy. | Chemical bonds are formed between adsorbate and surface of adsorbent. It takes place at a high temperature. It is irreversible. The extent of adsorption not related to liquefaction of the gas. It forms mono-molecular layers. It requires activation energy. |
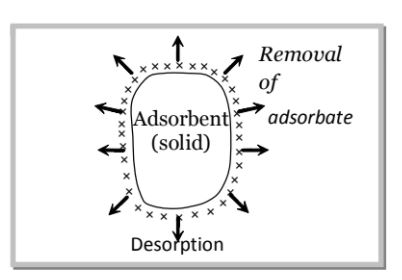
Desorption:
Desorption is the reverse of adsorption which involves the removal of the substance adsorbed from the surface. The process of desorption can be carried out by:
(i) Reducing the pressure and (ii) By heating
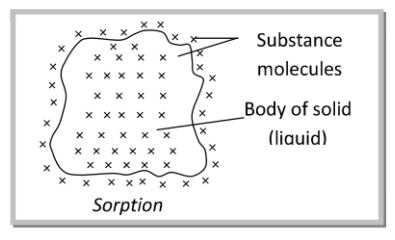
Sorption:
In absorption, the concentration of the molecules of a substance is more in the bulk than at the surface and the molecules in the bulk are uniformly distributed. If the concentration of the molecules of a substance is more in the bulk but molecules are not uniformly distributed, then it is called negative adsorption. When both adsorption and absorption takes place together and are indistinguish-able, then we use the term called Sorption.
Adsorption of gas on solid surface:
Almost all solids adsorb gases to some extent. Charcoal in gas mask adsorbs poisonous gases on its surface. Silica gel adsorbs moisture and is used for drying air.
The extent of adsorption of a gas on solid is expressed as x/m.
where, x = mass of the adsorbate, m = mass of the adsorbent
Factors affecting the extent of adsorption:
(i) Nature of the solid (adsorbent)
(ii) Nature of the gas (adsorbate)
(iii) Pressure of the gas
(iv) Temperature
(v) Activation of adsorbent
In short, adsorption depends on the nature of the adsorbent. More the roughness of a surface, more it has number of pores hence adsorbs more number of gases than the smooth surface. Most common adsorbents are silica gel and activated charcoal. The extent of adsorption also depends on the surface area of the solid. The greater the surface area of the solid, the greater would be the adsorption.
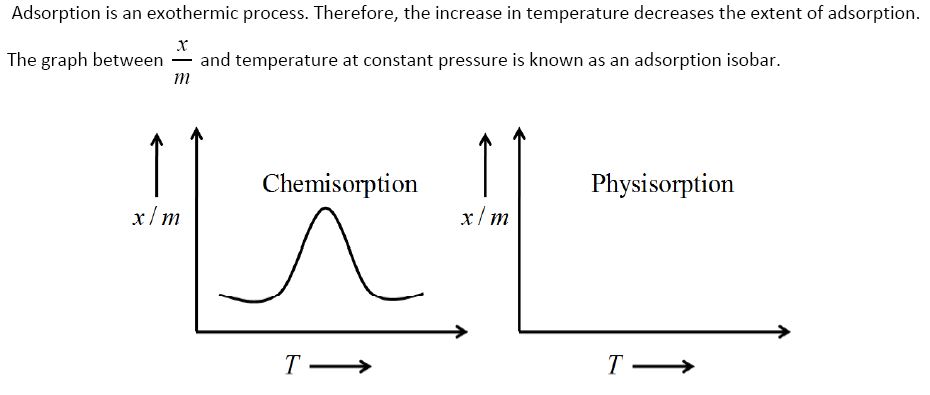
Adsorption isotherm:
The extent of adsorption of a gas on a solid is also affected by the pressure of the gas. A graph between x/m and the pressure P at constant temperature is known as the adsorption isotherm.
Freundlich Isotherm:
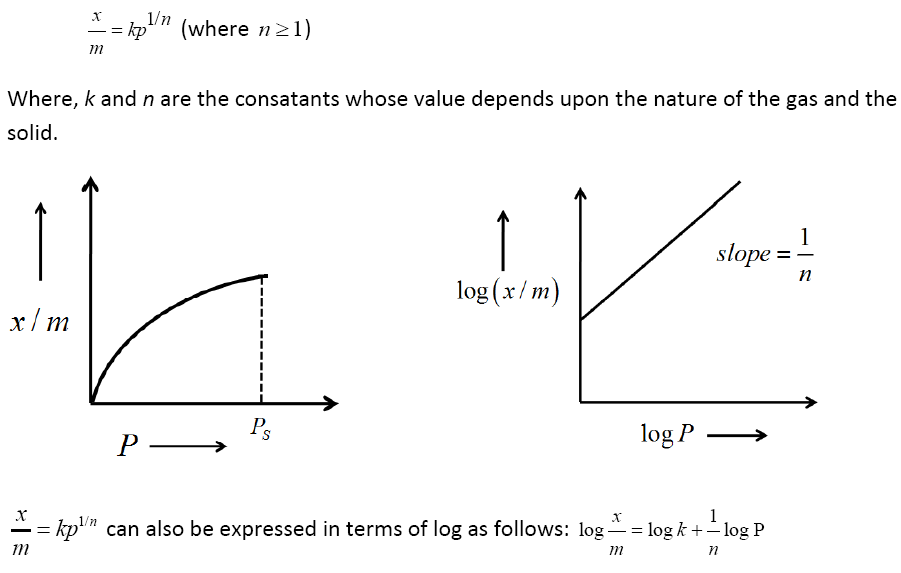
Adsorption isotherm:
Catalyst:
A catalyst is a substance which increases the rate of reaction without participating in the reaction. The catalyst is recovered chemically unchanged at the end of the reaction. Catalysts are always used in very small amounts and speed up the reaction by decreasing the potential energy activation barrier. A catalyst does not affect the equilibrium constant as it speeds up the reaction in both the directions whether forward or backward by the same factor.
Catalysis and its types:
The phenomenon of increase in the rate of a reaction with the help of a catalyst is known as catalysis.
It is of two types:
(i) Homogeneous catalysis: When the catalyst and the reactants are in the same phase then the type of catalysis is called the homogeneous catalysis.
(ii) Heterogeneous catalysis: When the reactants and the catalyst are in different physical states then the catalysis is termed as the heterogeneous catalysis.
Important features of the catalyst:
(i) Activity: It represents the capacity of s catalyst to speed up the reaction. It depends upon the strength of chemisorption to a large extent. It also depends on the surface area of the catalyst.
(ii) Selectivity: The ability of a catalyst to direct a reaction to yield a particular product excluding others.
Zeolites as shape selective catalyst:
The shape selective catalysis depends upon the structure of the pores present in the catalyst and the size of the reactant and product molecules. Zeolites are silicates with number of cages or pores. It catalysis only those reactant molecules having a shape and size similar to cages. Example is ZSM-5 used for converting alcohol directly into gasoline. Zeolites are mainly used in petrochemical industries for cracking of hydrocarbons and isomerisation.
Enzymes as biological catalysts:
Enzymes are biological catalysts which are more efficient than chemical catalysts. They can speed up the reaction rates by 108 to 1020 times. Enzymes are also very specific in their action.The specific action of enzyme is due to the presence of a specific and active site present on its surface. The substrate (reactant) binds to this active site through intermolecular forces. Enzymatic reaction follows lock-and-key mechanism.
In Part-I you discovered about the concepts of Surface Chemistry, Adsorption, Absorption, Catalysis etc.
Now in this Part – 2 of the revision notes on CBSE Class 12 Chemistry: Chapter 5 – Surface Chemistry you will study the following topics:
o Colloids
o Classification of colloids
• Physical state of dispersed phase and dispersion medium
• Nature of interaction between dispersed phase and dispersion medium
• Particles of dispersed phase
o Preparation of colloids
o Purification of colloids
o Properties of colloids
o Emulsions
o Applications of colloids
The notes of the chapter are as follow:
Colloid:
Depending upon the size of the solute particles the solutions can be categorised as true solution, suspension and colloids. The type of solution in which the size of the solute particle is in the range of 1 to 100 nm, is called a colloid. A colloid is a heterogeneous system in which one substance is dispersed (dispersed phase or colloidal particles) in another substance which is called dispersion medium.
Classification of Colloids:
On the basis of the physical state of the dispersed phase and the dispersion medium the colloidal solutions can be classified into eight types:
Dispersed phase | Dispersion medium | Type of colloid | Examples |
Solid | Solid | Solid sol | Gem stones, Coloured glasses |
Solid | Gas | Aerosol | Dust, Smoke |
Solid | Liquid | Sol | Paint |
Liquid | Liquid | Emulsion | Milk |
Liquid | Solid | Gel | Butter, Cheese |
Liquid | Gas | Aerosol | Fog, Cloud |
Gas | Liquid | Foam | Froth |
Gas | Solid | Solid sol | Rubber, Foam |
In short, if the dispersion medium is solid then the colloid is known as gel. If the dispersion medium is liquid or gas then the colloid is called sol. A colloid in which both the dispersed phase and dispersion medium are in the liquid form is known as emulsion.
On the basis of nature of interaction between dispersed phase and dispersion medium, colloids can be divided into two forms:
(a) Lyophilic Colloids: As the name lyophilic (liquid-loving or solvent attracting) indicates, lyophilic colloids are the colloids exhibiting a strong interaction between the two phases.
The substances like gum, gelatin, starch, when mixed with a suitable liquid as the dispersion medium, directly form the colloidal sol which is also named as lyophilic sol.
(b) Lyophobic Colloids: Lyophobic’ means ‘liquid hating’, which indicates that in these sols there is little or no interaction between the two phases. Substances like metals and their sulphides, when simply mixed with the dispersion medium do not form colloidal sol. They cannot be prepared by simply mixing the two phases. Such substances are called Lyophobic Sols.
CBSE Class 12th Chemistry Notes: Chemical Kinetics
Difference between Lyophilic sol and Lyophobic sol:
Lyophilic Sol | Lyophobic Sol |
These are reversible sols. | These are irreversible sols. |
They are quite stable and are not easily coagulated by electrolytes. | They are less stable and get coagulated by electrolytes, by heating or by agitating. |
They are obtained by simple solution method, e.g. starch solution. | They are prepared by indirect methods which are not so easy. |
They are obtained from organic material such as starch, gum, gelatin etc. | They are obtained from inorganic materials such as metals, sulphides, metal oxides etc. |
The particles are hydrated. | The particles are not hydrated. |
Preparation of Lyophilic and Lyophobic sols:
Lyophilic sols are prepared simply by the stirring dispersed phase with dispersion medium. Examples include sol of starch, gelatin, egg albumin.
Methods of preparation of Lyophobic sols can be prepared by two types of methods:
• Condensation
• Dispersion
Condensation methods are in turn of four different types
• Hydrolysis
• Reduction
• Oxidation
• Double decomposition method
Dispersion method involves breaking down of large particles of a substance into particles of colloidal size. There are three such methods:
• Mechanical dispersion
• Bredig’s arc method (to prepare metal sol)
• Peptisation method (to convert precipitate into particles of colloidal size using suitable peptising agent). The peptising agent used is usually an electrolyte.
On the basis of type of particles of the dispersed phase, colloids can be classified into following three types:
• Multimolecular colloids: They contains a large number of molecules in the form of aggregates, e.g., sulphur sol.
• Macromolecular colloids: These colloids consist of one single large size molecule as a dispersed phase, e.g., polythene.
• Associated colloids: These are the colloids which behave as normal electrolytes at low concentration but as a colloid at higher concentration. This is due to the formation of aggregates which are called micelles, e.g. soaps and detergents.
Purification of colloids:
Colloidal contains a number of electrolytic impurities. The following method are used to purify colloids:
• Dialysis (by using semipermeable membrane)
• Ultra-filtration (by using ultra fine quality filter papers)
• Ultra-centrifugation
Properties of colloidal solution:
The colloidal solution shows the following properties:
• Colligative properties: The properties of a solution which depends on the number of moles of solute particles present in the solution are called colligative properties like osmotic pressure, elevation in boiling point etc.
• Tyndall effect: The scattering of light by colloidal particles is known as Tyndall effect. True solutions do not show Tyndall effect.
• Brownian movement: The zigzag motion of the colloidal particles is termed as Brownian movement. This is due to the impact of the molecules of the dispersion medium on the molecules of the dispersed phase.
• Electrophoresis: The movement of colloidal particles towards their respective electrodes in the presence of electric field is known as electrophoresis. This is also known as cataphoresis. This helps in determining the charge present on the colloid.
Coagulation and Floculation:
The process of forming aggregates from colloidal particles by the addition of suitable electrolyte is called coagulation. The addition of an electrolyte to a lyophobic colloid results in its coagulation. At lower concentration of electrolyte, the aggregation of particles is called flocculation. Flocculation is reversible while coagulation is irreversible.
Hardy-Schulze’ s rules:
The precipitation or coagulating power of an electrolyte is determined by using Hardy-Schulze’ s rules:
• The effective ions of the electrolyte in bringing about coagulation are those which carry charge opposite to that of the colloidal particles. These ions are called coagulating ions.
• Coagulating power increases as the charge on the ion increases. Addition of AgNO3 to excess of KI results in the formation of yellow precipitate of AgI. AgI then adsorbs I‒ from the excess of KI preferentially. This adsorption of I‒ break down the precipitate of AgI into colloid particles acquiring a negative charge AgI/I−. This layer of negatively charged particles is balanced by the counter K+ ion. As a result the electric potential is developed. This is called Zeta potential or double layer potential or electro kinetic potential.
Emulsion:
Emulsions are colloids in which both the dispersed phase and the dispersion medium are in the liquid states.
Types of Emulsion:
• Oil in water: It is the emulsion in which dispersed phase is oil and dispersion medium is water. For example: Milk, vanishing cream.
• Water in oil: It is the emulsion in which dispersed phase is water and dispersion medium is oil. For example: Cold cream. butter, cod liver oil.
Emulsification:
The process of making emulsion is called emulsification.
Emulsifier or Emulsifying agent:
The emulsions are generally prepared by shaking strongly the mixture of two colloids these emulsions are generally unstable, e.g., oil and water are immiscible and form unstable emulsions. Thus a substance is added to stabilize the emulsions which named as emulsifiers or emulsifying agents.
For example: Protein casein is present in milk as an emulsifying agent.
Demulsification:
The process of converting the emulsion back into two distinct components, oil and water is called demulsification. This can be carried out by :
• Boiling
• Freezing
• Changing pH
• Electrostatic precipitation
Instruments
Application of colloids:
• Sewage disposal: Colloidal particles of the dirt, mud etc. carry electric charge, hence when sewage water is passed through the plates kept at a high potential, the colloidal particles are coagulated due to electrophoresis and the suspended matter gets removed.
• Cleansimg action of soap: Soap solution is colloidal in nature. It removes the dirt particles either by adsorption or by emulsifying the greasy matter sticking to the cloth.
• Rubber plating: Rubber plated articles are prepared by depositing negatively charged rubber particles over the article to be rubber plated by making that article an anode in a rubber plating bath.
• Medicines: Number of medicines are emulsions. Actually, medicines in colloidal form are easily adsorbed by the body tissues and hence are more effective.
• Artificial rain: Artificial rain can be caused by spraying oppositely charged colloidal dust or sand particles over a cloud. The colloidal water particles present in the cloud will be neutralized and coagulate to from bigger water drops causing artificial rain.
• Disinfectant: Certain disinfectants like Dettol and Lysol are formed of oil-in-water type emulsions.
• Froth floatation process: In the metallurgical processes, the concentration of ore by forth floatation process is based upon the treatment of the powdered ore with oil emulsion. The valuable particles of the ore form foam which comes to the surface and is skimmed off.
Surface Chemistry Class 12 Chemistry MCQs
1. The correct ascending order of adsorption of the following gases on the same mass of charcoal at same temperature and pressure is
(a) CH4 < H2 < SO2
(b) H2 < CH4 < SO2
(c) SO2 < CH4 < H2
(d) H2 < SO2 < CH4
Answer/Explanation
Answer: b
Explaination:
(b) The extent of adsorption increases with increase in polarity and molar mass.
2. The formation of micelles takes place only above
(a) Inversion temperature
(b) Boyle’s temperature
(c) Critical temperature
(d) Kraft temperature
Answer/Explanation
-Answer: d
Explaination:
(d) The temperature above which micelles formation takes place is called Kraft temperature.
3. Colloidion is 4% solution of which one of the following in alcohol-ether mixture.
(a) Nitroglycerin
(b) Cellulose acetate
(c) Glycol dinitrate
(d) Nitrocellulose
Answer/Explanation
Answer: d
Explaination:
(d) Nitrocellulose dissolved in 4% alcohol-ether mixture is called colloidion.
4. If V is amount of adsorbate and ‘/n’ is amount of adsorbent, which of the following is related to adsorption process?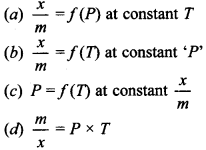
Answer/Explanation
Answer: d
Explaination:
(d) is not related to adsorption.
5. A plot of log \(\frac{x}{m}\) vs log p for adsorption of gas on a solid gives in straight line with slope equal to
(a) n
(b) \(\frac{1}{n}\)
(c) log k
(d) -log k
Answer/Explanation
Answer:
Explaination: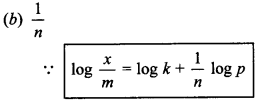
6. The protective power of lyophilic colloidal sol is expressed in terms of
(a) coagulation value
(b) gold number
(c) CMC (Critical Micelle Concentration)
(d) oxidation numbers
Answer/Explanation
Answer: b
Explaination:
(b) Gold number measures protective power of colloids. Lower the gold number more will be protective power, e.g., gelatin.
7. The coagulation values in millimoles per litre of the electrolyte for the coagulation of As2S3 sol are given
I. NaCl (52)
II. BaCl2 (0.69)
III. MgSO4 (0.22)
The correct order of coagulating power is
(a) I > II > III
(b) II > I > III
(c) III > II > I
(d) III > I > II
Answer/Explanation
Answer:
Explaination:
(c) III > II > I
∵ Lesser the coagulation value, more is coagulating power.
8. According to Freundlich adsorption isotherm, which of the following is correct?
(a) \(\frac{x}{m}\) ∝ p1
(b) \(\frac{x}{m}\) ∝ p1/n
(C) \(\frac{x}{m}\) ∝ p°
(d) All are correct of different ranges of pressure
Answer/Explanation
Answer:
Explaination:
(d) All are correct
(a) is correct at low pressure,
(b) is correct at moderate pressure and
(c) is correct at high pressure (saturation pressure).
9. 3g of activated charcoal was added to 50 ml of acetic acid solution (0.06 M) in a flask. After an hour it was filtered and the strength of filtrate was found to be 0.042 M. The amount of acetic acid adsorbed per gram of charcoal is
(a) 42 mg
(b) 54 mg
(c) 18 mg
(d) 36 mg
Answer/Explanation
Answer: b
Explaination:
(b) Molarity of CH3COOH adsorbed
= 0.06 – 0.042 = 0.018
Number of millimoles of CH3COOH
adsorbed = 0.018 × 50 ml = 0.90
Amount = 0.90 × 60 = 54 mg
[∵ molar mass of CH3COOH = 60 g mol-1]
10. During the adsorption of gas on the surface of solid, which of the following is true?
(a) ∆G < 0, ∆H > 0, ∆S < 0 (b) ∆G > 0, ∆H < 0, ∆S < 0
(c) ∆G < 0, ∆H < 0, ∆S < 0
(d) ∆G < 0, ∆H < 0, ∆S > 0
Answer/Explanation
Answer:
Explaination:
(c) ∆G < 0 means process is spontaneous because adsorption is exothermic, ∆H = -ve and entropy is decreasing, i.e., ∆S is -ve.
11. The best coagulant for the precipitation of Fe(OH)3 sol is
(a) Na2HPO3
(b) NaNO3
(c) Na3PO4
(d) Na2SO4
Answer/Explanation
Answer: c
Explaination:
(c) \(\mathrm{PO}_{4}^{3-}\) is most effective for +vely charged Fe(OH)3 sol due to higher charge.
12. Which is favourable for physical adsorption?
(a) High T and high P
(b) High T and low P
(c) Low T and high P
(d) T and P do not affect
Answer/Explanation
Answer: c
Explaination:
(c) Low temperature and high pressure favours physical adsorption because van der Waals’ forces of attraction will increase.
13. Identify the positively charged colloid.
(a) Haemoglobin
(b) As2S3
(c) Clay
(d) Gold sol
Answer/Explanation
Answer: a
Explaination:
(a) due to presence of Fe2+.
14. The stability of lyophobic sols is due to
(a) adsorption of covalent molecules on the colloid
(b) the size of the particles
(c) the charge on particles
(d) Tyndall effect.
Answer/Explanation
Answer: c
Explaination:
(c) same charge on particles cause repulsion and does allow particles to settle down.
15. Gold sol can be prepared by
(a) Hydrolysis of AUCl3
(b) Oxidation of Gold by aqua-regia
(c) Peptization
(d) Reduction of AUCl3 with HCHO solution.
Answer/Explanation
Answer: d
Explaination:
(d) 2AuCl3 + 3HCHO + 3H2O → 2Au + 3HCOOH + 6HCl
16. The term ‘sorption’ stands for ___________ . [NCERT Exemplar]
(a) absorption
(b) adsorption
(c) both absorption and adsorption
(d) desorption
Answer/Explanation
Answer: c
Explaination:
(c) When both adsorption and absorption takes place, it is called sorption, e.g., dying of cotton fibre.
17. Extent of adsorption of adsorbate from solution phase increases with ___________ . [NCERT Exemplar]
(a) increase in amount of adsorbate in solution.
(b) decrease in surface area of adsorbent.
(c) increase in temperature of solution.
(d) decrease in amount of adsorbate in solution.
Answer/Explanation
Answer: a
Explaination:
(a) \(\frac{x}{m}\) = k C1/n [where ‘C’ is concentration of solution]
18. Physical adsorption of a gaseous species may change to chemical adsorption with ___________ . [NCERT Exemplar]
(a) decrease in temperature
(b) increase in temperature
(c) increase in surface area of adsorbent
(d) decrease in surface area of adsorbent
Answer/Explanation
Answer: b
Explaination:
(b) At high temperature, covalent bond will be formed as activation energy will be provided.
19. On the basis of data given below predict which of the following gases shows least adsorption on a definite amount of charcoal? [NCERT Exemplar]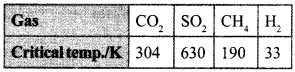
(a) CO2
(b) SO2
(c) CH4
(d) H2
Answer/Explanation
Answer: b
Explaination:
(b) SO2, higher the critical temperature, more will be van der Waal’s forces of attraction, more will be extent of adsorption.
20. In which of the following reactions heter-ogenous catalysis is involved? [NCERT Exemplar]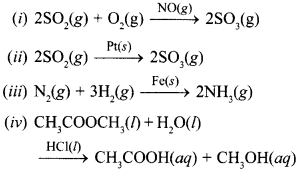
(a) (ii), (iii)
(b) (ii), (iii), (iv)
(c) (i), (ii), (iii)
(d) (iv)
Answer/Explanation
Answer: a
Explaination:
(a) (ii) and (iii) use solid catalyst in gaseous reaction.
∴ Heterogeneous catalysis.
21. In Freundlich adsorption isotherm x/m = Kp1/n, the value of ‘n’ at low pressure is
(a) more than one.
(b) less than one.
(c) equal to one.
(d) from zero to one.
Answer
Answer: c
22. According to adsorption theory of catalysis, the speed of the reaction increases because
(a) the concentration of the reactant molecules at the active centres of the catalyst becomes high due to adsorption.
(b) in the process of adsoption, the activation energy of the molecules becomes large.
(c) adsorption produces heat which increases the speed of the reaction.
(d) adsorption lowers the activation energy of the reaction.
Answer
Answer: d
23. Which shape selective catalyst is used to convert alcohol to gasoline?
(a) Trpsin
(b) Calgon
(c) ZSM-5
(d) Zeigler-Natta catalyst
Answer
Answer: c
24. Which of the following is an example of heterogenous catalyst?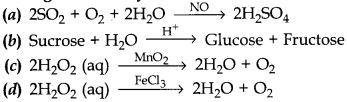
Answer
Answer: c
25. When a small amount of FeCl3 is added to a freshly precipitated Fe(OH)3, b reddish brown colloidal solution is obtained. This pheno¬menon is known as
(a) dialysis
(b) peptization
(c) protection
(d) dissolution
Answer
Answer: c
26. Lyophillic colloids are stable due to
(a) charge on the particles.
(b) large size of the particles.
(c) small size of the particles.
(d) layer of dispersion of medium on the particles.
Answer
Answer: d
27. Cottrell precipitator is used to
(a) precipitate mud from muddy water.
(b) precipitate carbon particles from smoke.
(c) purify the ordinary drinking water.
(d) precipitate salts in qualitative analysis.
Answer
Answer: b
28. The potential difference between the fixed charged layer and the diffused layer having opposite charge is called
(a) Zeta potential
(b) Electrokinetic potential
(c) Both (a) and (b)
(d) Streaming potential
Answer
Answer: a
29. Peptization is a process of
(a) precipitation of colloidal particles.
(b) purification of colloids.
(c) dispersing precipitate into colloidal solution.
(d) movement of colloidal particles in the electric field.
Answer
Answer: c
30. An emulsifier is a substance which
(a) stabilises the emulsion.
(b) homogenises the emulsion.
(c) Coagulates the emulsion.
(d) Accelerates the disperson of liquid in liquid.
Answer
Answer: a
Note: In the following questions two or more options may be correct. (Q.21 to Q.23)
31. Which of the following statements are correct about solid catalyst? [NCERT Exemplar]
(a) Same reactants may give different product by using different catalysts.
(b) Catalyst does not change AH of reaction.
(c) Catalyst is required in large quantities to catalyse reactions.
(d) Catalytic activity of a solid catalyst does not depend upon the strength of chemisorption.
Answer/Explanation
Answer: a
Explaination:
(a) It is called selectivity of catalyst.
(b) Catalyst changes only Ea (Activation energy)
32. Freundlich adsorption isotherm is given by the expression \(\frac{x}{m}\) = k p1/n which of the mfollowing conclusions can be drawn from this expression. [NCERT Exemplar]
(a) When \(\frac{1}{n}\) = 0, the adsorption is independent of pressure.
(b) When \(\frac{1}{n}\) = 0, the adsorption is directly proportional to pressure.
(c) When n = 0, \(\frac{x}{m}\) vs p graph is a line parallel to x-axis.
(d) When n = 0, plot of \(\frac{x}{m}\) vs p is a curve.
Answer/Explanation
Answer: a
Explaination:
(a) and (c) are correct, when n = 0.
∴ \(\frac{x}{m}\) vs p graph is line parallel to x-axis.
when \(\frac{1}{n}\) = 0, \(\frac{x}{m}\) is independent of pressure at high pressure.
33. H2 gas is adsorbed on activated charcoal to a very little extent in comparison to easily liquefiable gases due to. [NCERT Exemplar]
(a) very strong van der Waal’s interaction.
(b) very weak van der Waals forces.
(c) very low critical temperature.
(d) very high critical temperature.
Answer/Explanation
Answer: b
Explaination:
(b) and (c). It is due to weak van der Waals’ forces of attraction and very low critical temperature.
34. Method of formation of solution is given in Column I. Match it with the type of solution given in Column II. [NCERT Exemplar]
| Column I | Column II |
| (a) Sulphur passed through cold water | (i) Normal electrolyte solution |
| (b) Soap mixed water above critical micelle concentration | (ii) Molecular colloids |
| (c) White of egg whipped with water | (iii) Associated colloid |
| (d) Soap mixed water below critical micelle concentration | (iv) Macro molecular colloids |
Answer/Explanation
Answer:
Explaination:
(a) (ii)
(b) (iii)
(c) (iv)
(d) (i) It forms colloid above CMC.
35. Match the items given in Column I and Column II. [NCERT Exemplar]
| Column I | Column II |
| (a) Protective colloid | (i) FeCl3 + NaOH |
| (b) Liquid – liquid colloid | (ii) Lyophilic colloids |
| (c) Positively charged colloid | (iii) Emulsion |
| (d) Negatively charged | (iv) FeCl3 + hot water |
Answer/Explanation
Answer:
Explaination:
(a) (ii)
(b) (iii)
(c) (iv) Fe(OH)3/Fe3+
(d) (i) Fe(OH)3/OH–
36. Match the types of colloidal systems given in Column I with the name given in Column II. [NCERT Exemplar]
| Column I | Column II |
| (a) Solid in liquid | (i) Foam |
| (b) Liquid in solid | (ii) Sol |
| (c) Liquid in liquid | (iii) Gel |
| (d) Gas in liquid | (iv) Emulsio |
Answer/Explanation
Answer:
Explaination:
(a) (ii)
(b) (iii)
(c) (iv)
(d) (i)
37. In the following questions a statement of assertion followed by a statement of reason is given. Choose the correct answer out of the following choices.
(a) Assertion and reason both are correct and the reason is correct explanation of assertion.
(b) Assertion and reason both are correct but reason does not explain assertion.
(c) Assertion is correct but reason is incorrect.
(d) Both assertion and reason are incorrect.
(e) Assertion is incorrect but reason is ’ correct.
Assertion: An ordinary filter paper impregnated with collodion solution stops the flow of colloidal particles.
Reason: Pore size of the filter paper becomes more than the size of colloidal particle. [NCERT Exemplar]
Answer/Explanation
Answer:
Explaination:
(c) Assertion is correct but reason is incorrect because pore size less than size of colloidal particles.
38. Greater the valency of ion, more will be coagulating power is __________ rule.
Answer/Explanation
Answer:
Explaination: Hardy-Schulze rule
39. Emulsion can be __________ by change in pH.
Answer/Explanation
Answer:
Explaination: broken
40. __________ of kidney separates waste products from blood.
Answer/Explanation
Answer:
Explaination: Dialysis
41. Electrokinetic potential is difference in potential of fixed layer and diffused layer which are oppositely charged. [True/False]
Answer/Explanation
Answer:
Explaination: True
42. Enzyme work at specific pH and optimum temperature. [True/False]
Answer/Explanation
Answer:
Explaination: True
43. Lead chamber process for H2S04 manufactureis homogeneous catalysis. [True/False]
Answer/Explanation
Answer:
Explaination:![]()
44. What is the difference between absorption and adsorption?
Answer/Explanation
Answer:
Explaination:
In absorption, a substance is uniformly distributed all over the surface, e.g., cotton dipped in blue ink while in adsorption the existence of a substance at a surface in different concentration than in the adjoining bulk is called adsorption. For example, O2, H2 or SO2 get adsorbed on the surface of charcoal.
45. What is sorption? [Foreign 2013]
Answer/Explanation
Answer:
Explaination:
Sorption is a process in which adsorption and absorption take place simultaneously, e.g. dyeing of cotton fibres by azo dyes.
46. What is meant by desorpton?
Answer/Explanation
Answer:
Explaination:
The process of removing an adsorbed substance from a surface on which it is adsorbed is called desorption, i.e. silica gel removes water.
47. What type of forces are responsible for the occurrence of physisorption? [Foreign 2015]
Answer/Explanation
Answer:
Explaination:
Weak van der Waals’ forces of attraction are responsible for the occurrence of physisorption.
48. What is meant by chemisorption? [Delhi 2011(C)]
Answer/Explanation
Answer:
Explaination:
Chemisorption: If the forces holding the adsorbate are as strong as in chemical bonds, the adsorption process is known as chemical adsorption or chemisorption.
49. What is the effect of temperature on chemisorption? [AI2015 Panchkula; DoE]
Answer/Explanation
Answer:
Explaination:
Chemisorption first increases and then decreases with increase in temperature.
50. Why is chemisorption referred to as activated adsorption?
Answer/Explanation
Answer:
Explaination:
It is because chemisorption needs high activation energy as it involves first bond breaking and then bond formation.
51. Why do physisorption and chemisorption behave differently with rise in temperature?
Answer/Explanation
Answer:
Explaination:
It is because physical adsorption involves weak forces of attraction, whereas chemisorption involves strong covalent bonds.
52. Physisorption is reversible while chemisorption is irreversible. Why? [Foreign 2015]
Answer/Explanation
Answer:
Explaination:
In physisorption, the forces of attraction are weak and no new substance is formed, therefore, it is reversible, whereas in chemisorption, new substances are formed, therefore, it is irreversible.
53. Which has a higher enthalpy of adsorption, physisorption or chemisorption? [AI 2013]
Answer/Explanation
Answer:
Explaination:
Chemisorption has higher enthalpy of adsorption because there is a strong forces of attraction between adsorbate and adsorbent.
54. Why is it important to have clean surface in surface studies?
Answer/Explanation
Answer:
Explaination:
It facilitates the adsorption of desired species (adsorbate) on the clean surface of adsorbent.
55. Out of NH3 and N2, NH3 gas will be adsorbed more readily on the surface of charcoal. Why?
Answer/Explanation
Answer:
Explaination:
It is due to the fact, that NH3 has greater molecular size and more easily liquefiable as compared to N2.
56. What is the drawback of Freundlich Adsorption Isotherm.
Answer/Explanation
Answer:
Explaination:
It fails in case of high concentration and high pressure of adsorbate.
57. Write a mathematical expression showing the relationship between the amount of solute adsorbed per unit mass of the solid adsorbent and the concentration of the solute in the solution.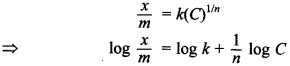
Answer/Explanation
Answer:
Explaination:
where \(\frac{x}{m}\) is the extent of adsorption, x is the mass of adsorbate, m is the mass of adsorbent, C is the concentration of solute in solution and is a constant.
58. Freundlich isotherm becomes independent of pressure at high pressure for a gas absorbed on a solid. Give reason. [Uttarakhand 2019]
Answer/Explanation
Answer:
Explaination:
\(\frac{x}{m}\) ∝ p° at high pressure because extent of adsorption becomes maximum and this pressure is called saturation pressure.
59. What are promoters? Give an example.
Answer/Explanation
Answer:
Explaination:
These are substances which enhance the activity of a catalyst. In Haber’s process, molybdenum (Mo)(i) act as a promoter for Fe(s) which is used as a catalyst.![]()
60. Which catalyst is used in Ostwald’s process for the manufacture of nitric acid?
Answer/Explanation
Answer:
Explaination:
Platinised asbestos at 573 K temperature.
61. Give a chemical reaction which involves heterogeneous catalysis.
Answer/Explanation
Answer:
Explaination:![]()
62. What is not explained by the adsorption theory of heterogeneous catalysis?
Answer/Explanation
Answer:
Explaination:
It does not explain the action of catalytic promoters and poisons.
63. What are two important features of solid catalysts?
Answer/Explanation
Answer:
Explaination:
Activity and selectivity of a solid catalyst.
64. Name an important zeolite catalyst used in the petroleum industry.
Answer/Explanation
Answer:
Explaination: ZSM-5
65. What is the optimum temperature and optimum pH range for enzymatic activity?
Answer/Explanation
Answer:
Explaination:
(i) 298 – 310 K (optimum temperature range)
(ii) 5-7 (optimum pH range).
66. A delta is formed at the meeting point of sea water and river water. Why? [AI 2015 Allahabad & Dehradun; DoE]
Answer/Explanation
Answer:
Explaination:
Muddy river water is a colloidal solution, gets coagulated by electrolytes present in sea water.
67. Write the name of the states of (i) dispersed phase {it) dispersion medium in the case of butter. [AI 2015 Patna]
Answer/Explanation
Answer:
Explaination:
(i) Water is dispersed phase and,
(ii) Oil is dispersion medium.
68. Write the dispersed phase and dispersion jnedium in smoke. [AI 2015 Guwahati; Delhi 2013]
Answer/Explanation
Answer:
Explaination:
Dispersed phase is solid (carbon particles) and dispersion medium is gas (air).
69. Give one example each of sol and gel. [Delhi 2014]
Answer/Explanation
Answer:
Explaination:
Starch in water is ‘sol’, whereas cheese is ‘gel’.
70. Give one example each of lyophobic sol and lyophilic sol. [Delhi 2014]
Answer/Explanation
Answer:
Explaination:
Starch in water is lyophilic sol, whereas AS2S3 in water is lyophobic sol.
71. Write a method by which lyophobic colloids can be coagulated. [AI 2015]
Answer/Explanation
Answer:
Explaination:
If we add electrolyte, lyophobic sols can be coagulated.
72. Give an example of multimolecular and macro-molecular colloid each.
Answer/Explanation
Answer:
Explaination:
(i) Multimolecular colloid – Sulphur sol
(ii) Macromolecular colloid – Starch
73. Based on the type of dispersed phase, what type of colloid is micelles? [CBSE Sample Paper 2018; Foreign 2014]
Answer/Explanation
Answer:
Explaination:
Associated colloids.
74. Name the temperature above which the formation of micelles takes place. [Foreign 2014]
Answer/Explanation
Answer:
Explaination:
Kraft temperature.
75. What is meant by critical micelle concentration?
Answer/Explanation
Answer:
Explaination:
The lowest concentration of the surfactant (e.g. soap solution) at which micelle formation takes place is called CMC (Critica Micelle Concentration). For soaps, the CMC is 10-4-10-3 mol L-1.
76. Write a chemical reaction involved in the preparation of colloid by double decomposition.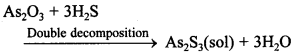
Answer/Explanation
Answer:
Explaination:
77. Name any two colloidal sols of metals which are prepared by electrical disintegration.
Answer/Explanation
Answer:
Explaination:
Gold and silver.
78. Name any one peptising agent.
Answer/Explanation
Answer:
Explaination: NaCl
79. Define Dialysis.
Answer/Explanation
Answer:
Explaination:
Dialysis: A process of removing a dissolved substance from a colloidal solution by means of diffusion through a suitable membrane is called dialysis.
80. Which process is used to made dialysis faster? Explain.
Answer/Explanation
Answer:
Explaination:
Electrodialysis: In this process, an electric field is applied if the dissolved substance in the impure colloidal solution is only an electrolyte.
81. What is the use of collodion solution in the process of ultrafiltration?
Answer/Explanation
Answer:
Explaination:
The pores of filter paper can be reduced in size by impregnating with collodion solution to stop the flow of colloidal particles.
82. Why the colligative properties are of small order in colloidal particles as compared to true solutions at same concentration?
Answer/Explanation
Answer:
Explaination:
This is because the number of particles in a colloidal solution is comparatively small as compared to a true solution.
83. True solution does not show Tyndall effect. Give reason. [Uttarakhand 2019]
Answer/Explanation
Answer:
Explaination:
Their particle size is too small (< lm), do not scatter light so do not show Tyndall effect.
84. What changes in colour are observed, as the size of particles increases in a red colour finest gold sol?
Answer/Explanation
Answer:
Explaination:
It appears purple, then blue and finally green.
85. Which property of colloidal solution is responsible for the stability of sols?
Answer/Explanation
Answer:
Explaination:
Brownian movement.
86. What will be the charge on Agl colloidal particles when it is prepared by addition of small amount of AgN03 to KI solution in water. What is responsible for development of this charge? [AI 2015 Bhubaneswar; Delhi 2012(C)]
Answer/Explanation
Answer:
Explaination:
It will be negatively charged due to the adsorption of I– ions as KI is in excess.
87. What is the ‘coagulation’ process? [AJ2017]
Answer/Explanation
Answer:
Explaination:
The conversion of a colloidal solution into precipitate is called coagulation. In other words, the process of settling of colloidal particles is called coagulation.
88. Out of BaCl2 and KC1, which one is more effective in causing coagulation of a negatively charged colloidal Sol? Give reason. [Delhi 2015]
Answer/Explanation
Answer:
Explaination:
BaCl2 will be more effective because Ba2+ has higher charge than K+.
89. Why are substances like platinum and palladium often used for carrying out electrolysis of aqueous solutions?
Answer/Explanation
Answer:
Explaination:
It is because they are inert, i.e. least reactive.
90. Give one example each of ‘oil in water’ and ‘water in oil’ emulsions. [AI 2017]
Answer/Explanation
Answer:
Explaination:
Milk is an example of oil in water and liquefied butter is an example of water in oil emulsions. [AI 2017]