Solid State : Notes and Study Materials -pdf
Notes and Study Materials
- Concepts of General Principles and Processes of Isolation of Elements
- Master File General Principles and Processes of Isolation of Elements
- NCERT Solutions for – General Principles and Processes of Isolation of Elements
- NCERT Exemplar Solutions for – General Principles and Processes of Isolation of Elements
- Mind Map of General Principles and Processes of Isolation of Elements
- Past Many 12th Board Years of General Principles and Processes of Isolation of Elements
Examples and Exercise
- General Principles and Processes of Isolation of Elements : Practice Paper 1
- General Principles and Processes of Isolation of Elements : Practice Paper 2
- General Principles and Processes of Isolation of Elements : Practice Paper 3
- General Principles and Processes of Isolation of Elements : Practice Paper 4
CBSE Class 12th Chemistry Notes: General Principles and Processes of Isolation of Elements
General Principles and Processes of Isolation of Elements is an important chapter of CBSE Class 12th Chemistry. This is chapter number 6th of NCERT Class 12th Chemistry text books. Questions based on this chapter are frequently asked in CBSE Class 12th board examinations.
General Principles and Processes of Isolation of Elements is the most important chapter of CBSE Class 12th Chemistry. This is chapter number 6th of NCERT Class 12th Chemistry text books. Questions based on this chapter are frequently asked in CBSE Class 12th board examinations. Here, you will get important notes of this chapter.
The main topics covered in this article are:
o Definition of
• Minerals
• Ore
• Gangue
• Metallurgy
o Extraction of metals
• Concentration of ore
• Extraction of crude metal from concentrated ore
o Purification of crude metal
o Mond’s process
o Van-Arkel method
o Predicting spontaneity of a reaction
o Ellingham diagrams
o Extraction of
• Zinc from zinc oxide
• Iron from iron oxide
• Copper from copper oxide
Notes on the chapter are as follows:
Mineral
The naturally occurring substances in the form of which the metals occur in the earth’s crust are called minerals.
Ore
The mineral from which a metal can be extracted profitably and conveniently is called an ore.
Gangue
The earthy impurities like sand, rock, etc., that surround the worthy mineral in a ore, are called called gangue.
Metallurgy
The scientific and technological process used for separating metal from its ore is known as metallurgy.
Extraction of metals
The extraction of metals involves three major steps:
(i) Concentration of the ore
(ii) Isolation of the metal from its concentrated ore
(iii) Purification of the metal
CBSE Class 12th Chemistry Notes: Surface Chemistry
Concentration of ores
The process of removal of unwanted materials from the ore is called concentration or benefaction of the ore.
It can be carried out by various steps as stated below:
Hydraulic washing: In this method, the lighter earthy impurities are washed away from the heavier ore particles. Thus this method of concentration of ore is based on the difference in specific gravities of the ore and gangue particles.
Magnetic separation: This method is based on the magnetic and non-magnetic properties of the ore components.
Froth flotation: This method is quite useful for the purification of the sulphide ores. The mineral particles are wetted by oils and the gangue particles by water. As a result. the ore particles become light and rise to the top in the form of froth while the gangue particles become heavy and settle down. The froth can be stabilised by the addition of stabilisers like aniline or cresols.
Leaching: This method is useful in case the ore is soluble in a suitable solvent. For example, Bauxite is leached with a hot concentrated solution of NaOH which dissolves aluminium while other oxides like Fe2O3, SiO2, etc., remain undissolved
Extraction of crude metal from concentrated ore
Concentrated ore is usually converted to oxide before reduction, as oxides are easier to reduce. Thus, extraction of crude metal from concentrated ore involves two major steps:
(i) Conversion to oxide.
(ii) Reduction of the oxides to metal
Conversion to oxide
It can be carried out by following two methods:
Calcination: It is the process of converting an ore into its oxide by heating it in a limited supply of air or in absence of air, below its melting point. The volatile matter is burnt away and the oxide of the metal is obtained. This process is useful for metal carbonates and hydroxides. For example: CaCO3 → CaO + CO2 and Al2O3.2H2O → Al2O3 + 2H2O.
Roasting: It is the process of converting an ore into its oxide by heating the ore in excess of oxygen (air). This process is commonly used for suiphide ores.
For example: 2PbS + 3O2 → PbO + 2 SO2 and 2 Cu2S + 3O2 → 2 Cu2O + 2 SO2
Reduction of metal oxides to metal
The metal present in metal oxide can be converted from cationic form to free by supplying electrons, i.e., by reduction of metal oxide. The nature of reducing agent used depends upon the activity of metal.
For example: If the metal is very reactive like Na, K, electrolytic reduction method is used whereas the less reactive metals like Cu, Sn, Fe can be reduced by chemical reducing agents like CO, H2, etc.
Reduction by carbon (Smelting):
The process of using carbon in form of coke, charcoal, CO to reduce metal oxides to respective metals, is termed as smelting.
For example: Fe2O3 + 3 CO → 2 Fe + 3CO2
Reduction by hydrogen:
Because of highly inflammable nature of H2 it is used as a reducing agent especially for oxides of Tungsten (W) and Molybdenum (Mo).
For example: MO3 + 3H2 → M + 3H2O
Reduction by Aluminium:
Reduction of metal oxides to respective metals by using aluminium is known as alumino thermite or Gold Schmidt thermite process. It is mainly used to reduce Cr2O3 or Fe2O3
For example: Cr2O3 + 2Al → Al2O3 + 2Cr
Electrolytic refining:
This method is used for Cu, Au, Ag, Pb, zinc, aluminium etc. The impure metal is made anode and pure metal is cathode. The following reaction takes place:
Anode: M → Mn+ + ne‒
Cathode: Mn+ + ne‒ → M
The impurities are collected at the bottom of anode and are called anode mud.
Zone refining method:
It is the method used to get elements of very high purity like Ge, Si, B, Ga. The method is based on the principle that the impurities are more soluble in the melt than in the pure metal. The method is specially useful for producing semiconductors of very high purity.
Distillation:
This method of purification is useful for low boiling metals. Examples are purification of zinc and mercury. Liquation In this method of purification, a low melting metal like tin can be made to flow on a sloping surface and thus separated from higher melting impurities.
Liquation
In this method a low melting metal like tin can be made to flow on a sloping surface. In this way it is separated from higher melting impurities.
Vapour phase refining
In this method, we convert the metal into its volatile compound which is then decomposed to give pure metal. For the application of this method:
(i) The metal should form a volatile compound with an available reagent
(ii) The volatile compound should be easily decomposable, so that the recovery is easy.
Chromatographic method
This method is based on the principle that different components of a mixture are differently adsorbed on an adsorbent. The method is suitable for such elements which are available only in minute quantities and the impurities are not very much different in their behaviour from the element to be purified.
Mond’s process
It is used for the refining of Ni. Nickel on heating with CO form volatile Ni(CO)4 which when subjected to high temperature decomposes to give pure nickel.
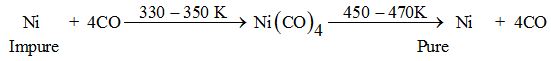
Van-Arkel method: This method is also used to purify Zr and Ti
![]()
Predicting spontaneity of a reaction
The spontaneity of a reaction is decided by the Gibbs energy change ΔG, which is given as, ΔG = ΔH ‒ TΔS
Where, ΔH is enthalpy change and ΔS is entropy change.
The sign of ΔG depends on the sign of ΔH, ΔS and the temperature. When the value of ΔG is negative, only then the reaction will proceed. If ΔS is positive, on increasing the temperature (T), the value of TΔS would increase (ΔH < TΔS) and then ΔG will become –ve.
The graphical representation of Gibbs energy is known as Ellingham diagram. Such diagrams help us in predicting the feasibility of thermal reduction of an ore.
The height of the line in an Ellingham diagram indicates the instability of the oxide (or the sulphide ore) since the higher the line, the more positive the ΔG, the less spontaneous the formation of the oxide (or the sulphide).
Example: Consider the reduction of Cr2O3 by Al.
The two equations involved in the formation of respective oxides of Cr and Al, are expressed as follows:
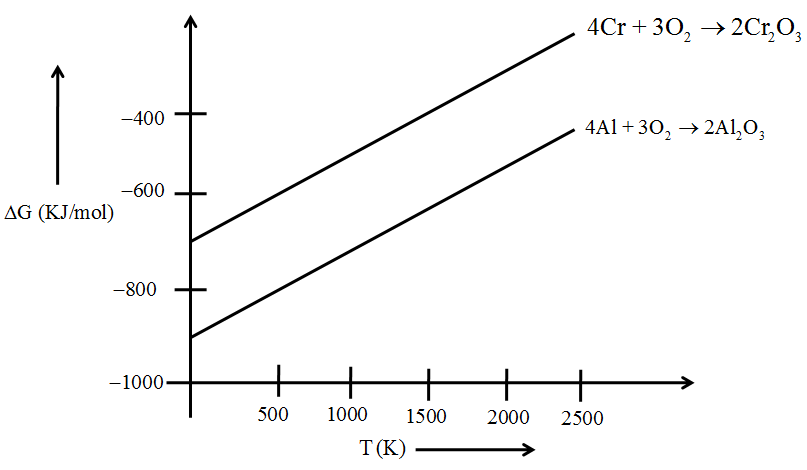
Here formation of aluminium oxide is represented by the lower line, i.e., ΔG is more negative for this reaction which means the oxide formed in this reaction is more stable. Thus Al can be used to reduce Cr2O3 to form more stable oxide, Al2O3. Cr2O3 + 2 Al → 2 Cr + Al2O3
Extraction of ZnO to Zn
The decomposition of ZnO to Zn and O2 does not occur until over 2000 K. However, ZnO can be reduced to Zn using CO at around 1200 K, because above 1200 K, ΔG for the reaction 2Zn + O2 → 2ZnO is more negative than foe the reacton 2 Zn + O2 → 2 ZnO
Extraction of Iron from its Oxides
The haematite ore (Fe2O3) is first calcined and then subjected to smelting. The charge consisting of calcined haematite, coke and limestone is fed into the blast furnace from its top. A blast of hot air is passed near the base of the blast furnace.
The coke undergoes combustion at the bottom of the furnace producing CO2 at about 1900 K. According to the Ellingham diagram, CO is more stable compared to CO2 to CO only above 1000 K , thus at 1900 K , CO is formed. It cools as it rises up the furnace and at a temperature of about 1000 K, the CO/CO2 line so that CO is able to reduce the iron oxide to iron.
Various reactions taking place inside the furnace at 500 – 800 K range are:
3Fe2O3 + CO → 2Fe3O4 + CO2
Fe3O4 + 4 CO → 3 Fe + 4 CO2
Fe2O3 + CO → 2 FeO + CO2
Pig iron: Iron obtained from a blast furnace is called pig iron and contains about 4% carbon and other impurities such as S, P, Si and Mn.
Cast iron: It is the iron that contains about 3% carbon, extremely hard, cannot be welded and brittle.
Wrought iron: Also known as malleable iron, it is the purest form of iron. It is prepared by oxidising the impurities in cast iron in a reverberatory furnace lined with haematile.
Extraction of Copper from Cuprous oxide
Cu2O can be easily redued to Cu directly by heating with coke. But in case if Cu ores are sulphides and contain some iron, the following methods are applied:
(i) Froth flotation of the sulphide ore
(ii) Roasting of the sulphide ore
2 Cu2S + 3O2 → 2 Cu2O + 2 SO2
2 FeS + 3 O2 → 2FeO + 2SO2
FeO can be removed by using SiO2 as flux
FeO + SiO2 → FeSiO3
(iii) The Cu2O above obtained is reduced to Cu by using Cu2S (Auto reduction)
2 Cu2O + Cu2S → 6Cu + SO2
The solidified copper obtained has blistered appearance due to the evolution of SO2 and so it is called blister copper.
General Principles and Processes of Isolation of Elements Class 12 Chemistry MCQs
1. Which of the following ore is best concentrated by froth floatation process?
(a) Magnetic
(b) Siderite
(c) Galena
(d) Malachite
Answer/Explanation
Answer: c
Explaination: (c) Galena is sulphide ore (PbS).
2. Extraction of gold and silver involves leaching with CN–. Silver is later recovered by
(a) Distillation
(b) Zone refining
(c) Displacement by Zn
(d) Liquation
Answer/Explanation
Answer: c
Explaination:
(c) 2[Ag(CN)2]– → [Zn(CN)4]2-+2Ag
3. Which one of the following ore is concentrated by chemical leaching method?
(a) Galena
(b) Copper pyrites
(c) Cinnabar
(d) Argentite
Answer/Explanation
Answer: d
Explaination:
(b) Ag2S + 4NaCN → 2Na[Ag(CN)2] = Na2S
4. According to Ellingham diagram, the oxidation reaction of carbon to carbon monoxide may be used to reduce which one of the following at lowest temperature?
(a) Al2O3
(b) Cu2O
(c) MgO
(d) ZnO
Answer/Explanation
Answer: b
Explaination:![]()
Cu2O can be easily reduced, is at top of diagrams.
5. The first step in extraction of copper from CuFeS2 is
(a) reduction by carbon
(b) electrolysis of ore
(c) roasting in O2
(d) magnetic separation
Answer/Explanation
Answer: c
Explaination:
(c) 2CuFeS2 + O2 → Cu2S + 2FeS + SO2
6. Roasted copper pyrites on smelting with sand produces
(a) FeSiO3 and Cu2S
(b) CaSiO2 and Cu2O
(c) Ca3(PO4)2 and Cu2S
(d) Fe3(PO4)2 and Cu2S
Answer/Explanation
Answer: a
Explaination:
(a) FeO + SiO2 → FeSiO3
2FeS + 3O2 → 2FeO + 2SO2
7. The composition of copper matte is
(a) Cu2S + FeS
(b) Cu2S + Cu2O
(c) Cu2S + FeO
(d) Cu2O + FeS
Answer/Explanation
Answer: a
Explaination:
(a) Cu2S and FeS is called copper matte.
8. ∆G° vs T plot in the Ellingham’s diagram slopes downward for the reaction.
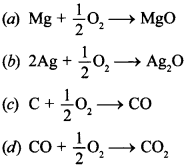
Answer/Explanation
Answer: c
Explaination:
(c) C + \(\frac{1}{2}\) O2 → CO slopes downward because ∆G° increases with increase in magnitude.
9. A number of elements are available in earth’s crust but most abundant elements are ___________ . [NCERT Exemplar]
(a) Al and Fe
(b) Al and Cu
(c) Fe and Cu
(d) Cu and Ag
Answer/Explanation
Answer: a
Explaination:
(a) Fe and Al are most abundant elements in the earth crust.
10. Brine is electrolysed by using inert electrodes. The reaction at anode is ___________ . [NCERT Exemplar]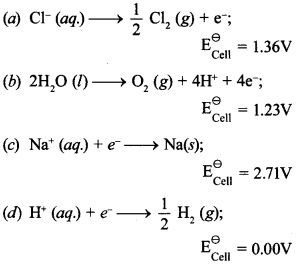
Answer/Explanation
Answer: a
Explaination:
(a) It has highest oxidation potential.
11. In the extraction of chlorine by electrolysis of brine ___________ . [NCERT Exemplar]
(a) oxidation of Cl– ion to chlorine gas occurs.
(b) reduction of Cl– ion to chlorine gas occurs.
(c) For overall reaction ∆G° has negative value.
(d) a displacement reaction takes place.
Answer/Explanation
Answer: a
Explaination:
(a) 2Cl– – 2e– → Cl2 (oxidation)
12. Which of the following reactions is an example of auto reduction? [NCERT Exemplar]
(a) Fe3O4 + 4CO → 3Fe + 4CO2
(b) Cu2O + C → 2Cu + CO
(c) Cu2+ (aq) + Fe (s) → Cu (s) + Fe2+ (aq)
(d) Cu20 + \(\frac{1}{2}\)Cu2S → 3Cu + \(\frac{1}{2}\) SO2
Answer/Explanation
Answer: d
Explaination:
(d) Autoreduction because Cu2S acts as reducing agent.
13. The electrolytic reduction technique is used in the extraction of
(a) Highly electronegative elements.
(b) Highly electropostive elements.
(c) Metalloids.
(d) Transition metals.
Answer
Answer: b
14. In the commercial electrochemical process for aluminium extraction, electrolyte used is
(a) Al(OH)3 is NaOH solution.
(b) An aqueous solution of Al2 (SO4)3.
(c) A molten mixture of Al2O3 and Na3AlF6.
(d) A molten mixture of Al2O3 and Al(OH)3.
Answer
Answer: c
15. Which ore can be best concentrated by froth floatation process?
(a) Malachite
(b) Cassiterite
(c) Galena
(d) Magnetite
Answer
Answer: c
16. Electrolytic reduction of Al2O3 to Al by Hall- Herault process is carried out
(a) in presence of NaCl.
(b) in presence of fluorite.
(c) in presence of cryolite which forms a melt with lower melting point.
(d) in presence of cryolite which forms a melt with high melting point.
Answer
Answer: c
17. The chemical composition of ‘slag’ formed during the melting process in the extraction of copper is
(a) Cu2O + FeS
(b) FeSiO3
(c) CuFeS2
(d) Cu2S + FeO
Answer
Answer: b
18. Bessemer converter is used in the manufacture of
(a) Pig iron
(b) Steel
(c) Wrought iron
(d) Cast iron
Answer
Answer: b
19. The method of zone refining of metals is based on the principle of
(a) greater mobility of the pure metal than that of the impurity.
(b) higher melting point of the impurity than that of the pure metal.
(c) greater noble character of the solid metal than that of impurity.
(d) greater solubility of the impurity in the molten state than in the solid.
Answer
Answer: d
20. In the leaching of Ag2S with NaCN, a stream of air is also passed. It is because
(a) The reaction between Ag2S and NaCN is reversible.
(b) to oxidise Na2S formed in the reaction to Na2SO4.
(c) to oxidise Ag2S to Ag2O.
(d) Both (a) and (b).
Answer
Answer: d
21. Purest form of iron is
(a) Cast iron
(b) Hard Steel
(c) Stainless steel
(d) Wrought iron
Answer
Answer: d
22. Consider the following reaction at 1000° C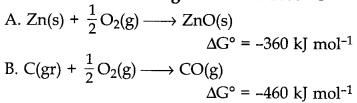
Choose the correct statement at 1000°C
(a) Zinc can be oxidised by carbon monoxide.
(b) Zinc oxide can be reduced by graphite.
(c) Both statements (a) and (b) are correct.
(d) Both statements (a) and (b) are false.
Answer
Answer: b
Note: In the following questions two or more options may be correct. (Q.13 to Q.17)
23. At the temperature corresponding to which of the points in Fig. FeO will be reduced to Fe by coupling the reaction 2FeO → Fe + O2 with all of the following reactions? [NCERT Exemplar]
(i) C + O2 > CO2
(ii) 2C + O2 > 2CO and
(Hi) 2CO + O2 > 2 CO2
(a) Point A
(b) Point B
(c) Point C
(d) Point D
Answer/Explanation
Answer: b
Explaination:
(b) and (d). Below these points all can reduce FeO.
2FeO → 2Fe + CO2
FeO + CO → Fe + CO
FeO + CO → Fe + CO2
24. Which of the following options are correct? [NCERT Exemplar]
(a) Cast iron is obtained by remelting pig iron with scrap iron and coke using hot ‘ air blast.
(b) In extraction of silver, silver is extracted as cationic complex.
(c) Nickel is purified by zone refining.
(d) Zr and Ti are purified by van Arkel method.
Answer/Explanation
Answer:
Explaination: (a) and (d) are correct.
25. In the extraction of aluminium by Hall-Heroult process, purified Al2O3 is mixed with CaF2 to [NCERT Exemplar]
(a) lower the melting point of Al2O3.
(b) increase the conductivity of molten mixture.
(c) reduce Al3+ into Al(s).
(d) acts as catalyst.
Answer/Explanation
Answer:
Explaination: (a) and (b) are correct.
26. Common impurities present in bauxite are [NCERT Exemplar]
(a) CuO
(b) ZnO
(c) Fe2O3
(d) SiO2
Answer/Explanation
Answer:
Explaination:
(c) and (d) Fe2O3 and SiO2 are impurities present.
27. Which of the following statements is correct about the role of substances added in the froth floation process? [NCERT Exemplar]
(a) Collectors enhance the non-wettability of the mineral particles.
(b) Collectors enhance the wettability of gangue particles.
(c) By using depressants in the process two sulphide ores can be separated.
(d) Froth stabilisers decrease wettability of gangue.
Answer/Explanation
Answer:
Explaination: (a) and (c) are correct.
28. Match the items of Column I with the items of Column II and assign the correct code
| Column I | Column II |
| (a) Pendulum | (1) Chrome steel |
| (b) Malachite | (2) Nickel steel |
| (c) Calamine | (3) Na3AlF6 |
| (d) Cryolitex | (4) CuCO3.Cu (OH)2 |
| (5) ZnCO3 |
Code:
(i) A (1) B (2) C (3) D (4)
(ii) A (2) B (4) C(5) D (3)
(iii) A (2) B (3) C (4) D (5)
(iv) A (4) B (5) C (3) D (2)
Answer/Explanation
Answer:
Explaination:
(ii) A (2) B (4) C (5) D (3)
29. Match the items of Column I with the items of Column II and assign the correct code:
| Column I | Column II |
| (A) Coloured bands | (1) Zone refining |
| (B) Impure metal to volatile complex | (2) Fractional distillation |
| (C) Purification of Ge and Si | (3) Mond Process |
| (D) Purification of mercury | (4) Chromato graphy |
| (5) Liquation |
Code:
(i) A (1) B (2) C (4) D (5)
(ii) A (4) B (3) C(l) D (2)
(iii) A (3) B(4) C (2) D (1)
(iv) A (5) B (4) C (3) D (2)
Answer/Explanation
Answer:
Explaination:
(ii) A (4) B(3) C (1) D (2)
30. Match items of Column I with the items of Column II and assign the correct code:
| Column I | Column II |
| (A) Cyanide process | (1) Ultrapure Ge |
| (B) Froth Floatation Process | (2) Dressing of ZnS |
| (C) Electrolytic reduction | (3) Extraction of A1 |
| (D) Zone | (4) Extraction of Au |
| (5) Purification of Ni |
Code:
(i) A (4) B (2) C (3) D (1)
(ii) A (2) B (3) C (1) D (5)
(iii) A (1) B (2) c (3) D (4)
(iv) A (3) B (4) C (5) D (1)
Answer/Explanation
Answer:
Explaination:
(i) A (4) B (2) C (3) D (1)
31. Match items of Column I with the items of Column II and assign the correct code:
| Column I | Column II |
| (A) Sapphire | (1) Al2O3 |
| (B) Sphalerite | (2) NaCN |
| (C) Depressant | (3) Co |
| (D) Corundum | (4) ZnS |
| (5) Fe2O3 |
Code:
(i) A (3) B (4) C (2) D (1)
(ii) A (5) B (4) C (3) D (2)
(iii) A (2) B (3) C (4) D (5)
(iv) A (1) B (2) C (3) D (4)
Answer/Explanation
Answer:
Explaination:
(i) A (3) B (4) C (2) D (1)
Note: In the following questions a statement of assertion followed by a statement of reason is given. Choose the correct answer out of the following choices. (Q.22 and Q.23)
(a) Both assertion and reason are true and reason is the correct explanation of assertion.
(b) Both assertion and reason are true but reason is not the correct explanation of assertion.
(c) Assertion is true but reason is false.
(d) Assertion is false but reason is true.
(e) Assertion and reason both are wrong.
32. Assertion: Hydrometallurgy involves dissolving the ore in a suitable reagent followed by precipitation by a more electropositive metal.
Reason: Copper is extracted by hydrometallurgy. [NCERT Exemplar]
Answer/Explanation
Answer: b
Explaination:
(b) Both assertion and reason are true but reason is not the correct explanation of assertion.
33. Assertion: Gold and Platinum occur in native state.
Reason: Gold and platinum are least reactive metals. [NCERT Exemplar]
Answer/Explanation
Answer: a
Explaination:
(a) Both assertion and reason are true and reason is the correct explanation of assertion.
34. Two reactions in which one has AG=+ve, other has AG = -ve but overall AG is -ve are called
Answer/Explanation
Answer:
Explaination: Coupled Reaction
35. PbS + O2 → ___________ + SO2
Answer/Explanation
Answer:
Explaination: PbO
36. FeS2 + O2 → ___________ + SO2
Answer/Explanation
Answer:
Explaination: Fe2A3
37. Mg metal can reduce Al2O3 but the process will be uneconomical. [True/False]
Answer/Explanation
Answer:
Explaination: True
38. The reduction of metal oxide easier if the metal formed is in liquid state at the temperature of reduction. [True/False]
Answer/Explanation
Answer:
Explaination: True
39. CO2 gas convert Na[Al(OH)J to Al203.xH20. [True/False]
Answer/Explanation
Answer:
Explaination: True
40. SiO2 reacts witl NaOH to form Na2SiO3. [True/False]
Answer/Explanation
Answer:
Explaination: True
41. Name the most abundant element on earth.
Answer/Explanation
Answer:
Explaination: Oxygen.
42. Which impurities are present in ruby and saphire?
Answer/Explanation
Answer:
Explaination: Cr is present in ruby and Co in sapphire.
43. What is the formula of calamine?
Answer/Explanation
Answer:
Explaination: ZnCO3.
44. What type of ores can be concentrated by magnetic separation method? [AI2011]
Answer/Explanation
Answer:
Explaination:
Those ores which are magnetic in nature, i.e. attracted by magnet, whereas impurities are non-magnetic in nature or vice versa are concentrated by magnetic separation method.
45. Name the method used for removing gangue from sulphide ore. [AI 2013]
Answer/Explanation
Answer:
Explaination: Froth floatation process.
46. Name the depressant which is used to separate ZnS and PbS ores in froth floatation process. [Foreign 2014]
Answer/Explanation
Answer:
Explaination: Sodium cyanide (NaCN).
47. Which reducing agent is employed to get copper from the leached low grade copper ore? [Delhi 2014; AI 2012,12(C); DoE]
Answer/Explanation
Answer:
Explaination:
Iron
Fe(s) + Cu2+(aq) → Fe2+ + Cu(s)
48. What is the role of zinc metal in the extraction of silver? [AI 2015 Ajmer; AI 2014]
Answer/Explanation
Answer:
Explaination:
Zinc acts as a reducing agent.
49. Write a chemical reaction involved in the process of calcination.
Answer/Explanation
Answer:
Explaination:![]()
50. Write a chemical reaction involved in the process of roasting.
Answer/Explanation
Answer:
Explaination:![]()
51. Give a chemical reaction involved in reduction with Coke (C)
Answer/Explanation
Answer:
Explaination: PbO + C(coke) → Pb + CO.
52. What is meant by the term ‘pyrometallurgy’?
Answer/Explanation
Answer:
Explaination:
The process of reducing a metal oxide with coke or any other reducing agent at high temperature is called pyrometallurgy. In other words, it is a thermal process of extracting a metal from its ore.
53. Write an application of ellingham diagram.
Answer/Explanation
Answer:
Explaination:
It helps in predicting the feasibility of thermal reduction of an ore.
54. Define wrought iron.
Answer/Explanation
Answer:
Explaination:
Wrought iron: It is the purest form of iron. It is also called malleable iron. It is prepared by oxidative refining of pig iron in reverberatory furnace lined with haematite which oxidises carbon to carbon monoxide.
55. Give the chemical reaction involved for extraction of zinc from zinc oxide.
Answer/Explanation
Answer:
Explaination:![]()
56. What is the role of CO2 in getting pure alumina (Al22O3) in the extraction of AI. [Delhi 2014(C)]
Answer/Explanation
Answer:
Explaination:
CO2 gas neutralises sodium aluminate and hydrated Al2O3 is precipitated.
57. State the principle of Liquation method of refining crude metals.
Answer/Explanation
Answer:
Explaination:
Liquation Method: Those metals which have impurities whose melting points are higher than metal can be purified by this method. Sn metal is purified by this method. Tin containing iron as impurity is heated on the top of sloping furnace. Tin melts and flows down the sloping surface, whereas iron is left behind and pure tin is obtained.
58. What type of metals are generally extracted by electrolytic method? [Delhi 2019]
Answer/Explanation
Answer:
Explaination:
Highly reacted metals are generally extracted by electrolytic method.
59. On what principle is the method of zone refining of metals based? [Foreign 2014]
Answer/Explanation
Answer:
Explaination:
It is based on the principle that impurities are more soluble in melt than pure metal.
60. How is Germanium refined? [Foreign & Delhi 2017]
Answer/Explanation
Answer:
Explaination: It is purified by zone refining.
61. Name the method of refining to obtain silicon of high purity. [AI 2015 Allahabad & Dehradun, Delhi 2019]
Answer/Explanation
Answer:
Explaination: Zone refining.
62. Name the method that is used for refining of nickel. [AI 2014]
Answer/Explanation
Answer:
Explaination: Mond’s process (vapour phase refining).
63. Define sintering.
Answer/Explanation
Answer:
Explaination:
Sintering is the process of crushing ore to suitable size before concentration of ore.
64. What is blister copper?
Answer/Explanation
Answer:
Explaination:
Blister copper: It is solidified copper extracted from cuprous oxide. It has blistered appearance due to the evolution of SO2.
65. Define smelting.
Answer/Explanation
Answer:
Explaination:
Smelting: It is a metal extraction process in which an ore (Cor Co) is heated at high temperature in an enclosed furnace. Reduction is takes place in this process.![]()