p- Block Elements : Notes and Study Materials -pdf
Notes and Study Materials
- Concepts of p- Block Elements
- Master File p- Block Elements
- NCERT Solutions for – p- Block Elements
- NCERT Exemplar Solutions for – p- Block Elements
- Mind Map of p- Block Elements
- Concept Map of p- Block Elements
- Past Many 12th Board Years of p- Block Elements
Examples and Exercise
CBSE Class 12th Chemistry Notes: The p-Block Elements
The p-Block Elements, is an important chapter of CBSE Class 12th Chemistry. This is chapter number 7th of NCERT Class 12th Chemistry textbook. Questions based on this chapter are frequently asked in CBSE Class 12th board examinations.
The p-Block Elements, is an important chapter of CBSE Class 12th Chemistry. This is chapter number 7th of NCERT Class 12th Chemistry textbook. Questions based on this chapter are frequently asked in CBSE Class 12th board examinations. Here you will get important notes on this chapter.
The main topics covered in these quick notes are:
• Definition of p-block elements
• Nitrogen family
• General properties of nitrogen family
• Anomalous properties of nitrogen
• Reactivity of group 15 elements towards:
o Hydrogen
o Oxygen
o Halogens
o Metals
• Preparation, properties and uses of dinitrogen (N2)
• Preparation, properties and uses of ammonia (NH3)
• Oxides of nitrogen
• Preparation, properties and uses of nitric acid (HNO3)
• Brown ring test
• Allotropes of phosphorus (P)
• Uses of phosphorus
• Preparation and properties phosphine (PH3)
• Phosphorus halides
The notes of the chapter as follow:
p-block elements
The group number 13 to 18, in which the last electrons or the valence electrons enter in the p-orbital are called the p-block elements. The general electronic configuration of p-block elements is ns2 np1 ‒ 6
Nitrogen family
The elements of group 15: nitrogen (N), phosphorus (P), arsenic (As), antimony (Sb) and bismuth (Bi) having general electronic configuration
ns2 np3, are known as the nitrogen family. The s-orbital in these elements is completely filled and p-orbitals are half-filled, making their electronic configuration extra stable.
General properties of nitrogen family:
Atomic and ionic radii: Covalent and ionic radii increase down the group. There is appreciable increase in covalent radii from N to P. However, from As to Bi only a small increase in covalent radius is observed.due to presence of completely filled d or f-orbitals in heavy elements.
Ionisation enthalpy: Ionisation enthalpy goes on decreasing down the group due to the increase in atomic size. Due to the stable electronic configuration with half filled p-orbital, group 15 elements have higher ionisation energy than group 16 elements. Also due to the smaller size of the elements, the group 15 elements have higher ionisation energy than group 14 elements.
Oxidation states: The common oxidation states are +3, +5, –3. The tendency to show –3 oxidation state decreases down the group due to increased size and hence decreased electronegativity. The stability of +5 oxidation state decreases whereas stability of +3 oxidation state increases due to inert pair effect. Nitrogen and phosphorus with oxidation states from +1 to +4 undergo oxidation as well as reduction in acidic medium. This process is called disproportionation.
For example: 3HNO2 → HNO3 + H2O + 2NO
Anomalous properties of nitrogen
Nitrogen has unique ability to form pπ ‒ pπ multiple bonds with itself and with other elements having small size and high electronegativity (e.g., C, O). Thus nitrogen exists as diatomc molecule, N2 with a triple bond between the two atoms. However, P cannot pπ ‒ pπ from bond, therefore P exists as P4 ‒ P4 is highly strained and is chemically reactive while N2 is chemically inert.
The behaviour of nitrogen differs from rest of the elements due to the following reasons:
(i) It has a small size
(ii) It does not have d – orbitals
(iii) It has high electronegativity
(iv) It has high ionization enthalpy
All the elements of Group 15 form trihydrides, MH3 having sp3 type hybridization.
Stability: The stability of hydrides decreases down the group due to decrease in bond dissociation energy down the group.
NH3 > PH3 > AsH3 > SbH3 > BiH3
Boiling point: Boiling point of hydrides (except NH3) increases down the group as with increase in size due to the Van der Waals forces also increase down the group. Boiling point of NH3 is highest due to the presence of hydrogen bonding.
PH3 < AsH3 < NH3 < SbH3 < BiH3
Bond angle: Electronegativity of N is highest. Therefore, the lone pairs will be towards nitrogen and hence more repulsion between bond pairs. Therefore bond angle is the highest. After nitrogen, the electronegativity decreases down the group.
NH3 (107.8°) > PH3 (93.6°) > AsH3 (91.8°) ≈ SbH3 (91.3°) > BiH3 (90°)
Basicity: Basicity decreases down the group.
NH3 > PH3 > AsH3 > SbH3 > BiH3.
Reactivity towards oxygen: All the group 15 elements form two types of oxides: trioxides, M2O3 and pentaoxides, M2O5. The oxide in the higher oxidation state of the element is more acidic than that of lower oxidation state. Their acidic character decreases down the group.
Reactivity towards halogens: Group 15 elements react to form two series of halides: trihalides, MX3 and pentahalides, MX5. Trihalides are sp3 hybridised with pyramidal shape whereas pentahalides are sp3d hybridized with trigonal bipyramidal shape. Nitrogen does not form pentahalide due to non-availability of the d– orbitals in its valence shell. All the trihalides of these elements except those of nitrogen are stable. In case of nitrogen, only NF3 is known to be stable.
Reactivity towards metals: All the group 15 elements react with metals to form binary compounds in –3 oxidation state.
Dinitrogen, N2
Preparation: N2 is produced commercially by the liquifaction of air followed by fractional distillation.
Liquid N2 distills out first leaving behind liquid oxygen. In the laboratory, dinitrogen is prepared by treating an aqueous solution of ammonium chloride with sodium nitrite.
NH4Cl (aq) + NaNO2 (aq) → N2 (g) + 2H2O (l) + NaCl (aq)
Small amounts of NO and HNO3 formed as impurities can be removed by passing the gas through aqueous H2SO4 containing K2Cr2O7.
(NH4)2Cr2O7 → N2 + 4 H2O + Cr2O3
Very pure nitrogen can be obtained by the thermal decomposition of sodium or barium azide
Ba (N3)2 → Ba + 3N2
Properties: Dinitrogen is a colourless, odourless gas, tasteless and non-toxic gas. It is chemically inert at room temperature due to the presence of triple bond with high bond dissociation energy.
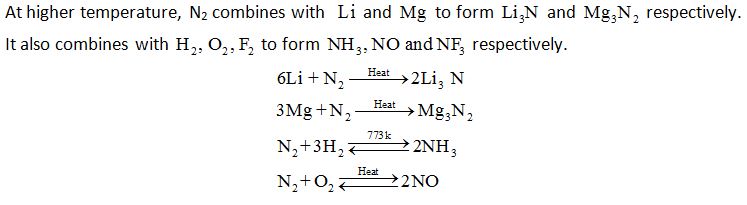
Uses: Some important uses of dinitrogen are as follows:
(i) Liquid N2 is used as a refrigerant
(ii) It is used to provide inert atmosphere in iron and steel industry
(iii) It is used in the manufacture of HNO3 and NH3.
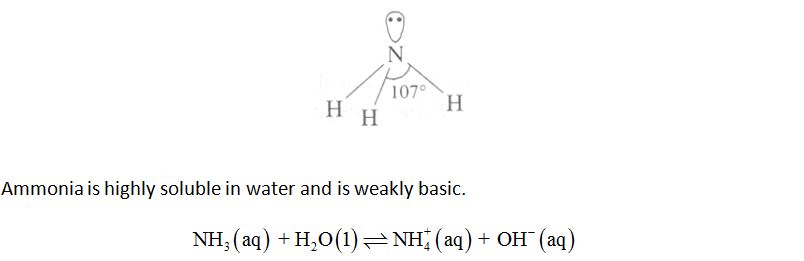
Ammonia, NH3
Ammonia molecule is trigonal pyramidal with nitrogen atom at the apex. It has 3 bond pairs and 1 lone pair. N is sp3 hybridised.
Preparation: Ammonia (NH3) is manufactured on the commercial scale by Haber’s process.
![]()
Pressure ‒ 200 × 105 Pa
Temperature ‒ 773 K
Catalyst ‒ Iron oxide with small amounts of K2O and Al2O3
In laboratory, ammonia is prepared by reacting NH4Cl with NaOH.
NH4Cl + NaOH → NaCl + H2O + NH3
Properties: Due to the presence of the lone pair of electrons on the nitrogen atoms, NH3 is a Lewis base. It can form coordinate covalent bond with the transition metal ion and form complexes, e.g.
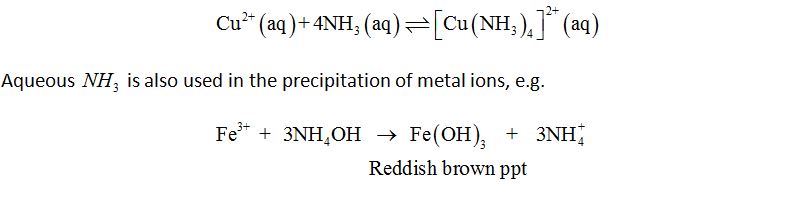
Uses: Some important uses of ammonia are:
(i) It is used as a refrigerant
(ii) It is used in the manufacture of nitric acid,
(iii) It is used in the production of nitrogenous fertilisers.
Oxides of nitrogen
Nitrogen forms a total of five oxides from +1 oxidation state to +5 oxidation state. The five oxides of nitrogen are: N2O, NO, N2O3, NO2 or N2O4, N2O5.
The following table gives the brief information of various oxides of nitrogen:
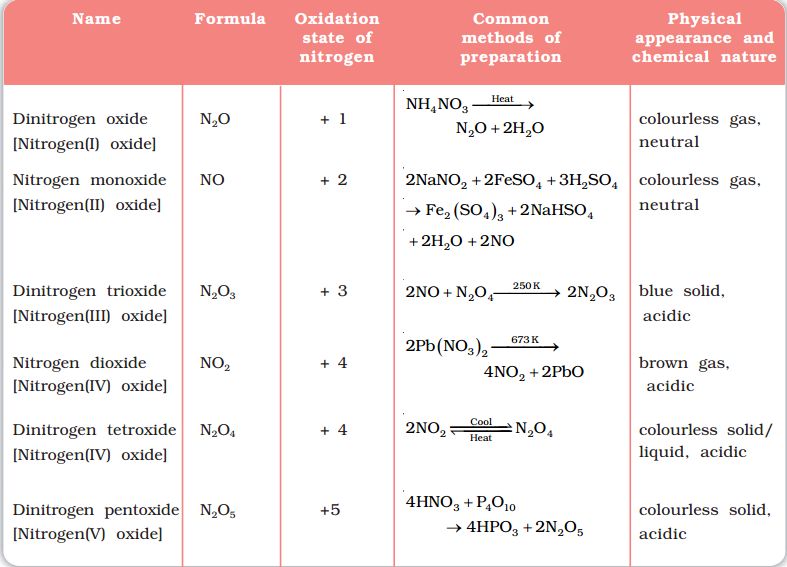
Image Source: NCERT Books
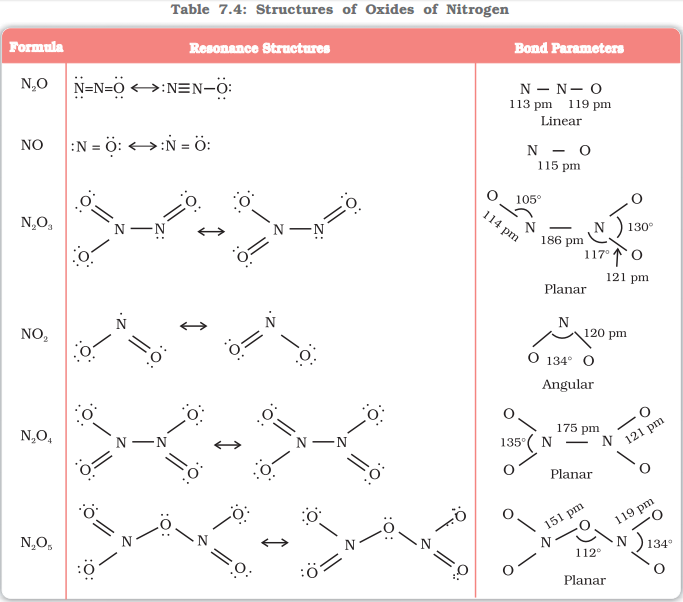
Image Source: NCERT Books
Nitric acid, HNO3
Nitric acid is the most important oxoacid formed by nitrogen. It is a colourless liquid. In the gaseous state, HNO3 exists as a planar molecule with the structure as shown below:
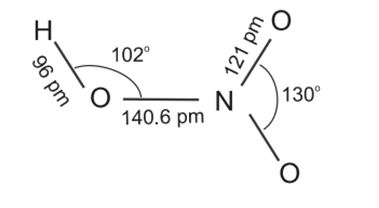
Preparation: Nitric acid is manufactured by the catalytic oxidation of ammonia in Ostwald process.
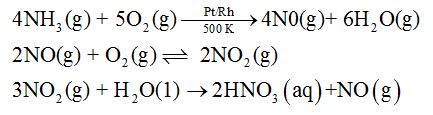
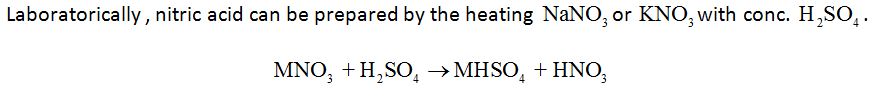
Properties: Concentrated HNO3 is a strong oxidising agent and attacks most metals except noble metals. Cr, Fe and AI do not dissolve in conc. HNO3 due to the formation of a passive film of oxide on the surface.
The oxidising action of HNO3 is depends on its concentration and the nature of the reducing agent. The principal product of reduction of HNO3 is NO when it is dilute but NO2 when it is concentrated.
For example:
3Cu + 8HNO3 (dil) → 3Cu(NO3)2 + 2NO + 4H2O
Cu + 4HNO3 (conc) → Cu(NO3)2 + 2NO2 + 2H2O
Brown ring test for the detection of nitrates:
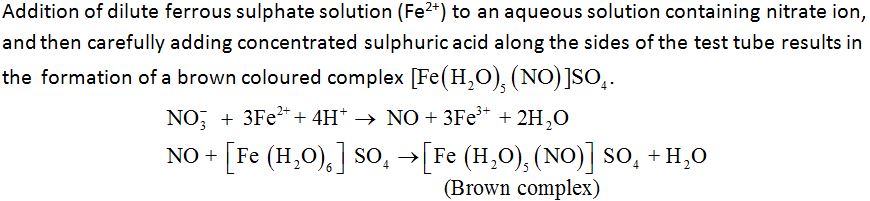
Uses: Some important uses of nitric acid are:
(i) HNO3 is used in the manufacture of fertilisers.
(ii) It is used in the formation of explosives, dynamites, TNT, etc.
(iii) It is also used in the etching of metals.
Phosphorus
Phosphorus is an essential constituent of elements and plants. It has many allotropic forms, the important ones are:
(i) White phosphorus
(ii) Red phosphorus
(iii) Black phosphorus
Properties of white phosphorus:
• Highly toxic and can ignite when exposed to air.
• Waxy, insoluble in H2O
• More reactive than the other solid phases due to the angular strain in the P4 molecule
• Glows in dark
• Discrete tetrahedral P4 molecules.
Properties of red phosphorus:
• Prepared by heating white phosphorus at 573K in an inert atmosphere
• Odourless, nonpoisonous and insoluble in H2O
• Less reactive than white phosphorus
• Polymeric structure consisting of chains of P4 units linked together
• Higher meltng point and density
Properties of black phosphorus:
• Prepared by heating white phosphorus at 473 K under high pressure.
• Exists in two forms – α black P and β black P
• Thermodynamically most stable, i.e., least reactive
• Has an opaque monoclinic or rhombohedral crystals
Uses: Some important uses of phosphorus are:
(i) Used in the manufacture of fertilisers and food grade phosphates.
(ii) Elemental P is used the manufacture of organo-phosphorus compounds used as pesticides.
Phosphine, PH3
Phosphine is a highly poisonous, colourless gas and has a smell of rotten fish.
Preparation: Phosphine is prepared by the reaction of calcium phosphide with water or dilute HCl.
Ca3P2 + 6H2O → 2PH3 + 3Ca(OH)2
Ca3P2 + 6HCl → 2PH3 + 3CaCl2
Properties: It is insoluble in water and is a weaker base than ammonia. Like ammonia, it gives phosphonium compounds with acids.
For example: PH3 + HBr → PH4Br
PH3 is non-inflammable when pure but becomes inflammable owing to the presence of P2H4 or P4 vapours.
In water, PH3 decomposes in the presence of light to give red phosphorus and H2.
Phosphorus halides
Phosphorus forms two types of halides, PX3 (X = F, Cl, Br, I) and PX5 (X = F, Cl, Br).
Phosphorus trichloride, PCl3
It is a colourless oily liquid.
Preparation:
It is obtained by passing dry chlorine over heated white phosphorus or by the action of thionyl chloride with white phosphorus.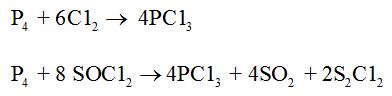
Properties: It has a pyramidal shape, in which phosphorus is sp3 hybridised.
It gets hydrolysed in the presence of moisture.
PCl3 + 3H2O → H3PO3 + 3HCl
Phosphorus pentachloride, PCl
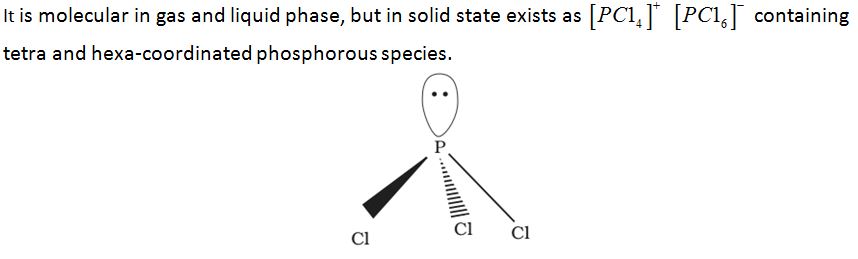
Preparation:
Phosphorus pentachloride is prepared by the reaction of white phosphorus with excess of dry chlorine or can be prepared by the action of SO2Cl2 on phosphorus.
P4 + 10 Cl2 → 4 PCl5
P4 + 10SO2Cl2 → 4 PCl5 + 10SO2
Properties:
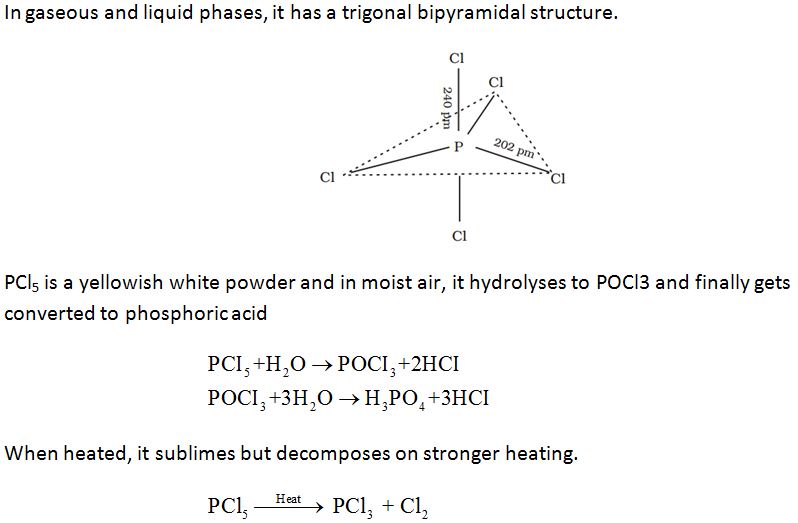
Oxides of nitrogen
Nitrogen forms a total of five oxides from +1 oxidation state to +5 oxidation state. The five oxides of nitrogen are: N2O, NO, N2O3, NO2 or N2O4, N2O5.
The following table gives the brief information of various oxides of nitrogen:
In Part-I we have studied about the Group 15 elements (Nitrogen family), their important compounds like Dinitrogen, Ammonia, Oxides of Nitrogen, Nitric acid, Phosphine, Phosphorus Halides, etc.
In Part-II, we will get acquainted with Group 16 elements. All the these quick notes are based on the latest CBSE syllabus for Class 12th Chemistry.
The main topics covered in these quick notes are:
• Oxygen family or Chalcogens
• General properties of oxygen family
• Anomalous properties of oxygen
• Reactivity of group 16 elements towards:
o Hydrogen
o Oxygen
o Halogens
• Preparation, properties and uses of dioxygen (O2)
• Simple Oxides
• Preparation, properties and uses of ozone (O3)
• Depletion of ozone layer
• Preparation, properties and uses of sulphur dioxide (SO2)
• Oxoacids of sulphur
• Preparation, properties and uses of sulphuric acid (H2SO4)
The notes of the chapter are as follow:
Oxygen family
The elements of group 15: oxygen (O), sulphur (S), selenium(Se), tellurium (Te) and polonium (Po) having general electronic configuration ns2np4, are known as the oxygen family. All these elements collectively are also known as chalcogens. Polonium is a radioactive element.
General properties of oxygen family
Atomic and ionic radii: Due to increase in the number of shells, atomic and ionic radii increase from top to bottom in the group.
Ionisation enthalpy: Due to the increase in size of the atoms the ionisation enthalpy decreases down the group. IE1of group 16 elements is less than the IE1 of group 15. This is because group 15 elements have extrastability due to half-filled p-orbitals.
Electron gain enthalpy: Due the compact nature of oxygen, it has less electron gain enthalpy than sulphur. After sulphur, the electron gain enthalpy decreases down the group.
Electronegativity: The electronegativity decreases down the group. This implies that the metallic character increases down the group from oxygen to polonium.
Melting and boiling point: The melting and boiling point increases with increase in atomic number down the group.
Oxidation states: Group 16 elements show ‒2, +2, +4, +6 oxidation states. The stability of ‒2 oxidation state decreases down the group due to increase in atomic size and decrease in electronegativity. O shows only ‒2 oxidation state except when it combines with the most electronegative F with which it shows positive oxidation states. S shows + 6 only with O and F.
Anomalous behaviour of oxygen
Oxygen forms strong hydrogen bonding in H2O which is not found in H2S. Also, the maximum covalency of oxygen is four, whereas in a case of other elements of the group, the valence shells can be expanded and covalency exceeds four.
Reasons for the anomalous behaviour of oxygen are:
• Small size and high electronegativity
• Absence of d-orbitals
Reactivity towards hydrogen: All the elements of Group 16 form hydrides of the type H2E (E = S, Se, Te, Po).
Thermal stability: Thermal stability of group 16 elements decreases down the group.
H2O > H2S > H2Se> H2Te > H2Po
This is because the H-E bond length increases down the group, hence the bond dissociation enthalpy decreases down the group.
Acidic nature: Due to the decreasing bond dissociation enthalpy, acidic character of group 16 elements increases down the group.
H2O < H2S < H2Se < H2Te
Reducing character: The reducing character also decreases down the group due to the decreasing bond dissociation enthalpy.
H2O < H2S < H2Se < H2Te < H2Po
Reactivity towards oxygen: All group 16 elements form oxides of the type EO2 and EO3 Reducing character of dioxides decreases down the group. Acidity also decreases down the group. Besides EO2 type, sulphur, selenium and tellurium also form EO3 type oxides. Both types of oxides are acidic in nature.
Reactivity with halogens: Elements of Group 16 form a large number of halides of the type, EX2 EX4 and EX6, where X is a halogen. The stability of halides decreases in the order F− > Cl− > Br− > I− . This is because E-X bond length increases with increase in size. Among hexa halides, hexafluorides are the most stable because of steric reasons. Dihalides are sp3 hybridised and have tetrahedral geometry. H2O is a liquid while H2S is a gas. Because in water due to the small size and high electronegativity of O, strong hydrogen bonding is present there.
Oxygen (O)
Oxygen is the first element of Group 16 with the electronic configuration of 1s2 2s2 2p4 in the ground state. Oxygen has two allotropes: dioxygen (O2) and trioxygen or ozone (O3).
Dioxygen (O2)
Oxygen usually exists in the form of dioxygen.
Preparation:
Dioxygen is prepared in the laboratory by thermal decompositions of oxygen rich compounds such as KClO3,
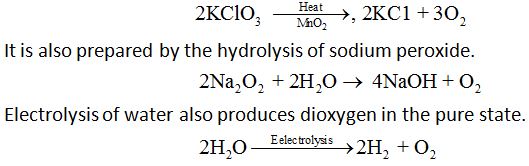
Properties:
(i) Oxygen is a colourless, odourless and is a highly reactive tasteless gas.
(ii) Due to the presence of pπ‒ pπ bonding, O2 is a discrete molecule and intermolecular forces are weak van der Waals forces, hence, O2 is a gas.
(iii) Dioxygen combines with metals and non-metals to form binary compounds called oxides.
Examples are:
2Ca + O2 → 2CaO
P4 + 5O2 → P4O10
Uses:
(i) Dioxygen is used in making steel.
(ii) It is used in the production of oxygen containing organic chemicals.
(iii) Dioxygen is also used for sewage treatment, river revival and paper pulp bleaching.
(iv) It is used as an oxidiser in underwater diving and in space shuttles.
Simple Oxides
Oxygen combines with majority of the elements of the periodic table to forms oxides (O2‒). There are three types of oxides:
(i) Acidic oxides: Oxides of non- metals are usually acidic in nature. For example, SO2 combines with water to give H2SO3, an acid.
SO2 + H2O → H2SO3
(ii) Basic oxides: Metallic oxides are mostly basic in nature. Basic oxides dissolve in water to give basic solution. For example, CaO combines with water to give Ca(OH)2, a base.
CaO + H2O → Ca(OH)2
(iii) Amphoteric oxides: Some metallic oxides show characteristics of both acidic as well as basic oxides. Such oxides are known as amphoteric oxides. For example, Al2O3 reacts with acids as well as alkalies
Al2O3 + 6HCl + 9H2O → 2 [Al(H2O)6]3+ + 6 Cl‒
Base Acid
Al2O3 + 6NaOH + 3H2O → 2Na3[Al(OH)6]
Acid Base
Ozone (O3)
Ozone is an allotropic form of oxygen. Ozone has angular structure with a bond angle of about 117o. Both O = O bonds are of equal bond length due to resonance.
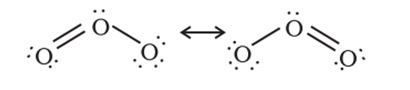
Preparation:
It is formed when dioxygen is irradiated with UV light or silent electric discharge.
3O2 (g) → 2O3 (g) ΔHo = +142 KJ/mol
Properties:
(i) Ozone is a pale blue gas with a characteristic pungent odour.
(ii) Ozone is diamagnetic in nature.
(iii) It is the second most powerful oxidising agent after fluorine. It liberates oxygen gas when acting as an oxidising agent.
2Fe2+ + O3 + 2H+ → 2Fe3+ + H2O + O2
PbS + 4O2 → PbSO4 + 4O2
Depletion of ozone layer:
Thinning of the ozone layer is termed as depletion of ozone layer. The depletion of ozone layer in the stratosphere is caused by the presence of chlorofluoro carbons. CFCs decomposed by UV radiation to produce chlorine which reacts with ozone and this causes a de-crease in the concentration of ozone at a rate faster than its formation from dioxygen. Another cause of depletion of ozone layer is the release of nitrogen oxides into the stratosphere by supersonic jet aeroplanes.
NO + O3 → NO2 + O2
Sulphur (S)
Sulphur exhibits allotropy, two important allotropes of which are:
(a) Yellow Rhombic (α – sulphur)
(b) Monoclinic (β- sulphur)
![]()
At 369 K both forms are stable. S8 puckered shape in both forms and has crown shape.
Sulphur Dioxide (SO2)
The molecule of SO2 is angular. It is a resonance hybrid of the two canonical forms:
![]()
Preparation:
Sulphur dioxide is formed together with a little (6-8%) sulphur trioxide when sulphur is burnt in air or oxygen:
S (g) + O2(g) → SO2(g)
In the laboratory it is readily generated by treating a sulphite with dilute sulphuric acid.
Na2SO3 + H2SO4 → Na2SO4 + H2O + SO2
Properties:
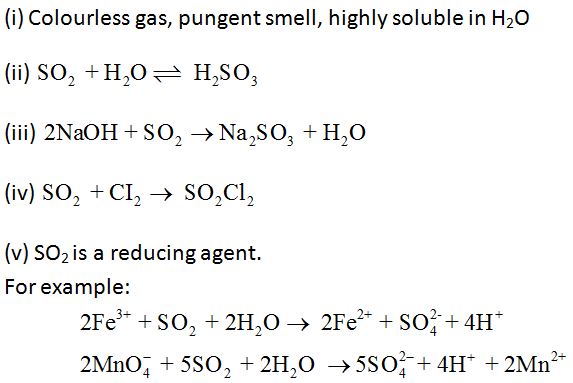
Uses:
(i) SO2 is used in refining petroleum and sugar
(ii) It is used in bleaching wool and silk
(iii) It is also used as a disinfectant and preservative.
(iv) It is used in the manufacture of sulphuric acid, sodium hydrogen sulphite and calcium hydrogen sulphite.
Oxoacids of sulphur
Sulphur forms variety of oxoacids. All oxoacids of sulphur are dibasic.
Sulphur forms a number of oxoacids such as H2SO3, H2S2O3, H2S2O4, H2S2O5, H2SxO6 (x = 2 to 5), H2SO4, H2S2O7, H2SO5, H2S2O8 . Some of these acids are unstable and cannot be isolated. They are known in aqueous solution or in the form of their salts. Structures of some important oxoacids are shown in figure given below
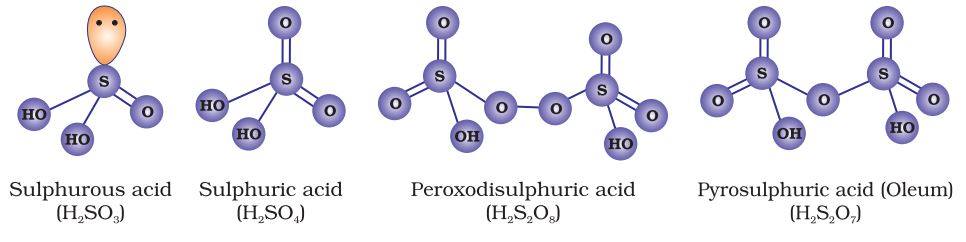
Image Source: NCERT Books
Sulphuric acid (H2SO4)
Preparation:
Sulphuric acid is manufactured by the Contact Process as follows:
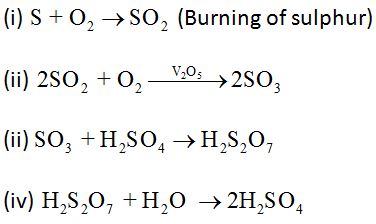
Properties:
(i) Sulphuric acid is a colourless, dense oily liquid.
(ii) It is dibasic acid or diprotic acid.
(iii) It is a strong dehydrating agent.
(iv) It is a moderately strong oxidizing agent.
Uses:
(i) H2SO4 is used in manufacture of fertilisers
(ii) It is also used in petroleum refining, manufacture of pigments, detergents, metallurgical application and storage batteries.
In Part-I and Part-II we have studied about the Group 15 elements (Nitrogen family) and Group 16 elements respectively. In Part-III, you will get acquainted with Group 17 and Group 18 elements. These notes are based on the latest CBSE syllabus for Class 12th Chemistry.
The main topics covered in these quick notes are:
• Halogen family
• General properties of halogen family
• Anomalous properties of fluorine
• Reactivity of halogens towards:
o Hydrogen
o Metals
o Other halogens (Interhalogens)
• Preparation, properties and uses of chlorine (Cl2)
• Preparation, properties and uses of hydrogen chloride (HCl)
• Oxoacids of halogens
• Noble gases
• General properties of noble gases
• Preparation and properties of xenon fluorine compounds
• Preparation and properties of xenon oxygen compounds
• Uses of noble gases
The notes are as follow:
Group 17 elements are fluorine (F), chlorine (Cl), bromine (Br), iodine (I) and astatine (At) are collectively known as halogens and are having the general electronic configuration of ns2, np5.
Atomic and ionic radii
The halogens have the smallest atomic radii in their respective periods due to maximum effective nuclear charge. Atomic and ionic radii increases down the group due to the addition of a new shell at each step.
Ionisation enthalpy
Due to their small size halogens have little tendency to lose electron. Thus they have very high ionisation enthalpy. Ionisation enthalpy decreases down the group due to the increase in atomic size.
Electron gain enthalpy
Electron gain enthalpy of halogens is very high as they are short of only one electron to attain noble gas configuration. Electron gain enthalpy becomes less negative as we move down the group. However F has less electron gain enthalpy than Cl due to its small size and high electron density.
Electronegativity
Electronegativity decreases down the group. F is the most electronegative element in the periodic table.
Melting and boiling point
The melting and boiling points increases down the group.
Bond dissociation enthalpy
Bond dissociation enthalpy decreases as we move down the group. F2 has less ΔHdiss. Then Cl2 due to small size and strong lone pair-lone pair repulsion.
Colour
All halogens exhibit colour due to the absorption of radiations in visible region of light due to which the electrons get excited to higher energy levels.
For example, F2 has yellow, Cl2 has greenish yellow, Br2 has red and I2 has violet colour.
Oxidation state
The most common oxidation state of halogens is −1. Cl, Br, I also shows positive oxidation states of +1, +3, + 5, + 7. F does not show positive oxidation states due to non-availability of d-orbitals.
Reactivity
All halogens are highly reactive and the reactivity decreases down the group.
Anomalous behaviour of fluorine
Fluorine is anomalous in many properties like, ionisation enthalpy, electronegativity, enthalpy of bond dissociation that are higher than expected from the regular trends among the halogens. Its ionic and covalent radii, melting and boiling points, and electron gain enthalpy is quite lower than expected.
Reasons for the anomalous behaviour of oxygen are:
• Small size and highest electronegativity
• Low F-F bond dissociation enthalpy
• Absence of d-orbitals
Reactivity towards hydrogen
All halogens reacts with H2 to form hydrogen halides (HX) and the reactivity towards H2 decreases down the group
Acidic strength
As the size of X increases and the strength of H─X bond decreases down the group, acidic strength dectreses down the group
HF < HCl < HBr < HI
Stability
As the bond dissociation enthalpy decreases down the group so the stability of hydrogen halides also decreases from HF to HI.
HF > HCl > HBr > HI
Boiling point
Due to the increase in size of halogens the van der Waals forces increases down the group resulting in the increase in boiling point from HCl to HI. HF has the highest boiling point due to the presence of strong intermolecular H bonding
HCl < HBr < HI < HF.
Ionic character
Due to the decrease in electronegativity down the group the ionic character of hydrogen halides also decreases down the group.
HF > HCl > HBr > HI
Dipole moment
Due to the decrease in electronegativity down the group the ionic character of hydrogen halides also decreases down the group.
HF > HCl > HBr > HI
Reducing power
As the bond dissociation enthalpy decreases, so it becomes easier to give out the hydrogen atom causing the reducing power to increase from HF to HI.
HF < HCl < HBr < HI
Reactivity towards metals:
Halogens react with metals to form metal halides of the form MX, where M is a monovalent metal.
Ionic character
Due to the decrease in electronegativity down the group the ionic character of metal halides also decreases down the group
MF > MCl > MBr > MI
Reactivity of halogens towards other halogens (Interhalogens):
Binary compounds of two different halogen atoms of general formula XX’n are called interhalogen compounds where n = 1, 3, 5, or 7. All the interhalogen compounds are covalent in nature.
Some properties of interhalogen compounds are given in the following table:
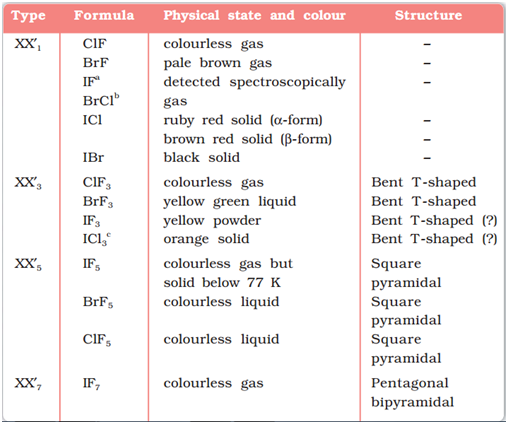
Image Source: NCERT Textbook
Chlorine (Cl)
Preparation:
Chlorine can be prepared by any of the following processes:
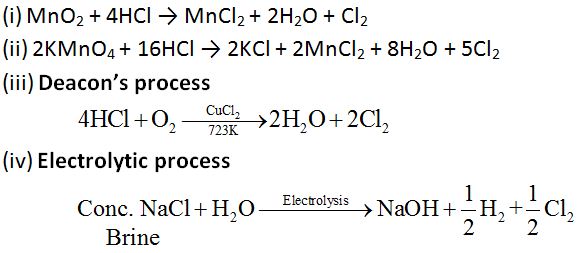
Properties:
• It is a greenish yellow gas with pungent and suffocating odour.
• It is soluble in H2O
• Reaction with metals and non-metals: Chlorine reacts with a number of metals and non-metals to form chlorides.
For example:
2 Al + 3Cl2 → 2AlCl3
S8 + 4Cl2 → 4S2Cl2
• Reaction with ammonia: When treated with excess ammonia, chlorine gives nitrogen and ammonium chloride whereas when excess chlorine reacts with ammonia, nitrogen trichloride is formed.
8NH3 + 3Cl2 → 6NH4Cl + N2
Excess
NH3 + Cl2 → NCl3 + 3 HCl
Excess
• Reaction with NaOH: Chlorine reacts differently with cold dilute NaOH and hot concentrated NaOH.
Cl2 + 2NaOH → NaCl + NaOCl + H2O
Cold dil.
Cl2 + 6NaOH → NaCl + NaOCl + H2O
Hot conc.
Reaction with slaked lime: Cl2 when treated With dry slaked lime it gives bleaching powder:
2Ca(OH)2 + 2Cl2 → Ca(OCl)2 + CaCl2 + 2H2O
Cl2 acts as a powerful bleaching agent and its bleaching action is due to its oxidizing nature.
Cl2 + H2O → 2HCl + O
Uses:
(i) Chlorine is used for bleaching woodpulp.
(ii) It is used in the extraction of gold and platinum
(iii) It is used in in sterilising drinking water.
(iv) It is used in the manufacture of dyes, drugs and organic compounds like CCl4, DDT, refrigerants, etc.
Hydrogen chloride (HCl)
Preparation:
It is prepared by heating sodium chloride with concentrated sulphuric acid.
2 NaCl + H2SO4 + Heat → Na2SO4 + HCl
Properties:
HCl is a colourless gas with pungent odour.
It is extremely soluble in water, HCl + H2O → H3O+ + Cl‒
It decomposes salts of weaker acids, Na2CO3 + 2HCl → 2NaCl + H2O + CO2
When treated with NH3, it gives white fumes of NH4Cl, NH3 + HCl → NH4Cl
3HCl : 1HNO3 is called aqua regia, which is used for dissolving noble metals.
Au + 4 H+ + NO3‒ + 4Cl‒ → AuCl4‒ + NO + 2 H2O
Uses:
(i) Hydrogen chloride is used in medicine and as a laboratory reagent.
(ii) It is used in the manufacture of chlorine, NH4Cl and glucose.
Oxoacids of halogens
Fluorine due to its small size and high electronegativity forms only one oxoacid HOF (Hypofluorous acid).
Other halogen form several oxoacids as given in the following table:
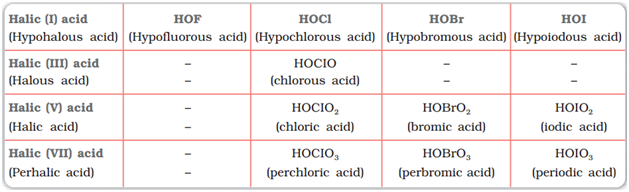
Image Source: NCERT Textbook
Group 18, Noble gases
Group 18 elements: helium (He), neon (Ne), argon (Ar), krypton (Kr), xenon (Xe) and radon (Rn) having the electronic configuration ns2 np6, are named as noble gases. All these are gases and chemically unreactive.
General properties of noble gases
Atomic radii:
Atomic radii of noble gases increases down the group due to the addition of a new shell at each step.
He < Ne < Ar < Kr < Xe < Rn
Ionisation enthalpy:
They have very high ionization enthalpy because of completely filled orbitals. Ionisation enthalpy decreases down the group because of increase in size.
He < Ne < Ar < Kr < Xe < Rn
Electron gain enthalpy:
Because of stable electronic configuration, noble gases have no tendency to accept the electron and therefore, have large positive values of electron gain enthalpy.
Melting and boiling point:
Due to the weak dispersion forces they have low melting and boiling point.
Xenon-fluorine compounds
Xenon forms three binary fluorides, XeF2, XeF4 and XeF6 .
Preparation:
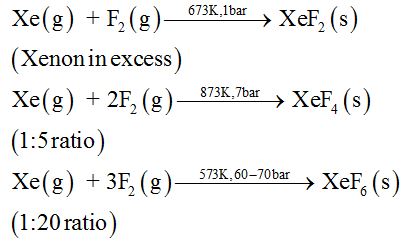
Properties:
XeF2 is linear, XeF4 is square planar and XeF6 is distorted octahedral.
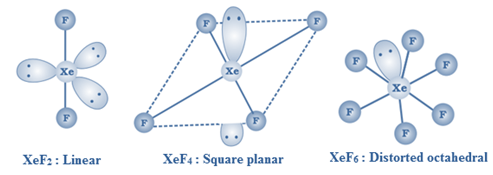
XeF2, XeF4 and XeF6 are colourless crystalline solids
They are readily hydrolysed
2XeF2(s) + 2H2O(l) → 2Xe(g) + HF(aq) + O2(g)
They react with fluoride ion acceptors to form cationic species and fluoride ion donors to form fluoroanions.
XeF2 + PF5 → [XeF] + [PF6]─
XeF4 + SbF5 → [XeF3]+ [SbF6]─
XeF6 + MF → M+ [XeF7]─
[Where, M = Na, K, Rb or Cs]
Xenon-oxygen compounds
Xenon forms some important compounds with oxygen like XeO3, XeOF4 XeO2F2.
Preparation:
Various xenon-oxygen compounds are prepared as follows:
6XeF4 + 12H2O → 4Xe + 2XeO3 + 24HF + 3O2
XeF6 + 3H2O → XeO3 + 6HF
Partial Hydrolysis XeOF4
XeF6 + H2O → XeOF4 + 2 HF
Partial Hydrolysis also gives XeO2F4
XeF6 + 2H2O → XeO2F4 + 4HF
Properties:
XeO3 is a colourless explosive solid having a trigonal pyramidal structure.
XeOF4 is a colourless volatile liquid with a square pyramidal
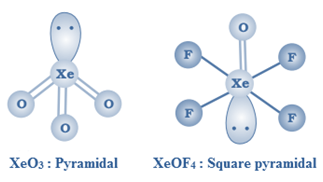
Uses of inert gases
Helium is used:
• Gas cooled Nuclear reactors
• In filling balloons for meteorological observations.
• In the oxygen mixture of deep sea divers
• In inflating aeroplane tyres
• Used to provide an inert atmosphere in melting and welding of easily oxidizable metals.
Neon is used:
• In discharge tubes and fluorescent bulbs used for advertising purposes
• In beacon lights for the safety of air navigators as the light can easily pass through the fog for a clear view.
Argon is used:
• To provide an inert atmosphere in high-temperature metallurgical processes (arc welding of metals or alloys)
• For filling electric bulbs.
• In the laboratory for handling substances that are air-sensitive.
• Xenon and Krypton are also used in light bulbs.
P Block Elements MCQ Chapter 7
The elements that can be found in the periodic table from group 13 to group 18 are called the P Block elements.
Below are some of the very important NCERT P Block Elements MCQ Class 12 Chemistry Chapter 7 with Answers. These P Block Elements MCQs have been prepared by expert teachers and subject experts based on the latest syllabus and pattern of CBSE Term 1 examination.
We have given these P Block Elements Class 12 Chemistry MCQs Questions with Answers to help students understand the concept.
The p-Block Elements Class 12 Chemistry MCQs
1. Among the following, which one is a wrong statement.
(a) PH5 and BiCl5 do not exist.
(b) pπ-dπ bonds are present in SO2
(c) SeF4 and CH4 have same shape.
(d) I3 has bent geometry.
Answer/Explanation
Answer: c
Explaination:
(c) SeF4 has see-saw shape where as CH4 is tetrahedral.
2. In which of the pair of ions, both species contain S—S bond?
Answer/Explanation
Answer: a
Explaination: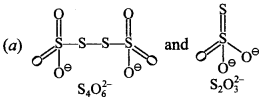
3. Which one of the following order is correct for the bond dissociation enthalpy of halogen molecule?
(a) Br2 > I2 > F2 > Cl2
(b) F2 > Cl2 > Br2 > I2
(c) I2 > Br2 > Cl2 > F2
(d) Cl2 > Br2 > F2 > I2
Answer/Explanation
Answer: d
Explaination:
(d) In F2, therefore, inter electronic repulsion, therefore, bond dissociation enthalpy is less.
4. Which is strongest acid in the following:
(a) HClO4
(b) H2SO3
(c) H2SO4
(d) HClO3
Answer/Explanation
Answer: a
Explaination:
(a) HClO4 is strongest because ‘Cl’ has +7 oxidation state.
5. In which of the following pairs, the two species are isostructural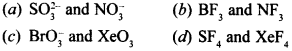
Answer/Explanation
Answer: c
Explaination:
(c) Both are pyramidal.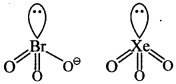
6. The correct order of oxidising power is
(a) HClO4 > HClO3 > HClO2 > HCIO
(b) HOCl > HClO2 > HClO3 > HClO4
(c) HClO3 > HClO4 > HClO2 > HClO
(d) HCIO2 > HOCl > HClO3 > HClO4
Answer/Explanation
Answer: b
Explaination:
(b) HOCl → HCl + [O]
It is strongest oxidising agent whereas HClO4 is weakest.
7. The correct order of acid strength is
(a) HClO4 < HClO3 < HClO2 < HClO
(b) HCIO < HClO2 < HClO3 < HClO4
(c) HClO4 < HClO < HClO2 < HClO3
(d) HClO2 < HClO3 < HClO4 < HClO
Answer/Explanation
Answer: b
Explaination:
(b) As oxidation state increases, acid strength increases.
8. Among the following which is strongest oxidising agent.
(a) Br2
(b) I2
(c) Cl2
(d) F2
Answer/Explanation
Answer: d
Explaination:
(d) F2 is best oxidising agent.
9. The correct order of bond angles in the following species is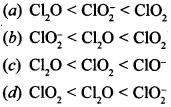
Answer/Explanation
Answer: b
Explaination:
(b) ClO2– < Cl2O < ClO2 is increasing order of bond angle.
10. Sulphur trioxide can be obtained by which of the following: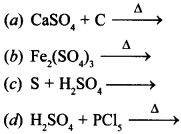
Answer/Explanation
Answer:
Explaination:![]()
11. When Cl2 reacts with hot and cone. NaOH, the oxidation number of chlorine changes from
(a) zero to +1 and zero to +5
(b) 0 to -1 and 0 to +5
(c) zero to -1 and zero to +3
(d) 0 to +1 and 0 to -3
Answer/Explanation
Answer: b
Explaination:
(b) Cl2 has oxidation number 0, in Cl (-1) and in ClO3(+5).
3Cl2 + 6NaOH(hot and cone.) → 5NaCl + NaClO3 + 3H2O
12. Acidity of diprotic acid in aqueous solution increases in the order.
(a) H2S < H2Se < H2Te
(b) H2Se < H2S < H2Te
(c) H2Te < H2S < H2Se
(d) H2Se < H2Te < H2S
Answer/Explanation
Answer: a
Explaination:
(a) Because bond dissociation enthalpy decreases as atomic size increases.
13. Which of the following statement is incorrect?
(a) ONF is isoelectronic with NO–2
(h) OF2 is an oxide of fluoride
(c) Cl2O7 is an anhydride of perchloric acid
(d) O3 molecule is bent
Answer/Explanation
Answer: b
Explaination:
(b) It is fluoride of oxygen.
∵ ‘F’ is more electronegative than O.
14. Chlorine reacts with excess of NH3 to form
(a) NH4Cl
(b) N2 + HCl
(c) N2 + NH4Cl
(d) NCl3 + HCl
Answer/Explanation
Answer: c
Explaination:
(c) 8NH3 + 3Cl2 → 6NH4Cl + N2
15. Which of the following reactions is an example of redox reaction?
(a) XeF4 + O2F2 → XeF6 + O2
(b) XeF2 + PF5 → [XeF]+ [PF6]–
(c) XeF6 + H2O → XeOF4 + 2HF
(d) XeF6 + 2H2O → Xeo2F2 + 2HF
Answer/Explanation
Answer: a
Explaination:
(a) Redox reaction becaueXe(+4) is getting oxidised to Xe(+6) and 0(+l) is reduced to zero.
16. On addition of cone. H2SO4 to a chloride salt, colourless fumes are evolved but in case of iodide salt, violet flames come out. This is because [NCERT Exemplar]
(a) H2SO4 reduces HI to I2
(b) HI is of violet colour
(c) HI gets oxidised to I2
(d) HI changes to HIO3
Answer/Explanation
Answer: c
Explaination:
(c) HI gets oxidised to I2
17. Which of the following pairs of ions are isoelectronic and isostructural? [NCERT Exemplar]
Answer/Explanation
Answer: a
Explaination:
(a) CO–3 and NO–3 are isoelectronic (32 electrons) and Planar.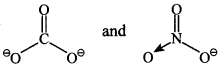
18. Affinity for hydrogen decreases in the group from fluorine to iodine. Which of the halogen acids should have highest bond dissociation enthalpy? [NCERT Exemplar]
(a) HF
(b) HCl
(c) HBr
(d) HI
Answer/Explanation
Answer: a
Explaination:
(a) HF has highest bond dissociation enthalpy due to smaller bond length.
19. Bond dissociation enthalpy of E—H (E = element) bonds is given below. Which of the compounds will act as strongest reducing agent? [NCERT Exemplar]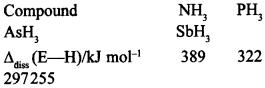
(a) NH3
(b) PH3
(c) AsH3
(d) SbH3
Answer/Explanation
Answer: d
Explaination:
(d) SbH3 due to lowest bond dissociation enthalpy.
20. Hot cone. H2SO4 acts as moderately strong oxidising agent. It oxidises both metals and non-metals. Which of the following element is oxidised by cone. H2SO4 into two gaseous products? [NCERT Exemplar]
(a) Cu
(b) S
(c) C
(d) Zn
Answer/Explanation
Answer: c
Explaination:
(c) C + 2H2SO4(conc.) → CO2 + SO2 + 2H2O
21. H2S is more acidic than H2O because
(a) oxygen is more electronegative than sulphur.
(b) atomic number of sulphur is higher than oxygen.
(c) H — S bond dissociation energy is less as compared to H — O bond.
(d) H — O bond dissociation energy is less also compared to H — S bond.
Answer
Answer: b
22. The boiling points of hydrides of group 16 are in the order
(a) H2O > H2Te > H2S > H2Se
(b) H2O > H2S > H2Se > H2Te
(c) H2O > H2Te > H2Se > H2S
(d) None of these
Answer
Answer: b
23. In the manufacture of sulphuric acid by contact process Tyndall box is used to
(a) convert SO2 and SO3
(b) test the presence of dust particles
(c) filter dust particles
(d) remove impurities
Answer
Answer: b
24. Fluorine differs from rest of the halogens in some of its properties. This is due to
(a) its smaller size and high electronegativity.
(b) lack of d-orbitals.
(c) low bond dissociation energy.
(d) All of the these.
Answer
Answer: b
25. The set with correct order of acidity is
(a) HClO < HClO2 < HClO3 < HClO4
(b) HClO4 < HClO3 < HClO2 < HClO
(c) HClO < HClO4 < HClO3 < HClO2
(d) HClO4 < HClO2 < HClO3 < HClO
Answer
Answer: b
26. When chlorine reacts with cold and dilute solution of sodium hydroxide, it forms
(a) Cl– and ClO–
(b) Cl– and ClO2–
(c) Cl– and ClO3–
(d) Cl– and ClO4–
Answer
Answer: a
27. The formation of O2+ [PtF6]– is the basis for the formation of first xenon compound. This is because
(a) O2 and Xe have different sizes.
(b) both O2 and Xe are gases.
(c) O2 and Xe have comparable electro-negativities.
(d) O2 and Xe have comparable ionisation enthalpies.
Answer
Answer: d
28. Partial hydrolysis of XeF4 gives
(a) XeO3
(b) XeOF2
(c) XeOF4
(d) XeF2
Answer
Answer: b
29. Helium is preferred to be used in balloons instead of hydrogen because it is
(a) incombustible
(b) lighter than hydrogen
(c) more abundant than hydrogen
(d) non polarizable
Answer
Answer: a
30. The increasing order of reducing power of the halogen acids is
(a) HF < HCl < HBr < HI
(b) HI < HBr < HCl < HF
(c) HBr < HCl < HF < HI
(d) HCl < HBr < HF < HI
Answer
Answer: a
Note: In the following questions two or more options may be correct. (Q 21. to Q 24)
31. Which of the following options are not in accordance with the property mentioned against them? [NCERT Exemplar]
(a) F2 > Cl2 > Br2 > I2 Oxidising power.
(b) MI > MBr > MCI > MF Ionic character of metal halide.
(c) F2 > Cl2 >Br2 > I2 Bond dissociation enthalpy.
(d) HI < HBr < HCl < HF Hydrogen-halogen bond strength.
Answer/Explanation
Answer:
Explaination:
(b) MF > MCl > HBr > MI Ionic character
(c) Cl2 > Br2 > F2 > I2
32. Which of the following statements are correct? [NCERT Exemplar]
(a) Among halogens, radius ratio between iodine and fluorine is maximum.
(b) Leaving F—F bond, all halogens have weaker X—X bond than X—X’ bond in interhalogens.
(c) Among interhalogen compounds maximum number of atoms are present in iodine fluoride.
(d) Interhalogen compounds are more reactive than halogen compounds.
Answer/Explanation
Answer:
Explaination:
(a), (c) and (d) are correct.
F2 is more reactive than interhalogen compounds.
(b) is not correct, other halogens are less reactive than interhalogen compounds
33. Which of the following statements are correct for SO2 gas? [NCERT Exemplar]
(a) It acts as bleaching agent in moist conditions.
(b) It’s molecule has linear geometry.
(c) It’s dilute solution is used as disinfectant.
(d) It can be prepared by the reaction of dilute H2S04 with metal sulphide.
Answer/Explanation
Answer:
Explaination:
(b) and (d). Its molecule is bent.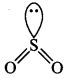
and Na2S + H2SO4 → Na2SO4 + H2S
34. Which of the following orders are correct as per the properties mentioned against each? [NCERT Exemplar]
(a) AS2O3 < SiO2 < P2O3 < SO2 Acid strength.
(b) AsH3 < PH3 < NH3 Enthalpy of vapourisation,
(c) S < O < Cl < F More negative electron gain enthalpy.
(d) H2O > H2S > H2Se > H2Te Thermal stability.
Answer/Explanation
Answer:
Explaination:
(a) and (d) are correct (b) and (c) are wrong.
(b) PH3 <ASH3 <NH3
(c) Cl > F > S > O
35. Match the compounds given in Column I with the hybridisation and shape given in Column II and mark the correct option. [NCERT Exemplar]
| Column I | Column II |
| (A) Xe F6 | (1) sp3d3 – distorted octahedral |
| (B) Xe O3 | (2) sp3d2 – square planar |
| (C) Xe OF4 | (3) sp3– pyramidal |
| (D) Xe F4 | (4) sp3 d2 – square pyramidal |
Code:
(a) A (1) B (3) C (4) D (2)
(b) A (1) B (2) C (4) D (3)
(c) A (4) B (3) C (1) D (2)
(d) A (4) B (1) C (2) D (3)
Answer/Explanation
Answer:
Explaination:
(a) A (1) B (3) C (4) D (2)
36. Match the items of Columns I and II and mark the correct option. [NCERT Exemplar]
| Column I | Column II |
| (A) H2SO4 | (1) Highest electron gain enthalpy |
| (B) CCl3NO2 | (2) Chalcogen |
| (C) Cl2 | (3) Tear gas |
| (D) Sulphur | (4) Storage batteries |
Answer/ExplanationCode:
(a) A (4) B (3) C (1) D (2)
(b) A (3) B (4) C (1) D (2)
(c) A (4) B (1) C (2) D (3)
(d) A (2) B (1) C (3) D (4)
Explaination:
(a) A (4) B (3) C (1) D (2)
Note: In the following questions a statement of assertion followed by a statement of reason is given. Choose the correct answer out of the following choices. (Q.27 to Q.29)
(a) Both assertion and reason are correct statements, and reason is the correct explanation of the assertion.
(b) Both assertion and reason are correct statements, but reason is not the correct explanation of the assertion.
(c) Assertion is correct, but reason is wrong statement.
(d) Assertion is wrong but reason is correct statement.
(e) Both assertion and reason are wrong statements.
37. Assertion: HI cannot be prepared by the reaction of KI with concentrated H2SO4.
Reason: HI has lowest H-X bond strength among halogen acids.[NCERT Exemplar]
Answer/Explanation
Answer: b
Explaination:
(b) Both assertion and reason are correct statements, but reason is not the correct explanation of the assertion. HI gets oxidised to I2 as H2SO4(conc.) is oxidising agent.
38. Assertion: Both rhombic and monoclinic sulphur exist as S8 but oxygen exists as O2.
Reason: Oxygen forms pπ – pπ multiple bond due to small size and small bond length but pπ – pπ bonding is not possible in sulphur. [NCERT Exemplar]
Answer/Explanation
Answer: a
Explaination:
(a) Both assertion and reason are correct statements, and reason is the correct explanation of the assertion.
39. Assertion: NaCl reacts with concentrated H2SO4 to give colourless fumes with pungent smell. But on adding MnO2 the fumes become greenish yellow.
Reason: Mn02 oxidises HC1 to chlorine gas which is greenish yellow. [NCERT Exemplar]
Answer/Explanation
Answer: a
Explaination:
(a) Both assertion and reason are correct statements, and reason is the correct explanation of the assertion.
NaCl + H2SO4(conc.) → NaHSO4 + HCl
4HCl + MnO2 → MnCl2 + Cl2 + 2H2O
40. The mixture of cone. HCl and anhydrous ZnCl2 is called ___________ .
Answer/Explanation
Answer:
Explaination: Lucas reagent
41. Out of H2O and H2S which has higher bond angle? ___________ .
Answer/Explanation
Answer:
Explaination: H20
42. Tin reacts with excess of chlorine gas to form ___________ .
Answer/Explanation
Answer:
Explaination: SnCl4
43. Lead sulphide is heated with air to form ___________ .
Answer/Explanation
Answer:
Explaination: PbO + SO2
44. I22 gets oxidised to ___________ by cone. HNO3.
Answer/Explanation
Answer:
Explaination: HIO3
45. Interhalogen compounds are more reactive than helogens except fluorine. [True/False]
Answer/Explanation
Answer:
Explaination: True. It is due to less effective overlapping.
46. C1F is neutral molecule isoelectronic withCIO–. [True/False]
Answer/Explanation
Answer:
Explaination: True. Both have 17 + 9 = 26 electrons.
47. NaF reacts with SbF6 to form Na+ [SbF7]–. [True/False]
Answer/Explanation
Answer:
Explaination:![]()
48. Hydrolysis of XeF6 is redox reaction. [True/False]
Answer/Explanation
Answer:
Explaination: It is false. XeF6 + 3H2O > XeO3 + 6HF.
49. Ozone is thermodynamically less stable than O2. [True/False]
Answer/Explanation
Answer:
Explaination: True
50. Oxygen does not show +4 and +6 oxidation states like sulphur. Why?
Answer/Explanation
Answer:
Explaination: It is due to the absence of vacant d-orbital in oxygen.
51. Draw the structure of 03 molecule.
Answer/Explanation
Answer:
Explaination: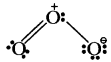
It is bent molecule.
52. Write one chemical reaction equation to show that SO2 acts as a reducing agent.
Answer/Explanation
Answer:
Explaination: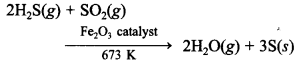
53. State reason for the following:
Sulphur has greater tendency for catenation than oxygen. [Delhi 2013; AI2016,12; DoE]
Or
O-O bond is weaker than S-S bond, why? [Chennai 2019]
Answer/Explanation
Answer:
Explaination:
It is because of strong S—S bond than 0—0 due to greater repulsion between valence electrons of smaller atoms of oxygen than sulphur atoms.
54. Write the formulae of any two oxoacids of sulphur. [AI 2015 Allahabad & Dehradun]
Answer/Explanation
Answer:
Explaination:
H2SO4 and H2SO3.
55. Write one chemical reaction equation to show that cone. H2S04 is a strong oxidising agent.
Answer/Explanation
Answer:
Explaination:
C + 2H2SO4(conc.) CO2+ 2H2O + 2SO2
56. Fluorine does not exhibit any positive oxidation state. Why? [Delhi 2013]
Answer/Explanation
Answer:
Explaination:
It is because Fluorine is most electronegative element and best oxidising agent.
57. Despite lower value of its electron gain enthalpy with negative sign, fluorine (F2) is a stronger oxidising agent than Cl2. [Delhi 2019; AI 2012; DoE]
Answer/Explanation
Answer:
Explaination:
It is due to higher standard reduction potential of F2 which is due to low bond dissociation energy of F—F bond due to inter electronic repulsion among small size F atoms, high electron gain enthalpy and highest hydration enthalpy.
58. Although the H-bonding in hydrogen fluoride is much stronger than that in water, yet water has a much higher boiling point than hydrogen fluoride. Why? [Foreign 2012]
Answer/Explanation
Answer:
Explaination:
It is because H20 molecules form H-bonds to more extent as compared to HF molecules.
59. Give a chemical reaction involved in chlorine with saturated hydrocarbon.
Answer/Explanation
Answer:
Explaination: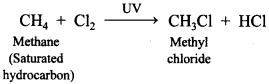
60. Explain giving a reason for the following situation:
In aqueous medium, HCl is a stronger acid than HF. [Foreign 2011]
Answer/Explanation
Answer:
Explaination:
It is because bond dissociation energy of H—Cl is lower than HF due to longer bond length.
61. Give the chemical formula of any one halic acid.
Answer/Explanation
Answer:
Explaination: H0BrO2 (Bromic acid)
62. Give a chemical reaction involved in the preparation of interhalogen compound.
Answer/Explanation
Answer:
Explaination:![]()
63. Which compound led to the discovery of the compounds of noble gas?
Answer/Explanation
Answer:
Explaination:
which was the first compound of noble gas.
64. Complete the following reaction:
Xe + PtF6 →
Answer/Explanation
Answer:
Explaination:
Xe + PtF6 → Xe+[PtF6]–
65. Sulphur disappears when boiled with an aqueous solution of sodium sulphite. Why?
Answer/Explanation
Answer:
Explaination:
It is due to the formation of sodium thiosulphate which is soluble in water, therefore, sulphur disappears.![]()
P Block Elements MCQ (1-10)
- Which one of the following oxides of nitrogen is a solid?
- NO2
- N2O
- N2O3
- N2O5
Answer/Explanation
1 . (4)
N2O5 exists as a solid under the temperature of 273 K. After this temperature, it starts too decompose.
- Which one of the following is stable?
- NCl3
- NBr3
- NI3
- NF3
Answer/Explanation
2. (4)
The unstable nature of NBr3, NCl3 and NI3 is due to low polarity of the N-X bond and large size difference between nitrogen and halogen atoms. The size of nitrogen and fluorine are comparable.
- The BCl3 is a planar molecule, whereas NCl3 is pyramidal because
- N-Cl bond is more covalent than B-Cl bond
- B-Cl bond is more polar than N-Cl bond
- BCl3 has no lone pair but NCl3 is a lone pair of electrons
- Nitrogen atom is smaller than boron
Answer/Explanation
3. (3)
Presence of bond pairs and lone pairs affect the shape of the molecule. This is due to the fact that lone pairs and bond pairs repel each other and affect the shape.
BCl3 has 3 bond pairs and 0 lone pair
NCl3 has 3 bond pairs and 1 lone pair
- Which of the following is tetrabasic acid?
- Metaphosphoric acid
- Orthophosphoric acid
- Hypophosphoric acid
- Hypophosphorous acid
Answer/Explanation
4. (3)
Tetrabasic acid means an acid having four hydrogens that can be replaced.
Hypophosphorous acid – H3PO2
Metaphosphoric acid – HPO3
Orthophosphoric acid – H3PO4
Hypophosphoric acid – H4P2O6
Hypophosphoric acid has 4 replaceable hydrogens.
- The reagent used to distinguish between hydrogen peroxide and ozone is
- PbS
- Starch and Iodine
- KMnO4
- Bleaching powder
Answer/Explanation
5. (2)
Iodine is used in the quantitative estimation of ozone.
- Ozone can be detected by using
- mercury
- silver
- sodium
- none of these
Answer/Explanation
6. (1)
Ozone reacts with mercury to form mercurous oxide, which sticks to the walls of the container. Therefore, the presence of ozone can be detected by mercury.
- The correct sequence of decrease in the bond angle of following hydride is
- NH3 > PH3 > AsH3 > SbH3
- NH3 > AsH3 > PH3 > SbH3
- SbH3 > AsH3 > PH3 > NH3
- PH3 > NH3 > AsH3 > SbH3
Answer/Explanation
7. (1)
As we move down the group, the radius of elements increases and electronegativity decreases so bond angle decreases.
- Among the following oxides, the least acidic is
- P4O6
- P4O10
- As4O6
- As4O10
Answer/Explanation
8. (3)
Acidic character of oxides decreases with the decrease in the oxidation state and also decreases down the group.
- Which of the following is paramagnetic?
- N2O3
- NO2
- N2O5
- All of these
Answer/Explanation
9. (2)
NO2 is paramagnetic due to the presence of an unpaired electron in the nitrogen.
- Concentrated H2SO4 is not used to prepare HBr from KBr because it
- oxidises HBr
- reduces HBr
- causes disproportionation of HBr
- reacts to slowly with KBr
Answer/Explanation
10. (1)
Conc. H2SO4 can not be used to prepare HBr from KBr because conc. H2SO4 oxidises HBr to bromine.
P Block Elements MCQ (11-20)
- Which of the following is thermally most stable ?
- H2S
- H2O
- H2Se
- H2Te
Answer/Explanation
11. (2)
H2O is the most stable as there is a formation of hydrogen bonds.
The order goes like : H2Te < H2Se < H2S < H2O
- Which of the following oxides react with both HCl and NaOH?
- N2O5
- ZnO
- CaO
- CO2
Answer/Explanation
12. (2)
ZnO is an amphoteric oxide, therefore it reacts with both HCl and NaOH.
- Which gas evolves when urea is treated with sodium hydroxide?
- Nitrogen
- Laughing gas
- Ammonia
- NO
Answer/Explanation
13. (3)
NH2CONH2 + 2NaOH → 2NH3 + NH2CO3
- Which of the following is an amphoteric oxide?
- Cr2O3
- Cl2O7
- SnO2
- None of these
Answer/Explanation
14. (1)
Cr2O3 is an amphoteric oxide.
- Which of the following allotropic forms of sulphur is thermodynamically most stable?
- β-monoclinic
- ℽ-monoclinic
- Plastic sulphur
- Orthorhombic
Answer/Explanation
15. (4)
Orthorhomic sulphur is the most stable form of sulphur having eight sulphur atoms arranged in an octahedron.
- Which of the following does not have a p-o-p bond?
- P4O6
- P4O10
- H4P2O6
- H4P2O7
Answer/Explanation
16. (3)
H4P2O6 (Hypophosphoric acid) doesn’t have any P-O-P bond.
- Which acid on heating produces phosphine?
- Phosphoric acid
- Phosphorous acid
- Peroxymonophosphoric acid
- Metaphosphoric acid
Answer/Explanation
17. (3)
H3PO5 (Peroxymonophosphoric acid) gives H3PO4 (orthophoric acid) and PH3 (phosphine).
- Xenon difluoride is
- linear
- angular
- trogonal
- pyramidal
Answer/Explanation
18. (1)
XeF2 has a linear shape.
- Which of the following is not a reducing agent
- Sulphur Dioxide
- Hydrogen Peroxide
- Carbon Dioxide
- Nitrogen Dioxide
Answer/Explanation
19. (2)
Carbon dioxide is an oxidising agent.
- Which of the following molecules does not possess a permanent dipole moment
- H2S
- SO2
- SO3
- CS2
Answer/Explanation
20. (4)
CS2 has a linear shape, hence doesn’t have a permanent dipole moment.
P Block Elements MCQ (21-30)
- Potassium chlorate on heating with concentrated sulphuric acid gives
- Chlorine dioxide
- HClO4
- KHSO4
- All of these
Answer/Explanation
21. (4)
3KClO3 + 3H2SO4 → 2KHSO4 + HClO4 + 2ClO2 + H2O
- The most abundant and common oxidation state of sulphur is
- -2
- +4
- +2
- +6
Answer/Explanation
22. (2)
Sulphur’s most common oxidation state is +4.
- Ozone is
- an unstable, dark blue, diamagnetic gas
- an unstable, dark blue, paramagnetic gas
- a stable, dark blue, paramagnetic gas
- found in the upper atmosphere where it absorbs UV radiation
Answer/Explanation
23. (1)
Ozone is an unstable, dark blue, diamagnetic gas.
- Structure of ClF3 is
- T-shape
- Octahedral
- Tetrahedral
- None of these
Answer/Explanation
24. (1)
ClF3 is of T-shape
- In two forms NCl3 whereas P can form both PCl3 and PCl5. Why?
- N atom has is larger than P in size
- P is more reactive towards Cl than N
- P has d orbitals which can be used for bonding but N2 does not have d orbitals
- None of these
Answer/Explanation
25. (3)
There is no vacant d-orbital in the outermost orbit of nitrogen. Thus nitrogen show valency only 3. There are vacant d-orbital is in the outermost orbit of phosphorus and hence it shows variable covalency 3 and 5 in ground state and excited state respectively. Hence, nitrogen forms only NCl3 but phosphorus forms PCl3 and PCl5 both.
- Which characteristic is not correct about sulphuric acid?
- Sulphonating agent
- Reducing agent
- Oxidizing agent
- Highly Vicious
Answer/Explanation
26. (2)
H2SO4 is a strong oxidising agent and can oxidise both metals and non-metals. It’s not a reducing agent.
- Which of the following is planar?
- XeF4
- XeO4
- XeO2F2
- XeOF4
Answer/Explanation
27. (1)
XeF4 has a square planar structure.
- Which of the following halide ions is the most basic?
- Cl–
- Br–
- I–
- F–
Answer/Explanation
28. (4)
F– ion is the most electronegative, most unstable and the most basic .
- Which of the following gives nitrogen on heating?
- Ammonium nitride
- Ammonium nitrate
- Ammonium nitrite
- Ammonium hydroxide
Answer/Explanation
29. (3)
Ammonium nitrite on thermal decomposition gives ammonia.
- One of the reasons why F-F bond is weak is that
- The repulsion between the non bonding pairs of electrons of the two fluorine atoms is high
- The F-F Bond distance is small and hence intermolecular repulsion is small
- The ionization enthalpy of the fluorine atom is very high
- The F-F Bond distances large
Answer/Explanation
30. (2)
The F-F Bond distance is small and hence intermolecular repulsion is small.
P Block Elements MCQ (31-40)
- The mixture of NaI and NaIO3 is treated with hot conc. H2SO4. The iodine-containing product formed is
- HIO3
- NaIO4
- I2
- HIO4
Answer/Explanation
31. (3)
5 NaI + NaIO3 + 3H2SO4 → 3Na2SO4 + 3I2 +3H2O
- When bleaching powder is treated with carbon dioxide
- Calcium chloride is formed
- No reaction occurs
- Chlorine is evolved
- It absorbs the gas
Answer/Explanation
32. (3)
When bleaching powder is treated with carbon dioxide chlorine is evolved.
- PtF6 converts O2 to
- O2–PtF6+
- O2+PtF6–
- F2+PtO2
- F2O+Pt
Answer/Explanation
33. (2)
O2+PtF6–
- Which of the following has the greatest reducing power?
- HBr
- HI
- HCl
- HF
Answer/Explanation
34. (2)
HI has the strongest reducing agent among halogen acids because of lowest bond dissociation enthalpy.
- XeF6 on complete hydrolysis gives
- XeO3
- Xe
- XeO2
- XeO4
Answer/Explanation
35. (1)
XeF2 + 3H2O → 6HF + XeO3
- Which of the following statements is not correct?
- Krypton is obtained during radioactive disintegration
- Argon is used in electric bulbs
- Half life of radon is only 3.8 days
- Helium is used in producing very low temperatures
Answer/Explanation
36. (1)
Krypton is not obtained during radioactive disintegration.
- Which of the following noble gas is not found in the atmosphere?
- Rn
- Kr
- Ne
- Ar
Answer/Explanation
37. (1)
Radon and helium is not found in the atmosphere.
- What is the colour produced when ammonia is passed through a solution of copper sulphate?
- Red
- Orange
- Yellow
- Blue
Answer/Explanation
38. (4)
The complex formed is [Cu(NH3)4]2+ which is square planar and paramagnetic and has a deep blue colour.
- What is the correct order of electron affinities among halogens?
- F2 > Cl2 > Br2 > I2
- Cl2 > Br2 > F2 > I2
- F2 < Cl2 < Br2 < I2
- I2 < Br2 < F2 < Cl2
Answer/Explanation
39. (2)
The electron affinity of chlorine is maximum in the periodic table and so the bond dissociation enthalpy. Fluorine has lower bond dissociation enthalpy than Br2 and Cl2. Due to small size electronic repulsion is very high. I2 has lowest bond dissociation enthalpy due to its quite larger size it is easiest to break the bond.
- What is the hybridisation of Xe in XeF2?
- sp3
- sp2
- sp2d
- sp3d
Answer/Explanation
40. (4)
Xe in XeF2 has sp3d hybridisation.
P Block Elements MCQ (41-50)
- Which one of the following undergoes thermal decomposition?
- XeF2
- XeF4
- ZeF6
- None of these
Answer/Explanation
41. (3)
XeF6 on thermal decomposition gives XeF2, XeF4 and F2
- Which of the following is the least oxidising in nature?
- HClO3
- HClO4
- HClO2
- HClO
Answer/Explanation
42. (2)
The oxidation state of chlorine in HClO4, HClO3, HClO2 and HClO is 7, 5, 3 and 1. The chlorine with the highest oxidation state has the lowest potential of getting oxidised.
- Which one of the following is the most stable thermally?
- HI
- HBr
- HCl
- HF
Answer/Explanation
43. (4)
The H-X bond strength decreases from HF to HI.
- The crystals of ferrous sulphate on heating give
- FeO + SO2 + H2O
- FeO + SO3 + H2SO4 + H2O
- Fe2O3 + SO2 + SO3 + H2O
- Fe2O3 + H2SO4 + H2O
Answer/Explanation
44. (3)
FeSO4 on heating gives Fe2O3 + SO2 + SO3 + H2O.
- Pure chlorine is obtained
- By heating MnO2 with HCl
- By heating bleaching powder with HCl
- By heating PtCl4
- By heating a mixture of NaCl and MnO2 with conc. H2SO4
Answer/Explanation
45. (3)
Pure chlorine is obtained on heating PtCl4.
- The element which forms oxides in all the oxidation states from +1 to +5 is
- N
- P
- As
- Sb
Answer/Explanation
46. (1)
Nitrogen has an oxidation state varying from +1 to +5.
- Which one of the following acts as an antichlor?
- MnO2
- Na2S2O3
- K2Cr2O7
- Na2SO4
Answer/Explanation
47. (2)
An antichlor is a substance used to decompose residual HCl or chlorine after chlorine-based bleaching in order to prevent ongoing reaction. Na2S2O3 acts as an antichlor.
- N2O3 is
- An acidic oxide and the anhydride of HNO2
- An acidic oxide and the anhydride of H2N2O2
- A neutral oxide and the anhydride of HNO3
- A basic oxide and the anhydride of HNO2
Answer/Explanation
48. (1)
Nitrogen in N2O3 has an oxidation state of +3 and so does in HNO2. Hence, N2O3 is an anhydride of HNO2. N2O3 is an acidic oxide.
- (NH4)2CrO7 on heating liberates a gas. The same gas will be obtained by
- Heating NH4NO3
- Treating Mg3N2 with H2O
- Heating NH4NO2
- Heating H2O2 on NaNO2
Answer/Explanation
49. (3)
(NH4)2CrO7 on heating liberates nitrogen gas which can also be obtained from heating NH4NO2.
- According to Le Chatlier’s principle, low temperature is required for more yield of ammonia in Haber’s process. What is the temperature commercially kept for the process?
- 350-450℃
- 450-550℃
- 250-350℃
- 150-250℃
Answer/Explanation
50. (2)
The temperature required for Haber’s process is 450-550℃.
Assertion and Reasoning MCQs
Codes
(a) Both A and R are true and R is the correct explanation of A
(b) Both A and R are true and but R is not a correct explanation of A
(c) A is true but R is false
(d) A is false, but R is true
1. Assertion (A) Valency of noble gas is 0.
Reason (R) Noble gases possess complete octet.
Answer/Explanation
1. (a)
Noble gases possess the electronic configuration ns1np6 and has 8 electrons in their outer shell, hence there valency is 0.
2. Assertion (A) F2 has lower bond dissociation enthalpy then Cl2.
Reason (R) Fluorine is more electronegative than chlorine.
Answer/Explanation
2. (b)
Flourine has low bond dissociation enthalpy due to small atomic size number of electrons create large repulsion in bonded electron.
3. Assertion (A) Acidic character of group 16 hydrides increases from H2O to H2Te.
Reason (R) Thermal stability of hydrides decreases down the group.
Answer/Explanation
3. (b)
The acidic character increases down the group and thermal stability of hydrides decreases down the group due to decrease in bond (H-E) dissociation enthalpy down the group.
4. Assertion (A) Interhalogen compounds are more reactive than halogens (except chlorine).
Reason (R) They all undergo hydrolysis giving halide ion derived from the smaller halogen.
Answer/Explanation
4. (b)
Interhalogen compounds are more reactive than halogens because X-X’ bond in interhalogens in weaker than X-X bond in halogen (except F-F bond).
5. Assertion (A) Halogens are not found in free state in nature.
Reason (R) Halogens are highly reactive compound.
Answer/Explanation
5. (a)
Halogens are highly reactive as they have seven electrons in their outermost orbit and they want to stabilize by acquiring an electron. therefore, they do not occur in free state.
6. Assertion (A) F2 has low reactivity.
Reason (R) F-F bonds ΔbondH.
Answer/Explanation
6. (d)
F2 is more reactive that other halogens because its valence electrons are more closer to nucleus and its more electronegative so, bonded electrons repel each other causing low bond dissociation enthalpy.
7. Assertion (A) Ozone layer in the upper region of atmosphere protect earth from UV radiation.
Reason (R) Ozone is a powerful oxidizing agent as compared to oxygen.
Answer/Explanation
7. (b)
Ozone layer filters the radiation coming from sun, hence serves as the protective layers.
8. Assertion (A) S shows paramagnetic nature, when present in vapour state.
Reason (R) S exists as S2 in vapour state.
Answer/Explanation
8. (a)
In vapor state S exists as S2 and it behaves like O2. It has 2 unpaired electrons in antibonding pi orbitals. Presence of these unpaired electrons make S paramagnetic.
9. Assertion (A) F2 is a strong oxidizing agent.
Reason (R) Electron gain enthalpy of fluorine is less negative.
Answer/Explanation
9. (b)
As F2 has low bond dissociation energy and high hydration energy than Cl2 but less electron gain enthalpy due to its smaller size. Due to these factors F2 wins in getting reduced fastly.
10. Assertion (A) PbI4 is not a stable compound.
Reason (R) Iodide stabilizes higher oxidizing state.
Answer/Explanation
10. (c)
Small highly elctronegative atoms such as F– can stabilise higher oxidation state.
11. Assertion (A) Solubility of noble gases in water decreases with increasing size of the noble gas.
Reason (R) Solubility of noble gases in water is due to dipole-induced dipole interaction.
Answer/Explanation
11. (d)
PbI4 is not a stable compound because Pb shows (II) oxidation state more frequently than Pb (IV) due to inert pait effect. Iodide cannot stabilize higher oxidation states.
Frequently Asked Questions (FAQs)
Q1. Why P Block elements called the P Block elements?
They are called so because their valence electrons are in the p-orbital.
Q2. Why group 16 elements called chalcogens?
They are called so because most ores of copper (Greek Chalcos) are oxides or sulphides and also traces of selenium and tellurium.
Q3. Why is nitrogen so inert?
It is due to the presence of the very strong triple bond present between the molecular state of nitrogen N2.