d and f Block Elements : Notes and Study Materials -pdf
Notes and Study Materials
- Concepts of d and f Block Elements
- Master File d and f Block Elements
- NCERT Solutions for – d and f Block Elements
- NCERT Exemplar Solutions for – d and f Block Elements
- Mind Map of d and f Block Elements
- Past Many 12th Board Years of d and f Block Elements
Examples and Exercise
CBSE Class 12th Chemistry Notes: The d- and f- Block Elements
In this article, you will get important notes on CBSE Class 12th Chemistry, Chapter 8: The d- and f-Block Elements. These notes are very helpful for quick revision before the exams.
This article provides you with the revision notes on CBSE Class 12 Chemistry: Chapter – 8: The d- and f-Block Elements. These notes will give you a quick glance of the chapter. These quick notes are prepared strictly according to the latest CBSE syllabus for Class 12th Chemistry.
• d –Block elements
• Features of d-block elements
• Transition metals
• General Properties of d –Block elements
• Some Important Compounds of Transition Elements
o Potassium dichromate, K2Cr2O7
o Potassium permanganate, KMnO4
The key notes of the chapter are as follows:
d –Block elements and transition metals
The elements lying in the middle of periodic table belonging to groups 3 to 12 are known as d – block elements.
Features of d-block elements:
• The general electronic configuration of d-block elements is (n −1)d1─10 ns1─2, where (n −1) stands for the inner d orbitals.
• In d-block, each horizontal row consists of ten elements as d-subshell can accommodate a maximum of 10 electrons.
• The d-block elements having incompletely filled d-subshell are called transition metals.
• Zinc, cadmium, mercury having the general electronic configuration as (n –1)d10 ns2, are not regarded as transition metals due to completely filled d – orbital.
• There are mainly three series of the transition metals:
• 3d series starts with Sc (Z = 21) and ends with Zn (Z = 30)
• 4d series starts with Y (Z = 39) and ends with Cd (Z = 48)
• 5d series starts with La (Z = 57) and ends with Hg (Z = 80)
General General Properties of transition elements:
Metallic character
Almost all the transition elements display metallic properties such as metallic luster, high tensile strength, ductility, malleability and high thermal and electrical conductivity.
In any row, the melting point of these metals rises to a maximum at d5 and after that as the electrons start pairing up so the melting point decreases regularly as the atomic number increases with an excepyion of Mn and Tc are exception.
Atomic and ionic radii
Due to the addition of new electron to a d-orbital each time the effective nuclear charge increases which causes the atomic radii to decrease in a series of transition elements. However, the atomic size of Fe, Co, Ni is almost the same because the attraction due to increase in nuclear charge is cancelled by the repulsion because of increased in shielding effect. The size of the 4d series elements is almost the same as the size of the 5d series elements. This phenomenon is associated with the intervention of the 4f orbitals which must be filled before the filling starts in 5d subshell.
Lanthanoid contraction
The filling of the 4f before the 5d orbital results in a regular decrease in size called lanthanoid contraction. This compensates for the expected increase in the atomic size with increasing atomic number. The net result of the lanthanoid contraction is is that the 4d and 5d series elements exhibit similar radii and show similarity in their physical and chemical properties.
Melting point
Due to the strong interatomic bonding which involves both (n−1)d and ns electrons participation, transition metals have high melting points.
Ionization enthalpy
In a particular transition series, there is an increase in ionization enthalpy from left to right which is due to the increase in effective nuclear charge along a series. But the trend is not very regular. The exceptions are chromium and copper which have notably larger ionization enthalpy than their neighbours. These exceptions are due to the extra stability associated with the half-filled and fully-filled set of d-orbitals.
Oxidation States
Transition metals show variable oxidation states due to tendency of (n-1)d as well as ns electrons to take part in bond formation.
For example:- Oxidation states of the first row transition metals are:
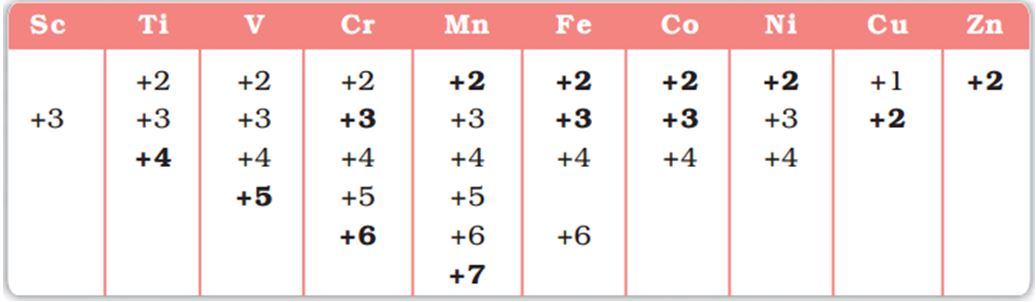
Image Source: NCERT Books
Enthalpy of atomization
The transition elements have high enthalpy of atomization which is due to the presence of strong metallic bonding. The elements with highest enthalpy of atomization tend to be noble metals. The elements of 4d and 5d series have greater enthalpies of atomization than the elements of 3d series. That’s why the elements of 4d and 5d series have more frequent metal-metal bonding in their compounds.
Reactivity
The metals of the 3d series are more reactive than the elements of the 4d or 5d series. All 3d series elements with the exception of Cu are highly reactive and are oxidized by 1 M H+. The tendency to form divalent cation decreases along the 3d series as indicated by their E°(M2+/M) values. The E° value does not follow a regular trend. This is due to irregularity in IE and the heat of atomisation.
Magnetic properties
Most of transition metals are paramagnetic in nature due to the presence of unpaired electrons. It increases from Sc to Mn due to the increased number of unpaired electrons and then starts decreasing as the number of unpaired electrons decreases.
Formation of complexes
The transition metals form a large number of complexes. This is due to their
o Comparatively small sizes of the metal ions.
o High ionic charges.
o Availability of vacant d atomic orbitals
Formation of coloured compounds
Transition metals and their compounds show colour. The colour is due to the excitation of electron from one d atomic orbital to higher energy d atomic orbital in the same subshell. The frequency of the light absorbed generally lies in the visible region. The colour observed is due to the colour of the complementary light. The colour is due to the presence of unpaired electrons. All Zn2+ compounds are white.
Formation of interstitial compounds
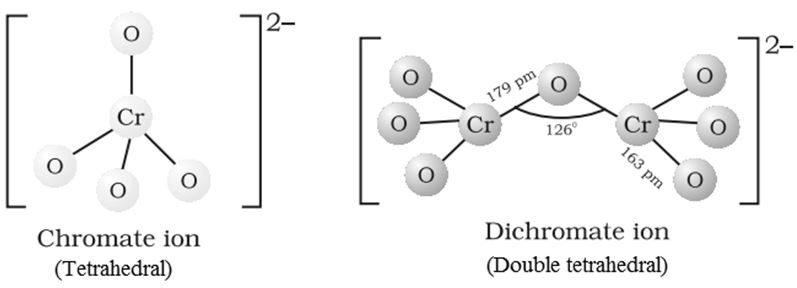
Transition metals have lattice structure in which the unoccupied space is called void or hole or interstices. Transition metals entrap smaller but highly electronegative elements in these interstices and results in the formation of interstitial compounds. Interstitial com-pounds have high melting points, hardness and retain metallic conductivity. The interstitial compounds are chemically inert. Examples are TiC, steel.
Formation of alloys
Alloy is a homogeneous mixture of two or more metals. Due to the comparable size of transition metals, one metal can displace other metal in the crystal lattice and this results in the alloy formation. The alloys so formed are hard and have high melting points. The best known are ferrous alloys; chromium, vanadium, tungsten, manganese are used for the production of variety of steels and stainless steels.
Catalytic properties
Most of transition metals are used as catalysts.
This is due to the
(i) presence of incomplete or empty d-orbitals,
(ii) large surface area and
(iii) variable oxidation state. For example Fe, Ni, V2O3, Pt, Mo, Co, etc., are used as catalyst.
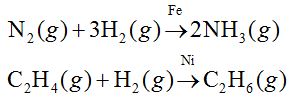
Formation of oxides
Transition metals form oxides on reaction with oxygen at elevated temperature. Transition metal form oxide in oxidation state of + 1 (in Ag2O) to + 7 (Mn2O7) to + 8 (in OsO4). As the oxidation number increases in case of same elements,
(a) The covalent character of oxides increases.
(b) The acidic strength of the oxides increases.
(c) The oxidizing power of oxides increases.
For example, Cr2O3 is amphoteric while CrO is basic and CrO3 is acidic.
Some Important Compounds of Transition Elements
Potassium dichromate, K2Cr2O7
Preparation:
It is prepared by fusion of chromate ore (FeCr2O4) with sodium carbonate in excess of air.
8Na2CO3 + 4FeCr2O4 + 7O2 → 8Na2CrO4 + 2Fe2O3 + 8CO2
Yellow
Na2CrO4 produced in the above reaction is then acidified to get sodium dichromate, Na2Cr2O7
Na2CrO4 produced in the above reaction is then acidified to get sodium dichromate, Na2Cr2O7
2Na2CrO4 + H2SO4 → Na2Cr2O7 + Na2SO4 + H2O
Orange
Solution of sodium dichromate treated with potassium chloride to get the final product, K2Cr2O7.
Na2Cr2O7 + 2KCl → K2Cr2O7 + 2NaCl
Orange Crystals
Structures of CrO42‒ and Cr2O72‒ ions:
Uses:
Potassium dichromate is used as a primary standard in volumetric analysis and as an oxidizing agent. In acidic medium, the oxidation state of Cr changes from + 6 in Cr2O72‒ to + 3 in Cr3+.
Cr2O72‒ + 14 H+ + 6e‒ → 2Cr3+ + 7H2O
Potassium permanganate, KMnO4
Preparation:
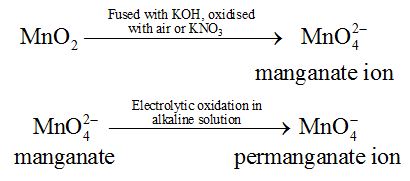
It is prepared by fusion of MnO4 with alkali metal hydroxide (KOH) in presence of O2 or oxidising agent like KNO3. It produces dark green coloured compound , K2MnO4 which undergoes oxidation as well as reduction in neutral or acidic solution to give permanganate.
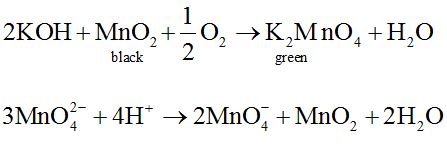
Commercially it is prepared by the alkaline oxidative fusion of MnO2 followed by the electrolytic oxidation of manganate (Vl).
Uses:
Potassium permanganate acts as a strong oxidizing agent in acidic, neutral or faintly basic medium.
In acidic medium:
MnO4‒ + 8H+ + 5e‒ → Mn2+ + 4 H2O
Oxidises Fe2+ to Fe3+:
Fe2+ → Fe3+ + e─
In an alkaline medium:
MnO4‒ + 2H2O + 3e‒ → MnO2 + 4OH‒
Oxidises I− to iodate IO3‒:
6OH‒ + I‒ → IO3‒ + 6e‒ + 3H2O
In Part-I you discovered about the d-block elements and their important compounds. In Part-II, you will get acquainted with the f-block elements. These quick notes are prepared strictly according to the latest CBSE syllabus for Class 12th Chemistry.
The main topics covered in this part are:
- f –Block elements or inner transition elements
- Lanthenoids
- Definition
- General properties
- Uses
- Definition
- General properties
- Actinoids
The key notes of the chapter are as follows:
f –Block elements or Inner Transition elements
The elements in which the differentiating electron enters the penultimate energy level i.e. (n−2)f, are called f-block elements. Due to such electronic configuration where the last electron enters the 4f or 5f orbitals that are lower than the outermost electrons, f-block elements are also named as inner transition elements.
Depending upon the fact whether the last electron enters the 4f or 5f-orbitals, f-block elements are differentiated into lanthanoids and actinoids.
1. Lanthanoids: The 14 elements immediately following lanthanum, i.e., Cerium (58) to Lutetium (71) are called lanthanoids. They belong to first inner transition series. Lanthanum (57) has similar properties.
General properties of lanthanoids:
- Electronic configuration: The general electronic configuration of the lanthanoids is
- Atomic and ionic Sizes: The decrease in in atomic and ionic radii from lanthanum to lutetium is not quite regular but there is a regularity in the size of M3+ ions. The regular decrease in size of M3+ ion is attributed to the imperfect shielding of one electron by another in the same 4f subshell. This regular decrease in size amongst lanthanides as atomic number increases is known as the lanthanoid contraction.
- Formation of coloured ions: Lanthanide form ions which are coloured in both solid state and in aqueous solutions. Colour of these ions may be attributed to the presence of f-electron.
Exception: Lu3+ ions do not show any colour due to the absence of any unpaired electron in the 4f subshell which is fully filled.
- Magnetic character: Lanthanide ions also show paramagnetism. In lanthanides, the magnetic moment is due to both spin magnetic moment as well as orbital magnetic moment.
- Oxidation state: The most common oxidation state of lanthanides is + 3 which is obtained by using two electrons in 6s and one electron from 5d subshell.
Exception: Some elements show +2 and +4 oxidation states. This irregularity arises mainly from the extra stability of empty, half-filled or filled f subshell.
For example:
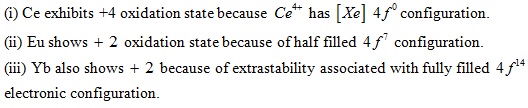
Physical properties of lanthanoids:
- All the lanthanoids are silvery white soft metals and tarnish rapidly in air, the hardness increases with increasing atomic number.
- The melting points range between 1000 to 1200 K but samarium melts at 1623 K.
- They are also good conductors of heat and electricity.
Chemical properties of lanthanoids:
Some important chemical reactions of lanthenoids are:
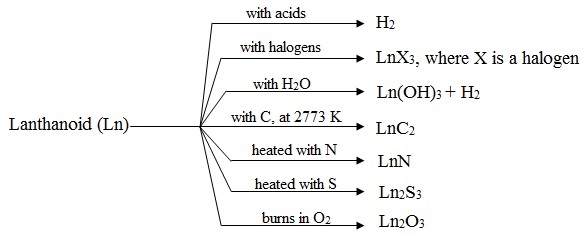
Formation of alloys: Lanthanoids are all used in steel industry for making alloy steels. Important and well-known alloy is misch-metal and it con-sists of lanthanoid (90-95%), iron (4-5%) and trace amount of S, C Ca and Al.
Uses:
(i) Misch-metal is used in making tracer bullets, shell and lighter flint.
(ii) Mixed oxides of lanthanoids are used as a catalyst in petroleum cracking. Some individual oxides of lanthanoids are used as phosphors in television screens and similar fluorescing surface.
2. Actinoids: The 14 elements immediately following actinium (89), with atomic numbers 90 (Thorium) to 103 (Lawrencium) are called actinoids. They belong to second inner transition series. In actinoids the filling of electrons takes place in the anti-penultimate subshell.
General properties of actinoids:
- Electronic configuration: The general electronic configurations for the actinoids is [Rn]5f1−146d0−17s2 , where Rn is the electronic configuration of the element Radium. The fourteen electrons are formally added to though not in Thorium but onwards from it and the 5f subshell is complete at Lr (Z = 103). The irregularities in the electronic con-figuration of the actinoids are releated to the stabilities of of empty, half-filled or filled f subshell.
- Oxidation state: The dominant oxidation state of actinoids is +3. However they also show variable oxidation states due to the comparable energy of 5f, 6d and 7s subshells.For example: The uranium shows oxidation states of and. The element neptunium (Z = 93) show an oxidation state upto +7.
- Magnetic character: Actinoids also show paramagnetism but their magnetic properties are much more complex than those of the lanthanoids.
- Atomic and ionic sizes: In actinides, the ionic radii decreases as we move down the series. This decrease in ionic radius is termed as actinide contraction. This effect is due to poor screening offered by 5f electrons.
- Ionisation enthalpy: The ionisation enthalpies of actinoids are lower than those of corresponding lanthanoids. This is because the orbitals in actinoids penetrate less into the inner core of electrons, and the electrons are more effectively shielded from the nuclear charge than are the electrons of the corresponding lanthanoids. As the outer electrons are less tightly held, less amount of energy is required to ionise an atom.
- Metallic character: The actinoids are all metals with silvery appearance. These metals are highly reactive when finely divided.
Chemical properties:
- They react with boiling water to give a mixture of oxide and hydride.
- All these metals are attacked by HCl but slightly affected by HNO3 due to the formation of a protective oxide layer on their surface.
- They combine with most of the non-metals at moderate temperature.
The d-and f-Block Elements Class 12 Chemistry MCQs
1. In which of the following pairs, both the ions are coloured in aqueous solutions?
(a) Sc3+, Ti
(b) Sc3+, Co2+
(c) Ni2+, Cu+
(d) Ni2+, Ti3+
[Atomic no of Sc = 21, Ti = 22, Ni = 28, Co = 27, Cu = 29]
Answer/Explanation
Answer: d
Explaination:
(d) Ni2+, Ti3+ are coloured due to presence of unpaired electrons.
2. Which of the following is most stable in aqueous solution?
(a) Mn2+
(b) Cr3+
(c) V3+
(d) Ti3+
Answer/Explanation
Answer: b
Explaination:
(b) Cr3+
∵ t2g3+ (half filled p-orbitals) are more stable.
3. The number of moles of KMnO. that will be needed to react with one mole of SO32- in acidic solution.
(a) 1
(b) 3/5
(c) 4/5
(d) 2/5
Answer/Explanation
Answer: d
Explaination:
(d) 2 MnO4– + 5SO32 + 16H+ → 2Mn2+ + 5SO42+ + 8H20
5 moles of SO32- needs 2 moles of KMnO4
1 mole of SO32- needs 2/5 moles of KMnO4
4. The correct order of decreasing second ionisation enthalpy of Ti(22), V(23), Cr(24) Mn(25)
(a) V > Mn > Cr > Ti
(b) Mn > Cr > Ti > V
(c) Ti > V > Cr > Mn
(d) Cr > Mn > V > Ti
Answer/Explanation
Answer: d
Explaination:
(d) ∵ Cr+ (4s°3d5), Mn+ 4s13d5, V1(4s+3d3), Ti+4s13d2
5. Which of the following pairs has the same ionic size?
(a) Zr4+, Hf4+
(b) Zn2+, Hf4+
(c) Fe2+, Ni2+
(d) Zr4+, Ti4+
Answer/Explanation
Answer: a
Explaination:
(a) Zr4+, Hf have similar size due to lanthanoid contraction.
6. Acidified K2Cr207 solution turns green when S02 gas is passed through it due to formation of
(a) Cr2(SO4)3
(b) CrO42-
(c) Cr2(SO3)3
(d) CrSO4
Answer/Explanation
Answer: a
Explaination: (a) It is due to formation of chromium sulphate.
7. The stability of Mn2+, Fe2+, Cr2+, Co2+ is in order of (At No. of Mn = 25, Fe = 26, Cr = 24, Co = 27)
(a) Mn2+ > Fe2+ > Cr2+ > Co2+
(b) Fe2+ > Mn2+ > Co2+ > Cr2+
(c) Co2+ > Mn2+ > Fe2+ > Cr2+
(d) Cr2+ > Mn2+ > Co2+ > Fe2+
Answer/Explanation
Answer: a
Explaination:
(a) Mn2+ (3d5) is most stable, Fe2+ (3d6), Cr2+(3d4 Co2+(3d1)
8. Which of the following does not give 02 on heating?
(a) K2Cr2O7
(b) (NH4)2 Cr2O7
(c) KClO3
(d) Zn(ClO3)2
Answer/Explanation
Answer:
Explaination:![]()
9. Which of the following lanthanoid ion is diamagnetic? (At No. of Ce = 58, Sm = 62, Eu = 63 Yb = 70)
(a) Eu2+
(b) Yb2+
(c) Ce2+
(d) Sm2+
Answer/Explanation
Answer: b
Explaination:
(b) Yb2+ (4f14) does not have unpaired election, therefore, diamagnetic.
10. The reaction of acidified KMnO. and FLO, gives
(a) Mn4+ and O2
(b) Mn2+ and O2
(c) Mn2+ and O3
(d) Mn4+ and MnO2
Answer/Explanation
Answer: b
Explaination:
(b) 2MnO4 + 6H4 + 5H2O2 → 2Mn2+ + 8H2O + 5O2
11. Magnetic moment of 2.83 BM is given by which of the following ion?
(a) Ti3+
(b) Ni2+
(c) Cr3+
(d) Mn2+
Answer/Explanation
Answer: b
Explaination:
(b) Ni2+ has 2 unpaired electrons.![]()
12. The colour of KmnO, is due to
(a) L → M charge transfer transition
(b) a → σ* transition
(c) M → L charge transfer transition
(d) d → d transition.
Answer/Explanation
Answer:
Explaination:
(a) It is due to L → M charge transfer transition by absorbing light from visible region and radiates purple colour.
13. KMnO4 is not acidified by HCl instead of H2SO4 because
(a) H2 SO4 is stronger acid than HCl
(b) HCl is oxidised to Cl2 by KMnO4
(c) H2SO4 is dibasic acid
(d) rate is faster in presence of H2SO4
Answer/Explanation
Answer: b
Explaination:
(b) 2KMnO4 + 16 HCl → 2KCl + 2MnCl2 + 5Cl2 + 2H2O
14. Out of Mn2O7 V2O3, V2O5, CrO, Cr2O3, the basic oxides are
(a) Mn2O7, V2O3
(b) V2O3, V2O5
(c) V2O5, CrO
(d) V2O3 and CrO
Answer/Explanation
Answer: d
Explaination:
(d) V2O3 and CrO are basic oxides due to lower, oxidation states.
15. The oxidation state of Cr in final product formed by reaction of KI and acidified dichromate solution is
(a) +4
(b) +6
(c) +2
(d) +3
Answer/Explanation
Answer: d
Explaination:
(d) Cr3+ is formed.
16. KMnO4 gets reduced to
(a) K2MnO4 in neutral medium
(b) MnO2 in acidic medium
(c) Mn2+ in alkaline medium
(d) MnO2 in neutral medium
Answer/Explanation
Answer:
Explaination:![]()
17. The electronic configuration of Cu(II) is 3d9 whereas that of Cu(I) is 3d10. Which of the following is correct? [NCERT Exemplar]
(a) Cu(II) is more stable
(b) Cu(II) is less stable
(c) Cu(I) and Cu(II) are equally stable
(d) Stability of Cu(I) and Cu(II) depends on nature of copper salts
Answer/Explanation
Answer: a
Explaination:
(a) Cu(II) is more stable due to higher hydration energy.
18. Metallic radii of some transition elements are given below. Which of these elements will have highest density? [NCERT Exemplar]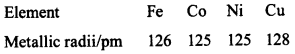
(a) Fe
(b) Ni
(c) Co
(d) Cu
Answer/Explanation
Answer: d
Explaination:
(d) Cu has highest density due to greater atomic mass.
19. Generally transition elements form coloured salts due to the presence of unpaired electrons. Which of the following compounds will be coloured in solid state?
(NCERT Exemplar]
(a) Ag2SO4
(b) CuF2
(c) ZnF2
(d) Cu2Cl2
Answer/Explanation
Answer: b
Explaination:
(b) CUF2 is coloured due to presence ofunpaired electron in d-orbital.
20. On addition of small amount of KMnO4 to concentrated H2SO4, a green oily compound is obtained which is highly explosive in nature. Identify the compound from the following. [NCERT Exemplar]
(a) Mn2O7
(b) MnO2
(c) MnSO4
(c) Mn2O3
Answer/Explanation
Answer: a
Explaination:
(a) It is due to formation of Mn2O7.
21. Which of the following reactions are dispro-portionation reactions? [NCERT Exemplar]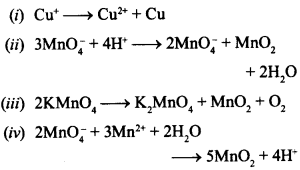
(a) (i), (ii)
(b) (i), (ii), (iii)
(c) (ii), (iii)
(iv) (d) (i), (iv)
Answer/Explanation
Answer: b
Explaination:
(b) (i), (ii), (iii) are disproportionation because same substance is oxidised as well as reduced.
22. When KMnO. solution is added to oxalic acid solution, the decolourisation is slow in the beginning but becomes instantaneous after some time because [NCERT Exemplar]
(a) CO2 is formed as the product.
(b) Reaction is exothermic.
(c) MnO4– catalyses the reaction.
(d) Mn2+ acts as autocatalyst.
Answer/Explanation
Answer: d
Explaination:
(d) Mn2+ acts as autocatalyst.
23. Anomalous electronic configuration in the 3d series are of
(a) Cr and Fe
(b) Cu and Zn
(c) Fe and Cu
(d) Cr and Cu
Answer
Answer: d
24. Which of the following are d-block elements but not regarded as transistion elements?
(a) Cu, Ag, Au
(b) Zn, Cd, Hg
(c) Fe, Co, Ni
(d) Ru, Rh, Pd
Answer
Answer: b
25. CuSO4. 5H2O is blue is colour because
(a) It contains water of crystallization.
(b) SO42- ions absorb red light.
(c) Cu2+ ions absorb orange red light.
(d) Cu2+ ions absorb all colours except red from the white light.
Answer
Answer: c
26. Transistion elements form alloys easily because they have
(a) Same atomic number
(b) Same electronic configuration
(c) Nearly same atomic size
(d) None of the above
Answer
Answer: c
27. Which one of the following characteristics of the transistion metals is associated with higher catalytic activity?
(a) High enthalpy of atomisation
(b) Paramagnetic behaviour
(c) Colour of hydrate ions
(d) Variable oxidation states
Answer
Answer: d
28. Which of the following has the maximum number of unpaired electrons?
(a) Mg2+
(b) Ti3+
(c) V3+
(d) Fe2+
Answer
Answer: d
29. The property which is not characteristic of transistion metals is
(a) variable oxidation states.
(b) tendency to form complexes.
(c) formation of coloured compounds.
(d) natural radioactivity.
Answer
Answer: d
30. Which of the following is incorrect for KMnO4 to be used as an oxidising agent?
(a) HCl cannot be used because some KMnO4 is consumed in the reaction.
(b) Nitric acid is not used for the above purpose because it itself acts as a self oxidising agent and will react with the reducing agent.
(c) The equivalent weight of KMnO4 in basic medium is 158.
(d) The number of electrons involved in oxidation of KMnO4 in acidic medium is 3.
Answer
Answer: d
31. Transistion metals, despite high E° oxidation, are poor reducing agents. The incorrect reason is
(a) high heat of vaporization.
(b) high ionization energies.
(c) low heats of hydration.
(d) complex forming nature.
Answer
Answer: d
32. Which of the following has magnetic moment value of 5.9?
(a) Fe2+
(b) Fe3+
(c) Ni2+
(d) Cu2+
Answer
Answer: b
Note: In the following questions two or more options may be correct. (Q.23 to Q.26)
33. In the form of dichromate, Cr (VI) is a strong oxidising agent in acidic medium but Mo (VI) in Mo03 and W (VI) in W03 are not because . [NCERT Exemplar]
(a) Cr (VI) is more stable than Mo(VI) and W(VI).
(b) Mo( VI) and W( VI) are more stable than Cr(VI).
(c) Higher oxidation states of heavier members of group-6 of transition series are more stable.
(d) Lower oxidation states of heavier *> members of group-6 of transition series are more stable.
Answer/Explanation
Answer:
Explaination:
(b) and (c) higher oxidation states are more stable.
34. Which of the following actinoids show oxidation states upto +7? [NCERT Exemplar]
(a) Am
(b) Pu
(c) U
(d) Np
Answer/Explanation
Answer:
Explaination:
(b) and (d) Pu and Np show oxidation state upto +7.
35. General electronic configuration of actionoids is (n – 2)f1 – 14 (n – 1)d0 – 2 ns².Which of the following actinoids have one electron in 6d orbital? [NCERT Exemplar]
(a) U (Atomic no. 92)
(b) Np (Atomic no. 93)
(c) Pu (Atomic no. 94)
(d) Am (Atomic no. 95)
Answer/Explanation
Answer:
Explaination:
(a) and (b) U and Np, U (5f36d17s2), Np (5f46d17S2)
36. Which of the following lanthanoids show +2 oxidation state besides the characteristic oxidation state +3 of lanthanoids? [NCERT Exemplar]
(a) Ce
(b) Eu
(c) Yb
(d) Ho.
Answer/Explanation
Answer:
Explaination:
(b) and (c) Eu2+ (4f7) and Yb2+ (4f14) are more stable.
37. Match the catalysts given in Column I with the processes given in Column II. [NCERT Exemplar]
Answer/Explanation
Answer:
Explaination:
(a) (iii)
(b) (iv)
(c) (ii)
(d) (v)
(e) (i)
38. Match the compounds/elements given in Column I with uses given in Column II.
Answer/Explanation
Answer:
Explaination:
(a) (ii)
(b) (i)
(c) (iv)
(d) (v)
(e) (iii)
39. Match the properties given in Column I with the metals given in Column II. [NCERT Exemplar]
Answer/Explanation
Answer:
Explaination:
(a) (iii)
(b) (i)
(c) (ii) Cr due to maximum number of unpaired electrons.
40. Match the statements given in Column I with the oxidation states given in Column II. [NCERT Exemplar]
Answer/Explanation
Answer:
Explaination:
(a) (iii)
(b) (i)
(c) (v)
(d) (ii)
41. In the following questions a statement of assertion followed by a statement of reason is given. Choose the correct answer out of the following choices.bvg
(a) Both assertion and reason are true, and reason is the correct explanation of the assertion.
(b) Both assertion and reason are true but reason is not the correct explanation of assertion.
(c) Assertion is not true but reason is true.
(d) Both assertion and reason are false. Assertion: Cu2+ iodide is not known.
Reason: Cu2+ oxidises I- to iodine.
Answer/Explanation
Answer: a
Explaination:
(a) Both assertion and reason are true, and reason is the correct explanation of the assertion.
Cu2+ 2I– → 2Cu+ +I2
42.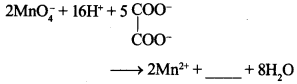
Answer/Explanation
Answer:
Explaination: 10CO2
43. Cr2O72- + 14H+ + 6Isup>- → 2Cr3++ ____ +7H2O
Answer/Explanation
Answer:
Explaination: 3I2
44. Cr2O72-+ 14H+ + 6Fe2+ → 2Cr3+ + ______ + 7H2O
Answer/Explanation
Answer:
Explaination: 6Fe3+
45. Cu2+ is reduced by CN– to Cu+ which forms the complex [Cu(CN)4]3-. [True/False]
Answer/Explanation
Answer:
Explaination: True.
46. The number of moles of Mohr’s salt required per mole of dichromate ion are 6. [True/False]
Answer/Explanation
Answer:
Explaination: True.
47. The colour of light absorbed by an aqueous solution of CuSO4 is orange red. [True/False]
Answer/Explanation
Answer:
Explaination: True.
48. There is hardly any increase in atomic size with increasing atomic numbers in a series of transition metals. Give reason. [AI2012]
Answer/Explanation
Answer:
Explaination:
It is because effective nuclear charge does not increase appreciably as pairing of electrons in d-orbitals take place which causes repulsion after Mn(25).
49. On what ground can you say that scandium (Z = 21) is a transition element but zinc (Z = 30) is not? [NCERT Example]
Answer/Explanation
Answer:
Explaination:
It is because Sc (21) has incompletely filled rf-orbital, that is why it is transition element, whereas Zn(30) does not have incompletely filled rf-orbitals, therefore, it is not regarded as transition element.
50. What is the lattice structure of Tc?
Answer/Explanation
Answer:
Explaination: hep.
51. Density of rf-block elements is quite high. Why?
Answer/Explanation
Answer:
Explaination:
This is due to the fact that their small atomic volume high nuclear charge and mass.
52. Ni(II) compounds are thermodynamically more stable than Pt(II) compounds. Why?
Answer/Explanation
Answer:
Explaination:
This is because that sum of ionisation energies (E1 + E2) is less in case of Ni than Pt.
53. Why do transition metals show variable oxidation states? [Delhi 2016,14(C)]
Answer/Explanation
Answer:
Explaination:
It is because electrons from both ‘s’ and d-orbitals can take part in bond formation.
54. Why the value of standard electrode potentials (E°) for Ni is more negative?
Answer/Explanation
Answer:
Explaination:
This is due to its highest value of hydration enthalpy (∆hydH°).
55. Which type of magnetic behaviour is generally shown by transition elements?
Answer/Explanation
Answer:
Explaination: Paramagnetism.
56. What is the colour of Mn2+ ions in aqueous solution?
Answer/Explanation
Answer:
Explaination: Pink.
57. Name two complex compounds formed by transition metals.
Answer/Explanation
Answer:
Explaination:
[FeCCN6)]4- and [Cu(NH3)4]2+
58. Name a catalyst used in contact process.
Answer/Explanation
Answer:
Explaination: Vanadium (V) oxide (V2O5).
59. Write any two interstitial compounds.
Answer/Explanation
Answer:
Explaination: Fe3H, TiC.
60. Give two physical properties of alloy formed by transition metals.
Answer/Explanation
Answer:
Explaination:
(i) They are usually hard.
(ii) They have often high melting points.
61. What is the geometry of chromate ion?
Answer/Explanation
Answer:
Explaination: Tetrahedral.
62. How will you convert Fe3+ ion (yellow) from Fe2+ ion (green)? Write chemical reaction equation.
Answer/Explanation
Answer:
Explaination:![]()
63. Among lanthanoids, Ln(III) compounds are predominant. However, occasionally in solutions or in solid compounds, +2 and +4 ions are also obtained. Give reason. [AI 2012]
Answer/Explanation
Answer:
Explaination:
Lanthanoids show +3 oxidation state mostly as 2 electrons from outer 6s orbital and one electron from 5 d orbital take part in bond formation. Some show +2 and +4 oxidation states due to stability of half-filled and completely filled 4f orbitals.
64. Lanthanoids form primarily +3 ions, while the actinoids usually have higher oxidation states in their compounds, +4 or even +6 being typical. Give reason. [Delhi 2012]
Answer/Explanation
Answer:
Explaination:
In Actinoids, 5f, 6d and 7s orbitals have comparable energies and electrons from these orbitals can take part to show higher oxidation states.
65. Name an element oflanthanoid series which is well known to shown +4 oxidation state. Is it a strong oxidising agent or reducing agent? [Chennai 2019]
Answer/Explanation
Answer:
Explaination: Ce4+, it is good oxidising agent.